Abstract
This paper describes printing of microscale fibroblast-laden matrices using an aqueous two-phase approach that controls thrombin-mediated enzymatic crosslinking of fibrin. Optimization of aqueous two-phase formulations enabled polymerization of consistent sub-microliter volumes of cell-laden fibrin. When plasminogen was added to these micro-scaffolds, the primary normal human lung fibroblasts converted it to plasmin, triggering gradual degradation of the fibrin. Time-lapse live-cell imaging and automated image analysis provided readouts of time to degradation of 50% of the scaffold as well as maximum degradation rate. The time required for degradation decreased linearly with cell number while it increased in a dose-dependent manner upon addition of TGF-β1. Fibroblasts isolated from idiopathic pulmonary fibrosis patients showed similar trends with regards to response to TGF-β1 stimulation. Addition of reactive oxygen species slowed fibrinolysis but only in the absence of TGF-β1, consistent with published studies demonstrating that pro-fibrotic cellular phenotypes induced by TGF-β1 are mediated, at least in part, through increased production of reactive oxygen species. FDA-approved and experimental anti-fibrosis drugs were also tested for their effects on fibrinolysis rates. Given the central role of fibrinolysis in both normal and pathogenic wound healing of various tissues, the high-throughput cell-mediated fibrinolysis assay described has broad applicability in the study of many different cell types and diseases. Furthermore, aqueous two-phase printing of fibrin addresses several current limitations of fibrin bio-inks, potentially enabling future applications in tissue engineering and in vitro models.
Keywords: fibrinolysis, phenotypic assay, aqueous two-phase system, fibrin, wound healing, fibrosis
1. Introduction
Degradation of the provisional fibrin matrix is a key process in wound healing [1]. Following tissue damage, fibrin serves as a temporary scaffold that enables fibroblasts to migrate to the injury site for matrix remodeling [2]. Accelerated fibrin degradation can delay healing by hindering cells’ ability to migrate into the wound [3], while suppressed fibrin degradation can promote fibrotic scarring by contributing to excessive collagen accumulation [3, 4]. Properly regulated fibrinolysis is crucial to wound resolution; however, few phenotypic assays are available to evaluate cell-mediated fibrin degradation [5]. Due to the variety of cell-produced proteases and inhibitors as well as biomechanical cellular processes that combinatorially influence fibrinolysis [6], there exists the need for improved phenotypic assays for cell-mediated fibrin degradation.
Many techniques to evaluate cell-mediated fibrinolysis focus on quantifying mRNA and protein expression for specific components of the fibrinolytic system; however, these approaches are likely to neglect the fibrinolytic contributions of any components that are not specifically evaluated [7–10]. Cell-mediated modulation of the fibrinolytic system occurs predominantly through regulating conversion of the proenzyme plasminogen into the active enzyme plasmin, which degrades fibrin into soluble degradation products. This activation of plasmin is controlled by cellular expression of a variety of proteases and inhibitors, enabling cells to both positively and negatively regulate fibrinolysis [11–15]. Due to this large number of regulators involved in fibrinolysis, experimental approaches that focus on specific contributors to cell-mediated fibrin degradation are liable to overlook unexpected changes to the fibrinolytic system.
Here, we analyze human lung fibroblast-mediated fibrinolysis with a focus on idiopathic pulmonary fibrosis (IPF), a disorder of unknown etiology where repeated small injuries have been implicated in pathogenesis. This chronic fibroproliferative disease presents as progressive loss of lung function due to fibrotic remodeling; where epigenetics, senescence, and environmental factors have all been proposed as potential contributing factors [16]. The progression of IPF results in a progressive decline in lung capacity that ultimately leads to respiratory failure and death [17]. A growing body of evidence indicates that dysregulation of the fibrinolytic system may be a contributor to IPF pathogenesis [18–20]. Current FDA-approved drugs for IPF are incapable of curing or reversing fibrosis, and can only slow the progression of fibrotic scarring throughout the lungs [21]. However, both of these therapeutics target fibroblast proliferation, thus it is feasible that targeting different phenotypes in the fibrotic process may hold the key to the development of more effective therapeutics. A high-throughput phenotypic assay for fibrinolysis may contribute to efforts to find therapeutic compounds.
There are currently few phenotypic approaches to evaluate fibrinolysis. One implementation of cell-laden fibrin gels was able to differentiate between mesenchymal stem cells sourced from different tissues by comparing relative amounts of degraded matrix [5]. Importantly, this approach implemented a fibrin hydrogel, which enabled the biophysical feedback of matrix interactions to contribute to fibrin remodeling [22, 23]. However, this fibrinolysis assay faces limitations due to its large volume format. A high-throughput adaptation of this fibrin-based fibrinolysis assay would require establishing a microplate-compatible technique to generate the cell-laden fibrin scaffolds. This poses an engineering hurdle due to difficulties controlling thrombin-mediated crosslinking for low volume fibrin structures [24]. No prior methods to fabricate microscale fibrin scaffolds were suitable for a high-throughput assay.
