Keywords: Ca2+ imaging, medulla, rat, respiration, swallow
Abstract
Swallow is a primitive behavior regulated by medullary networks, responsible for movement of food/liquid from the oral cavity to the esophagus. To investigate how functionally heterogeneous networks along the medullary intermediate reticular formation (IRt) and ventral respiratory column (VRC) control swallow, we electrically stimulated the nucleus tractus solitarius to induce fictive swallow between inspiratory bursts, with concurrent optical recordings using a synthetic Ca2+ indicator in the neonatal sagittally sectioned rat hindbrain (SSRH) preparation. Simultaneous recordings from hypoglossal nerve rootlet (XIIn) and ventral cervical spinal root C1-C2 enabled identification of the system-level correlates of 1) swallow (identified as activation of the XIIn but not the cervical root) and 2) Breuer–Hering expiratory reflex (BHE; lengthened expiration in response to stimuli during expiration). Optical recording revealed reconfiguration of respiration-modulated networks in the ventrolateral medulla during swallow and the BHE reflex. Recordings identified novel spatially compact networks in the IRt near the facial nucleus (VIIn) that were active during fictive swallow, suggesting that the swallow network is not restricted to the caudal medulla. These findings also establish the utility of using this in vitro preparation to investigate how functionally heterogeneous medullary networks interact and reconfigure to enable a repertoire of orofacial behaviors.
NEW & NOTEWORTHY For the first time, medullary networks that control breathing and swallow are recorded optically. Episodic swallows are induced via electrical stimulation along the dorsal medulla, in and near the NTS, during spontaneously occurring fictive respiration. These findings establish that networks regulating both orofacial behaviors and breathing are accessible for optical recording at the surface of the sagittally sectioned rodent hindbrain preparation.
INTRODUCTION
Swallow is a primitive function found, in some form, in animals ranging from single-cell organisms to mammals. As vertebrates grew more complex, some evolved a shared oropharyngeal space that is used for both food and air (1–6). This requires precise coordination with other oropharyngeal/laryngeal behaviors such as breathing, coughing, sneezing, sniffing, and vocalizing (7–16). Both food ingestion and breathing must be functional immediately at birth.
Brainstem medullary networks control a diverse repertoire of functionally interdependent orofacial behaviors, autonomic functions, and respiratory rhythms. A variety of preparations have delineated the broad outlines of the functional anatomy of these networks (17–26). The motor output is controlled by interneuronal networks in ventrolateral medulla (27, 28), which provide drive to pools of (pre-) motoneurons. These networks are modulated by the nucleus tractus solitarius (NTS) in the dorsomedial medulla, which integrates descending drive (29) and afferent feedback (30, 31) to initiate or modulate motor patterns. Control of orofacial motor actions depends on activation of sensors and brainstem central pattern generators (CPGs) that are influenced by a fast, coordinated rhythmic component which is phase-locked to exploratory sniffing/whisking and can be reset at the premotor level by the respiratory control network as the master oscillator (15, 32, 33). However, because swallow arrests inspiration (34, 35), it could have hierarchical control over breathing as well as the orofacial behaviors that are reset by respiration.
Swallow can be divided into three phases: 1) oral; 2) pharyngeal, which directs food/liquid past the larynx and into the esophagus using a highly regulated series of bilateral muscle contractions (>20) in ∼500 ms; and 3) esophageal (34, 36–38). The swallow pattern generator (SPG) networks, described by Marckwald (39), Jean and colleagues (40–42), Ertekin and Aydogdu (43), and Bieger (44), are specific to the pharyngeal phase of swallow (herein termed “swallow”) and are thought to reside in the caudal brainstem. They are described with two major components. First, the dorsal swallowing group (DSG) in the NTS and adjacent reticular formation (RF) receives information from peripheral afferents and higher brain centers (41, 45). Then the swallow command is sent to the ventral swallowing group (VSG) in the caudal ventrolateral medulla adjacent to the nucleus ambiguus (NA) (46, 47). The VSG is thought to function as a switching ensemble to distribute the swallow command sequentially to various motoneuron pools (including those for cranial nerves V, VII, X, and, XII; NA, and C1–C6).
There is, however, a large area of RF neurons that may help to strictly regulate oropharyngeal and laryngeal muscle onset. The medullary RF spans the rostrocaudal length of the medulla, provides input to cranial motoneuron pools, and can be roughly divided into several main zones. Amirali et al. (48) demonstrated c-fos labeling in each of these zones in response to repeated swallow: the intermediate zone of the reticular formation (IRt), the parvocellular reticular formation (pCRt), and the gigantocellular reticular formation. The CPGs for chewing, whisking/sniffing, licking, and their associated premotor regions are found primarily within the IRt in the same regions thought to be activated by swallow; Kleinfeld, Deschênes, Moore, Wang, and colleagues propose a role for the medullary RF in the overall organization of behavioral orofacial control (15, 33, 49). Although its role in swallow has not been thoroughly investigated, the medullary IRt may be important for the generation of swallow and its coordination with breathing and other orofacial behaviors.
The relative inaccessibility of the brainstem in vivo has rendered a detailed mapping of orofacial network connectivity difficult. Although in vitro preparations allow access to network constituents and tight control of experimental conditions, the repertoire of behaviors is then limited to fictive respiration generated as a feed-forward pattern. These networks are preserved in the tilted sagittally sectioned rodent hindbrain (SSRH) preparation. A diagonal cut is made (50) to expose the NTS (51), much of the NA and IRt, and the ventral respiratory column for optical recording (52, 53), while removing relatively little brainstem tissue (Fig. 1A). To investigate how networks along the IRt and VRC control swallow and breathing-related behaviors, we electrically stimulated the DSG (42) to induce fictive swallow between inspiratory bursts, with concurrent optical recordings using a synthetic Ca2+ indicator. This preparation allowed us to test the hypothesis that the SPG spans the medulla and incorporates part of the IRt.
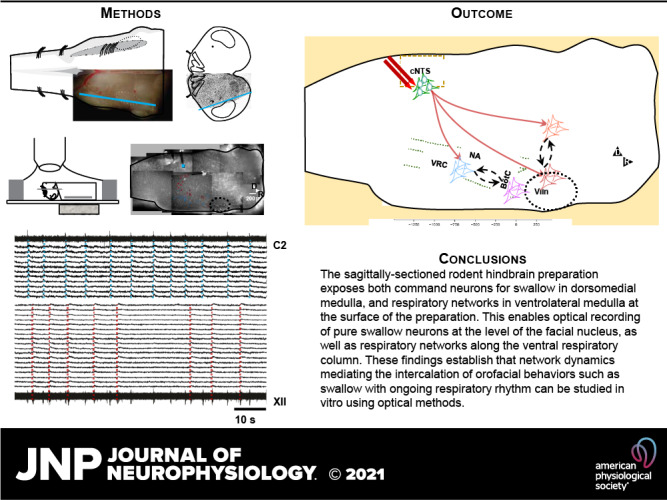
Figure 1.
Neurons activated during fictive swallow are interdigitated with respiration modulated neurons along the ventral respiratory column. A: cartoon of the preparation. Left panel shows the tilted sagittal face of the preparation, which tapers medial toward its caudal margin and tapers lateral toward its ventral margin (black triangles dm, vl), with functional anatomical landmarks added. BötC, Bötzinger complex; cNTS, caudal nucleus tractus solitarii; NA, nucleus ambiguus; VIIn, facial nucleus; VRC, ventral respiratory column). The vertical line through VIIn indicates the level of section of the transverse plane cartoon (right); the yellow diagonal line in the transverse view indicates the surface exposed for optical recording. B: traces generated by respiratory neurons (top traces, aligned to C2 motor output), and neurons activated during fictive swallow (bottom traces, aligned to fictive swallow bursts in XII motor output). C: a tiled image of one preparation, showing stimulus location (blue square), optical recording field of view (bordered by a broken yellow line) and location of stimulus-activated neurons (red dots) and respiration-modulated neurons (blue dots). The VIIn is outlined in a broken black line. D: location of the center of mass of the ROI generated by somatic Ca2+ transients associated with swallow-activated (red crosses, n = 65) and respiration-modulated neurons (blue dots, n = 34), in and dorsal to the VRC, pooled from 3 experiments, aligned to VIIc (x-axis), and ventral surface (y-axis).
METHODS
Experimental Protocols
Two experimental protocols were performed to investigate network coordination of fictive swallow and BHE reflex, using optical recording and electrophysiology. 1) Identify and describe swallow-related neurons and their anatomical location, and the coordination of swallow with breathing (n = 7 animals; n = 498 neurons). 2) Generate a functional-anatomical map of locations in the dorsal brainstem, which stimulated swallow or Breuer–Hering expiratory reflex (n = 4).
Brainstem Preparation
In accordance with methods approved by the Institutional Animal Care and Use Committee, neonate Sprague-Dawley rat pups (Charles River, P0-P2) were anesthetized with isoflurane. Because of their age, animals could not be sexed. The neuraxis was transected at the level of the cerebellar peduncles, and hindbrain and spinal cord were rapidly isolated in cold (5°C–10°C) artificial cerebrospinal fluid (aCSF) made up of (in mM) 128.0 NaCl, 3.0 KCl, 1.2 CaCl2, 1.0 MgSO4, 21.0 NaHCO3, 0.5 NaH2PO4, and 30.0 glucose, equilibrated with 95% O2–5% CO2. The hindbrain was then pinned out on a custom chuck designed to enable sectioning at compound angles (50). A sagittal section cut at an 18.6° ventrodorsal tilt relative to the dorsal hindbrain surface and an 8.5° rostrocaudal tilt relative to the midline, at the lateral margin of the small oval convexity produced by the facial nucleus on the ventral surface of brain stem (50, 54), with the goal of transecting the ventral respiratory column along its major axis (Fig. 1A). In adult rats, the minor axis of the VRC is estimated at 500 µm (55); as a consequence, systematic investigation of changes in network constituents along the mediolateral axis in neonate rat brainstems is impossible using a vibratome, given the minimal thickness of slices (200 µm) that can reliably be sectioned. Because only one diagonal section was made through the tissue, this hemisected preparation is not a conventional slice preparation; it preserves all of the tissue in one half of the brainstem, and much of the tissue in the other half.
