Abstract
Background
This study aimed to evaluate the clinical safety of delayed antibiotic prescribing for upper respiratory tract infections (URTIs), which is recommended in treatment guidelines for less severe cases.
Methods
Two population-based cohort studies used the English Clinical Practice Research Databank and Welsh Secure Anonymized Information Linkage, containing electronic health records from primary care linked to hospital admission records. Patients with URTI and prescriptions of amoxicillin, clarithromycin, doxycycline, erythromycin, or phenoxymethylpenicillin were identified. Patients were stratified according to delayed and immediate prescribing relative to URTI diagnosis. Outcome of interest was infection-related hospital admission after 30 days.
Results
The population included 1.82 million patients with an URTI and antibiotic prescription; 91.7% had an antibiotic at URTI diagnosis date (immediate) and 8.3% had URTI diagnosis in 1–30 days before (delayed). Delayed antibiotic prescribing was associated with a 52% increased risk of infection-related hospital admissions (adjusted hazard ratio, 1.52; 95% confidence interval, 1.43–1.62). The probability of delayed antibiotic prescribing was unrelated to predicted risks of hospital admission. Analyses of the number needed to harm showed considerable variability across different patient groups (median with delayed antibiotic prescribing, 1357; 2.5% percentile, 295; 97.5% percentile, 3366).
Conclusions
This is the first large population-based study examining the safety of delayed antibiotic prescribing. Waiting to treat URTI was associated with increased risk of hospital admission, although delayed antibiotic prescribing was used similarly between high- and low-risk patients. There is a need to better target delayed antibiotic prescribing to URTI patients with lower risks of complications.
Keywords: antibiotics, primary care, effectiveness, upper respiratory tract infections, epidemiology
Delayed antibiotic prescribing in patients with upper respiratory tract infections was associated with a 52% increased risk of infection-related hospital admissions in 2 large population-based cohorts. Delayed antibiotic prescribing should be targeted only to patients with lower risks of complications.
Antibiotics are frequently prescribed for the treatment of upper respiratory tract infections (URTIs; including sore throat, cough, and colds). In the United Kingdom, about 40% of antibiotics are given to patients with URTIs [1, 2]. Overuse of antibiotics is a major public health concern as it can lead to antimicrobial resistance [3]. Several initiatives have been implemented to reduce the levels of antibiotic prescribing by UK clinicians. In primary care in England, these include development and implementation of the TARGET (Treat Antibiotics Responsibly, Guidance, Education, Tools) toolkit [4] and treatment guidelines. The toolkit helps influence prescribers’ and patients’ personal attitudes, social norms, and perceived barriers for optimal antibiotic prescribing [4]. In 2008, a treatment guideline was introduced in the United Kingdom for self-limiting respiratory tract infections. It recommends that no antibiotic- or a delayed antibiotic–prescribing strategy should be used in URTIs except in more severe cases [5]. An editorial proposed that delayed antibiotic prescribing should be embraced in order to reduce antibiotic use in respiratory infections [6]. A Cochrane review of 11 randomized trials including 3555 patients with a variety of respiratory infections concluded that delayed antibiotic prescribing reduced antibiotic use compared with immediate antibiotic prescribing and there were no differences between these strategies in symptom control and clinical complications [7]. However, major clinical complications, including risk of infection-related hospital admission, were not evaluated. An observational study found that suppurative complications were rare with sore throat (1.2% in antibiotic users) [8], with limited statistical power to detect a difference between the immediate and delayed antibiotic groups. The objectives of this study were to evaluate in 2 large cohorts the clinical safety of delayed antibiotic prescribing for URTIs and the targeting to patients with low risk of infection-related complications.