To consistently print microscale cell-laden fibrin scaffolds with standard liquid handling equipment, this work details a new approach that controls crosslinking during the polymerization process by introducing fibrinogen and thrombin within separate aqueous phases. We utilize an aqueous two-phase system (ATPS), in which soluble polymers thermodynamically drive aqueous systems to form two distinct phases. Our research group has previously developed ATPS assays to evaluate collagen contraction with living cells [25]. In this prior publication, the ATPS was simply used to localize collagen to the dextran (DEX) phase while the slow collagen gelation process was taking place. Here, the DEX phase is used to localize fibrinogen, similar to our prior work, but with the additional use of the PEG phase as a source of thrombin that evenly diffuses into the DEX phase, but only after droplet formation, to enable successful polymerization of sub-microliter volumes of cell-laden fibrin scaffolds. These microgels can be directly degraded through addition of plasmin, or fibrinolysis can occur through cell-mediated activation of exogenous plasminogen. Fibrinolysis rates vary in response to pro-fibrotic stimuli and anti-fibrotic therapeutics, as determined through label-free brightfield microscopy. We demonstrate our fibrin-printing technology as a simple, versatile, and easily managed approach to fabricate precise microscale scaffolds, and this technology was used for phenotypic evaluation of cell-mediated fibrinolysis.
2. Materials and methods
2.1. Cell culture and ATPS reagents
A stock solution of DEX (20% w/w dextran T500; Sigma) was prepared in phosphate buffered saline (PBS) on a rocker overnight. A stock solution of PEG (6% w/w, 35k MW; Sigma) was prepared in fully supplemented culture media with 10% deionized water to balance osmolality. Both stock solutions were passed through a 0.22 μm sterilizing syringe filter before storage. PEG working solutions were stored for up to 2 weeks at 4°C. Thrombin (Human Alpha Thrombin; Enzyme Research Labs) was also added to the PEG solution at a concentration of 0.1 U/mL immediately preceding experiments. Fibrinogen-DEX solutions were prepared by diluting fibrinogen stock solution (human fibrinogen 3; Enzyme Research Labs) to a final concentration of 4 mg/mL in a sterile solution of 4% 10x DMEM, 15% DEX stock solution (to a final concentration of 3% dextran), and 50% cell suspension in growth media. For all experiments excluding cell concentration evaluation, the cell suspension was diluted for 1000 cells per microliter in the final fibrinogen-DEX solution.
2.2. Cell preparation
Normal human lung fibroblasts (NHLF lot#0000580583; Lonza) from a 79 year old female with a history of smoking, and idiopathic pulmonary fibrosis fibroblasts (IPF lot#0000627840; Lonza) from a 52 year old male were cultured in fibroblast growth media (FGM; Lonza). Cells were passaged at 80–90% confluence, and were sub-cultured in 1:3 ratios by trypsinization. When at the desired confluence, cells were washed with PBS and 0.05% trypsin solution was added to the flask. Cells were incubated for 2 min, diluted with fibroblast growth media, and then harvested and centrifuged (200 × g, 5min) in a conical tube. The supernatant was aspirated and the cell pellet was re-suspended in serum-free culture media. When used in fibrin degradation experiments, cells were re-suspended at 2x the final desired concentration (1000 cells/μl unless otherwise indicated). All experiments were conducted with cells at or below passage 12. In all experiments, media was changed every 48 hours and any media additives (plasminogen, TGF-β1, drugs, etc.) were included.
2.3. ATPS printing of fibrin microgels
Working solutions of PEG with 0.1 U/mL of thrombin were warmed to 37°C and pipetted into a 96-well plate. For production of droplets, fibrinogen-DEX solutions with cell suspension were maintained at 37°C and pipetted directly into the PEG-thrombin media using either a manual pipette or a semi-automated 96-channel pipette (Viaflo-96; Integra). All assays utilized a volume of 1 μl unless otherwise noted. Following dispensing of the DEX phase, the plates were placed in an incubator at 37°C for 30 min to allow the thrombin to enzymatically crosslink the fibrinogen into a fibrin matrix (figure 1a). The PEG-enriched media was then washed four times by removing, then replacing half of the media with PEG-free media. When applicable, the final media addition was supplemented with stimuli as detailed in section 2.5. For the duration of each experiment, assay plates were imaged every 2 hours at 4x with an automated cell culture monitoring system (Incucyte S3; Essen Biosystems). After one day of culture, plasminogen (50 μg/mL) (Human Glu-Plasminogen; Enzyme Research Labs) was added as a 10x concentrated solution to each well in order to initiate assay degradation (figure 1b), unless otherwise noted for specific conditions. Fresh plasminogen was included with each subsequent media addition. Positive controls with active plasmin (1 U/ml) (Human Plasmin; Enzyme Research Labs) and negative controls without plasminogen were included in each experiment. As cells activated plasminogen, the fibrin scaffold progressively degraded as illustrated in figure 1c.
Figure 1.
ATPS fibrin printing and cell-mediated degradation: (a) Illustration of the enzymatic control enabled by ATPS printing of fibrin scaffolds, whereby thrombin from the PEG phase diffuses into the DEX phase and crosslinks the fibrinogen into fibrin during the incubation period. (b) Process schematic of ATPS generation of microscale fibrin droplets and subsequent fibrinolysis. (c) Characteristic brightfield microscope images (taken at 4x magnification) illustrate the assay progression when stimulated with 0.5 ng/mL of TGF-β1, showing an opaque fibrin matrix and progressive degradation. Scale bars are 1 mm.
In order to print fibrin into letters and arbitrary shapes, a 6% PEG solution containing 0.1 U/ml of thrombin was pipetted into a 6-well plate and warmed to 37°C. A 6% DEX solution containing 8 mg/mL fibrinogen was pipetted directly into the PEG phase to manually draw the desired shapes. After 30 min, darkfield images were taken on a stereoscope (Leica S6 E) to visualize the printed fibrin scaffold.