Following 1-h incubation in an aerated solution containing the Ca2+ indicator Cal 520 AM (10 μg, Kd = 320 nM, AAT Bioquest), solubilized in 20 μL 5% pluronic F-127 in DMSO (2 g/10 mL DMSO; Invitrogen), and diluted in 1 mL aCSF to a final concentration of ∼10 μM, the preparation was then transferred to the recording chamber (JG 23 W/HP; Warner Instruments, Hamden, CT) mounted on an upright microscope (Axioskop 2 FS; Zeiss Instruments, Jena, DE). The preparation was perfused at 4 mL/min with aCSF warmed to 24°C–27°C and aerated with a 95%–5% O2–CO2 gas mixture.
Stimulation
Whole nerve signals were amplified and filtered (100–1,000 Hz; Grass model P511s), and then rectified and integrated (20 ms) using Spike2 (Cambridge Electronic Design; Cambridge, UK). To elicit fictive swallow, a bipolar electrode (Pt:Ir, 0.1 MΩ, 125-μm tip separation, MicroProbes) was positioned at an origin 100 μm ventral from the dorsal edge of the exposed face of the SSRH preparation, at the caudal pole of the medulla. Starting from this location, stimuli were applied across the respiratory cycle (20 Hz, 8 V, 2 ms pulses 0.5–1 s train; S88 84T50D, Grass Instruments) to elicit changes in hypoglossal nerve (XIIn) and ventral root output. In experiments in which synthetic indicator was applied to the preparation, brief optical recordings were carried out during these exploratory stimuli. The electrode was displaced in 200 μm steps until a burst of activity at XIIn but not cervical root (fictive swallow) was elicited. In subsequent experiments, a more complete mapping of the effect of stimulation along the dorsal half of the medulla was carried out without optical recording, enabling more accurate delineation of regions capable of either advancing or delaying inspiratory burst onset, as well as swallow.
Based on an earlier study of the first synapse of the pathway conveying afferent feedback from slowly adapting lung mechanoreceptors to central respiratory networks (30), we were able to elicit expiratory lengthening in response to midexpiratory stimuli, consistent with the Breuer–Hering expiratory (BHE) reflex, matching earlier in vitro studies in the isolated rat medulla, in which lung mechanoreceptors were mechanically activated (56, 57).
Data Acquisition
Respiratory activity was recorded via suction electrode from ventral roots C1-C2; fictive swallow and respiratory activity was recorded from the hypoglossal nerve (XIIn) and digitized at 20 kHz. Optical signals, visualized at ×10, ×20, or ×40 (Achroplan 10×/0.3 W Ph1, Achroplan 20×/0.5W, Achroplan40×/0.75W; Zeiss), illuminated using an LED lamp (LCS-0470-50-48; Mightex, Ontario, Canada), were recorded using a large format (18.7 mm diagonal, 11 µm × 11 µm pixels) sCMOS camera (Prime 95B, Teledyne Photometrics, Tucson, AZ) that sampled a 1,300 μm × 1,300 μm (×10) field of view, with pixels capturing photons over 2.3 μm of tissue (×10 with 2 × 2 binning), resulting in a spatial resolution of >9 pixels/soma, at a sampling rate of 20 Hz. Image acquisition (Active Silicon PHX-D48CL, Chelmsford, MA) was triggered off voltage acquisition (20 kHz; PCI-6221, National Instruments, Austin, TX). In addition, the square-pulse waveform used to trigger the lamp was also saved, ensuring precise time registration between image and voltage recordings. Camera control, voltage, and image acquisition was integrated in custom software (LabView; National Instruments, Austin, TX).
Signal Processing
Somatic Ca2+ transients were extracted using semiautomated methods (58) implemented in LabView (National Instruments, Austin, TX). As described elsewhere (52), only signals with signal-to-noise ratio greater than −5 dB were included for analysis. Optical traces were high-pass filtered (>0.05 Hz), then respiration-modulated and swallow-mediating neurons were identified by inspection of the averaged trace triggered off ventral cervical root (for respiration) or onset of the electrical stimulus artifact (for swallow). In both cases, identification was straightforward: for inspiratory neurons, time-varying signals with S/N > −5 dB displayed the characteristic steep rise, followed by exponential roll-off during each inspiratory burst, which was further enhanced in the burst-triggered average; only neurons with strong activation during stimulation were included in swallow-activated neurons, which was most clearly discernible for stimuli applied during midexpiration. The half-width of the stimulus triggered average was quantified, estimated by measuring the steep rise from baseline to maximum luminance value, finding the midpoint of this upward deflection, and measuring the duration that the luminance value remained above this midpoint. The stimulus-triggered half-width was quantified to identify the rate of adaptation of swallow-activated neurons to electrical stimulus. Because stimulus durations varied between experiments, half-widths were normalized by dividing by the longest half-width.
RESULTS
Simultaneous Optical Recording and Electrical Stimulation
Optical recording at the level of the ventral respiratory column (VRC) revealed respiration-modulated neurons along the VRC as well as neurons activated during fictive swallow (n = 11, n = 19, respectively, Fig. 1, B and C). As in the raw voltage traces, XII activity reflected both respiratory drive and stimulus-induced swallow, whereas ventral root activity (C2) reflected only respiratory drive (Fig. 2B). Pooled data revealed that fictive swallow consistently activated networks overlapping with the IRt, NA, along the ventral margin of the VRC, and immediately caudal to VIIn (Fig. 1D).
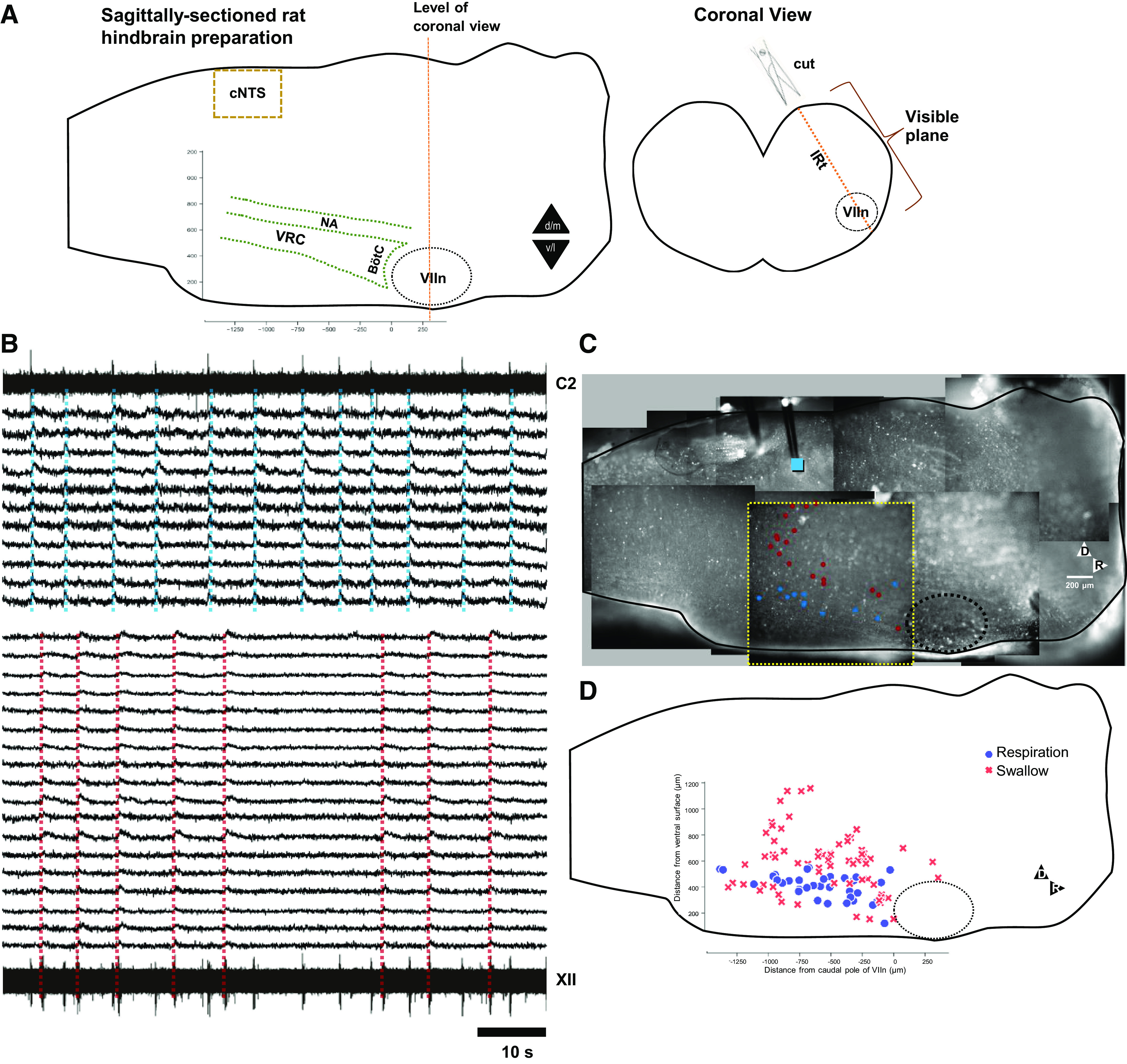
Figure 2.
