Abstract
Background
Human immunodeficiency virus (HIV)–exposed uninfected (HEU) infants in endemic settings are at high risk of tuberculosis (TB). For infants, progression from primary Mycobacterium tuberculosis (Mtb) infection to TB disease can be rapid. We assessed whether isoniazid (INH) prevents primary Mtb infection.
Methods
We conducted a randomized nonblinded controlled trial enrolling HEU infants 6 weeks of age without known TB exposure in Kenya. Participants were randomized (1:1) to 12 months of daily INH (10 mg/kg) vs no INH. Primary endpoint was Mtb infection at end of 12 months, assessed by interferon-γ release assay (QuantiFERON-TB Gold Plus) and/or tuberculin skin test (TST, added 6 months after first participant exit).
Results
Between 15 August 2016 and 6 June 2018, 416 infants were screened, with 300 (72%) randomized to INH or no INH (150 per arm); 2 were excluded due to HIV infection. Among 298 randomized HEU infants, 12-month retention was 96.3% (287/298), and 88.9% (265/298) had primary outcome data. Mtb infection prevalence at 12-month follow-up was 10.6% (28/265); 7.6% (10/132) in the INH arm and 13.5% (18/133) in the no INH arm (7.0 vs 13.4 per 100 person-years; hazard ratio, 0.53 [95% confidence interval {CI}, .24–1.14]; P = .11]), and driven primarily by TST positivity (8.6% [8/93] in INH and 18.1% [17/94] in no INH; relative risk, 0.48 [95% CI, .22–1.05]; P = .07). Frequency of severe adverse events was similar between arms (INH, 14.0% [21/150] vs no INH, 10.7% [16/150]; P = .38), with no INH-related adverse events.
Conclusions
Further studies evaluating TB preventive therapy to prevent or delay primary Mtb infection in HEU and other high-risk infants are warranted.
Clinical Trials Registration
Keywords: infant, tuberculosis, prevention, isoniazid, HIV-exposed
HIV-exposed uninfected infants are at high risk of tuberculosis (TB). For infants, progression from primary Mycobacterium tuberculosis infection to TB disease can be rapid. We assessed whether isoniazid prevents primary M. tuberculosis infection.
Tuberculosis (TB) is a leading cause of morbidity and mortality in children under 5 globally [1–3]. With human immunodeficiency virus (HIV) treatment success, most children born to mothers living with HIV will be HIV exposed and uninfected (HEU), but remain at high risk for Mycobacterium tuberculosis (Mtb) infection and TB disease [4–10]. For young infants, progression from Mtb infection to TB disease can be rapid, with 19%–50% estimated to develop TB without intervention [9, 11–13]. Preventing Mtb infection during this vulnerable window may be beneficial.
Isoniazid preventive therapy (IPT) is used routinely to prevent TB for people living with HIV (PLHIV) >1 year of age, regardless of exposure, and in children under 5 after known TB exposure [14–18]. For infants without reported TB exposure, no empiric chemoprophylaxis is recommended, including those with HIV exposure or infection in TB-endemic settings. This is despite recent estimates that suggest most TB transmission (70%–90%) to young children occurs outside the household without identified exposure [6]. These guidelines reflect uncertainty regarding IPT benefit in young children, based on 3 trials yielding conflicting data whether isoniazid (INH) prevents TB disease or mortality among children living with HIV [19–21]. Only 1 study, the P1041 trial in South Africa and Botswana (2004–2008), evaluated HEU children and found no protective effect of INH in decreasing Mtb infection or a composite outcome of Mtb infection, TB disease, or death [5]. This trial was completed prior to extensive antiretroviral therapy (ART) availability and the World Health Organization recommendation regarding TB preventive therapy for PLHIV. Whether INH provides effective primary prevention of Mtb infection in HEU children under contemporary conditions of universal ART and widespread programmatic IPT for PLHIV is unknown.
In the infant TB Infection Prevention Study (iTIPS), we hypothesized that INH would reduce the risk of primary Mtb infection and be safe in young HEU infants at potentially high risk of Mtb infection and TB disease.
METHODS
Study Design and Participants
We conducted a randomized nonblinded controlled trial in western Kenya, an area of high HIV and TB burden [3, 22]. Eligible infants 6–10 weeks of age, born to HIV-infected mothers, with birth weight >2.5 kg, and ≥37 weeks’ gestation, were enrolled from prevention of maternal-to-child transmission of HIV clinics. We excluded infants with known TB exposure (including maternal TB diagnosed in past year), or enrollment in other TB prevention studies. Trial design and procedure details have been previously published [23].
Randomization and Masking
Eligible infants were randomized 1:1 to 12 months’ daily INH (10 mg/kg dose) with pyridoxine or no INH. After randomization assignment, neither participants nor study staff were blinded to the assigned intervention groups. Infants randomized to the control arm (no INH) did not receive placebo.