For cell viability measurements, 1 μl fibrin scaffolds were printed each containing 5000 cells total. The fibroblast-laden scaffolds were maintained in serum free media for 24 hours before live/dead staining (ReadyProbes™ Cell Viability Imaging Kit; Invitrogen). NucBlue and NucGreen (staining for total cells and dead cells respectively) were applied according to manufacturer directions. Dead control scaffolds were treated with 70% ethanol for 15 min prior to staining. Scaffolds were fluorescently imaged to assess viability. For calculation of percent viability, the density counting workflow in ilastik was used to count the number of total cells and dead cells.
2.4. High-throughput brightfield image analysis
After each experiment, brightfield images for every time point were downloaded in jpeg format from the automated cell culture monitoring system. Python’s OpenCV library was implemented for the masking approach illustrated in figure 2a. First, a threshold was set at 50% of the maximum intensity (128 for 8-bit integer pixel values) in order to isolate the darker pixels of semi-opaque fibrin hydrogel from the background of the image. A closing morphological filter with a 25×25 kernel was then applied to each mask in order to remove noise. This masking approach was applied to the initial time point from every experimental condition in order to establish the relevant assay area for downstream measurements. As fibrin degrades during an experiment, the average pixel intensity within the masked area increases accordingly (figure 2b). The automated live-cell imager (Incucyte S3; Essen Biosystems) automatically adjusts brightness to maintain consistent white balance between images. For experiments involving multiple assay volumes, image brightness was scaled to maintain consistent background intensity (supplemental figure 1).
Figure 2.
High-throughput quantification of fibrin degradation: (a) Automated image processing and analysis utilized Python’s OpenCV library for thresholding and morphological filtering in order to establish an initial mask for each individual assay that was applied to all assay images for that well. Scale bars are 1 mm. (b) The average pixel intensity within masked regions was plotted for time course evaluation, as illustrated here with different plasminogen addition times, where dotted lines indicate the plasminogen addition time and error bars represent the standard deviation between experimental replicates. (c) An example measurement demonstrates image metric extraction by fitting a logistic function to time course pixel intensity data with least squares regression. The time point for 50% degradation (d) and maximum slope from the sigmoid centroid (e) were determined using logistic functions fit for each experimental replicate. Note that the 50% degradation time (vertical axis) is indicated here as days after plasminogen addition, while the plasminogen addition time (horizontal axis) is in hours. (Statistical significance for (d, e) P < 0.01 by ANOVA. ab = P < 0.01; bc = P < 0.05; ac = P < 0.1 by post-hoc Tukey test. N = 5 for all conditions)
For each experimental replicate, a sigmoid curve was fit using the curve_fit function from the SciPy library in Python. The logistic function given by the equation in figure 2c enabled automated extraction of the time point for 50% degradation, as well as the maximum slope at the equation’s centroid (figures 2d and 2e).
2.5. Phenotypic evaluation of stimuli
In order to evaluate fibrin degradation rate with a known anti-fibrinolytic stimulus, various concentrations of transforming growth factor type β1 (Human Recombinant TGF-β1; Peprotech) were added to the assay media after ATPS polymers were rinsed out of the microplates.
To evaluate the capability of this assay to test the fibrinolytic effects of therapeutic stimuli, a variety of drug compounds were introduced to the fibrinolysis assays after the wash step. This included 400 μM pirfenidone (Selleck Chem), 0.4 μM nintedanib (Selleck Chem), 100 μM hydrogen peroxide (Sigma), and 20 μM diethyl-pythiDC (AOBIOUS). These concentrations were established in preliminary experiments that evaluated a range of concentrations used in prior literature. Stimuli were freshly mixed for each media change during experiments, and a minimum of four replicates were tested per experimental condition.
2.6. Statistical Analysis
All experimental values are reported as means ± standard deviation. ANOVA tests were performed using the statsmodels library in Python 3 with the Tukey test for post-hoc pairwise comparisons.
3. Results and discussion
The development and characterization of the cell-mediated fibrinolysis assay was focused on establishing a microplate-compatible fibroblast-laden fibrin scaffold and verifying the ability to distinguish between subtly different fibrinolytic environments. First, we implemented an ATPS approach to enable accurate printing of unprecedentedly small cell-laden fibrin scaffolds. Then, an automated image processing approach quantified fibrin degradation data from label-free brightfield images. Next, the established fibrinolytic effects of cell density and TGF-β1 were used to validate the assay’s capability to distinguish between conditions. Finally, the microscale cell mediated fibrinolysis assay was implemented to evaluate the effects of anti-fibrotic therapeutics on fibroblasts from normal and diseased donors.
3.1. Fabrication of microscale fibrin scaffolds
Biological environments establish fibrin matrices through coagulation, where a cascade of clotting factors activates thrombin, which enzymatically crosslinks fibrinogen into fibrin [2]. Similarly, in vitro fibrin scaffolds are formed by exposing monomeric fibrinogen to thrombin [26]. Fibrin has been used extensively in a wide variety of tissue engineering applications, but it is generally implemented as a bulk cast hydrogel. The conventional bulk casting procedure mixes thrombin and fibrinogen solutions by micropipette; however, this method cannot consistently handle small volumes (under 100 μl) due to adhesion of the partially coagulated mixture to pipette tips.
There have been a few applications of fibrin bio-printing that control crosslinking by alternating between layers of fibrinogen and thrombin, but this poses limitations to accuracy and reproducibility due to lack of control over fibrinogen’s exposure to thrombin [26–28]. There have also been a variety of applications for fibrin microbeads where oil immersions were used to disperse microbeads during crosslinking in oil-suspended droplets, but this results in inconsistent size and cells must be added separately after the microbeads have been washed [29, 30]. Reliable microscale volume and microplate compatibility were necessary to enable high-throughput adaptation in this assay. Precise control over cell seeding density was also vital for our approach due to its effect on remodeling rate.