IRt networks at the dorsal edge of, and dorsal to, the facial nucleus are activated during stimulus-induced fictive swallow. A: tiled image of representative preparation, showing stimulus location (blue square), optical recording field of view (bordered by a broken yellow line), and location of ROIs from which traces were extracted (red dots). Bi: motor output (XII, C2) and optical recordings during four fictive swallows. Stimuli evoked XII bursts, but not C2 bursts. High-pass-filtered optical traces of 136 somata are sorted from rapidly to slowly adapting units. Bii: stimulus-triggered averages (STAs) of traces shown in Bi highlight variability in neuronal responses. At the top are superimposed stimulus-triggered averages of the most rapidly (red trace) and second-most slowly adapting (green trace) neurons. C: histogram of normalized STA half-widths reveals a skewed distribution, with a small number of slowly adapting neurons. D: pooled data from three experiments, aligned to VIIc (x-axis) and ventral surface (y-axis; n = 394 neurons), color coded from fast- (0.0–0.39, AU) to slow-adapting (1.0, AU) does not reveal any parcellation of slowly adapting neurons. Each point represents the center of mass of the ROI generated by somatic Ca2+ transients. Histograms (inset) of cell numbers reveal a bimodal distribution in the ventrodorsal axis, suggesting the existence of two distinct networks, one centered at the dorsal margin of VIIn (peak at 400 μm from the ventral surface) and the other centered at 800 μm from the ventral surface. At the sampling rates used here, no differences in onset times or pattern of activity between groups could be detected. AU, arbitrary units; IRt, intermediate reticular formation; ROI, region of interest.
Optical recording at the level of the facial nucleus (VIIn) revealed intense activation of a large population of neurons dorsal to VIIn, as well as a smaller population at the dorsal edge of VIIn (Fig. 2A, Supplemental Video S1; https://doi.org/10.6084/m9.figshare.13087763). Traces of time varying luminance (Fig. 2B), as well as stimulus-triggered averages (Fig. 2C) revealed that activated neurons were mostly rapidly adapting, but included slowly adapting neurons. Data were pooled (n = 498 neurons) to quantify relative numbers of slowly and rapidly adapting neurons. A histogram of the normalized half-width of each neuron’s stimulus-triggered average revealed heterogeneous rates of adaptation that included a small number of very slowly adapting neurons (Fig. 2C). A plot of the distribution of swallow-activated neurons color-coded from fast- to slow-adapting response fails to reveal a clear distribution based on adaption rate (Fig. 2D). Histograms (Fig. 2D, inset) of rostrocaudal and ventrodorsal distributions suggest that swallow-activated neurons are proportioned ventrodorsally into two groups. Higher resolution videos from ventral and dorsal populations reveal qualitatively different neuronal morphologies (Supplemental Fig. S1; https://doi.org/10.6084/m9.figshare.13194929; Supplemental Videos S2 and S3; https://doi.org/10.6084/m9.figshare.13087772; https://doi.org/10.6084/m9.figshare.13087781).
Electrical Stimulation Mapping
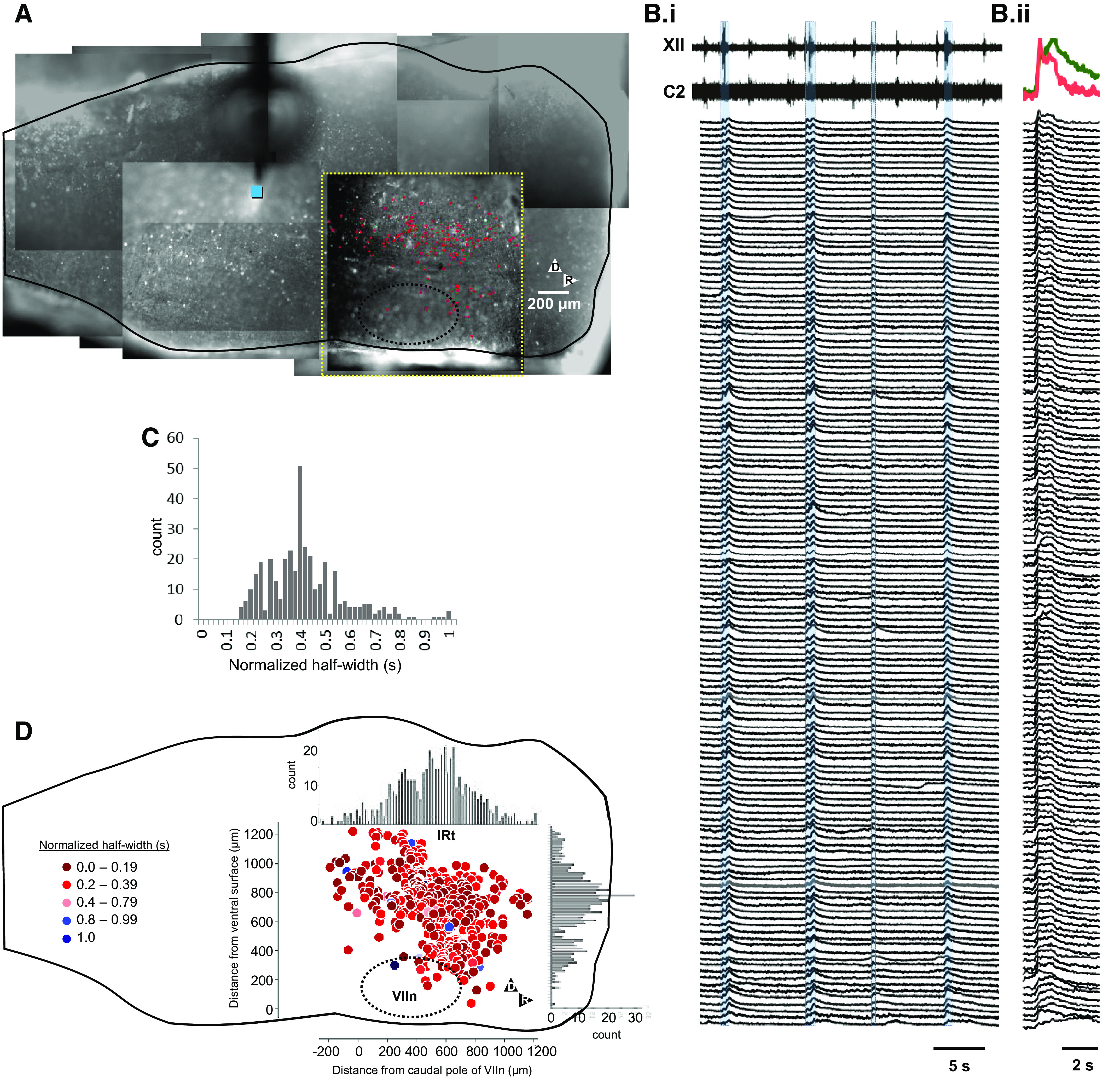
The effect of electrical stimulation on phrenic and hypoglossal activity was systematically explored by displacing the stimulation electrode in 200 μm steps to generate a functional-anatomical map of the dorsal half of the medulla (n = 4 animals). The effect of stimuli in locations shown in Fig. 3A are shown in Fig. 3Bi–vi. These include swallow accompanied by delayed inspiratory onset (Fig. 3Bi), swallow with advanced inspiratory onset (Fig. 3Bii), and swallow without effect on inspiratory onset time (Fig 3Biii). In a subset of cases, swallow was accompanied with weak, sporadic coactivation of C2, consistent with schluckatmung (swallow-related inspiratory activity) (7–9, 16, 39, 45, 59–66). In addition, stimulation at other locations resulted in expiratory lengthening that persisted after stimulus offset. This is consistent with an in vivo study locating the first synapse of slowly adapting lung mechanoreceptors in caudal dorsal medulla (30). Figure 3Biv demonstrates stimulation locations where inspiratory bursts occurred immediately after stimulus offset, consistent with a brake on motor outflow. Finally, stimuli applied to the dorsal and caudal margin of the pre-Bötzinger complex (PBC) elicited inspiratory bursts, and enabled entrainment of the respiratory rhythm to phasic stimulation at frequencies close to the preparation’s native frequency (Fig. 3Bvi). Over much of the neuraxis, stimuli had no effect. Figure 3C demonstrates regions where stimulus-evoked swallow were obtained. Of note, in one experiment, rostrocaudal tracks of stimuli all evoked XII activation (white triangles, Fig. 3C), consistent with tract activation (see Supplemental Video S4; https://doi.org/10.6084/m9.figshare.13093496).
Figure 3.
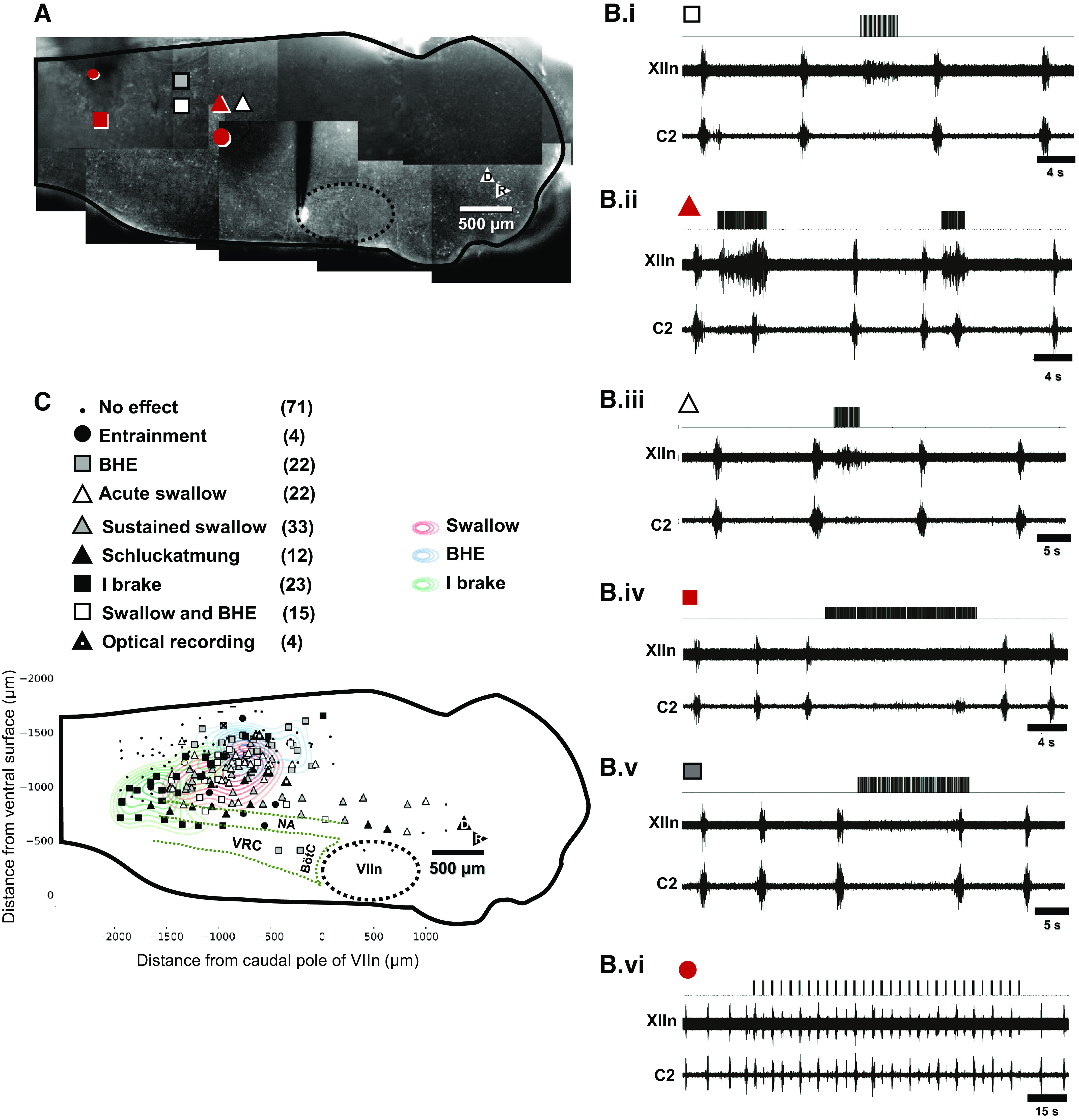
Identification of regions along dorsal and midbrainstem capable of evoking swallow, the Breuer–Hering expiratory reflex (BHE), and respiratory entrainment. A: tiled image of one preparation with symbols indicating stimulus location. B: effects of stimuli at the locations indicated in A. Bi: swallow with lengthened expiration (white square). Bii: swallow with shortened expiration (black triangle). Biii: swallow that had no effect on expiratory duration (white triangle). Biv: in addition, stimuli at more caudal locations elicited expiratory lengthening after stimulus offset consistent with Breuer–Hering expiratory reflex (gray square). Bv: suppression of inspiration throughout the duration of the stimulus (inspiratory brake, black square). Bvi: finally, in regions caudal and dorsal to the pre-Bötzinger complex, stimuli evoked inspiratory bursts, enabling entrainment of respiratory rhythm to a frequency slightly faster than the free-running behavior (black circle). I brake, inspiratory brake.