Procedures
Standardized questionnaires regarding sociodemographic, clinical, obstetric, and HIV-related factors, TB history and exposure, and TB symptom screen [24] were administered by study staff. For infants randomized to INH, liver function tests (LFTs) were performed at enrollment and 1 month following INH initiation, graded per the National Institutes of Health Division of AIDS (DAIDS) toxicity cutoffs [25]. Infants with baseline LFTs grade 2 or lower initiated INH. Adherence was assessed by caregiver questionnaire at follow-up visits conducted at 10 and 14 weeks and 6, 9, and 12 months of age coinciding with Kenyan immunization schedules.
Primary endpoint was Mtb infection at end of 12 months, assessed by interferon-γ release assay (IGRA; QuantiFERON-TB Gold Plus [QFT-Plus]) and/or tuberculin skin test (TST). Initially, QFT-Plus was used alone. TST was added as a composite primary endpoint in February 2018, approximately 6 months after first study exit, per data safety and monitoring board (DSMB) recommendation due to lower than anticipated QFT-Plus endpoints, and to optimize sensitivity since TST is recommended for children aged <2 years. Study investigators were blinded to QFT-Plus results by arm when this decision was made. TSTs were placed and read by study staff who were not blinded by study arm. De-identified data were entered in real time into password-protected and encrypted tablets using Research Electronic Data Capture software (REDCap, Vanderbilt University, Nashville, Tennessee) [26].
Outcomes
The primary endpoint was Mtb infection, defined as positive QFT-Plus or TST after 12 months following enrollment. QFT-Plus results were considered positive with either TB1-Nil or TB2-Nil ≥0.35 IU/mL per the manufacturer’s recommendations [27]. TST of ≥10 mm induration was considered positive [15]. Secondary outcomes included grade 3 or higher severe adverse events by DAIDS scales [25]. Exploratory outcomes included a composite endpoint of Mtb infection, TB diagnosis, and/or death.
Statistical Analysis
We estimated that INH would decrease Mtb infection risk by 65%, consistent with TB prevention efficacy among TST-positive adult PLHIV and historical community-based trials [18, 28, 29], but undefined for Mtb infection prevention. We assumed 20% annual cumulative incidence without intervention based on 10% prevalence of Mtb infection in 6-month-old HIV-exposed infants in Kenya, detected by IGRA on cryopreserved peripheral blood mononuclear cells (PBMCs) [30]. We determined that enrollment of 250 infants (125 per arm) would provide 80% power with a 2-sided α of .05 to detect 65% decrease in Mtb infection. We increased the sample size by 20% to 300 infants (150 per arm) to account for loss to follow-up, nonadherence, and INH resistance.
Baseline characteristics were summarized by arm. We used a modified intention-to-treat approach including all randomized participants with at least 1 Mtb infection measure, excluding children found to be HIV DNA positive after enrollment. We compared proportions of infants with Mtb infection by arm with a χ 2 test and estimated relative risk (RR) by generalized linear model with log link and Poisson family and cumulative Mtb infection incidence with a Cox proportional hazards regression model, given that early infancy enrollment with Mtb infection assessment after 12 months of follow-up provides reasonable approximation of incident infection over the first year of life. For this model, Mtb infection timing was estimated as midpoint between enrollment and end of follow-up for those in whom infection was detected; those without infection were censored at 12-month follow-up completion.
For secondary, exploratory, subgroup, and sensitivity analyses, we compared proportions by arm using a χ 2 test. All hypothesis testing was 2-sided. Analyses were conducted using Stata version 15 software (StataCorp, College Station, Texas).
An external independent DSMB monitored operational aspects, safety, and effectiveness, and made recommendations regarding study continuation or modifications. Futility was not considered a basis for discontinuation because of the trial’s anticipated value in understanding epidemiologic and immunologic correlates of Mtb infection in HEU infants. This study is registered at ClinicalTrials.gov (NCT02613169).
Ethical Considerations
Written informed consent was obtained from caregivers. Trial procedures were approved by the University of Washington Institutional Review Board, the Kenyatta National Hospital/University of Nairobi, the Jaramogi Oginga Odinga Teaching and Referral Hospital ethics and research committees, and the Kenya Pharmacy and Poisons Board.
RESULTS
Participant Characteristics
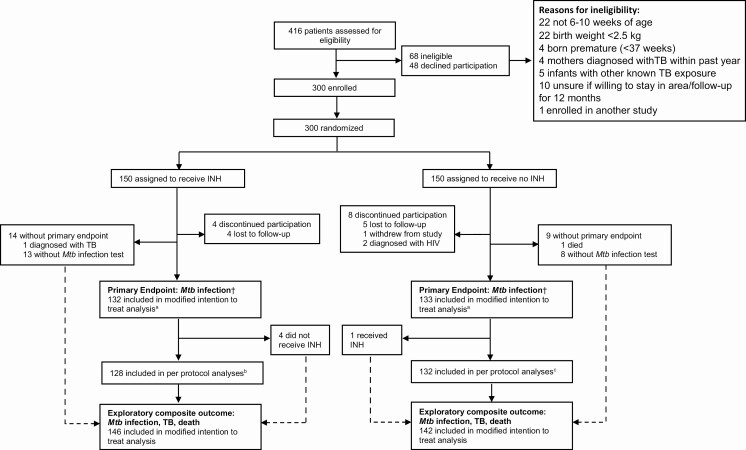
From August 2016 through June 2018, we screened 416 HIV-exposed infants; 300 (72.1%) were enrolled and randomized to INH or no INH (150 per arm) (Figure 1). Two participants were subsequently excluded due to HIV diagnosis by 10 weeks of age, 9 were lost to follow-up, 1 withdrew from the study, and 1 died, with 287 (96.3%) participants completing study follow-up. Two-hundred sixty-five infants (88.9%) were assessed in the modified intention-to-treat analysis of Mtb infection and 288 (96.3%) for the composite outcome of Mtb infection, TB diagnosis, or death.