We established a new approach to maintain fibrinogen in a distinct droplet and control diffusion of thrombin into fibrinogen during the polymerization process by implementing an ATPS with PEG and DEX. Above their critical concentrations, these soluble polymers thermodynamically drive aqueous systems to form two distinct phases [31]. A previous ATPS microscale adaptation from our lab for collagen contraction demonstrated consistency in response between the conventional 100 uL assay and ATPS microscale volumes. This work specifically took advantage of the short length scales for time-dependent and burst stimulation profiles, which would not be possible with conventional approaches due to diffusion constraints [25]. A similar ATPS adaptation suited our approach, and enabled fabrication of microscale fibrin scaffolds with standard liquid handling equipment to facilitate microplate compatibility.
During the initial optimization of PEG and DEX concentrations, we found that lower concentrations were unstable and resulted in fissure of the ATPS droplet. In order to maintain stable separation of phases during polymerization, minimum assay concentrations of 6% 35 kDa PEG and 3% T500 dextran were determined for stability during crosslinking (figure 1a). As a demonstration of the printing capabilities enabled by controlled enzymatic crosslinking, this formulation was pipetted into specific letters and shapes (supplemental figure 2). Fibroblast viability has previously been verified at these ATPS concentrations in a prior microscale assay adaptation of collagen scaffolds [25]. Fibroblast viability was evaluated for this fibrin ATPS procedure, which demonstrated 88.5 ± 0.6% viability (supplemental figure 3).
The necessity for this ATPS environment in our microscale fibrin degradation assay comes from the capability of aqueous two-phase partitioning to control the timing of thrombin diffusion into the fibrinogen droplet. This control over timing restricts enzymatic crosslinking of cell-laden fibrin matrices until after the droplets have been dispensed (figure 1a). After a 30 minute incubation period, the fibrin was sufficiently polymerized and the ATPS solutions could be removed and replaced with growth media and stimulants for specific conditions (figure 1b). Bioengineered tissues were incubated for an additional period of 24 hours in regular growth media in order to allow cells to anchor themselves to the fibrin matrix before adding exogenous plasminogen.
After plasminogen was added to the wells, various activators and inhibitors produced by cells regulate the conversion of plasminogen into plasmin [11–15]. Control conditions for each experiment verified rapid matrix degradation with the addition of exogenous plasmin and no matrix degradation when plasminogen is omitted (supplemental video 1). As the assay proceeds, the fibrin matrix gradually degrades with activated plasmin cleaving fibrin into soluble fibrin degradation products (figure 1c). This is visually evident by the disappearance of the opaque fibrin matrix. The following section is focused on implementing an image processing and analysis approach that enabled automated quantification of differences in fibrin degradation between conditions.
3.2. Label-free quantification of fibrin degradation
Due to the relative opacity of our fibrin scaffolds, pixels within the assay area are significantly darker than those in the background of microscope images. This enabled an analysis approach based on pixel intensities within the assay area. Many established hemostasis assays take advantage of fibrin’s attenuation of light for quantification. These assays generally implement plate readers to measure absorbance during coagulation and fibrinolysis [32, 33]. Evaluation of this assay in a microplate reader may therefore serve as an alternative to brightfield analysis. However, our approach favored evaluation of pixel intensity from brightfield images so that the micrographs could serve as validation of assay progression. Unfortunately, the commercial image analysis package embedded in our live cell imaging system was not able to reliably discern the microprinted fibrin scaffold. We therefore developed an alternative image analysis pipeline using Python’s OpenCV library.
In order to isolate the assay area from background, a thresholding approach was sufficient because of the significant difference in pixel brightness. Here, any pixels brighter than the specified threshold were classified as background. A closing morphological filter was applied to the thresholded images to remove noise left by the thresholding process. Figure 2a demonstrates mask generation and its implementation at later time points as the fibrin matrix degrades. After isolation of the assay area, average pixel intensity within masked regions was plotted in order to visualize time-course fibrin degradation (figure 2b). Fitting time-course data from each individual well with a sigmoidal curve facilitated extraction of the time point for 50% matrix degradation, as well as the maximum slope at the sigmoid’s centroid (figure 2c).
Figure 2d shows changes in the 50% degradation time point in response to different plasminogen addition times. The 50% degradation time point is shown as days since plasminogen addition. Increases in bar height indicate slower cell-mediated fibrinolysis. ANOVA indicated statistical significance of these differences in degradation time (P < 0.01), and post-hoc pairwise analysis with the Tukey test demonstrated statistically significant differences between specific conditions (figure 2d). The increase in time to 50% degradation for later plasminogen additions indicates significant changes in the scaffold or cells in the first 24 hours. It has previously been demonstrated that cell-matrix interactions influence the rate of fibroblast-mediated fibrinolysis, so additional time before plasminogen addition may have influenced rates observed here through similar pathways [23]. Hence, it was important in subsequent studies to evaluate cell-mediated fibrin degradation with a consistent plasminogen addition time. A plasminogen addition time at 24 hours was implemented so that fibroblasts could initiate cell-matrix interactions. This 24-hour addition of plasminogen was chosen to allow cells to recover from trypsinization [34] and minimize residual trypsin activity [35].