METHODS
Database
Two large population-based cohort studies were delineated from the Clinical Practice Research Datalink (CPRD GOLD) and the Secure Anonymized Information Linkage (SAIL) databank. The CPRD contains longitudinal, anonymized, patient-level electronic health records (EHRs) from general practices in the United Kingdom representing approximately 8% of the UK population [9]. SAIL contains data from general practices in Wales covering approximately 75% of the population in Wales [10, 11]. The EHRs contain information on start and end of registration (including date of death), clinical diagnoses, medications prescribed, vaccination histories, diagnostic testing, lifestyle information, clinical referrals, as well as age, sex, ethnicity, smoking history, and body mass index (BMI). These primary care EHRs were linked to hospital admission data (using the Hospital Episode Statistics [HES]) based on unique patient identifiers. The hospital data contained information on the date of hospital admission and diagnostic codes at and during admission. The general practices in this study were restricted to those that had been linked to the hospital admission data including about half of the CPRD practices (all located in England) and all the SAIL practices (all in Wales). Patient-level socioeconomic information was available through linkage of the postal code of a patient’s residence to the Index of Multiple Deprivation (IMD). Patient-level IMD was aggregated into quintiles for the current analysis. Prescriptions were classified using the British National Formulary (BNF) sections. The period of data collection in CPRD GOLD was between 1 January 2000 and 29 June 2015; for SAIL, this time was between 1 January 2000 and 31 December 2017. The start of study eligibility was 1 year after the date of start of practice data collection or date of patients’ registration at the practice, whichever date came last (with the exception of newly born infants where this started at registration).
Study Population
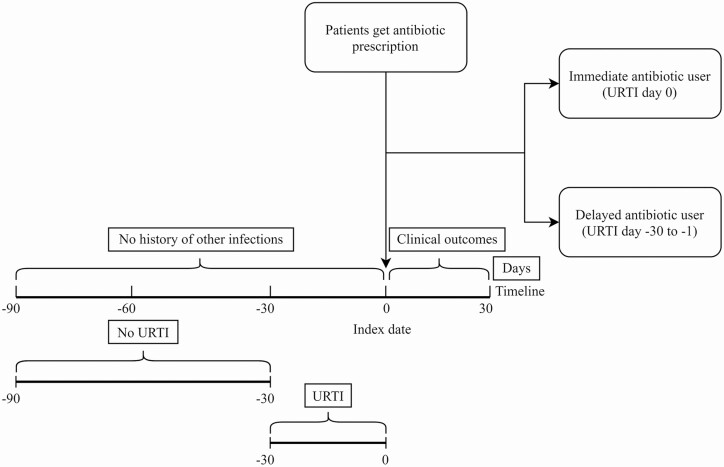
The overall source population consisted of antibiotic users of any age who were prescribed an antibiotic for the first time in 3 months. It was then restricted to patients who had an URTI record (outpatient diagnosis) either on the date of the antibiotic prescription or in the 30 days before. Upper respiratory tract infections also included sore throat, cold, and cough. Patients with an URTI record in the 30 to 90 days before were excluded (in order to help ensure selection of acute URTI at the date of the antibiotic prescription). A further restriction was to only include patients who were prescribed amoxicillin, clarithromycin, doxycycline, erythromycin, or phenoxymethylpenicillin (ie, the most frequently used antibiotic types for URTI in the United Kingdom [12]). The final restriction was to exclude patients with a record of another common infection on the date of the antibiotic prescription or in the 3 months before. These other infections used for exclusion included lower respiratory tract infection (LRTI), otitis externa, otitis media, sinusitis, urinary tract infection, exacerbation of asthma or chronic obstructive pulmonary disease, or skin or renal infection. Figure 1 shows a diagrammatic representation of the main inclusion criteria in this study. Follow-up for the clinical outcomes of interest started at the first day after the antibiotic prescription and ended 30 days after antibiotic prescribing. Antibiotic prescribing was classified as immediate or delayed, where immediate antibiotic use as defined as an URTI diagnosis on the same date as the antibiotic prescription; delayed antibiotic use was defined as an URTI diagnosis in the 1–30 days before. Given the length of the study follow-up, patients could contribute multiple observations over time (ie, if they consulted the general practitioner [GP] multiple times at least 3 months apart) with follow-up starting at the date of each antibiotic prescription. Patients were censored at the date of the first outcome of interest, death, or end of data of collection, whichever date came first.
Figure 1.
Diagrammatic representation of the main inclusion criteria in this study. Abbreviation: URTI, upper respiratory tract infection.