Figure 1.
Detailed trial profile. †Mycobacterium tuberculosis (Mtb) infection by interferon-γ release assay (QuantiFERON-TB Gold Plus) or tuberculin skin test. aExcludes 2 children diagnosed with human immunodeficiency virus and 23 without primary Mtb infection endpoint. bExcludes 4 infants in the isoniazid (INH) arm with a primary outcome who did not receive INH. cExcludes 1 infant in the no INH arm who received INH due to tuberculosis exposure. Abbreviations: HIV, human immunodeficiency virus; INH, isoniazid; Mtb, Mycobacterium tuberculosis; TB, tuberculosis.
Among 300 randomized infants, median age at enrollment was 6.3 (interquartile range [IQR], 6.0–6.6) weeks, 158 (52.7%) were male, and 282 (94.0%) previously received Bacillus Calmette–Guérin (BCG) vaccination (Table 1). All mothers were on antiretroviral therapy (ART); 297 (99.0%) infants received antiretrovirals for HIV prevention. Median maternal CD4 count was 478 (IQR, 330–668) cells/μL and most mothers (226 [79.3%]) had an undetectable HIV RNA viral load (<20 copies/mL). Two-hundred twenty-four (74.7%) mothers previously received programmatic IPT, including 63 (28.1%) at enrollment. Thirty-two mothers (10.7%) reported history of TB. Baseline characteristics were well balanced between arms.
Table 1.
Participant Characteristics at Baseline
| Characteristic | All Participants (N = 300) |
INH (n = 150) |
No INH (n = 150) |
|---|---|---|---|
| Infant characteristics | |||
| Infant age, wk, median (IQR) | 6.3 (6.0–6.6) | 6.3 (6.0–6.6) | 6.3 (6.0–6.4) |
| WAZ, kg, median (IQR)a | 0.20 (−0.52 to 0.91) | 0.24 (−0.54 to 0.85) | 0.19 (−0.44 to 1.0) |
| Underweight (WAZ < −2) | 6 (2.0) | 3 (2.0) | 3 (2.0) |
| Male sex | 158 (52.7) | 79 (52.7) | 79 (52.7) |
| Breastfed on enrollment | 295 (98.3) | 146 (97.3) | 149 (99.3) |
| Received ARVs for PMTCT | 297 (99.0) | 148 (98.7) | 149 (99.3) |
| BCG vaccination | 282 (94.0) | 141 (94.0) | 141 (94.0) |
| Maternal sociodemographic characteristics | |||
| Maternal age | 27 (24–31) | 27 (24–31) | 28 (24–32) |
| Secondary education started | 140 (46.7) | 75 (50.0) | 65 (43.3) |
| Parity, median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| Maternal HIV/TB | |||
| Maternal partner HIV statusb | |||
| Positive | 149 (54.8) | 70 (50.0) | 79 (59.9) |
| Negative | 91 (33.5) | 47 (33.6) | 44 (33.3) |
| Unknown | 32 (11.8) | 23 (16.4) | 9 (6.8) |
| Maternal ARVs | |||
| Initiated before pregnancy | 219 (73.0) | 113 (75.3) | 106 (70.7) |
| Initiated during pregnancy | 79 (26.3) | 36 (24.0) | 43 (28.7) |
| Initiated after pregnancy | 2 (0.7) | 1 (0.7) | 1 (0.7) |
| Maternal ARV regimen | |||
| Efavirenz based | 220 (73.3) | 112 (74.7) | 108 (72.0) |
| Nevirapine based | 62 (20.7) | 31 (20.7) | 31 (20.7) |
| Protease inhibitor based | 18 (6.0) | 7 (4.7) | 11 (7.3) |
| Maternal CD4 count, cells/μL, median (IQR)c | 478 (330–668) | 482 (330–683) | 472 (334–655) |
| Maternal HIV RNA, copies/mL, median (IQR)d | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| HIV viral load undetectablee | 226 (79.3) | 106 (75.2) | 120 (83.3) |
| HIV viral load >1000 copies/mL | 16 (5.6) | 9 (6.4) | 7 (4.9) |
| Maternal history of TB | 32 (10.7) | 15 (10.0) | 17 (11.3) |
| Maternal history of IPTf | 224 (74.7) | 107 (71.3) | 117 (78.0) |
| Initiated before pregnancy | 94 (42.2) | 42 (39.3) | 52 (44.8) |
| Initiated during pregnancy | 110 (49.3) | 55 (51.4) | 55 (47.4) |
| Initiated after pregnancy | 19 (8.5) | 10 (9.4) | 9 (7.8) |
| Residential characteristics | |||
| Crowding | |||
| Persons in household, median (IQR) | 4 (4–6) | 4 (4–6) | 4.5 (4–6) |
| Rooms in household, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Housing | |||
| Pit latrine use | 248 (82.7) | 125 (83.3) | 123 (82.0) |
| Electricity in home | 222 (74.0) | 108 (72.0) | 114 (76.0) |
| Running water in home | 77 (25.7) | 40 (26.7) | 37 (24.7) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ARV, antiretroviral; HIV, human immunodeficiency virus; INH, isoniazid; IPT, isoniazid preventive therapy; IQR, interquartile range; PMTCT, prevention of maternal-to-child transmission; TB, tuberculosis; WAZ, weight-for-age z score.