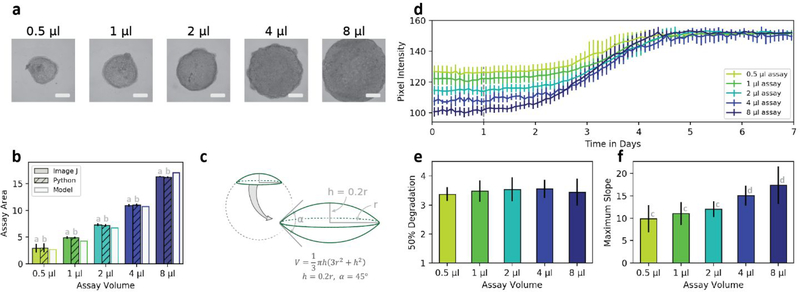
The effects of assay volume were also evaluated. Assay volumes between 0.5 μl and 8 μl could be consistently printed and viewed within the field-of-view of a 4x microscope objective (figure 3a). The Python-based image masking approach was compared against a manual approach that outlined the assay area in image J with no significant differences in cross sectional area between techniques (figure 3b). Cross sectional area was also compared to volume through evaluation of geometric models. Compared against spheres, hemispheres, and spherical caps; a doubled spherical cap fit the volume and area data most closely as determined through least squares regression (figure 3c).
Figure 3.
Assay volume consistency: (a) ATPS printing of fibrin scaffolds demonstrated consistency in assay shape and texture between volumes. Scale bars are 1 mm. (b) Cross sectional area of assays was compared between image J, Python generated masks, and a geometric model of assay volume. (c) A doubled spherical cap demonstrated the best fit of the geometric volume models evaluated (including sphere, hemisphere, and single spherical cap). (d) Time course data shows changes in average pixel intensity for different assay volumes to demonstrate consistency in fibrin degradation time between volume conditions. Different initial pixel intensity values between conditions indicate varied transmission of light through different volume constructs. The 50% degradation time (e) and maximum slope (f) further demonstrate these trends. (Statistical significance for (b, f) P < 0.01 by ANOVA. ab = P > 0.2; cd = P < 0.05 by post-hoc Tukey test. N = 4 for all conditions)
In our prior microscale adaptation of collagen contraction, we found that different assay volumes maintained consistent contraction rates as long as cell density was maintained [25]. Fibrinolysis trends in our experiments also depend on cell density rather than assay volume. While the pixel intensity of higher volume assays had lower starting values, this reflected the presence of more fibrin which resulted in decreased transmission of light through those assays (figure 3d). Time points for 50% degradation, as determined by a sigmoid fit, showed no significant difference in degradation timing between different volume conditions (figure 3e). This consistency in degradation timing indicates similar rates of cell-mediated fibrinolysis between different volume conditions. Differences in maximum slope between conditions followed the same trend as differences in initial pixel intensity, resulting from the decreased transmitted light through higher volume fibrin scaffolds.
The consistency in degradation rates between volume conditions indicates uniformity in fibrin organization. Fibrin network morphology has a significant impact on fibrinolysis rate, where tight fibrin conformations degrade at a slower rate than scaffolds with looser fibrin conformations and thicker fibers [36]. This suggests that at the concentration of thrombin used in our assays, ATPS-mediated control over the diffusion of thrombin into the fibrinogen-containing phase results in consistent fibrin organization across the range of assay volumes tested.
3.3. Effect of cell seeding density and TGF-β1
Cell seeding density was also evaluated. Conditions with higher seeding densities demonstrated significantly faster fibrinolysis (figure 4), with decreased time points for 50% degradation and increased maximum slope (figures 4b and c; P < 0.05 for all pairwise comparisons). The linear relationship between rate of fibrinolysis and cell number is consistent with a cell-mediated step being rate limiting in this process. This also highlights the importance of consistent cell-seeding density in fibrin printing applications. Our ATPS printing technique is uniquely capable of establishing microscale cell-laden fibrin scaffolds with a consistent seeding density [26, 30]. However, seeding densities higher than 5000 cells per microliter could not be consistently established due to an increased viscosity that interfered with pipetting.
Figure 4.
Cell density and TGF-β1 effects: (a) Time course pixel intensity data demonstrates changes in fibrin degradation between different densities of cells within a 1 μl assay. The 50% degradation time (b) and maximum slope (c) demonstrate decreased fibrinolysis time and increased slope with higher cell counts. (d) Pixel intensity data for various concentrations of TGF-β1 indicates delays in fibrin degradation in response to the stimulus. The 50% degradation time (e) and maximum slope (f) show increases in fibrinolysis time but no significant changes in slope with higher concentrations of TGF-β1. (Statistical significance P < 0.01 by ANOVA. In (b, c, e, f) P < 0.05 by post-hoc Tukey test between all bars with different lettered labels. N = 4 for all conditions)
TGF-β1 is an established pro-fibrotic stimulus with well-characterized anti-fibrinolytic effects [37]. Various concentrations of TGF-β1 were used to stimulate NHLF cells in the fibrin assays (figure 4d). Increasing concentrations resulted in longer time delays before fibrinolysis. The time points for 50% degradation further demonstrate this trend (figure 4e). All pairwise differences in 50% degradation time between conditions were significant with P < 0.05 (figure 4f). Interestingly, these differences in fibrinolysis profile appear as a delay before initiation of fibrin degradation. Prior studies have linked elevated PAI-1 with delayed fibrinolysis [38], and TGF-β1 stimulation is closely associated with upregulation of PAI-1 [39]. However, TGF-β1 is also involved in fibroblast proliferation and matrix production, so a variety of factors are likely involved in the altered fibrin degradation.
In NHLF cells, we also noticed a significant effect of cell passage number on fibrinolysis (supplemental figure 4a). Higher passage numbers exhibited progressively longer 50% degradation times with slower fibrin degradation rates (supplemental figure 4b). These incidental observations are consistent with prior studies which demonstrate inhibition of fibrinolysis in senescent fibroblasts in vivo and in vitro due in part to the upregulation of PAI-1 [40–42].