In addition, we compared the motor pattern of inspiratory bursts before and after a stimulated swallow, with at least three breaths between stimulations (n = 4; paired t test). There was no significant effect on the subsequent inspiratory burst duration of XII (P = 0.99) or C2 (P = 0.87); time from burst onset to peak of XII (P = 1.0) or C2 (P = 0.77); or burst amplitude (% of maximum) of XII (P = 0.88) or C2 (P = 0.65).
DISCUSSION
These findings from network activity recorded optically in the SSRH preparation allow us to infer a more complete description of swallow pattern generator (SPG) networks. Consistent with earlier studies in decerebrate rats in vivo (42, 67), stimuli at discrete loci in caudal NTS (the region of the DSG) reproducibly elicited fictive swallow, defined as a burst of activity at XIIn, but not at C2. In addition, the optical recordings revealed that stimulus-induced XIIn activity was accompanied by activation of networks dorsal to VIIn (Fig. 1), at the dorsomedial margin of the ventral respiratory column (VRC), and in the intermediate reticular formation (IRt). This is consistent with earlier results from Amirali et al. (48) and hypotheses by Neuhuber and Bieger (68) regarding the SPG, and enables characterization of swallow network-level mechanisms. Stimuli at various electrode locations produced an assortment of fictive respiratory and swallow motor pattern interactions (Fig. 3).
In general, the swallow motor pattern proceeds in a stereotypic ballistic rostrocaudal wave of muscle contractions, caused by precisely timed activation of various motoneuronal nuclei (including those for cranial nerves V, VII, IX, X, and XII), yet its initiation is subject to modulation by afferent inputs (9, 40, 64, 65, 69–87). This muscular wave pattern is thought to be preceded by an initial strong inhibition of all swallow motor pools (45). This is followed by sequential disinhibition and/or excitation of selected motor pools to accomplish the precisely timed muscle contractions required for the peristaltic oropharyngeal and subsequent esophageal swallow phases (88). This coordinated switching and distribution of drive is proposed to be accomplished by the VSG located in the ventral medulla near the nucleus ambiguus (NA). The VSG is thought to be activated by the DSG, which is located in the NTS and adjacent reticular formation (RF) and receives afferent information to initialize the swallow command (41, 45). As expected, our optical recordings showed that neurons in the VSG and DSG regions were active during fictive swallow (Fig. 1C and D). We also found other swallow-activated regions throughout the rostrocaudal span of the medulla, particularly the parafacial region of the IRt (Figs. 1 and 2). Electromyogram studies have consistently described a pattern in which the sequential activation of the swallow muscles themselves proceeds in a rostrocaudal direction, starting in the oral cavity and proceeding through the pharynx and then esophagus (37, 38, 89). However, the cranial motoneuron pools responsible for activating these muscles do not have a corresponding sequential myotopic rostrocaudal arrangement. For example, oral muscles are innervated by cranial nerves V, VII, IX, X, and XII, and these muscles are activated at various points throughout the entire swallow cycle, whereas laryngeal muscles are innervated almost exclusively by the recurrent laryngeal branch of X, but are also activated at different times throughout the swallow cycle. Thus, specific portions of the different cranial motoneuron pools must be precisely activated at different times to accomplish swallow, and this activation would not proceed in a strict “in-order” rostrocaudal direction in the brainstem, as the pools are distributed “out-of-order” throughout the entire length of the medulla.
Fictive swallow evoked strong activation of networks dorsal to VIIn, and at the rostrodorsal margin of VIIn (Fig. 2A). These regions have significant overlap with the IRt. The bimodality of the histogram describing the ventrodorsal distribution of neurons activated in this region during fictive swallow (Fig. 2D) may reflect functional-anatomical parcellation. In the context of sniffing/whisking behaviors, retrograde labeling identified neurons at the dorsal margin of VIIn as premotoneurons controlling nose motion during sniffing (90). Thus, the anatomical overlap between premotor neuronal pools mediating nose motion during sniffing and those activated during swallow suggests that this is a locus for integrating upper airway behaviors and breathing. The IRt provides input to various cranial motoneuron pools, and is thus a premotor region that spans a large dorsoventral and rostrocaudal area (91–94). Rostrolateral medullary reticular formation premotor neurons project to multiple orofacial motor nuclei and receive input from the NTS (95–99). A recent study demonstrated a dense network of chewing premotor neurons in the IRt between the level of the V and XII motor nuclei; these neurons provided both excitatory and inhibitory input to chewing motor pools (92). At the rostral margin of the IRt in mouse, the postinspiratory complex (PiCo) has been identified and is thought to coordinate postinspiratory laryngeal activity (100), and the pre-Bötzinger complex [preBötC; generates inspiratory rhythm, see Del Negro et al. (101)] has collaterals within the IRt that rise ventrally (including to regions of other putative orofacial CPGs) spanning several premotor neuron populations (15, 102–104). A recent retrograde tracing study by Yang et al. (105) found monosynaptic excitatory and inhibitory connections to the preBötC from the IRt dorsal to VIIn.
The parvocellular reticular formation (PCRt) and the trigeminal zone of the intermediate reticular formation (tIRt) are premotor to the trigeminal motor nucleus (Vm). (106). But rather than being located near Vm, these premotor tIRt/PCRt formations are located parafacially at about the rostrocaudal level of the VIIn (107, 108). This arrangement in which a more caudal premotor nucleus acts on a more rostral motor nucleus is true in general for these premotor orofacial formations. The whisking premotor area (vibrissa intermediate reticular formation; vIRt) acts on VIIn, but it is not located parafacially; rather it is much more caudal, near the preBötC at the paravagal level. The suckling/chewing pattern generator is also thought to be parafacial. The chewing pattern generator was first identified in the medial medullary RF in 1986 (108), and although some controversy remains over its exact location (109), chewing premotor neurons are located in the IRt between the level of the V and XII motor nuclei (92).
In hierarchical control, the respiratory oscillator (whether discrete or a distributed network) is a master that can reset, pace, or otherwise influence most orofacial rhythms such as suckling/chewing (110, 111), rhythmic licking (112), whisking, and sniffing (15, 113). However, in conditions in which the airway requires protection, the respiratory oscillator is overridden (34). Although breathing and chewing appear to be asynchronous (114, 115), both are delayed by swallow (35, 116). Although chewing can powerfully influence breathing in certain situations, respiration and masticatory processes are not strongly coupled (116–118).
We propose that the IRt acts as a multimodal integration hub to receive afferent information from various sources and to distribute selective commands to various premotor and motor nuclei. By distributing through the IRt, the swallow command could override/reset all other orofacial behaviors. The SPG could directly override other orofacial pattern generators individually or could act indirectly (except on mastication) via the respiratory oscillator(s). Because respiration can strongly reset most orofacial behaviors, but not chewing/suckling, the SPG would have to influence the masticatory pattern generator separately (114–118). The large number of neurons dorsal to VIIn (the putative masticatory pattern generator) that were activated by fictive swallow was somewhat surprising (Fig. 2), as that region has not traditionally been considered part of the SPG. However, the results support those of Amirali et al. (48) and hypotheses by Neuhuber and Bieger (68), and this region appears to provide some pharyngeal nerve innervation (119). This would be consistent with a requirement for the SPG to directly influence the masticatory premotor region in the IRt to selectively influence muscles to arrest or modify masticatory movements and/or to position the jaw/oral cavity ideally for effective swallow (118). Similarly, Bautista et al. (120) proposed that the medullary gigantocellular reticular nucleus could be a candidate region for the integration of swallow and breathing, based on results from Feroah et al. (60). This is also supported by studies demonstrating that this region is an essential functional and paucisynaptic anatomical conduit for respiratory responses to activation of vestibular or cerebellar fastigial nuclei, suggesting a role for the medullary RF in coordinating respiration with other behaviors such as locomotion, posture, vomiting, and autonomic output (121–125).