an = 299 (150 INH, 149 no INH). Caregiver of 1 infant in the no INH arm withdrew from study after consent but before anthropometric assessment.
bn = 272 (140 INH, 132 no INH). Twenty-eight caregivers of participants who did not have a current partner were excluded.
cn = 257 (133 INH, 124 no INH). CD4 data were collected from routine programmatic data; 43 missing due to changing guidelines regarding CD4 monitoring in Kenya.
dn = 285 (141 INH, 144 no INH). HIV viral load data collected from routine programmatic data, 15 missing.
eHIV viral load lower level of detection by routine Ministry of Health testing in Kenya is 20 copies/mL.
fMissing timing of programmatic maternal IPT initiation for 1 participant in the no INH arm.
Of 150 infants randomized to INH, 145 received at least 1 dose. Of 150 infants randomized to no INH, 1 received subsequent INH due to TB exposure (Figure 1). Two hundred sixty-five (88.9%) infants had QFT-Plus or TST performed (132 INH, 133 no INH), with 3 of 249 (1.2%) QFT-positive and 25 of 187 (13.4%) TST-positive (Supplementary Tables 1 and 2). Among children completing follow-up, the most common reasons for missing the primary endpoint was difficult blood draw or insufficient volume for QFT-Plus, and caregiver travel and or inability to return for TST read within the allotted window. Baseline characteristics of infants either with or missing an Mtb infection endpoint were similar (data not shown), as was proportion of infants per arm with QFT-Plus and TST results (Supplementary Table 1). Among 132 children randomized to INH with available primary outcome assessment, 53.0% (70/132) reported optimal use (≥90% adherence) for the entire study period, with self-reported optimal adherence ranging from 77% to 91% across visits.
Primary Outcome Mtb Infection
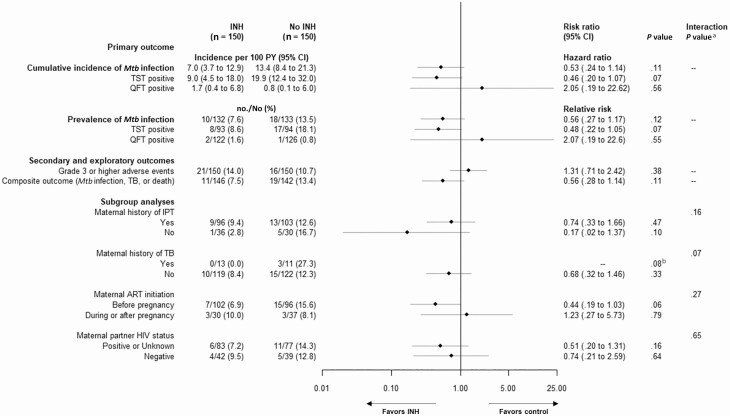
The primary Mtb infection endpoint occurred in 10.6% (28/265) of participants with QFT-Plus or TST results for a cumulative Mtb infection incidence of 10.1 (95% confidence interval [CI], 7.0–14.6) per 100 person-years (PY), and was driven by TST positivity (13.4% [25/187]; 14.4 [95% CI, 9.7–21.2] per 100 PY). In the modified intention-to-treat analysis, Mtb infection prevalence (by TST or QFT-Plus) was 7.6% (10/132) in the INH arm and 13.5% (18/133) in the no INH arm (7.0 vs 13.4 per 100 PY; hazard ratio [HR], 0.53 [95% CI, .24–1.14]; P = .11) (Figure 2). Restricted to TST alone, 8.6% (8/93) of children randomized to INH were TST positive vs 18.1% (17/94) of those randomized to no INH (9.0 vs 19.9 per 100 PY; HR, 0.46 [95% CI, .20–1.07]; P = .07).
Figure 2.
Primary, secondary, and exploratory outcomes including subgroup analyses. The primary outcome is Mycobacterium tuberculosis (Mtb) infection (either tuberculin skin test or QuantiFERON-TB Gold Plus) 12 months after randomization utilizing a modified intention-to-treat approach including all participants who underwent randomization irrespective of receipt of trial medication with at least 1 measure of Mtb infection, excluding 2 children found to be human immunodeficiency virus DNA positive in the no isoniazid arm during the study. Exploratory composite outcome of Mtb infection, death, or tuberculosis includes all infants with known vital or tuberculosis status at the end of the trial. Subgroup analyses are based on baseline characteristics at the trial entry and include only participants with available primary outcome Mtb assessment. aInteraction between study group and subgroup. bBy Fisher exact test due to zero cases in the isoniazid arm. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; INH, isoniazid; IPT, isoniazid preventive therapy; Mtb, Mycobacterium tuberculosis; PY, person-years; QFT, QuantiFERON-TB Gold Plus; TB, tuberculosis; TST, tuberculin skin test.