3.4. Evaluation of hydrogen peroxide, therapeutics and IPF fibroblasts
Having established baseline cell response measurements for fibrinolysis of the bioprinted fibrin micro-scaffolds, we next compared fibrinolytic profiles between normal and diseased lung fibroblasts with a number of stimulants and inhibitors. Hydrogen peroxide is a reactive oxygen species (ROS) known to be produced by cells in response to TGF-β1 stimulation, while nintedanib and pirfenidone are the only two FDA-approved therapies for IPF. Diethyl-pythiDC, an experimental anti-fibrotic drug, is an inhibitor of certain prolyl 4-hydroxylases that play a role in post-translational modification of collagen and other proteins [43]. The plasmin control condition was included in graphs for reference, but was excluded from statistical analysis in the interest of focusing on therapeutic conditions of interest.
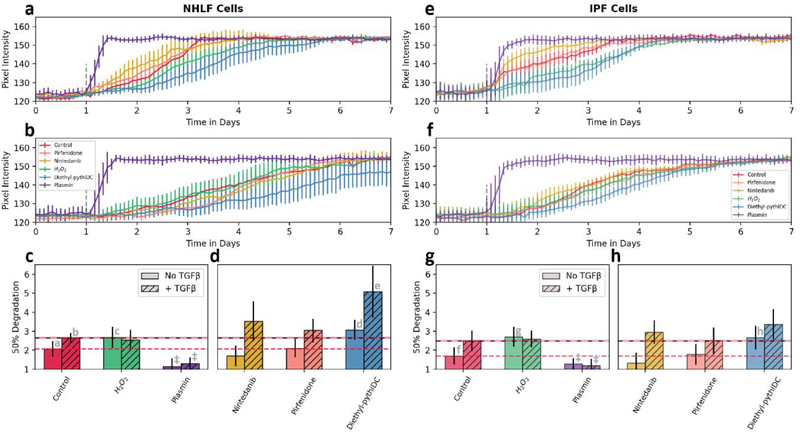
A general comparison between normal and diseased fibroblasts (figure 5a–h) demonstrates that cells from the IPF donor consistently degraded fibrin significantly faster than the normal fibroblasts (P < 0.01 by two-way ANOVA). However, prior research indicates that diseased fibroblasts from IPF donors express elevated levels of PAI-1 and should consequently exhibit slower fibrin degradation [44]. This unexpected decrease in fibrinolysis time in IPF fibroblasts may be due to the cells’ extended removal from the diseased microenvironment. In the diseased lung, overactive epithelial cells secrete several growth factors, cytokines, and chemokines involved in migration, proliferation, and activation of fibroblasts [45]. Additionally, the donor for these NHLF cells does not fit the typical profile for healthy lung tissue. This particular donor was a 79 year old female with a history of smoking. Age related cellular senescence and tobacco use have both been associated with increased levels of PAI-1, so the fibrinolytic system in these “normal” fibroblasts may be dysregulated compared to a younger non-smoking donor [46].
Figure 5.
Cell donor and drug stimulation: Time-course pixel intensity data show the effects on fibrin degradation of several different stimulants, with NHLF cells on the left and diseased IPF cells on the right. The upper pixel intensity graphs have no TGF-β1 (a and e) while the lower graphs contain 2 ng/ml TGF-β1 (b and f). Sigmoid fits were used to determine 50% degradation time (c, d, g and h) from the above pixel intensity graphs. Dotted lines show mean value from control conditions for comparison. (Statistical significance P < 0.01 by two-way ANOVA: As the positive control, plasmin was excluded from ANOVA. ‡ = P < 0.01; ad, be = P < 0.05; ac, fg, fh = P < 0.1 by post-hoc Tukey test. N = 4 for all conditions)
Stimulation with hydrogen peroxide (H2O2) alone demonstrated highly significant decreases in the rates of fibrinolysis (P < 0.1) suggesting a critical role of ROS in the process of cell-mediated fibrinolysis. In contrast, conditions that included TGF-β1 showed no significant difference upon further stimulation with exogenous hydrogen peroxide. This non-additive effect is consistent with a notion that the effects of adding exogenous H2O2 and exogenous TGF-β1 converge [47]. That is, TGF-β1-triggered increase in endogenous H2O2 production [48, 49], may mask effects of any exogenous H2O2 addition. Such effects may also work in concert with ROS-induced reduction in TGF-β1 receptors [43].
The two FDA-approved IPF drugs, nintedanib and pirfenidone, did not show a significant impact on fibrinolysis. These therapeutics have established anti-fibrotic effects, so these results indicate that the mechanism of action for nintedanib and pirfenidone has little relation to fibrinolytic activity of lung fibroblasts. Given the limitation that nintedanib and pirfenidone can slow but not stop or reverse IPF, the ability to test IPF-relevant pathways such as fibroblast-mediated fibrinolysis-associated processes [50] that these drugs do not target may provide opportunities for developing co-therapeutics or alternatives with enhanced efficacy. The experimental drug diethyl-pythiDC significantly (P < 0.05) delayed fibrinolysis. Diethyl-pythiDC is a selective inhibitor of prolyl 4-hydroxylase, an enzyme best known for structure-stabilizing modifications of collagen [43] that also acts on a variety of proteins [50] including hypoxia inducible factor 1 [47, 51]. The ability of diethyl-PythiDC to reduce fibroblast-mediated fibrinolysis is a novel finding and demonstrates the utility of our assay. Given the many physiological factors present in blood or expressed by many types of cells positively (e.g. proteases such as uPA, tPA, cathepsins, FXIa, FXIIa, kallikreins) and negatively (e.g. serpins such as PAI-1 and α2-antiplasmin, α2-macroglobulin) impact fibrinolysis [15, 52–54], we believe this fibrin printing and fibrinolysis assay to be of broad utility.