Swallow preferentially occurs within the tidal volume range, has a preference for the expiratory (E) phase of breathing (10, 14, 64, 126–129), and is in part mediated by pulmonary stretch receptors and airway proprioceptor spinal afferents (9). Swallow during the inspiratory phase risks aspiration of material into the lungs, and swallow or laryngeal afferent stimulation can terminate inspiration by inhibiting inspiratory neurons or by activating postinspiratory (post-I) neurons in the VRC (130, 131), not unlike the inspiratory Hering–Breuer reflex. The apneic response to laryngeal afferent stimulation is thought to be mediated by the DSG and strong post-I inhibition from the BötC (45, 131, 132).
To more precisely define the boundaries of regions in which electrical stimulation elicited fictive swallow, we conducted a final series of experiments in which electrode locations were documented by taking images that were later tiled, but no optical recordings of Ca2+ transients were made, enabling a more rapid and comprehensive mapping of the effect of stimulation on motor output recorded at C2 and XIIn. We were able to induce fictive swallow accompanied by expiratory lengthening (Fig. 3Bi), strong XIIn activation accompanied by delayed C2 activation (swallow-related inspiratory activity: schluckatmung, Fig. 3Bii), and swallow without any change in expiratory duration (Fig. 3Biii). In addition, stimuli either suppressed inspiratory bursts (I-brake, Fig. 3Biv), delayed inspiratory onset (BHE, Fig. 3Bv), or elicited inspiratory bursts, leading to entrainment of respiratory rhythm to periodic stimulus trains (Fig. 3Bvi). Although swallow-evoking stimulus locations clustered in caudal NTS (red ovals, Fig. 3C), fictive swallows were evoked by stimuli over a wide area well ventral to the NTS. This observation is consistent with evoked swallow elicited by stimulation along the ventral swallow group in cat (46, 133). Similarly, stimulus-evoked expiratory lengthening consistent with the Breuer–Hering expiratory (BHE) reflex (lengthened expiration in response to stimulus during expiration) was clustered in caudal NTS, consistent with published reports in vivo (30); stimuli applied in caudal VRC suppressed inspiratory drive (I brake, Fig. 3Biv), consistent with inhibitory drive from decrementing expiratory neurons in this region (134, 135). Although expiratory lengthening was evoked by stimulation in regions congruent with those identified as the first synapse for lung mechanoreceptor afferents in vivo (30), we cannot exclude the possibility that the first synapse for chemoreceptor afferents rather than mechanoreceptor afferents was activated. Taken together, these observations suggest that projections from dorsal networks that act as relays for sensory feedback or descending drive that modulates respiratory rhythm are preserved in the SSRH and are amenable to selective activation under optical recording conditions.
Stimuli in specific paravagal anatomical regions produced multiple motor patterns. The pooled data reveal stimulation of the VSG (approximate area) produced swallow or inspiratory-brake (Fig. 3C). Stimulus in the approximate area of the DSG produced swallow or BHE. This is unsurprising, as this region of the NTS receives dense peripheral afferent innervation, including laryngeal and pulmonary afferents that stimulate swallow, BHE, and other reflexes. The partial overlap of these regions suggests a potential multimodal function.
Horton et al. (130) posit that coordination of breathing with swallow is partly independent of the traditional BötC “inspiratory off-switch,” with SPG neurons sharing reciprocal inhibitions with populations of both I and E neurons to form a “central swallow gate” that operates in a state-dependent fashion to surveil afferent information to optimally regulate swallow initiation and swallow-breathing coordination. In a different model extended by Toor et al. (132), during eupnea, post-I timing originates in the pons and relays to the BötC inspiratory off-switch, but also relays to the VSG/IRt to recruit post-I activity in laryngeal motor and sympathetic premotor outputs. However, during stimulation of laryngeal afferent fibers, post-I pons activity is not required to activate the BötC or the VSG/IRt. Instead, second-order NTS neurons trigger swallow via activation of the DSG. The DSG then recruits BötC neurons to arrest respiratory rhythm and activates the VSG in the IRt to distribute swallow-related rhythmic activity to respiratory and sympathetic outputs as well. In their experiments, the fictive swallows produced by SLN stimulation also resulted in swallow-locked oscillations in sympathetic nerve activity. This required activation of the IRt, demonstrating that SPG activity is distributed via the VSG to vasomotor outputs in addition to motor outputs. Thus, they propose that VSG/IRt neurons may serve as a premotor integration site, transmitting input to common motor outputs that are recruited in the postinspiratory period. This may include distinct neuron classes that perform each function or overlapping populations that perform both functions. Although our results cannot address the models of Horton et al. (130) or Toor et al. (132) specifically, their results with ours are consistent with a role of the VSG/IRt as a premotor integration site and distribution hub for postinspiratory (or other oromotor) commands. The anatomical distribution of swallow-related neurons throughout the NTS, VRC, and IRt would also allow for interactions between the SPG and inspiratory and expiratory neurons as proposed by Horton et al. (130), but do not reveal anything regarding the possible existence of a swallow gate.
By enabling recording in parallel from networks of neurons during fictive swallow, optical recording of the SSRH permitted observation of the variability of stimulus-induced activity patterns. This revealed that most neurons activated during fictive swallow returned to baseline upon stimulus offset, but a minority were slowly adapting (Fig. 2, B and C), suggesting that these networks are functionally heterogeneous. Because slowly adapting neurons showed no anatomical parcellation (Fig. 2D), these slowly adapting neurons do not appear to constitute a functional-anatomical structure. Possible roles for adaptation times may include: regulation of muscle burst duration, different motoneuron/fiber types [i.e., fast-twitch vs. slow-twitch regulation, and/or a precise temporal switching role (interburst interval)]. Regardless, it suggests the possibility that processing the swallow pattern requires more nuanced control than had been previously assumed based on the ballistic stereotyped nature of the motor pattern.
The heterogeneity and in vivo inaccessibility of brainstem medullary networks justify our in vitro optical recording methods; nonetheless, these methods have important limitations. In vitro preparations isolate networks from some central modulation and afferent inputs; we have demonstrated that some afferent feedback and central modulation can be emulated using stimulating electrodes. However, this approach must be validated by a more comprehensive characterization of orofacial behaviors, such as vocalization (136) and sniffing/whisking (32, 33), whose networks are likely retained in this preparation, so as to delineate the degree of overlap of the interneuronal populations mediating these diverse behaviors. Similarly, the utility of these methods for studying the convergence patterns of visceral afferent feedback (137) onto medullary respiratory and cardiovascular networks remains to be discovered. The limitations of Ca2+ transient optical recording must also be considered: the signals we recorded represent a convolved low-pass filtered record of the action potentials (138). Furthermore, the synthetic indicators are subject to photobleaching and give rise to phototoxicity (139), representing tradeoffs between optimization of signal-to-noise ratio, and maintenance of quasistationary network dynamics. Thus, although this method permits recording of heterogeneous networks in parallel, it offers limited temporal resolution, such that estimations of coupling based on changes in instantaneous firing rate extracted via deconvolution methods are possible, at best (52).
In this study, as well as in earlier in vivo studies, qualitatively similar changes in motor output could be elicited by stimuli applied over large areas. Previously, the first synapse for the slowly adapting lung afferents was identified over a 700-μm region (30), and loci from which swallow could be elicited extended over 2 mm (42) in adult rat. Similarly, in the present study, regions over which swallow or expiratory lengthening could be elicited were large. This is possibly because cell body and tract stimulation elicit qualitatively similar motor outputs. In the brief optical recordings that accompanied stimuli used to locate the DSG, we found that a given change in motor output was accompanied by tract activation, and so long as the electrode was displaced along the tract, a qualitatively similar motor output was observed. In the experiments in which a more detailed functional anatomical map was generated, no optical recordings were carried out, because photobleaching would have occurred over their duration. Thus, in the mapping experiments, it was impossible to determine whether neuronal soma or axon tracts were stimulated. These anecdotal observations suggest that to differentiate between somatic and tract activation, stimulus protocols that incorporate anterograde labeling may be necessary to identify the location of the cell bodies of neurons that trigger swallow or Breuer–Hering reflexes, rather than the tracts that convey this drive.
The anatomical overlap between the identified swallow-activated neurons and networks hypothesized to coordinate breathing, sniffing, chewing, and suckling raises the question of whether these anatomical regions contain functionally distinct neurons that are interdigitated, or—exemplifying degeneracy (140, 141)—contain multifunctional neurons that participate in diverse behaviors. Disambiguating between these alternatives may be possible in this preparation, because optical recording enables the functional screening of relatively large numbers of neurons in parallel, and allows for targeting of functionally identified neurons for detailed electrophysiological recording and labelling. By reincorporating the more complex repertoire of behaviors latent in this in vitro preparation, a more accurate functional description of these sometimes poorly understood and inaccessible networks may be feasible in future studies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL111215 (T. Pitts) and National Institute of Neurological Disorders and Stroke Grant NS110169 (T. Pitts).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.P., A.H., and N.M. conceived and designed research; A.H., M.R., and N.M. performed experiments; M.R. and N.M. analyzed data; T.P., A.H., M.R., and K.I. interpreted results of experiments; M.R. and N.M. prepared figures; N.M. drafted manuscript; T.P., A.H., K.I., and N.M. edited and revised manuscript; T.P., A.H., M.R., K.I., and N.M. approved final version of manuscript.
