Short abstract
Background
The biconcave (B2) glenoid is characterized by preservation of the anterior portion of the native glenoid with asymmetric wear of the posterior glenoid. Surgical options for glenoid correction have evolved. The goal of shoulder arthroplasty is to place the implants in such a manner to return the humeral head to a centered position and restore the joint line to a neutral position. There is no current consensus on method of treatment and correction.
Methods
The current and historical literature on total shoulder arthroplasty was used to examine technique viability.
Results
Asymmetric remaining can be used to address up to 15° of version correction without compromise of cortical bone. It is important to have the proper presurgical planning, to understand the limitations of correction, and to have other options available to treat the biconcave glenoid.
Keywords: biconcave (B2) glenoid, total shoulder arthroplasty, humeral head, component loosening, preoperative planning
Introduction
In 1999, Walch classified the changes in morphology of the glenoid during glenohumeral osteoarthritis. 1 The biconcave (B2) glenoid is characterized by preservation of the anterior portion of the native glenoid with asymmetric wear of the posterior glenoid. The development of such deformity is related to many factors, including a significant increase in native retroversion of the glenoid. 2 The percentage of the affected glenoid can be highly variable. In addition, there can be a range of depth of the posterior concavity and erosion. The humeral head translates posteriorly into the defect and progressively subluxates. When the humeral head is subluxated posteriorly, patients are likely to have lower final outcome scores, more pain, and decreased active external rotation following either total shoulder arthroplasty (TSA) or hemiarthroplasty. 3
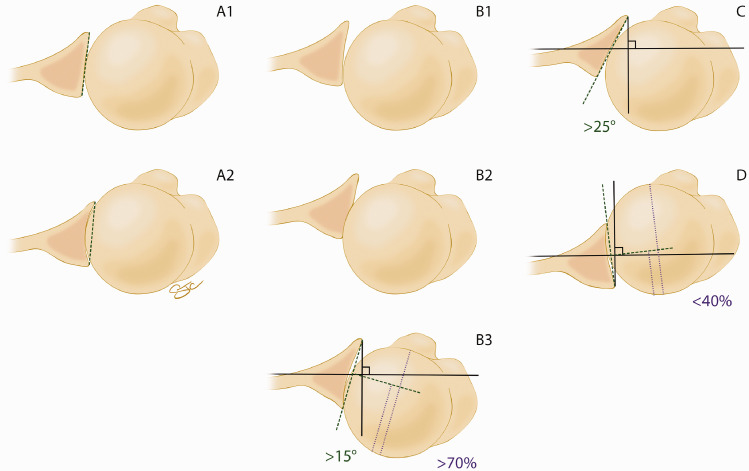
The Walch classification of glenoid morphology has aided providers in differentiating posterior erosion versus dysplasia. 1 It subsequently has aided the decision-making process in terms of treatment of osteoarthritis of the glenohumeral joint based upon its morphology. However, it has also had varying reported rates of intraobserver and interobserver reliability since its conception in 1999.4–6 Because of these varying results, Bercik et al. 7 developed the modified Walch classification (Figure 1) that has most recently been shown to have a substantial intraobserver reliability (cohen κ coefficient of .77) and a moderate interobserver reliability (cohen κ coefficient of .55). 8
Figure 1.
Artist rendering of the Modified Walch Classification based on the original work of Walch and subsequent work or Berick et al.1,7 Reproduced with permission from The Curators of the University of Missouri (copyright 2019 by The Curators of the University of Missouri).
In general, arthroplasty in the setting of the B2 glenoid has been associated with higher rates of glenoid component loosening and premature failure. A systematic review addressing B2 glenoids showed 42% of patients having some loosening of the glenoid component at mean follow-up of 55 months. 9 Correction of significant retroversion is required to avoid glenoid component malposition with subsequent early loosening and failure. 10 Addressing the B2 glenoid requires careful preoperative planning.