4. Conclusions
This work describes an approach for ATPS-based printing of microscale cell-laden fibrin scaffolds. A droplet comprised of the heavier phase partitions cells and fibrinogen while the bulk phase provides thrombin to promote localized enzymatic crosslinking, leading to controlled production of microliter-scale fibrin constructs. Automated label-free image processing quantified rates of cell-mediated fibrin degradation from time-course brightfield images. We found that primary human lung fibroblasts degrade the fibrin scaffold at a rate dependent on source of cells, cell density, and the presence of soluble factors. Given the variety of contributors to dysregulation of fibrinolysis seen in cancer, fibrosis, and metabolic disease; this phenotypic assay for cell-mediated fibrin degradation provides a potentially valuable research tool for further studies in these and other fields. Additionally, the technique developed here for aqueous two-phase printing of cell-laden fibrin by in situ enzymatic cross-linking can be broadly applied in bio-printing and tissue engineering applications.
Supplementary Material
Acknowledgements
We thank the NIH (R21 AG061687 and R01 HL136141) for funding. The authors also gratefully acknowledge funding by the Defense Threat Reduction Agency (DTRA) under Space and Naval Warfare Systems Center Pacific (SSC PACIFIC) Contract No. N66001-13-C-2027. The content is solely the responsibility of the authors and does not necessarily represent the official views of the awarding agency. This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship Program to J.C. and E.P. (DGE-1650044).
References
- 1.Mosesson MW, Fibrinogen and fibrin structure and functions. J Thromb Haemost, 2005. 3(8): p. 1894–904. [DOI] [PubMed] [Google Scholar]
- 2.Laurens N, Koolwijk P, and de Maat MP, Fibrin structure and wound healing. J Thromb Haemost, 2006. 4(5): p. 932–9. [DOI] [PubMed] [Google Scholar]
- 3.Moriwaki H, et al. , Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res, 2004. 95(6): p. 637–44. [DOI] [PubMed] [Google Scholar]
- 4.Flevaris P and Vaughan D, The Role of Plasminogen Activator Inhibitor Type-1 in Fibrosis. Semin Thromb Hemost, 2017. 43(2): p. 169–177. [DOI] [PubMed] [Google Scholar]
- 5.Chaires-Rosas CP, et al. , Differential adhesion and fibrinolytic activity of mesenchymal stem cells from human bone marrow, placenta, and Wharton's jelly cultured in a fibrin hydrogel. J Tissue Eng, 2019. 10: p. 2041731419840622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collen D, Ham-Wasserman lecture: role of the plasminogen system in fibrin-homeostasis and tissue remodeling. Hematology Am Soc Hematol Educ Program, 2001: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 7.Muhleder S, et al. , The role of fibrinolysis inhibition in engineered vascular networks derived from endothelial cells and adipose-derived stem cells. Stem Cell Res Ther, 2018. 9(1): p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roussel BD, et al. , HMGB-1 promotes fibrinolysis and reduces neurotoxicity mediated by tissue plasminogen activator. J Cell Sci, 2011. 124(Pt 12): p. 2070–6. [DOI] [PubMed] [Google Scholar]
- 9.Castillo O, et al. , Endothelial fibrinolytic response onto an evolving matrix of fibrin. BMC Hematol, 2016. 16: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanamaru Y, et al. , Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. J Immunol, 2006. 176(9): p. 5607–15. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman-Maus G and Hajjar KA, Molecular mechanisms of fibrinolysis. Br J Haematol, 2005. 129(3): p. 307–21. [DOI] [PubMed] [Google Scholar]
- 12.Kucharewicz I, et al. , The plasmin system in airway remodeling. Thromb Res, 2003. 112(1–2): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 13.Levi M, et al. , Assessment of the relative contribution of different protease inhibitors to the inhibition of plasmin in vivo. Thromb Haemost, 1993. 69(2): p. 141–6. [PubMed] [Google Scholar]
- 14.Bouma BN and Mosnier LO, Thrombin activatable fibrinolysis inhibitor (TAFI)--how does thrombin regulate fibrinolysis? Ann Med, 2006. 38(6): p. 378–88. [DOI] [PubMed] [Google Scholar]
- 15.Douglas SA, et al. , Human cathepsins K, L, and S: Related proteases, but unique fibrinolytic activity. Biochim Biophys Acta Gen Subj, 2018. 1862(9): p. 1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selman M and Pardo A, Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med, 2014. 189(10): p. 1161–72. [DOI] [PubMed] [Google Scholar]
- 17.King TE Jr., Pardo A, and Selman M, Idiopathic pulmonary fibrosis. Lancet, 2011. 378(9807): p. 1949–61. [DOI] [PubMed] [Google Scholar]
- 18.Loskutoff DJ and Quigley JP, PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest, 2000. 106(12): p. 1441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sisson TH and Simon RH, The plasminogen activation system in lung disease. Curr Drug Targets, 2007. 8(9): p. 1016–29. [DOI] [PubMed] [Google Scholar]
- 20.Swaisgood CM, et al. , The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol, 2000. 157(1): p. 177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahluwalia N, Shea BS, and Tager AM, New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med, 2014. 190(8): p. 867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chester D and Brown AC, The role of biophysical properties of provisional matrix proteins in wound repair. Matrix Biol, 2017. 60–61: p. 124–140. [DOI] [PubMed] [Google Scholar]