The primary outcome of interest was hospital admission for infection-related complications (as recorded in HES) that occurred in the 30 days after the antibiotic prescription (excluding the date of the antibiotic prescription). The hospital admissions for infection-related complications were based on the primary admission diagnosis (International Classification of Diseases, 10th revision) for a broad set of infection-related complications (such as hospital admission for LRTI, pneumonia, sepsis) (a list of predefined codes is provided in the Supplementary Material). The secondary outcomes of interest included infection-related complications as recorded by the GP in the EHR, death, and repeat antibiotic prescribing in the 30 days after. The GP-recorded infection-related complications included pneumonia, sepsis, quinsy, mastoiditis, empyema, bacterial meningitis, intracranial abscess, and Lemierre’s syndrome, as previously defined [13]. The analyses of infection-related complications (leading to hospital admission or those recorded by GPs) were based on the first case in the 30 days after the antibiotic prescription; cases were excluded if there was an infection-related hospital admission in the preceding 6 months. Code lists used in this study are available on the Clinical Codes repository https://clinicalcodes.rss.mhs.man.ac.uk/.
Statistical Analysis
The main analysis compared the different antibiotic-prescribing strategies for the risk of infection-related hospital admission in the 30 days after the antibiotic prescription. Time-to-event Cox proportional hazard regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) with delayed antibiotic prescribing. In addition to an indicator of delayed prescribing, the regression models included calendar year and month of the date of antibiotic prescription, Charlson comorbidity index (composite score of history of chronic conditions [14]), BMI categories, smoking history, socioeconomic status (IMD quintiles), flu vaccination, outpatient referral, and hospitalization in the previous year. These variables were possible predictors for hospital admission or adjusted for seasonality in antibiotic prescribing. An indicator for missing values was used for BMI and smoking history. Analyses were stratified by 5-year age bands and sex (ie, comparisons were made within these groups). Tests for proportionality of HRs over follow-up were conducted (indicating statistically proportional HRs in CPRD and SAIL with P values of .07 and .24, respectively).
The second analysis visualized the incidence of infection-related hospital admissions. Negative binomial regression models were fitted to estimate incidence rate ratios (IRRs) at each day after an antibiotic prescription, with as reference the last week in the immediate prescribing group. The IRRs were subject to higher variability due to the small number of cases at each time point. The way of dealing with this problem involves “smoothing” the IRR estimates, removing the noise (ie, it smooths out the random variation) and shows more clearly the pattern over time [15].
The third analysis compared the probability of delayed antibiotic prescribing with predicted risk of infection-related hospital admissions. Cox proportional hazard regression was used to estimate patient-level risks at day 30 using the predictors of the main model. Supplementary Tables 1 and 2 and Supplementary Figures 1 and 2 provide details on the prediction model. The attributable risks (ie, the difference between predicted risks of delayed and immediate antibiotic prescriptions) were then estimated. The inverse of the attributable risk is the number needed to harm (NNH). This is an epidemiological measure that indicates how many persons need to be exposed to a risk factor to cause harm in 1 additional person [16]. This calculation assumed that the effect of delayed antibiotic prescribing as observed in this study (HR) was causal (ie, not explained by unmeasured confounding) and the NNH estimates were conditional on the variables included in the risk prediction model. The interpretation of this NNH is that a lower number may indicate more harm with delayed antibiotic prescribing and a higher number indicates fewer adverse clinical effects.
The effect of delayed antibiotic prescribing level from CPRD and SAIL were pooled using inverse variance weighting [17]. Fixed-effects models rather than random-effects models were selected as the analysis of the 2 cohorts yielded broadly similar results. SAS (version SAS/STAT 13.2; SAS Institute) and R (version 3.6; R Foundation for Statistical Computing) were used.
RESULTS
There were 1.54 million patients with a first URTI and antibiotic prescription over a 3-month period in CPRD. They were given a total of 2.78 million antibiotic prescriptions. After excluding patients with other common infections, there were 1.45 million patients. A further restriction to the antibiotic types commonly used for URTI resulted in the final CPRD study population of 1.40 million patients with URTI (given 2.38 million antibiotic prescriptions). The final SAIL population included 0.42 million patients (given 0.69 million antibiotic prescriptions). Table 1 shows the characteristics of the 2 study populations at the dates of the antibiotic prescription. The mean age of patients was lower in the delayed antibiotic–prescribing compared with the immediate antibiotic-prescribing groups (in CPRD: 31.7 and 36.9 years, respectively). The mean predicted risk of infection-related hospital admission (without the effects of delayed prescribing) was 0.16% in both groups of patients. The frequency of delayed prescribing was 8.3% in CPRD and 9.2% in SAIL. For patients with delayed antibiotic prescribing, the median time between antibiotic and URTI diagnosis was 8 days (25th percentile, 3 days; 75th percentile, 16 days).