The initial deformity assessment is typically performed with plain radiographs. The axillary view is used to assess humeral head position and glenoid morphology (Figure 2). Further imaging may be required when any degree of humeral subluxation or glenoid asymmetry are present. Surgeons tend to overestimate glenoid retroversion with plain radiographs; therefore, 2-dimensional (2D) computed tomography (CT) scans can be helpful in assessing glenoid shape and preoperative planning (Figure 3). 11 Magnetic resonance imaging (MRI) has also been comparable to CT for measuring version12,13 and can be used on the occasion the patient presents already having an MRI (Figure 4).
Figure 2.
Axillary x-ray of B2 glenoid secondary to previous instability surgery and excessive anterior tightening.
Figure 3.
A, Corresponding computed tomography showing biconcavity with retroversion. B, Planning drawn with Friedman’s line (red) and intermediate glenoid line and corresponding paleo glenoid angles in yellow.
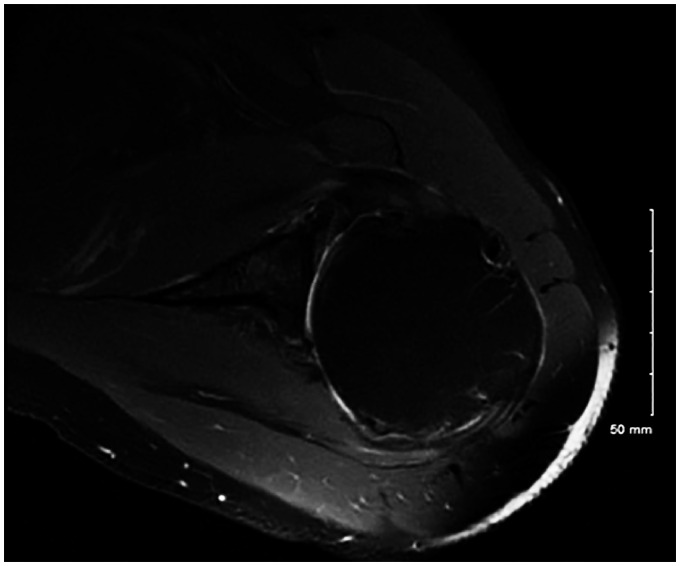
Figure 4.
T2 axial magnetic resonance imaging from another patient showing biconcavity and subluxation of the humeral head. There is mild bony deformity and existing anterior glenoid cartilage.
Advancements in 3-dimensional (3D) imaging have also allowed for improved surgical planning and simulation of glenoid placement. Moreover, 3D reconstruction is valuable for surgeons to accurately determine the location of glenoid bone loss and improve surgical decision-making. 14 The addition of surgical planning with 3D imaging further improves glenoid correction to within 10° of desired version. 15 Many commercially available systems are in use for CT 3D planning (Figure 5).
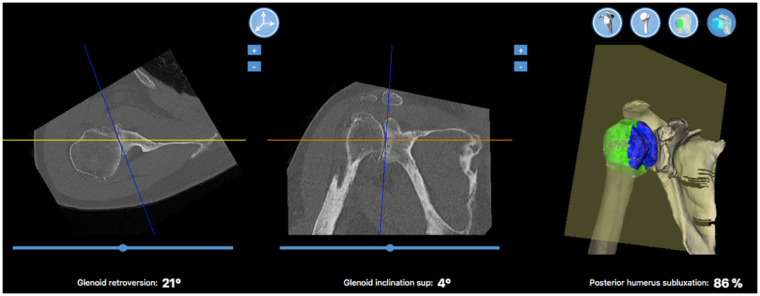
Figure 5.
Surgical planning software with 3D rendering (Wright Medical Group, Blueprint™). From left to right are calculated values for glenoid retroversion, glenoid inclination, and posterior humeral head subluxation.
Surgical options for glenoid correction have evolved. The goal of shoulder arthroplasty is to place the implants in such a manner to return the humeral head to a centered position and restore the joint line to a neutral position. There is no current consensus on method of treatment and correction. For completeness, the following options are briefly discussed: hemiarthroplasty with corrective reaming (ream and run), posterior bone grafting, implant augmentation, and reverse TSA. The remainder of this article will focus on eccentric reaming with placement of anatomic shoulder arthroplasty.