- 23.Lorimier S, et al. , The rate of fibrinolysis is increased by free retraction of human gingival fibroblast populated fibrin lattices. Int J Biochem Cell Biol, 1997. 29(1): p. 181–9. [DOI] [PubMed] [Google Scholar]
- 24.Murphy SV, Skardal A, and Atala A, Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A, 2013. 101(1): p. 272–84. [DOI] [PubMed] [Google Scholar]
- 25.Moraes C, et al. , Aqueous two-phase printing of cell-containing contractile collagen microgels. Biomaterials, 2013. 34(37): p. 9623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed TA, Dare EV, and Hincke M, Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev, 2008. 14(2): p. 199–215. [DOI] [PubMed] [Google Scholar]
- 27.Guillotin B, et al. , Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials, 2010. 31(28): p. 7250–6. [DOI] [PubMed] [Google Scholar]
- 28.Xu T, et al. , Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials, 2006. 27(19): p. 3580–8. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, et al. , Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. ScientificWorldJournal, 2015. 2015: p. 685690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorodetsky R, et al. , Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. J Invest Dermatol, 1999. 112(6): p. 866–72. [DOI] [PubMed] [Google Scholar]
- 31.Johansson HO, et al. , Driving forces for phase separation and partitioning in aqueous two-phase systems. J Chromatogr B Biomed Sci Appl, 1998. 711(1–2): p. 3–17. [DOI] [PubMed] [Google Scholar]
- 32.Pratt CW and Monroe DM, Microplate coagulation assays. Biotechniques, 1992. 13(3): p. 430–3. [PubMed] [Google Scholar]
- 33.Ilich A, Bokarev I, and Key NS, Global assays of fibrinolysis. Int J Lab Hematol, 2017. 39(5): p. 441–447. [DOI] [PubMed] [Google Scholar]
- 34.Huang HL, et al. , Trypsin-induced proteome alteration during cell subculture in mammalian cells. J Biomed Sci, 2010. 17: p. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kocholaty W, Ellis WW, and Jensen H, Activation of plasminogen by trypsin and plasmin. Blood, 1952. 7(9): p. 882–90. [PubMed] [Google Scholar]
- 36.Collet JP, et al. , Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol, 2000. 20(5): p. 1354–61. [DOI] [PubMed] [Google Scholar]
- 37.Samarakoon R, Overstreet JM, and Higgins PJ, TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal, 2013. 25(1): p. 264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuan TL, et al. , Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. J Invest Dermatol, 1996. 106(5): p. 1007–11. [DOI] [PubMed] [Google Scholar]
- 39.Verrecchia F, Chu ML, and Mauviel A, Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem, 2001. 276(20): p. 17058–62. [DOI] [PubMed] [Google Scholar]
- 40.Mu XC and Higgins PJ, Differential growth state-dependent regulation of plasminogen activator inhibitor type-1 expression in senescent IMR-90 human diploid fibroblasts. J Cell Physiol, 1995. 165(3): p. 647–57. [DOI] [PubMed] [Google Scholar]
- 41.Martens JW, et al. , Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb Haemost, 2003. 89(2): p. 393–404. [PubMed] [Google Scholar]
- 42.Serrano M, et al. , Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell, 1997. 88(5): p. 593–602. [DOI] [PubMed] [Google Scholar]
- 43.Vasta JD, et al. , Selective Inhibition of Collagen Prolyl 4-Hydroxylase in Human Cells. ACS Chem Biol, 2016. 11(1): p. 193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang WT, et al. , Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis. Exp Gerontol, 2015. 61: p. 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardo A and Selman M, Lung Fibroblasts, Aging, and Idiopathic Pulmonary Fibrosis. Ann Am Thorac Soc, 2016. 13 Suppl 5: p. S417–S421. [DOI] [PubMed] [Google Scholar]
- 46.Cesari M, Pahor M, and Incalzi RA, Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther, 2010. 28(5): p. e72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorlach A, et al. , Reactive oxygen species modulate HIF-1 mediated PAI-1 expression: involvement of the GTPase Rac1. Thromb Haemost, 2003. 89(5): p. 926–35. [PubMed] [Google Scholar]
- 48.He T, et al. , Oxidative exposure impairs TGF-beta pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. Age (Dordr), 2014. 36(3): p. 9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasuda K, Aoshiba K, and Nagai A, Transforming growth factor-beta promotes fibroblast apoptosis induced by H2O2. Exp Lung Res, 2003. 29(3): p. 123–34. [DOI] [PubMed] [Google Scholar]
- 50.Gorres KL and Raines RT, Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol, 2010. 45(2): p. 106–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruick RK and McKnight SL, A conserved family of prolyl-4-hydroxylases that modify HIF. Science, 2001. 294(5545): p. 1337–40. [DOI] [PubMed] [Google Scholar]
- 52.Al-Horani RA, Serpin regulation of fibrinolytic system: implications for therapeutic applications in cardiovascular diseases. Cardiovasc Hematol Agents Med Chem, 2014. 12(2): p. 91–125. [DOI] [PubMed] [Google Scholar]
- 53.Zorio E, et al. , Fibrinolysis: the key to new pathogenetic mechanisms. Curr Med Chem, 2008. 15(9): p. 923–9. [DOI] [PubMed] [Google Scholar]
- 54.Singh S, Houng A, and Reed GL, Releasing the Brakes on the Fibrinolytic System in Pulmonary Emboli: Unique Effects of Plasminogen Activation and alpha2-Antiplasmin Inactivation. Circulation, 2017. 135(11): p. 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.