Table 1.
Baseline Characteristics of the Study Population Stratified by Immediate or Delayed Antibiotic Prescribing (in CPRD and SAIL)
| CPRD | SAIL | |||
|---|---|---|---|---|
| Immediate Antibiotic (n = 2 185 492) | Delayed Antibiotic (n = 197 522) | Immediate Antibiotic (n = 624 483) | Delayed Antibiotic (n = 63 287) | |
| Time, mean (SD) from antibiotic prescription until censoring, years | 5.5 (3.7) | 5.3 (3.7) | 5.1 (4.0) | 5.0 (4.1) |
| Age, mean (SD), years | 36.9 (24.6) | 31.7 (26.6) | 28.5 (24.3) | 22.1 (25.0) |
| Age, n (%) | ||||
| 0–17 years | 623 552 (28.5) | 80 342 (40.7) | 267 056 (42.8) | 36 998 (58.5) |
| 18–59 years | 1 080 113 (49.4) | 78 464 (39.7) | 266 614 (42.7) | 18 756 (29.6) |
| ≥60 years | 481 827 (22.0) | 38 716 (19.6) | 90 813 (14.5) | 7533 (11.9) |
| Women, n (%) | 1 251 759 (57.3) | 115 439 (58.4) | 347 963 (55.7) | 35 270 (55.7) |
| Indication, n (%) | ||||
| URTI | 481 313 (22.0) | 76 365 (38.7) | 124 870 (20.0) | 27 631 (43.7) |
| Cough /cold | 907 769 (41.5) | 114 000 (57.7) | 235 066 (37.6) | 34 029 (53.8) |
| Sore throat | 819 195 (37.5) | 60 490 (30.6) | 270 473 (43.3) | 18 446 (29.1) |
| Charlson comorbidity index, n (%) | ||||
| No (score 0) | 1 504 443 (68.8) | 141 461 (71.6) | 596 526 (95.5) | 60 970 (96.3) |
| Low (1–2) | 565 661 (25.9) | 46 273 (23.4) | 23 026 (3.7) | 1907 (3) |
| Moderate (3–4) | 88 553 (4.1) | 7515 (3.8) | 3755 (0.6) | 318 (0.5) |
| High (5–6) | 19 789 (0.9) | 1704 (0.9) | 1058 (0.2) | 81 (0.1) |
| Very high (≥7) | 7046 (0.3) | 569 (0.3) | 118 (0) | 11 (0%) |
| Flu vaccination year before, n (%) | 489 251 (22.4) | 43 392 (22.0) | 104 514 (16.7) | 10 421 (16.5) |
| Hospital admission year before, n (%) | 35 936 (1.6) | 4007 (2.0) | 10 426 (1.7) | 1411 (2.2) |
| Calendar year, n (%) | ||||
| 2000–2004 | 589 076 (27.0) | 49 742 (25.2) | 86 702 (13.9) | 7604 (12.0) |
| 2005–2009 | 883 375 (40.4) | 79 254 (40.1) | 176 492 (28.3) | 16 751 (26.5) |
| 2010–2015 | 713 041 (32.6) | 68 526 (34.7) | 361 289 (57.9) | 38 932 (61.5) |
Characteristics at each antibiotic prescription are shown.
Abbreviations: CPRD, Clinical Practice Research Datalink; SAIL, Secure Anonymized Information Linkage; URTI, upper respiratory tract infection.