Ream and Run
Humeral hemiarthroplasty with concentric glenoid reaming is an additional technique that can be used to address the B2 glenoid. This technique is also referred to as the “ream-and-run” technique. It involves concentrically reaming the glenoid to remove biconcavity, but does not address the glenoid version. This avoids excessive glenoid bone loss and also gives the humeral head a surface with a single concavity for joint contact. 16 Recent studies have shown that this technique is capable of improving pain and function in the short term for primary glenohumeral arthritis17,18 and has also shown promising results in the management of the B2 glenoid. 19 The arthroplasty should be anatomic and not oversize the joint to prevent asymmetric loading. There remains concern that painful glenoid wear continues in the face of hemiarthroplasty. 20
Bone Grafting
Bone grafting is a useful technique for addressing excessive glenoid retroversion that is beyond 15°. This method of correction is also technically demanding. It involves creating a uniform step with fixation of autograft onto the glenoid face to correct excessive glenoid retroversion prior to fixation of the glenoid component. This technique relies on graft incorporation in addition to the glenoid component fixation. 21 Therefore, several studies have shown that this technique has failures secondary to failure of graft fixation. 22 Hill and Norris 23 showed 5 failures out of a cohort of 21 patients treated with bone grafting at the time of TSA, and only one of these failures was secondary to graft fixation failure. They did show a loss of correction in 3 patients secondary to graft nonunion or dissolution. Some favorable results have been shown despite radiographic evidence of incomplete graft incorporation or glenoid component failure. 24
Augmented Glenoid Components
The advent of augmented glenoid components avoids many of the drawbacks of the bone grafting techniques. The augmented glenoid does not have to rely on bone graft incorporation. Wedge-augmented glenoid components are designed for addressing a B2 glenoid component in which the neoglenoid involves >50% of the glenoid face, while step-augmented glenoid components are useful in addressing B2 glenoids with a neoglenoid that involves <50% of the glenoid face. These methods likely allow for greater preservation of glenoid bone stock. 21 Although long-term follow-up data are not available for these components, there is promising short-term data showing the ability to correct version with less joint line medialization compared to standard TSA with the use of an all polyethylene stepped glenoid component. 25 Augmented glenoid designs show promising results with improved clinical function and pain in several short-term studies with some components showing radiolucencies of no clinical consequence.24–26
Reverse TSA
Reverse TSA is also an option for the management of the B2 glenoid, especially in the elderly, low-demand patient. The reverse TSA can be used in patients with excessive retroversion of the glenoid, and it is also useful for patients in which an augmented glenoid component that may result in peg perforation. Mizuno et al. have demonstrated favorable outcomes in the treatment of the biconcave glenoid with reverse TSA in patients with an intact rotator cuff. 26