REFERENCES
- 1.Bosma J, Fletcher S. The upper pharynx. A review. II. Physiology. Ann Otol Rhinol Laryngol 71: 134–157, 1962. doi: 10.1177/000348946207100113. [DOI] [PubMed] [Google Scholar]
- 2.Dullemeijer P. The functional morphology of the head of the common viper. Arch Néerl Zool 11: 387–497, 1956. doi: 10.1163/036551656X00139. [DOI] [Google Scholar]
- 3.Gupta O. Studies on the morphology, histology and the swallowing mechanism of the digestive tract of a carnivorous fish, Xenentodon cancila (Ham.). Okajimas folia Anat Jpn 48: 29–51, 1971. doi: 10.2535/ofaj1936.48.1_29. [DOI] [PubMed] [Google Scholar]
- 4.Herring SW, Scapino RP. Physiology of feeding in miniature pigs. J Morphol 141: 427–460, 1973. doi: 10.1002/jmor.1051410405. [DOI] [PubMed] [Google Scholar]
- 5.Miller AJ. Deglutition. Physiol Rev 62: 129–184, 1982. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- 6.Negus V. The mechanism of swallowing. J Laryn Otol 58: 46–59, 1943. doi: 10.1017/S0022215100156427. [DOI] [Google Scholar]
- 7.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngol Clin North Am 46: 957–964, 2013. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonis J, Neumueller S, Marshall B, Krause K, Qian B, Pan L, Hodges M, Forster H. The effects of lesions in the dorsolateral pons on the coordination of swallowing and breathing in awake goats. Respir Physiol Neurobiol 175: 272–282, 2011. doi: 10.1016/j.resp.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huff A, Reed MD, Iceman KE, Howland DR, Pitts T. Sex-specific vagal and spinal modulation of swallow and its coordination with breathing. PLoS One 15: e0234194, 2020. doi: 10.1371/journal.pone.0234194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huff A, Reed MD, Smith BK, Brown EH, Ovechkin AV, Pitts T. Strategies for the integration of cough and swallow to maintain airway protection in humans. Lung 196: 601–608, 2018. doi: 10.1007/s00408-018-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly B, Huckabee M, Jones R, Frampton C. The early impact of feeding on infant breathing-swallowing coordination. Respir Physiol Neurobiol 156: 147–153, 2007. doi: 10.1016/j.resp.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Kendall KA, Leonard RJ. Bolus transit and airway protection coordination in older dysphagic patients. Laryngoscope 111: 2017–2021, 2001. doi: 10.1097/00005537-200111000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Martin B, Logemann J, Shaker R, Dodds W. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. J Appl Physiol (1985) 76: 714–723, 1994. doi: 10.1152/jappl.1994.76.2.714. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol (1985) 94: 1735–1743, 2003. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 15.Moore JD, Deschenes M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature 497: 205–210, 2013. doi: 10.1038/nature12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitts T, Rose MJ, Mortensen AN, Poliacek I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol 189: 543–551, 2013. doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes BJ, Tuong CM, Mellen NM. Functional imaging reveals respiratory network activity during hypoxic and opioid challenge in the neonate rat tilted sagittal slab preparation. J Neurophysiol 97: 2283–2292, 2007. doi: 10.1152/jn.01056.2006. [DOI] [PubMed] [Google Scholar]
- 18.Dhingra RR, Furuya WI, Bautista TG, Dick TE, Galan RF, Dutschmann M. Increasing local excitability of brainstem respiratory nusclei reveals a distributed network underlying respiratory motor pattern formation. Front Physiol 10: 887, 2019. doi: 10.3389/fphys.2019.00887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol 35: 429–450, 1990. doi: 10.1016/0301-0082(90)90030-K. [DOI] [PubMed] [Google Scholar]
- 20.Lumsden T. Observations on the respiratory centres in the cat. J Physiol 57: 153–160, 1923. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onimaru H, Arata A, Homma I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Jpn J Physiol 47: 385–403, 1997. doi: 10.2170/jjphysiol.47.385. [DOI] [PubMed] [Google Scholar]
- 22.Paton JF. The ventral medullary respiratory network of the mature mouse studied in a working heart-brainstem preparation. J Physiol 493: 819–831, 1996. doi: 10.1113/jphysiol.1996.sp021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol 100: 93–107, 1982. [DOI] [PubMed] [Google Scholar]
- 24.Segers LS, Shannon R, Lindsey BG. Interactions between rostral pontine and ventral medullary respiratory neurons. J Neurophysiol 54: 318–334, 1985. doi: 10.1152/jn.1985.54.2.318. [DOI] [PubMed] [Google Scholar]
- 25.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol 354: 173–183, 1984. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellenberger HH, Feldman JL. Origins of excitatory drive within the respiratory network: anatomical localization. Neuroreport 5: 1933–1936, 1994. doi: 10.1097/00001756-199410000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res 403: 380–384, 1987. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- 29.Gasparini S, Howland JM, Thatcher AJ, Geerling JC. Central afferents to the nucleus of the solitary tract in rats and mice. J Comp Neurol 528: 2708–2728, 2020. doi: 10.1002/cne.24927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonham A, McCrimmon D. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol 427: 261–280, 1990. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal sensory neuron subtypes that differentially control breathing. Cell 161: 622–633, 2015. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschenes M, Kurnikova A, Elbaz M, Kleinfeld D. Circuits in the ventral medulla that phase-lock motoneurons for coordinated sniffing and whisking. Neural Plast 2016: 7493048, 2016. doi: 10.1155/2016/7493048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McElvain LE, Friedman B, Karten HJ, Svoboda K, Wang F, Deschenes M, Kleinfeld D. Circuits in the rodent brainstem that control whisking in concert with other orofacial motor actions. Neuroscience 368: 152–170, 2018. doi: 10.1016/j.neuroscience.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doty R, Bosma J. An electromyographic analysis of reflex deglutition. J Neurophysiol 19: 44–60, 1956. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 35.Miller FR, Sherrington CS. Some observations on the bucco-pharyngeal stage of reflux deglutition in the cat. Quart J Exp Physiol 9: 147–186, 1915. doi: 10.1113/expphysiol.1915.sp000201. [DOI] [Google Scholar]
- 36.Doty R. Neural organization of deglutition. In: Handbook of Physiology. Washington, DC: American Physiological Society, 1968, p. 1861–1902. [Google Scholar]
- 37.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol 102: 1017–1025, 2009. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol (1985) 102: 587–600, 2007. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 39.Marckwald M. The Movements of Respiration and Their Innervation in the Rabbit: With a Supplement on the Relation of Respiration to Deglutition, and on the Question of the Existence of Respiratory Centres in the Spinal Cord. London, UK: Blackie & Son, 1888. [Google Scholar]
- 40.Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review. J Auton Nerv Syst 10: 225–233, 1984. doi: 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- 41.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 42.Kessler J, Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res 57: 256–263, 1985. doi: 10.1007/BF00236530. [DOI] [PubMed] [Google Scholar]
- 43.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol 114: 2226–2244, 2003. doi: 10.1016/S1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 44.Bieger D. Rhombencephalic pathways and neurotransmitters controlling deglutition. Am J Med 111: 85–89, 2001. doi: 10.1016/S0002-9343(01)00824-5. [DOI] [PubMed] [Google Scholar]
- 45.Gestreau C, Milano S, Bianchi AL, Grelot L. Activity of dorsal respiratory group inspiratory neurons during laryngeal-induced fictive coughing and swallowing in decerebrate cats. Exp Brain Res 108: 247–256, 1996. doi: 10.1007/BF00228098. [DOI] [PubMed] [Google Scholar]
- 46.Oku Y, Tanaka I, Ezure K. Activity of bulbar respiratory neurons during fictive coughing and swallowing in the decerebrate cat. J Physiol 480: 309–324, 1994. doi: 10.1113/jphysiol.1994.sp020361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito Y, Tanaka I, Ezure K. Morphology of the decrementing expiratory neurons in the brainstem of the rat. Neurosci Res 44: 141–153, 2002. doi: 10.1016/S0168-0102(02)00095-0. [DOI] [PubMed] [Google Scholar]
- 48.Amirali A, Tsai G, Weisz D, SchrAder N, Sanders I. Mapping of brain stem neuronal circuitry active during swallowing. Ann Otol Rhinol Laryngol 110: 502–513, 2001. doi: 10.1177/000348940111000603. [DOI] [PubMed] [Google Scholar]
- 49.Kleinfeld D, Moore JD, Wang F, Deschenes M. The brainstem oscillator for whisking and the case for breathing as the master clock for orofacial motor actions. Cold Spring Harb Symp Quant Biol 79: 29–39, 2014. doi: 10.1101/sqb.2014.79.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mellen NM. A vibrating microtome attachment for cutting brain slice preparations at reproducible compound angles relative to the midline. J Neurosci Methods 168: 113–118, 2008. doi: 10.1016/j.jneumeth.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol 164: 3–11, 2008. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gourevitch B, Mellen N. The preBotzinger complex as a hub for network activity along the ventral respiratory column in the neonate rat. NeuroImage 98: 460–474, 2014. doi: 10.1016/j.neuroimage.2014.04.073. [DOI] [PubMed] [Google Scholar]
- 53.Mellen NM, Mishra D. Functional anatomical evidence for respiratory rhythmogenic function of endogenous bursters in rat medulla. J Neurosci 30: 8383–8392, 2010. doi: 10.1523/JNEUROSCI.5510-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mellen NM, Funk GD. The sagittally-sectioned rat hindbrain preparation: improved access to the brainstem respiratory network. In: Multidisciplinary Tools for Investigating Synaptic Plasticity, edited by Nguyen PV. New York: Springer-Verlag, 2013, p. 257–268. [Google Scholar]