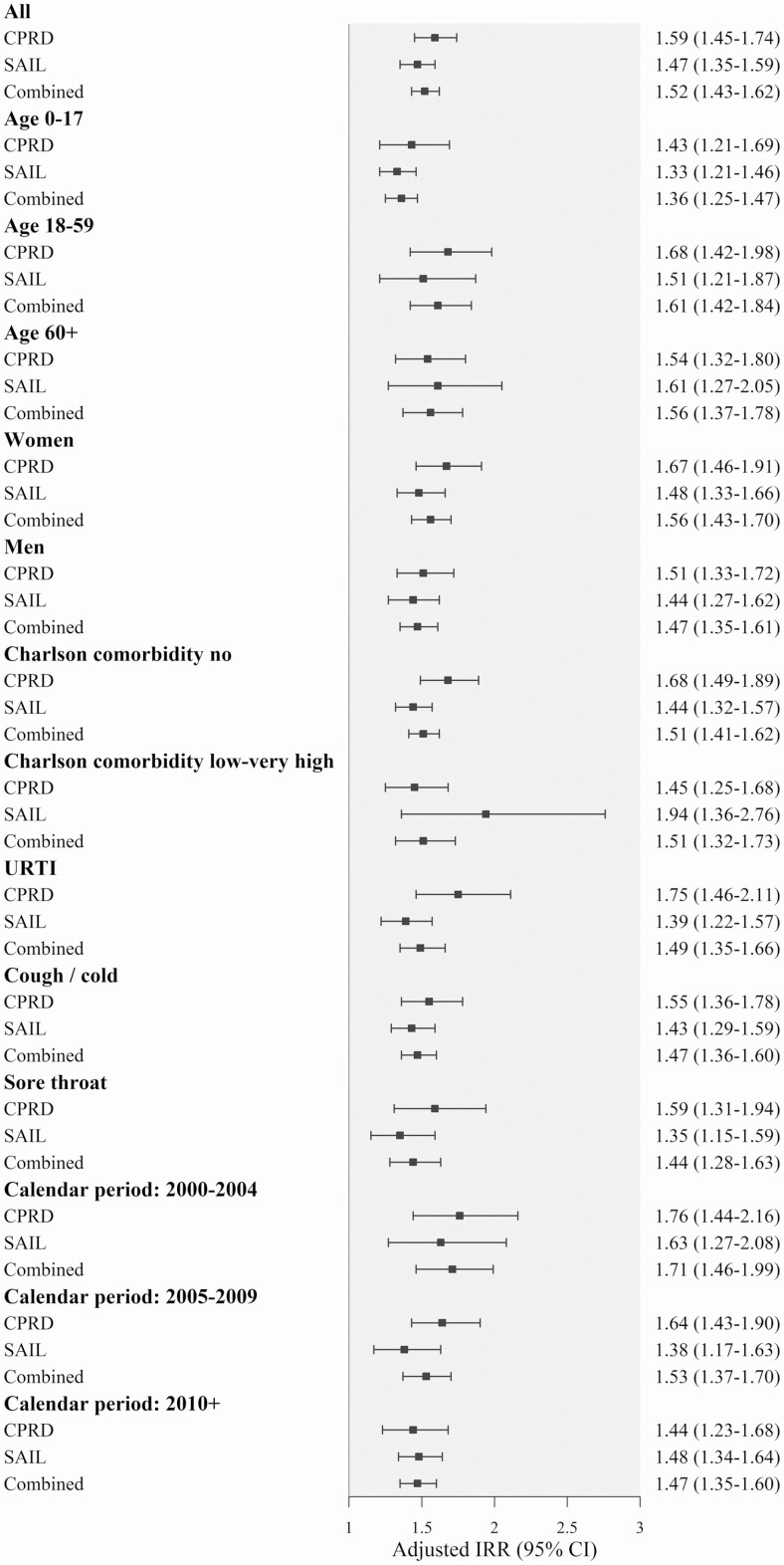
As shown in Table 2, the crude incidence of infection-related hospital admissions in the 30 days after was 0.15 per 100 person-months in CPRD and 0.67 in SAIL (totals of 3247 cases in CPRD and 4242 in SAIL). Delayed antibiotic prescribing was associated with a 52% increased risk of infection-related hospital admissions combining the results of both datasets (adjusted HR, 1.52; 95% CI, 1.43–1.62) (Figure 2). The effects of delayed antibiotic prescribing were lowest in children (adjusted HR, 1.36; 95% CI, 1.25–1.47) and highest in adults aged 18–59 years (HR, 1.61; 95% CI, 1.42–1.84). Most of the infection-related hospital admissions concerned respiratory diseases (96.8% in CPRD).