However, a recent review from the German registry (SEPR) showed a higher revision rate with reverse arthroplasty compared to anatomic arthroplasty (21% vs 12%) with higher instability rates among reverse arthroplasty for B2 glenoids. The anatomic shoulders did show an 11% glenoid loosening rate compared to 3% loosening of the reverse arthropalsties. 27
Eccentric Reaming and Anatomic TSA
The historic difficulty with B2 glenoids involved restoring a neutral joint line to recenter the humeral head and avoid glenoid component loosening. If retroversion is not corrected, osteolysis around the central peg is more likely to occur. 28 Each of the options previously discussed have limitations in treatment and may, in some cases, dramatically increase the cost of implants over traditional anatomic implants. Asymmetric reaming is generally considered the least difficult method of correction from a technical standpoint; however, using this method, standard total shoulder implants have been shown to have a high complication rate. Premature glenoid loosening and posterior instability are well documented. One study even found a 16% revision rate at a mean interval of 96 months. 29
Obtaining a CT scan may allow for preoperative planning and the use of patient-specific instrumentation. Traditional methods to correct moderate to severe glenoid retroversion in order to place the glenoid component are not consistent. Retroversion greater or equal to 20° makes it difficult to place a pegged glenoid component perpendicular to the plane of the scapula by reaming alone without risking peg perforation. 3 A study by Hendel et al. in 2012 found more accurate reaming (less overreaming), more appropriate version correction, and lower incidence of peg perforation when patient-specific instrumentation was used. 30
Frequently, the anterior (paleo) glenoid portion has unworn cartilage, and simply removing cartilage may correct the version to some degree and reveal a minimal amount of bony reaming prior to final implant placement. When 50% or more of the native glenoid is intact, simple pin guides and corrective reaming are very reliable. More wear and deformity secondary to osteophyte formation make pin placement more difficult. 21
In addition, there is likely a limit to the amount of correction that can be obtained by reaming the anterior, high side. Studies have suggested that reaming can make up for up to 8 mm of bone loss of the posterior glenoid and version corrected up to about 15°. 31 Reaming beyond that may compromise cortical bone. A recent study showed virtual version correction as small as 10° reduced the density of bone available for glenoid fixation. Increased reaming resulted in gradual bone loss from the anterior glenoid 32 or medical subsidence with loss of bony support. 33 Other studies have shown that medial reaming likely decreases the anteroposterior dimension of the glenoid, further decreasing support of a polyethylene glenoid component. 34
Further concerns over reaming the glenoid include the possibility of excessive medialization of the joint line, therefore decreasing rotator cuff efficiency and increasing the likelihood of peg perforations. To avoid such complications, incomplete version correction may be required. Research has shown correction within 6° of retroversion (rather than 0) may be beneficial 35 and falls within the 10° of remaining retroversion that is favored biomechanically. 36