Distribution of TST and QFT-Plus results including quantitative values are provided in Supplementary Figures 1 and 2 and Supplementary Table 4). Among 171 with both QFT-Plus and TST performed, agreement between TST and QFT-Plus results was 84.2% based primarily on negative results with no overlap between positive QFT-Plus (2/171 [1.1%]) and TST (25/171 [14.6%]) (κ = −0.02 [95% CI, −.05 to .01; P = .72; Supplementary Table 2). In sensitivity analyses restricted to children with both TST and QFT-Plus results, the direction of effect was similar (INH, 9/83 [10.8%] vs no INH, 18/88 [20.5%]; RR, 0.53 [95% CI, .25–1.11]; P = .10).
Sensitivity analyses of QFT-Plus and TST positivity and of the primary outcome of Mtb infection by varied cutoffs, and best- and worst-case scenarios for missing endpoints are detailed in Supplementary Tables 2 and 3.
Secondary Safety and Exploratory Outcomes
Severe adverse event frequency was similar between arms (INH, 14.0% [21/150] vs no INH, 10.7% [16/150]; P = .38); there were no INH-related severe adverse events (Table 2). One child randomized to INH had grade 2 elevation of LFTs on enrollment prior to INH initiation, which remained grade 2 after 1 month of INH. One child was diagnosed with TB (INH arm), and 1 died of non-TB-related causes (no INH arm) (Figure 1). The exploratory composite endpoint of Mtb infection, TB, and/or death was similar to the primary outcome, with 7.5% (11/146) of participants in the INH arm and 13.4% (19/142) in the no INH arm (RR, 0.56 [95% CI, .28–1.14]; P = .11) and driven almost exclusively by Mtb infection (Figure 2).
Table 2.
Adverse Events of Grade 3 or Higher
| Adverse Event | All Participants (N = 300) |
INH (n = 150) |
No INH (n = 150) |
|---|---|---|---|
| Total participants with SAEsa | 37 (12.3) | 21 (14.0) | 16 (10.7) |
| Total SAEsb | 40 (13.3) | 24 (16.0) | 16 (10.7) |
| Deathc | 1 (0.3) | 0 (0) | 1 (0.7) |
| HIV infection | 2 (0.7) | 0 (0) | 2 (1.3) |
| Hepatotoxicity | 0 (0) | 0 (0) | 0 (0) |
| Peripheral neuropathy | 0 (0) | 0 (0) | 0 (0) |
| Hospitalization | 38 (12.7) | 24 (16.0)c,d,e,f,g | 14 (9.3)h,i,j |
| Pneumonia/URTI | 10 (3.3) | 5 (3.3) | 5 (3.3) |
| Gastroenteritis | 11 (3.7) | 8 (5.3) | 3 (2.0) |
| Malaria | 18 (6.0) | 12 (8.0) | 6 (4.0) |
| Other | 4 (1.3) | 3 (2.0)g | 1 (0.7)j |
Data are presented as no. (%).
Abbreviations: INH, isoniazid; SAE, severe adverse event; URTI, upper respiratory tract infection.
aGrade 3 or higher. P = .38 between the INH and no INH arms.
b P = .17 between the INH and no INH arms.
cFour infants hospitalized with multiple diagnoses: malaria and flu (n = 1), malaria and pneumonia (n = 1), malaria and gastroenteritis (n = 2).
dOne infant hospitalized for malaria on 2 separate occasions.
eOne infant hospitalized for malaria and gastroenteritis and then hospitalized for pneumonia on a later date.
fOne infant hospitalized for corrective surgery for encephalocele and then hospitalized for URTI on a later date.
gCorrective surgery for cleft palate (n = 1), encephalocele (n = 1), or burns (n = 1).
hOne infant who was hospitalized for pneumonia and died is included as both a death and a hospitalization.
iOne infant hospitalized for malaria and URTI on the same occasion.
jCorrective surgery for spina bifida.
Per protocol analyses of infants with any INH compared to no INH use yielded similar results (INH, 7.8% [10/128] vs no INH, 13.6% [18/136]; HR, 0.57 [95% CI, .27–1.20]; P = .14) (Supplementary Table 3).
In subgroup analyses, there was a trend for lower prevalence of Mtb infection for infants with mothers with a history of TB (INH, 0% [0/13] vs no INH, 27.3% [3/11]; interaction P = .07) (Figure 1).
DISCUSSION
In this trial, INH did not provide a statistically significant reduction in risk of primary Mtb infection among HEU infants in a high TB/HIV-endemic setting under contemporary conditions of universal maternal ART and high programmatic IPT uptake [31]. Although approximately half as many children randomized to INH had Mtb infection (HR, 0.53; P = .11), lower than anticipated rates of Mtb infection likely limited our power to demonstrate a significant protective INH effect. As with previous pediatric TB prevention trials, INH was safe [5, 14, 19–21], with no drug-related severe adverse events, and similar severe adverse event frequency reported between arms.