Table 2.
Frequency of Delayed Antibiotic Prescribing and Incidence Rates of Infection-related Hospital Admissions Overall and Stratified by Age and Sex (in CPRD and SAIL)
| CPRD | SAIL | |||
|---|---|---|---|---|
| Characteristic | Delayed Antibiotic Prescribing, % | Incidence Rate (No. of Cases)a | Delayed Antibiotic Prescribing, % | Incidence Rate (No. of Cases)a |
| All | 8.3 | 0.15 (3247) | 9.2 | 0.67 (4242) |
| Age | ||||
| 0–17 years | 11.4 | 0.12 (790) | 12.2 | 0.94 (2694) |
| 18–59 years | 6.8 | 0.12 (1300) | 6.6 | 0.36 (960) |
| ≥60 years | 7.4 | 0.24 (1157) | 7.7 | 0.67 (588) |
| Sex | ||||
| Women | 8.1 | 0.17 (1606) | 9.2 | 0.57 (2049) |
| Men | 8.4 | 0.13 (1641) | 9.2 | 0.77 (2193) |
Abbreviations: CPRD, Clinical Practice Research Datalink; SAIL, Secure Anonymized Information Linkage.
aNumber of cases per 100 person-months.
Figure 2.
HRs of infection-related hospital admission in patients with delayed compared with immediate antibiotic prescribing overall and stratified by age, sex, Charlson comorbidity, type of infection, and calendar time period. x axis: fully adjusted HR over follow-up (95% CI). Models included delayed prescribing, calendar year, and month of the date of antibiotic prescription, Charlson comorbidity index, BMI categories, smoking history, IMD quintiles, flu vaccination, outpatient referral, and hospitalization in the previous year. Abbreviations: BMI, body mass index; CI, confidence interval; CPRD, Clinical Practice Research Datalink; HR, hazard ratio; IMD, Index of Multiple Deprivation; IRR, incidence rate ratio; SAIL, Secure Anonymized Information Linkage.
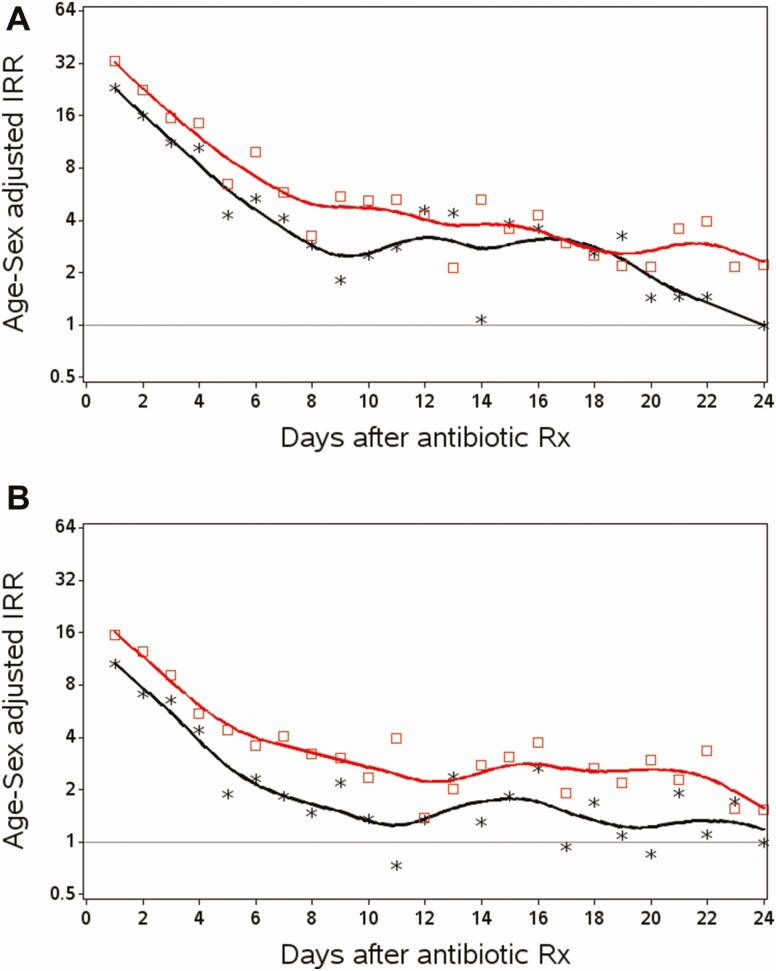
Figure 3 describes the IRRs of infection-related hospital admissions by day after the antibiotic prescription. The IRRs were highest in the first days after the antibiotic prescription and then quickly dropped over time more distant from the antibiotic prescription (as expected given URTI recovery). The IRRs in the patients with delayed antibiotic prescribing were generally higher at most time points compared with those with immediate antibiotic prescribing, although the patterns were comparable over time.