Asymmetric reaming for placement of an anatomic component continues to be a viable and common method for treating the B2 glenoid. When glenoid retroversion is greater than 15°, there may actually be an advantage to reaming. A biomechanical study on loosening demonstrated micromotion of polyethylene glenoids placed with uncorrected reaming at 2000 cycles, while posterior augmented specimens showed micromotion at 10 000 cycles. Both showed significantly more micromotion than glenoids implanted with corrective reaming for the same number of cycles. 37
It must be emphasized that preparation with reaming, especially in the face of asymmetry, must result in a perfectly congruent surface. Remaining biconcavity prevents complete component seating, and inadequate bone preparation leads to a lack of support. Edge loading of a prosthetic component that does not have full backside support leads to warping of the implant as well as increased micromotion. Lucent lines and early glenoid component failure are directly related to initial seating. 34
A recent clinical study on anatomic TSA described treatment of an average 18° of preoperative retroversion and 67% humeral head posterior subluxation yielded good clinical outcomes. Fifty-nine patients reported improved American Shoulder and Elbow Surgeon (ASES) and Simple Shoulder Test scores with no overall difference in progression of radiolucencies. Using a threshold of 20° of preoperative retroversion, there was no difference in radiographic lucency <20° versus >20° and no revisions secondary to loosening or instability at a mean of 50-month follow-up. 38
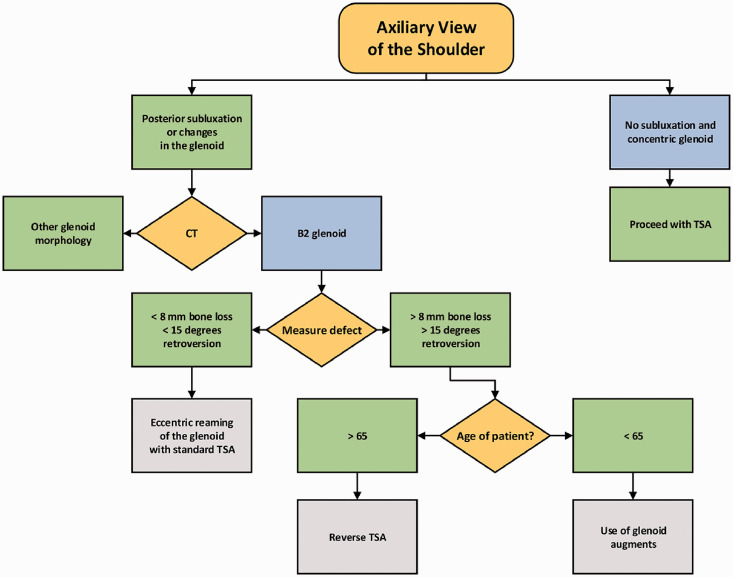
It is our current practice to obtain standard axillary radiographs on all patients with glenohumeral osteoarthritis. With any glenoid deformity or humeral subluxation, dedicated shoulder CT with 1 mm cuts and 3D reconstructions are obtained. We also regularly use planning, including commercially available software, to accurately assess depth of the posterior (neo) glenoid. If less than 8 mm of the anterior (paleo) glenoid remains, and retroversion is 15° or less, we rely on asymmetric reaming of the high side of the glenoid and standard component placement. Figure 6 shows the post-operative x-rays on the same patient shown in Figures 2 and 3. We have also found that position of the humeral head serves an important part in planning, and we prefer not to aggressively correct remaining retroversion when the humeral head remains centered. If the shoulder CT shows greater than 8 mm difference between the posterior (neo) glenoid and anterior (paleo) glenoid along with a need for correction of greater than 15° of retroversion, we then turn our attention to the age of the patient. Patients with an age greater than 65 years are considered for reverse TSA. Patients with an age less than 65 years are considered for TSA with use of augmented glenoid techniques, either bone grafting or augmented glenoid components. A summary of this treatment algorithm is demonstrated in Figure 7. Using this algorithm, we identified 29 patients with at least 1 year of clinical and radiographic follow-up who had B2 glenoids and retroversion of at least 10° corrected with asymmetric reaming and standard TSA. Preoperative ASES and visual analog scale scores averaged 39 and 7.1. At final follow-up, scores averaged 76.7 and 1.1, respectively. Average forward elevation improved from 91.2° to 148.7°.
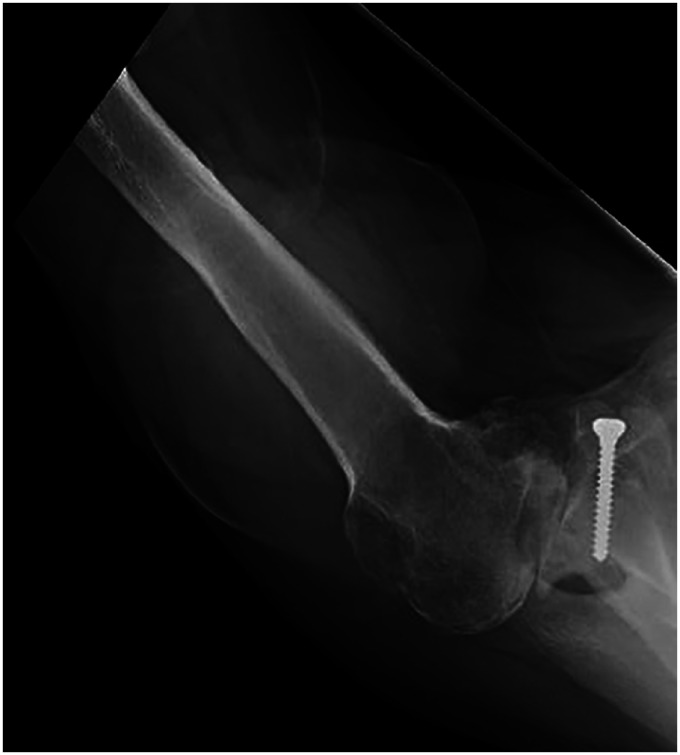
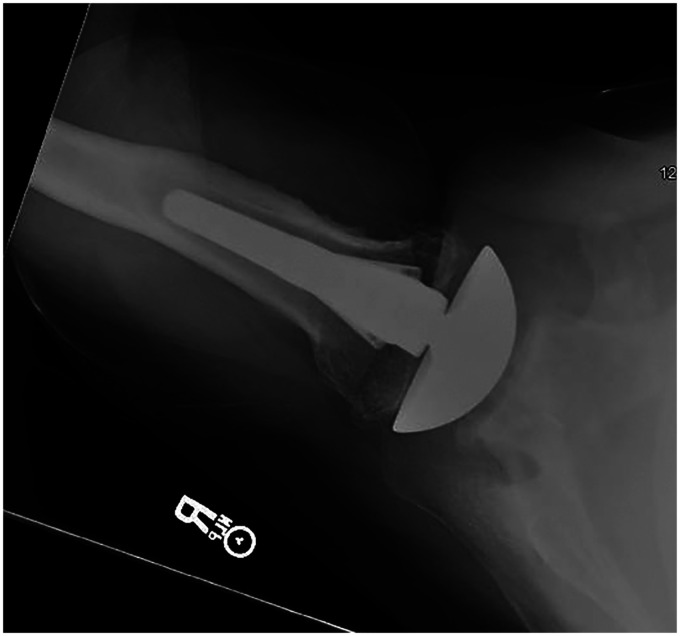
Figure 6.