Our trial has several novel features including an emphasis on HEU children and prevention of primary Mtb infection. HEU infants remain at high risk of Mtb infection and TB disease in endemic settings, even in the absence of known Mtb exposure, with markedly increased risk compared to HIV-unexposed children [4, 5, 30, 32, 33]. In young children who lack preexisting adaptive immune responses, Mtb infection can progress rapidly to TB disease [7–10, 34, 35]. A recently published systematic review and meta-analysis estimated an approximately 20% TB risk within 2 years of exposure for young children, with most (83% <5 years of age) developing TB disease within weeks of initial contact investigation [13]. Although contact tracing focused strategies are important, they are unlikely to prevent the preponderance of pediatric TB. A strategy that prevents or delays primary Mtb infection during the first year of life could protect infants during this particularly vulnerable window.
There are distinctions that may account for the differences between our study and P1041, the placebo-controlled randomized trial in South Africa and Botswana that randomized children living with HIV and HEU children enrolled at a median of 96 days of life to INH or placebo for 96 weeks; that study found no evidence of protection from a combined endpoint of TB disease or death in either group or Mtb infection (as measured by TST) in HEU children [5]. Infants in our study were enrolled at a younger age (median age, 44 weeks). In P1041, Mtb assessment was part of a composite outcome at 96 weeks and only 2.6% of HEU children had Mtb infection. Our study observed a 3- to 4-fold higher prevalence of TST positivity at study end at approximately 56 weeks of age (10.8% among all children with an infection endpoint, and 13.6% when limited to children with an available TST result). While the initially reported outcome of Mtb infection by TST was lower in P1041 compared to our study, Mtb infection may have been underestimated in P1041 as evidenced in a subsequently published substudy in Cape Town, which reported that 14% (19/132) of HEU infants were QFT positive upon exit from the parent study [36]. Last, while mortality was similarly low (<1%) among HEU children in both studies (1/300 vs 6/804 in P1041), incident TB disease was lower in our study (0.03% [1/300] vs 7.3% [59/804]), potentially due to contemporary community ART and IPT use.
Our trial has limitations. We estimated a substantive INH (65%) effect based on studies of TB disease prevention among TST-positive adult PLHIV and community trials in HIV-negative populations; meta-analyses of INH efficacy to prevent pediatric TB disease have ranged from 35% to 63% [13, 14, 18, 28]. Our observed cumulative incidence was lower than our estimate extrapolated from a historical HIV-exposed Kenyan infant cohort [30], potentially due in part to the high proportion of mothers who had received IPT during pregnancy and early postpartum [31]. These issues likely limited our power and precision to detect differences between trial arms. There is no gold standard to diagnose Mtb infection; both TST and IGRA rely on immune-mediated responses to detect infection [37]. We initially chose QFT-Plus to test for Mtb infection based on the potential for higher sensitivity and specificity (due to lack of cross-reactivity with BCG administration) than TST, though data at trial inception were limited in young infants [27, 37, 38]. TST was incorporated later per DSMB recommendations due to low accrued IGRA endpoints to optimize sensitivity and because it is still recommended for children <2 years of age. TST-positivity may have been due to BCG cross-reactivity [37]. Study staff were not blinded to TST result, and TST reading may have been affected by terminal digit bias with potential misclassification of endpoint. Delayed TST implementation, the primary driver of endpoints, may have led to underestimation of primary outcome in both arms. The proportion of children assessed by QFT-Plus and or TST was similar in both arms, and therefore was unlikely to have biased outcomes differentially by study arm. We used recommended cutoffs for QFT-Plus and TST but evaluated additional cutoffs in sensitivity analyses. There was low correlation between TST positive and QFT-Plus results, with only 2 positive QFTs for which TST results were available, though overall agreement was high because most children were negative on both tests. Recent studies indicate that some individuals have IFN-γ–independent immune responses to Mtb antigens [39]. We plan to explore these alternate cytokine responses in QFT-Plus supernatants. Although we cannot exclude an INH effect on BCG efficacy, we delayed INH initiation until 6 weeks after birth BCG administration to mitigate this. Finally, Mtb infection may have occurred prior to study entry. We plan to assess BCG and Mtb-specific T-cell responses utilizing cryopreserved PBMCs from enrollment to detect potential early Mtb infection and possible BCG-induced immune correlates of Mtb infection [40].
With the current trial results, we cannot ascertain durability of INH effect; however, an extended observational period with assessment of Mtb infection at 24 months of age is ongoing. Similar to recent TB prevention trials in adults, participants were not blinded to the intervention, and adherence was based on participant (albeit caregiver) report [41, 42]. During the trial, the Kenyan public health sector experienced multiple healthcare worker strikes (250 days over 11 months in 2016–2017), limiting clinical services [43] and potentially impacting participant follow-up at visits aligned with Kenyan routine immunization schedules, resulting in gaps of INH coverage despite overall high retention (>96%), contributing to the relatively low proportion of children reporting optimal adherence. As such, adherence to INH more closely resembled that of a programmatic setting. Poor adherence may have reduced the effect size of our intervention.