Figure 3.
IRRs of infection-related hospital admissions at each day of follow-up in patients with delayed or immediate antibiotic prescribing (age- and sex-matched cohorts). x axis: days after an antibiotic prescription; y axis: age- and sex-adjusted IRR. *Immediate antibiotic prescribing; □, delayed antibiotic prescribing; reference is days 24–30 after antibiotic prescribing in patients with immediate antibiotic prescribing. Abbreviation: IRR, incidence rate ratio. Panel A = CPRD (Clinical Practice Research Datalink); panel B = SAIL (Secure Anonymized Information Linkage).
Supplementary Table 3 shows the results of sensitivity analyses with different outcomes (infection complications as recorded by GPs, death, and repeat antibiotic prescribing), analyses restricting to antibiotics prescribed before 2008, and stratifying by extent of delay in antibiotic prescribing and Kaplan-Meier plots.
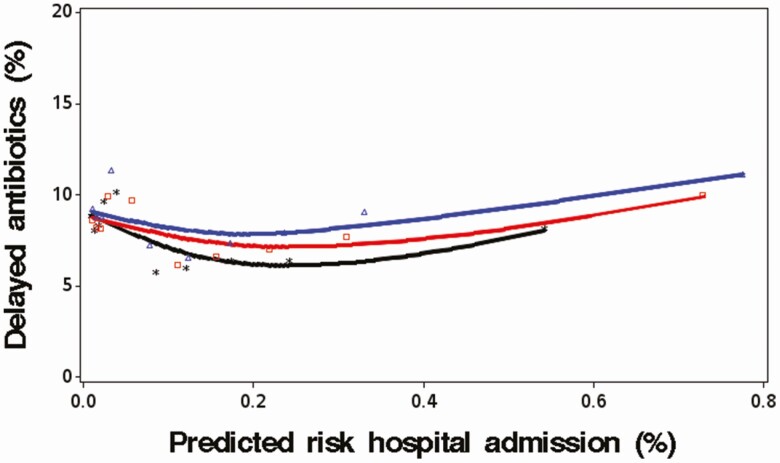
The analyses of NNH with delayed antibiotic prescribing showed considerable variability across different patient groups. The median NNH with delays in antibiotic prescribing was 1357 (2.5% percentile, 295; 97.5% percentile, 3366). Figure 4 shows the level of delayed antibiotic prescribing by deciles of predicted risk of infection-related hospital admission. It was found that patients with higher predicted risks of being admitted to the hospital were broadly as likely to get a delayed antibiotic prescription compared with patients with very low risks. There were no substantial changes over calendar time.
Figure 4.
Percentage of patients with URTI who received a delayed antibiotic prescription in patient subgroups with different predicted risks of infection-related hospital admission (stratified by calendar time). y axis: percentage of delayed antibiotic prescribing; x axis: deciles of predicted risk of infection-related hospital admission (%) (in CPRD based on the risk prediction model developed in patients with immediate antibiotic prescribing; details on the prediction model are found in the Supplementary Material). *black, 2000–2004; □ red, 2005–2009; ∆ blue, 2010+. Abbreviations: CPRD, Clinical Practice Research Datalink; URTI, upper respiratory tract infection.