Two-year postoperative image with screw removal and total shoulder with centered humeral head.
Figure 7.
Treatment algorithm used by the authors in the treatment of the B2 glenoid. CT, computed tomography; TSA, total shoulder arthroplasty.
Conclusion
Asymmetric remaining can be reliable in the appropriate clinical setting. It is important to have the proper presurgical planning, to understand the limitations of correction, and to have other options available to treat the biconcave glenoid.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C. M. Loftis and N. W. Skelley report no outside conflicts of interest. M. J. Smith reports the following: Arthrex, Inc: paid presenter or speaker; research support; Ignite Orthopedics: stock or stock options; Tornier: research support; Zimmer: paid consultant.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Statement of Ethics
Our institution did not require board approval to conduct this review.
Statement of Patient Consent
Patient consent was not required to conduct this review.
References
- 1.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999; 14:756–760. [DOI] [PubMed] [Google Scholar]
- 2.Knowles NK, Ferreira LM, Athwal GS. Premorbid retroversion is significantly greater in type B2 glenoids. J Shoulder Elbow Surg. 2016; 25:1064–1068. [DOI] [PubMed] [Google Scholar]
- 3.Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012; 21:48–55. [DOI] [PubMed] [Google Scholar]
- 4.Aronowitz JG, Harmsen WS, Schleck CD, Sperling JW, Cofield RH, Sánchez-Sotelo J. Radiographs and computed tomography scans show similar observer agreement when classifying glenoid morphology in glenohumeral arthritis. J Shoulder Elbow Surg. 2017; 26:1533–538. [DOI] [PubMed] [Google Scholar]
- 5.Nowak DD, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Interobserver and intraobserver reliability of the Walch classification in primary glenohumeral arthritis. J Shoulder Elbow Surg. 2010; 19:180–183. [DOI] [PubMed] [Google Scholar]
- 6.Scalise JJ, Codsi MJ, Brems JJ, Iannotti JP. Inter-rater reliability of an arthritic glenoid morphology classification system. J Shoulder Elbow Surg. 2008; 17:575–577. [DOI] [PubMed] [Google Scholar]
- 7.Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016; 25:1601–1606. [DOI] [PubMed] [Google Scholar]
- 8.Shukla DR, McLaughlin RJ, Lee J, Cofield RH, Sperling JW, Sánchez-Sotelo J. Intraobserver and interobserver reliability of the modified Walch classification using radiographs and computed tomography. J Shoulder Elbow Surg. 2019; 28:625–630. [DOI] [PubMed] [Google Scholar]
- 9.Luedke C, Kissenberth MJ, Tolan SJ, Hawkins RJ, Tokish JM. Outcomes of anatomic total shoulder arthroplasty with b2 glenoids: a systematic review. JBJS Rev. 2018; 6:e7. [DOI] [PubMed] [Google Scholar]
- 10.Hendel MD, Werner BC, Camp CL, et al. Management of the biconcave (B2) glenoid in shoulder arthroplasty: technical considerations. Am J Orthop. 2016; 45:220–227. [PubMed] [Google Scholar]
- 11.Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003; 12:493–496. [DOI] [PubMed] [Google Scholar]
- 12.Aygün Ü, Duran T, Oktay O, Sahin H, Calik Y. Comparison of magnetic resonance imaging and computed tomography scans of the glenoid version in anterior dislocation of the shoulder. Orthopedics. 2017; 40:e687–e692. [DOI] [PubMed] [Google Scholar]
- 13.Lowe JT, Testa EJ, Li X, Miller S, DeAngelis JP, Jawa A. Magnetic resonance imaging is comparable to computed tomography for determination of glenoid version but does not accurately distinguish between Walch B2 and C classifications. J Shoulder Elbow Surg. 2017; 26:669–673. [DOI] [PubMed] [Google Scholar]
- 14.Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008; 90:2438–2445. [DOI] [PubMed] [Google Scholar]