- 55.Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Botzinger complex. J Comp Neurol 455: 499–512, 2003. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- 56.Mellen NM, Feldman JL. Vagal stimulation induces expiratory lengthening in the in vitro neonate rat. J Appl Physiol (1985) 83: 1607–1611, 1997. doi: 10.1152/jappl.1997.83.5.1607. [DOI] [PubMed] [Google Scholar]
- 57.Mellen NM, Feldman JL. Phasic vagal sensory feedback transforms respiratory neuron activity in vitro. J Neurosci 21: 7363–7371, 2001. doi: 10.1523/JNEUROSCI.21-18-07363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mellen NM, Tuong CM. Semi-automated region of interest generation for the analysis of optically recorded neuronal activity. NeuroImage 47: 1331–1340, 2009. doi: 10.1016/j.neuroimage.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feroah TR, Forster H, Fuentes CG, Lang IM, Beste D, Martino P, Pan L, Rice T. Effects of spontaneous swallows on breathing in awake goats. J Appl Physiol (1985) 92: 1923–1935, 2002. doi: 10.1152/japplphysiol.01079.2000. [DOI] [PubMed] [Google Scholar]
- 60.Feroah TR, Forster H, Fuentes CG, Wenninger J, Martino P, Hodges M, Pan L, Rice T. Contributions from rostral medullary nuclei to coordination of swallowing and breathing in awake goats. J Appl Physiol (1985) 93: 581–591, 2002. doi: 10.1152/japplphysiol.01268.2001. [DOI] [PubMed] [Google Scholar]
- 61.Gestreau C, Grelot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Exp Brain Res 130: 27–34, 2000. doi: 10.1007/s002210050003. [DOI] [PubMed] [Google Scholar]
- 62.Hårdemark Cedborg AI, Sundman E, Bodén K, Hedström HW, Kuylenstierna R, Ekberg O, Eriksson LI. Co‐ordination of spontaneous swallowing with respiratory airflow and diaphragmatic and abdominal muscle activity in healthy adult humans. Exp Physiol 94: 459–468, 2009. doi: 10.1113/expphysiol.2008.045724. [DOI] [PubMed] [Google Scholar]
- 63.Pitts T, Poliacek I, Rose MJ, Reed MD, Condrey JA, Tsai H-W, Zhou G, Davenport PW, Bolser DC. Neurons in the dorsomedial medulla contribute to swallow pattern generation: evidence of inspiratory activity during swallow. PLoS One 13: e0199903, 2018. doi: 10.1371/journal.pone.0199903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pitts T, Rose M, Poliacek I, Condrey J, Davenport PW, Bolser D. Effect of laparotomy on the swallow–breathing relationship in the cat. Lung 193: 129–133, 2015. doi: 10.1007/s00408-014-9662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spearman DG, Poliacek I, Rose MJ, Bolser DC, Pitts T. Variability of the pharyngeal phase of swallow in the cat. PLoS One 9: e106121, 2014. doi: 10.1371/journal.pone.0106121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Umezaki T, Shiba K, Sugiyama Y. Intracellular activity of pharyngeal motoneurons during breathing, swallowing, and coughing. J Neurophysiol 124: 750–762, 2020. doi: 10.1152/jn.00093.2020. [DOI] [PubMed] [Google Scholar]
- 67.Kessler JP, Cherkaoui N, Catalin D, Jean A. Swallowing responses induced by microinjection of glutamate and glutamate agonists into the nucleus tractus solitarius of ketamine-anesthetized rats. Exp Brain Res 83: 151–158, 1990. doi: 10.1007/BF00232203. [DOI] [PubMed] [Google Scholar]
- 68.Neuhuber W, Bieger D. Brainstem control of deglutition: brainstem neural circuits and mediators regulating swallowing. In: Principles of Deglutition, edited by Shaker R, Belafsky P, Postma G, Easterling C. New York: Springer, 2013, p. 89–113. doi: 10.1007/978-1-4614-3794-9_7. [DOI] [Google Scholar]
- 69.Ali GN, Laundl TM, Wallace KL, deCarle DJ, Cook IJS. Influence of cold stimulation on the normal pharyngeal swallow response. Dysphagia 11: 2–8, 1996. doi: 10.1007/BF00385791. [DOI] [PubMed] [Google Scholar]
- 70.Borggreven PA, Leeuw IV, Rinkel RN, Langendijk JA, Roos JC, David EF, De Bree R, Leemans CR. Swallowing after major surgery of the oral cavity or oropharynx: a prospective and longitudinal assessment of patients treated by microvascular soft tissue reconstruction. Head & Neck 29: 638–647, 2007. doi: 10.1002/hed.20582. [DOI] [PubMed] [Google Scholar]
- 71.Butler SG, Postma GN, Fischer E. Effects of viscosity, taste, and bolus volume on swallowing apnea duration of normal adults. Otolaryngol Head Neck Surg 131: 860–863, 2004. doi: 10.1016/j.otohns.2004.06.706. [DOI] [PubMed] [Google Scholar]
- 72.Chee C, Arshad S, Singh S, Mistry S, Hamdy S. The influence of chemical gustatory stimuli and oral anaesthesia on healthy human pharyngeal swallowing. Chem Senses 30: 393–400, 2005. doi: 10.1093/chemse/bji034. [DOI] [PubMed] [Google Scholar]
- 73.Ertekin C, Kiylioglu N, Tarlaci S, Keskin A, Aydogdu I. Effect of mucosal anaesthesia on oropharyngeal swallowing. Neurogastroenterol Motil 12: 567–572, 2000. doi: 10.1046/j.1365-2982.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- 74.Hamdy S, Jilani S, Price V, Parker C, Hall N, Power M. Modulation of human swallowing behaviour by thermal and chemical stimulation in health and after brain injury. Neurogastroenterol Motil 15: 69–77, 2003. doi: 10.1046/j.1365-2982.2003.00390.x. [DOI] [PubMed] [Google Scholar]
- 75.Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia 16: 128–135, 2001. doi: 10.1007/s004550011001. [DOI] [PubMed] [Google Scholar]
- 76.Huff A, Day TA, English M, Reed MD, Zouboules S, Saran G, Leacy JK, Mann C, Peltonen JDB, O'Halloran KD, Sherpa MT, Pitts T. Swallow-breathing coordination during incremental ascent to altitude. Respir Physiol Neurobiol 265: 121–126, 2019. doi: 10.1016/j.resp.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kitagawa J, Nakagawa K, Hasegawa M, Iwakami T, Shingai T, Yamada Y, Iwata K. Facilitation of reflex swallowing from the pharynx and larynx. J Oral Sci 51: 167–171, 2009. doi: 10.2334/josnusd.51.167. [DOI] [PubMed] [Google Scholar]
- 78.Kitagawa J, Shingai T, Takahashi Y, Yamada Y. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol 282: R1342–R1347, 2002. doi: 10.1152/ajpregu.00556.2001. [DOI] [PubMed] [Google Scholar]
- 79.Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Hear Res 38: 556–563, 1995. doi: 10.1044/jshr.3803.556. [DOI] [PubMed] [Google Scholar]
- 80.Paterson W. Alteration of swallowing and oesophageal peristalsis by different initiators of deglutition. Neurogastroenterol Motil 11: 63–68, 1999. doi: 10.1046/j.1365-2982.1999.00131.x. [DOI] [PubMed] [Google Scholar]
- 81.Pommerenke WT. A study of the sensory areas eliciting the swallow reflex. Am J Physiol 84: 36–41, 1928. doi: 10.1152/ajplegacy.1928.84.1.36. [DOI] [Google Scholar]
- 82.Rademaker AW, Pauloski BR, Colangelo LA, Logemann JA. Age and volume effects on liquid swallowing function in normal women. J Speech Lang Hear Res 41: 275–284, 1998. doi: 10.1044/jslhr.4102.275. [DOI] [PubMed] [Google Scholar]
- 83.Shingai T, Shimada K. Reflex swallowing elicited by water and chemical substances applied in the oral cavity, pharynx, and larynx of the rabbit. Jpn J Physiol 26: 455–469, 1976. doi: 10.2170/jjphysiol.26.455. [DOI] [PubMed] [Google Scholar]
- 84.Sifrim D, Lefebvre R. Role of nitric oxide during swallow-induced esophageal shortening in cats. Dig Dis Sci 46: 822–830, 2001. doi: 10.1023/a:1010760619615. [DOI] [PubMed] [Google Scholar]
- 85.Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, Dziewas R. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci 8: 62, 2007. doi: 10.1186/1471-2202-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weerasuriya A, Bieger D, Hockman C. Interaction between primary afferent nerves in the elicitation of reflex swallowing. Am J Physiol 239: R407–R414, 1980. doi: 10.1152/ajpregu.1980.239.5.R407. [DOI] [PubMed] [Google Scholar]
- 87.Yamamura K, Kitagawa J, Kurose M, Sugino S, Takatsuji H, Mostafeezur RM, Zakir HM, Yamada Y. Neural mechanisms of swallowing and effects of taste and other stimuli on swallow initiation. Biol Pharm Bull 33: 1786–1790, 2010. doi: 10.1248/bpb.33.1786. [DOI] [PubMed] [Google Scholar]
- 88.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol 281: G1246–G1263, 2001. doi: 10.1152/ajpgi.2001.281.5.G1246. [DOI] [PubMed] [Google Scholar]
- 89.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol 101: 1386–1393, 2009. doi: 10.1152/jn.90847.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurnikova A, Deschenes M, Kleinfeld D. Functional brain stem circuits for control of nose motion. J Neurophysiol 121: 205–217, 2019. doi: 10.1152/jn.00608.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isokawa-Akesson M, Komisaruk BR. Difference in projections to the lateral and medial facial nucleus: anatomically separate pathways for rhythmical vibrissa movement in rats. Exp Brain Res 65: 385–398, 1987. doi: 10.1007/BF00236312. [DOI] [PubMed] [Google Scholar]
- 92.Stanek ET, Cheng S, Takatoh J, Han BX, Wang F. Monosynaptic premotor circuit tracing reveals neural substrates for oro-motor coordination. eLife 3: e02511, 2014. doi: 10.7554/eLife.02511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol 220: 280–298, 1983. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- 94.Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol 488: 28–47, 2005. doi: 10.1002/cne.20604. [DOI] [PubMed] [Google Scholar]
- 95.Amri M, Car A, Roman C. Axonal branching of medullary swallowing neurons projecting on the trigeminal and hypoglossal motor nuclei: demonstration by electrophysiological and fluorescent double labeling techniques. Exp Brain Res 81: 384–390, 1990. doi: 10.1007/BF00228130. [DOI] [PubMed] [Google Scholar]
- 96.Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res 557: 265–279, 1991. doi: 10.1016/0006-8993(91)90143-J. [DOI] [PubMed] [Google Scholar]