While newer short course preventive therapy regimens (primarily rifamycin-based) have improved adherence and completion rates with fewer adverse events [41, 44, 45], it is unclear due to their short duration if they would exert durable protection throughout the period of risk in infants. Additionally, rifamycins significantly reduce concentrations of nevirapine, which is routinely used for HIV prevention for HIV-exposed children [46].
In conclusion, INH for the prevention of primary Mtb infection in HEU children warrants further study as part of a comprehensive approach to address the global burden of pediatric TB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. G. J.-S., B. A. R., J. K., and S. M. L. designed the randomized clinical trial. S. M. L., G. J.-S., B. A. R., T. R. H., L. M. C., J. K., D. M., A. W., and E. M.-O. developed the study protocol. G. J.-S. is the principal investigator and protocol chair and T. R. H. is the immunology principal investigator. J. K. is the protocol co-chair and country principal investigator. E. M.-O. is the Pediatric Clinical Tuberculosis lead. G. J.-S., B. A. R., and S. M. L. were responsible for the statistical design of the trial. S. M. L. and J. N. E. performed the data analysis. J. M. managed the study data and conducted interim analyses. S. M. L. is the project director and wrote the initial draft of the manuscript. S. M. L., D. M., A. W., J. K., J. M., and G. J.-S. participated in trial implementation. All authors read and approved the manuscript.
Acknowledgments. The authors acknowledge the iTIPS Study Clinic Staff, the Kisumu and Siaya County Directors of Health, health facility staff, University of Washington (UW)–Kenya, and Kenyatta National Hospital Research and Programs operational staff. The authors also thank Qiagen for providing discounted QFT-Plus test kits, and the Kenya Medical Research Institute (KEMRI)/Centers for Disease Control and Prevention (CDC) in Kisumu, Kenya, for performing the QFT-Plus assays. The authors appreciate the thoughtful oversight of the data safety and monitoring board: Drs Grace Montepiedra (Chair), Rashida Ferrand, Helena Rabie, and Evans Amukoye, and the UW Global Center for the Integrated Health of Women, Adolescents, and Children for comments and insights provided during study design and manuscript development. Most of all, the authors thank the families who have participated in the study.
Disclaimer. The funders had no role in the design, collection of data, data analysis and interpretation, or decision to submit the manuscript for publication.
Financial support. This work was supported by the Thrasher Research Fund; the National Institute of Allergy and Infectious Diseases (NIAID); the Fulbright program awarded to the Northern Pacific Global Health Fellows Program by the Fogarty International Center of the National Institutes of Health (NIH/Fogarty); and the National Center for Advancing Translational Sciences at the NIH (grant numbers Thrasher to G. J. S.; NIH/NIAID K23AI120793 to S. M. L.; NIH/NIAID 2K24AI137310 to T. R. H.; NIH/Fogarty R25TW009345 to A. W.; and NIH UL1TR000423 for REDCap).
Potential conflicts of interest. L. M. C. reports grant number K23AI143479 from NIH/NIAID, outside the submitted work. G. J.-S. reports grants from NIH and CDC, and personal fees from UpToDate and UW, outside the submitted work. B. A. R. reports personal fees from Gilead and PATH, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 2017; 5:e898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Global tuberculosis report 2019. Available at: https://www.who.int/tb/global-report-2019. Accessed 5 February 2020.
- 4. Cotton MF, Schaaf HS, Lottering G, Weber HL, Coetzee J, Nachman S; PACTG 1041 Team . Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis 2008; 12:225–7. [PubMed] [Google Scholar]
- 5. Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med 2011; 365:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez L, Lo NC, Cords O, et al. Paediatric tuberculosis transmission outside the household: challenging historical paradigms to inform future public health strategies. Lancet Respir Med 2019; 7:544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moyo S, Verver S, Mahomed H, et al. Age-related tuberculosis incidence and severity in children under 5 years of age in Cape Town, South Africa. Int J Tuberc Lung Dis 2010; 14:149–54. [PubMed] [Google Scholar]
- 8. Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 1974; 99:131–8. [DOI] [PubMed] [Google Scholar]
- 9. Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:278–85. [PubMed] [Google Scholar]
- 10. Starke JR. Childhood tuberculosis during the 1990s. Pediatr Rev 1992; 13:343–53. [DOI] [PubMed] [Google Scholar]
- 11. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 12. Gedde-Dahl T. Tuberculous infection in the light of tuberculin matriculation. Am J Hyg 1952; 56:139–214. [DOI] [PubMed] [Google Scholar]
- 13. Martinez L, Cords O, Horsburgh CR, Andrews JR; Pediatric TB Contact Studies Consortium . The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet 2020; 395: 973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ayieko J, Abuogi L, Simchowitz B, Bukusi EA, Smith AH, Reingold A. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta-analysis. BMC Infect Dis 2014; 14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Academy of Pediatrics. Tuberculosis. In: Kimberlin DW, Brady MT, Jackson MA, Long SA. Red Book 2018–2021 Report of the Committee on Infectious Diseases; American Academy of Pediatrics. 31st ed. Elk Grove Village, IL: AAP, 2018:879–53. [Google Scholar]
- 16. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. Available at: http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed 1 February 2020. [PubMed]
- 17. Kenya Ministry of Health. National guidelines on management of tuberculosis in children, 3rd ed. Available at: https://www.chskenya.org/wp-content/uploads/2018/03/National-Guidelines-on-Management-of-Tuberculosis-in-Children.pdf. Accessed 5 February 2020. [Google Scholar]
- 18. Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; 1:CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ 2007; 334:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gray DM, Workman LJ, Lombard CJ, et al. Isoniazid preventive therapy in HIV-infected children on antiretroviral therapy: a pilot study. Int J Tuberc Lung Dis 2014; 18:322–7. [DOI] [PubMed] [Google Scholar]
- 21. Zunza M, Gray DM, Young T, Cotton M, Zar HJ. Isoniazid for preventing tuberculosis in HIV-infected children. Cochrane Database Syst Rev 2017; 8:CD006418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kenya Ministry of Health. Kenya tuberculosis survey. Available at: https://www.nltp.co.ke/survey-reports-2/. Accessed 5 February 2020.