- 15.Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg Am. 2015; 97:651–658. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013; 22:1298–1308. [DOI] [PubMed] [Google Scholar]
- 17.Mercer DM, Gilmer BB, Saltzman MD, Bertelsen A, Warme WJ, Matsen FA. A quantitative method for determining medial migration of the humeral head after shoulder arthroplasty: preliminary results in assessing glenoid wear at a minimum of two years after hemiarthroplasty with concentric glenoid reaming. J Shoulder Elbow Surg. 2011; 20:301–307. [DOI] [PubMed] [Google Scholar]
- 18.Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: an analysis of 176 consecutive cases . J Bone Joint Surg Am. 2012; 94:e102. [DOI] [PubMed] [Google Scholar]
- 19.Matsen FA, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015; 473:2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons IM, IV, Millett PJ, Warner JJ. Glenoid wear after shoulder hemiarthroplasty: quantitative radiographic analysis. Clin Orthop Relat Res. 2004; 421:120–125. [DOI] [PubMed] [Google Scholar]
- 21.Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016; 9:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabesan V, Callanan M, Ho J, Iannotti JP. Clinical and radiographic outcomes of total shoulder arthroplasty with bone graft for osteoarthritis with severe glenoid bone loss. J Bone Joint Surg Am. 2013; 95:1290–1296. [DOI] [PubMed] [Google Scholar]
- 23.Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001; 83-A:877–883. [PubMed] [Google Scholar]
- 24.Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014; 23:1066–1072. [DOI] [PubMed] [Google Scholar]
- 25.Youderian AR, Napolitano JA, Davidson IU, Iannotti JP. Management of glenoid bone loss with the use of a new augmented all-polyethylene glenoid component. Tech Shoulder Elbow Surg. 2012; 13:163–169. [Google Scholar]
- 26.Mizuno N, Denard P, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013; 95:1297–1304. [DOI] [PubMed] [Google Scholar]
- 27.Magosch P, Habermeyer P, Lichtenberg S, et al. [Results from the German shoulder- and elbow arthroplasty register (SEPR): anatomic or reverse shoulder arthroplasty in B2-glenoids?]. Orthopade. 2017; 46:1063–1072. [DOI] [PubMed] [Google Scholar]
- 28.Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013; 95:e82. [DOI] [PubMed] [Google Scholar]
- 29.Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012; 21:1526–1533. [DOI] [PubMed] [Google Scholar]
- 30.Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg Am. 2012; 94:2167–2175. [DOI] [PubMed] [Google Scholar]
- 31.Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009; 18:680–688. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Reddy AS, Kontaxis A, et al. Version correction via eccentric reaming compromises remaining bone quality in b2 glenoids: a computational study. Clin Orthop Relat Res. 2017; 475:3090–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walch G, Young AA, Boileau P, Loew M, Gazielly D, Molé D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012; 94:145–150. [DOI] [PubMed] [Google Scholar]
- 34.Matsen FA, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008; 90:885–896. [DOI] [PubMed] [Google Scholar]
- 35.Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014; 23:964–973. [DOI] [PubMed] [Google Scholar]
- 36.Farron A, Terrier A, Büchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006; 15:521–526. [DOI] [PubMed] [Google Scholar]
- 37.Sowa B, Bochenek M, Braun S, et al. Replacement options for the B2 glenoid in osteoarthritis of the shoulder: a biomechanical study. Arch Orthop Trauma Surg. 2018; 138:891–899. [DOI] [PubMed] [Google Scholar]
- 38.Orvets ND, Chamberlain AM, Patterson BM, et al. Total shoulder arthroplasty in patients with a B2 glenoid addressed with corrective reaming. J Shoulder Elbow Surg. 2018; 27:S58–S64. [DOI] [PubMed] [Google Scholar]