- 97.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 98.Li YQ, Takada M, Mizuno N. Premotor neurons projecting simultaneously to two orofacial motor nuclei by sending their branched axons. A study with a fluorescent retrograde double-labeling technique in the rat. Neurosci Lett 152: 29–32, 1993. doi: 10.1016/0304-3940(93)90475-Z. [DOI] [PubMed] [Google Scholar]
- 99.Ter Horst GJ, Copray JC, Liem RS, Van Willigen JD. Projections from the rostral parvocellular reticular formation to pontine and medullary nuclei in the rat: involvement in autonomic regulation and orofacial motor control. Neuroscience 40: 735–758, 1991. doi: 10.1016/0306-4522(91)90009-D. [DOI] [PubMed] [Google Scholar]
- 100.Anderson TM, GarciaAJ , 3rd, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM. A novel excitatory network for the control of breathing. Nature 536: 76–80, 2016. doi: 10.1038/nature18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci 19: 351–367, 2018. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci 28: 2353–2365, 2008. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moore JD, Kleinfeld D, Wang F. How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends Neurosci 37: 370–380, 2014. doi: 10.1016/j.tins.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of preBotzinger complex neurons in adult rats. J Comp Neurol 518: 1862–1878, 2010. doi: 10.1002/cne.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang CF, Kim EJ, Callaway EM, Feldman JL. Monosynaptic projections to excitatory and inhibitory preBötzinger complex neurons. Front Neuroanat 14: 58, 2020. doi: 10.3389/fnana.2020.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei in the rat using pseudorabies virus. II. Facial muscle motor systems. Brain Res Brain Res Rev 25: 276–290, 1997. doi: 10.1016/S0165-0173(97)00027-1. [DOI] [PubMed] [Google Scholar]
- 107.Chandler SH, Tal M. The effects of brain stem transections on the neuronal networks responsible for rhythmical jaw muscle activity in the guinea pig. J Neurosci 6: 1831–1842, 1986. doi: 10.1523/JNEUROSCI.06-06-01831.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nozaki S, Iriki A, Nakamura Y. Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol 55: 806–825, 1986. doi: 10.1152/jn.1986.55.4.806. [DOI] [PubMed] [Google Scholar]
- 109.Kolta A, Brocard F, Verdier D, Lund JP. A review of burst generation by trigeminal main sensory neurons. Arch Oral Biol 52: 325–328, 2007. doi: 10.1016/j.archoralbio.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 110.Katakura N, Jia L, Nakamura Y. NMDA-induced rhythmical activity in XII nerve of isolated CNS from newborn rats. Neuroreport 6: 601–604, 1995. doi: 10.1097/00001756-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 111.Nakamura Y, Katakura N, Nakajima M. Generation of rhythmical ingestive activities of the trigeminal, facial, and hypoglossal motoneurons in in vitro CNS preparations isolated from rats and mice. J Med Dent Sci 46: 63–73, 1999. [PubMed] [Google Scholar]
- 112.Chen Z, Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. Am J Physiol Regul Integr Comp Physiol 285: R68–R83, 2003. doi: 10.1152/ajpregu.00054.2003. [DOI] [PubMed] [Google Scholar]
- 113.Kleinfeld D, Deschênes M, Wang F, Moore JD. More than a rhythm of life: breathing as a binder of orofacial sensation. Nat Neurosci 17: 647–651, 2014. doi: 10.1038/nn.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.German RZ, Crompton AW, Thexton AJ. The coordination and interaction between respiration and deglutition in young pigs. J Comp Physiol A 182: 539–547, 1998. doi: 10.1007/s003590050201. [DOI] [PubMed] [Google Scholar]
- 115.Matsuo K, Palmer JB. Coordination of mastication, swallowing and breathing. Jpn Dent Sci Rev 45: 31–40, 2009. doi: 10.1016/j.jdsr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McFarland DH, Lund JP. An investigation of the coupling between respiration, mastication, and swallowing in the awake rabbit. J Neurophysiol 69: 95–108, 1993. doi: 10.1152/jn.1993.69.1.95. [DOI] [PubMed] [Google Scholar]
- 117.Matsuo K, Palmer JB. Coordination of oro-pharyngeal food transport during chewing and respiratory phase. Physiol Behav 142: 52–56, 2015. doi: 10.1016/j.physbeh.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 118.McFarland DH, Lund JP. Modification of mastication and respiration during swallowing in the adult human. J Neurophysiol 74: 1509–1517, 1995. doi: 10.1152/jn.1995.74.4.1509. [DOI] [PubMed] [Google Scholar]
- 119.Kobler JB, Datta S, Goyal RK, Benecchi EJ. Innervation of the larynx, pharynx, and upper esophageal sphincter of the rat. J Comp Neurol 349: 129–147, 1994. doi: 10.1002/cne.903490109. [DOI] [PubMed] [Google Scholar]
- 120.Bautista TG, Sun QJ, Pilowsky PM. The generation of pharyngeal phase of swallow and its coordination with breathing: interaction between the swallow and respiratory central pattern generators. Prog Brain Res 212: 253–275, 2014. doi: 10.1016/B978-0-444-63488-7.00013-6. [DOI] [PubMed] [Google Scholar]
- 121.Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci 23: 4657–4666, 2003. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol (1985) 106: 138–152, 2009. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mori RL, Bergsman AE, Holmes MJ, Yates BJ. Role of the medial medullary reticular formation in relaying vestibular signals to the diaphragm and abdominal muscles. Brain Res 902: 82–91, 2001. doi: 10.1016/S0006-8993(01)02370-8. [DOI] [PubMed] [Google Scholar]
- 124.Xu F, Zhou T, Gibson T, Frazier DT. Fastigial nucleus-mediated respiratory responses depend on the medullary gigantocellular nucleus. J Appl Physiol (1985) 91: 1713–1722, 2001. doi: 10.1152/jappl.2001.91.4.1713. [DOI] [PubMed] [Google Scholar]
- 125.Xu F, Zhuang J, Zhou TR, Gibson T, Frazier DT. Activation of different vestibular subnuclei evokes differential respiratory and pressor responses in the rat. J Physiol 544: 211–223, 2002. doi: 10.1113/jphysiol.2002.022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin-Harris B, McFarland D, Hill EG, Strange CB, Focht KL, Wan Z, Blair J, McGrattan K. Respiratory-swallow training in patients with head and neck cancer. Arch Phys Med Rehabil 96: 885–893, 2015. doi: 10.1016/j.apmr.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martin-Harris B, McFarland DH. Coordination of deglutition and respiration. In: Principles of Deglutition, edited by Shaker R, Belafsky P, Postma G, Easterling C. New York: Springer, 2013, p. 25–34. doi: 10.1007/978-1-4614-3794-9_3. [DOI] [Google Scholar]
- 128.Wheeler Hegland K, Huber JE, Pitts T, Davenport PW, Sapienza CM. Lung volume measured during sequential swallowing in healthy young adults. J Speech Lang Hear Res 54: 777–786, 2011. doi: 10.1044/1092-4388(2010/09-0237. [DOI] [PubMed] [Google Scholar]
- 129.Wheeler Hegland KM, Huber JE, Pitts T, Sapienza CM. Lung volume during swallowing: single bolus swallows in healthy young adults. J Speech Lang Hear Res 52: 178–187, 2009. doi: 10.1044/1092-4388(2008/07-0165. [DOI] [PubMed] [Google Scholar]
- 130.Horton KK, Segers LS, Nuding SC, O'Connor R, Alencar PA, Davenport PW, Bolser DC, Pitts T, Lindsey BG, Morris KF, Gestreau C. Central respiration and mechanical ventilation in the gating of swallow with breathing. Front Physiol 9: 785, 2018. doi: 10.3389/fphys.2018.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun QJ, Bautista TG, Berkowitz RG, Zhao WJ, Pilowsky PM. The temporal relationship between non-respiratory burst activity of expiratory laryngeal motoneurons and phrenic apnoea during stimulation of the superior laryngeal nerve in rat. J Physiol 589: 1819–1830, 2011. doi: 10.1113/jphysiol.2010.203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toor R, Sun QJ, Kumar NN, Le S, Hildreth CM, Phillips JK, McMullan S. Neurons in the intermediate reticular nucleus coordinate postinspiratory activity, swallowing, and respiratory-sympathetic coupling in the rat. J Neurosci 39: 9757–9766, 2019[Erratum inJ Neurosci41: 1617, 2021]. doi: 10.1523/JNEUROSCI.0502-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ezure K, Oku Y, Tanaka I. Location and axonal projection of one type of swallowing interneurons in cat medulla. Brain Res 632: 216–224, 1993. doi: 10.1016/0006-8993(93)91156-M. [DOI] [PubMed] [Google Scholar]
- 134.Ezure K, Tanaka I, Kondo M. Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J Neurosci 23: 8941–8948, 2003. doi: 10.1523/JNEUROSCI.23-26-08941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Segers LS, Nuding SC, Ott MM, Dean JB, Bolser DC, O'Connor R, Morris KF, Lindsey BG. Peripheral chemoreceptors tune inspiratory drive via tonic expiratory neuron hubs in the medullary ventral respiratory column network. J Neurophysiol 113: 352–368, 2015. doi: 10.1152/jn.00542.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hernandez-Miranda LR, Ruffault PL, Bouvier JC, Murray AJ, Morin-Surun MP, Zampieri N, Cholewa-Waclaw JB, Ey E, Brunet JF, Champagnat J, Fortin G, Birchmeier C. Genetic identification of a hindbrain nucleus essential for innate vocalization. Proc Natl Acad Sci USA 114: 8095–8100, 2017. doi: 10.1073/pnas.1702893114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lutcke H, Gerhard F, Zenke F, Gerstner W, Helmchen F. Inference of neuronal network spike dynamics and topology from calcium imaging data. Front Neural Circuits 7: 201, 2013. doi: 10.3389/fncir.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsien RY, Ernst LA, Waggoner AS. Fluorophores for confocal microscopy: photophysics and photochemistry. In: Handbook of Confocal Microscopy, edited byPawley J.New York: Plenum Press, 2010, p. 351–365. doi: 10.1007/978-0-387-45524-2_16. [DOI] [Google Scholar]
- 140.Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proc Natl Acad Sci USA 98: 13763–13768, 2001. doi: 10.1073/pnas.231499798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mellen NM. Degeneracy as a substrate for respiratory regulation. Respir Physiol Neurobiol 172: 1–7, 2010. doi: 10.1016/j.resp.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