- 23. LaCourse SM, Richardson BA, Kinuthia J, et al. Infant TB Infection Prevention Study (iTIPS): a randomised trial protocol evaluating isoniazid to prevent M. tuberculosis infection in HIV-exposed uninfected children. BMJ Open 2020; 10:e034308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Available at: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. Accessed 5 February 2020.
- 25. Division of AIDS National Institute of Allergy and Infectious Diseases. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. Available at: http://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf. Accessed 10 February 2020.
- 26. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiagen. QuantiFERON-TB Gold Plus (QFT-Plus) ELISA Package Insert. Available at: http://www.quantiferon.com/irm/content/PI/QFT/PLUS/2PK-Elisa/UK.pdf. Accessed 1 February 2020.
- 28. Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis 1967; 95:935–43. [DOI] [PubMed] [Google Scholar]
- 29. Salazar-Austin N, Dowdy DW, Chaisson RE, Golub JE. Seventy years of tuberculosis prevention: efficacy, effectiveness, toxicity, durability, and duration. Am J Epidemiol 2019; 188:2078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cranmer LM, Kanyugo M, Jonnalagadda SR, et al. High prevalence of tuberculosis infection in HIV-1 exposed Kenyan infants. Pediatr Infect Dis J 2014; 33:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. LaCourse SM, Wagner AD, Cranmer LM, et al. Brief report: high programmatic isoniazid preventive therapy (IPT) use in pregnancy among HIV-infected women. J Acquir Immune Defic Syndr 2019; 82:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marquez C, Chamie G, Achan J, et al. Tuberculosis infection in early childhood and the association with HIV-exposure in HIV-uninfected children in rural Uganda. Pediatr Infect Dis J 2016; 35:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bekker A, Du Preez K, Schaaf HS, Cotton MF, Hesseling AC. High tuberculosis exposure among neonates in a high tuberculosis and human immunodeficiency virus burden setting. Int J Tuberc Lung Dis 2012; 16:1040–6. [DOI] [PubMed] [Google Scholar]
- 34. Donald PR, Maher D, Qazi S. A research agenda to promote the management of childhood tuberculosis within national tuberculosis programmes. Int J Tuberc Lung Dis 2007; 11:370–80. [PubMed] [Google Scholar]
- 35. Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ 2018; 362:k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cranmer LM, Draper HR, Mandalakas AM, et al. High incidence of tuberculosis infection in HIV-exposed children exiting an isoniazid preventive therapy trial. Pediatr Infect Dis J 2018; 37:e254–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014; 27:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rozot V, Patrizia A, Vigano S, et al. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis 2015; 60: 432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu LL, Smith MT, Yu KKQ, et al. IFN-gamma-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med 2019; 25:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warr AJ, Shah JA, LaCourse SM, et al. Mycobacterium tuberculosis antigen-specific T-cell responses in HIV-infected mothers and their infants. In: Keystone Symposia on Tuberculosis: Translating Scientific Findings for Clinical and Public Health Impact. Whistler, Canada, 15–19 April, 2018. [Google Scholar]
- 41. Swindells S, Ramchandani R, Gupta A, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med 2019; 380:1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med 2018; 379:440–53. [DOI] [PubMed] [Google Scholar]
- 43. Irimu G, Ogero M, Mbevi G, et al. Tackling health professionals’ strikes: an essential part of health system strengthening in Kenya. BMJ Glob Health 2018; 3:e001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. [DOI] [PubMed] [Google Scholar]
- 45. Diallo T, Adjobimey M, Ruslami R, et al. Safety and side effects of rifampin versus isoniazid in children. N Engl J Med 2018; 379:454–63. [DOI] [PubMed] [Google Scholar]
- 46. McIlleron H, Denti P, Cohn S, et al. Prevention of TB using rifampicin plus isoniazid reduces nevirapine concentrations in HIV-exposed infants. J Antimicrob Chemother 2017; 72:2028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.