Discussion
This study found that delayed antibiotic prescribing was associated with increases in the risk of infection-related hospital admissions and repeat antibiotic prescribing in patients with URTI. The NNH with delayed antibiotic prescribing was found to vary considerably between different patient groups. However, the probability of delayed prescribing was unrelated to patient risks of being admitted to the hospital for infection-related complications.
Treatment guidelines and the Cochrane review provide limited recommendations on when to use delayed antibiotic prescribing. The UK URTI guideline states the decision between immediate or delayed/no antibiotic is to be based on the clinical assessment of the infection severity [5]. The sore throat guideline recommends a “back-up” antibiotic prescription in case of an intermediate score with FeverPAIN or CENTOR (which are prediction rules of bacterial infection infections based on a sum of symptom scores) [18]. However, it has been found that the predictive values of these rules were poor and most complications occurred with low scores [19]. The Cochrane review recommended that a delayed antibiotic strategy could be used when it is clinically safe not to prescribe immediately [20]. The present study found that delayed antibiotic prescribing was not targeted to patients with lower risks of complications. This indicates that further research is needed to optimize the targeting of delayed antibiotic prescribing in order to provide more specific guidance when and when not to use this strategy. Clinical models to predict the risk of infection-related hospitalizations, in addition to clinical symptoms, could help inform decisions of antibiotic prescribing in primary care [21].
An important consideration is the balancing of any increased risk of infection-related complication with reductions in antibiotic use. The Cochrane review of trials that randomized patients with respiratory infections reported a substantive reduction in antibiotic use with delayed compared with immediate prescribing (odds ratio of 0.04 with delayed compared with immediate prescribing) [7]. However, trials in the Cochrane review may have enrolled patients who would not have received antibiotics outside the trial. In the 3 larger trials that evaluated delayed antibiotic prescribing, one enrolled patients only if there was reasonable clinical doubt as to whether to treat with an antibiotic [22], the second if patients were not very ill [23], and the third if immediate antibiotics were not needed [24]. Further evidence may be needed to assess the extent of reduction in antibiotic usage with a delayed prescribing strategy.
This study has several strengths and limitations. The main strengths were that this study was population based from representative general practices from England and Wales [9] and that it included a large number of patients with replication of findings in 2 independent databases. One major limitation was that patients had not been randomized to the different antibiotic strategies. The observed increase in the risk of clinical outcomes with delayed antibiotic prescribing could be related to either more severe infection or to underlying differences in patient characteristics. More severe infections in patients with delayed antibiotic prescribing would be a causal explanation of our findings (ie, delaying treatment may lead to more severe infections in some patients, increasing the risk of hospital admission). On the other hand, differences in patient characteristics (ie, subgroups of patients at higher risks of hospital admission with a similar infection severity) could have biased and confounded the study results. However, the risk factors as measured in this study did not indicate higher underlying risks of hospital admission in patients with delayed antibiotic prescribing. Important predictors for hospital admissions (such as age, comorbidity score, and mean predicted risk of infection-related hospital admission of 0.16% in both groups) were either comparable or lower in delayed compared with immediate antibiotic-prescribing groups. Another limitation was that this study evaluated actual delays in antibiotic prescribing irrespective of whether this was intended or not by the clinicians at the initial URTI diagnosis. However, the chance of patients getting an antibiotic in the United Kingdom for URTI did not change substantially from 2000 to 2015 [1]. Delayed antibiotic prescribing was first proposed in treatment guidelines in 2008 and there may been misclassification in 2008 onwards [5] (with antibiotic prescriptions recorded on the date of the URTI consultation but with the advice to delay intake [25]). However, the HRs did not change over time and no effect of such misclassification was observed. A further limitation was that the incidence rates of the clinical outcomes were different between SAIL and CPRD, with higher rates in Wales. It may not be unexpected that the incidence of infections and complications varies between regions (the largest differences were found among young children aged <5 years). In recent years, there has been a measles epidemic in Wales [26]; measles can lead to hospital admissions [27] and this may possibly partly explain these differences. The relative differences with delayed antibiotic prescribing were mostly consistent between SAIL and CPRD. Finally, no external validation of the risk prediction model was conducted, as the objective of this analysis was to describe the distribution of risks in the study population (rather than to use it for risk prediction in different populations). The model performance indicated that the model was properly fitted to the data. Another limitation was that there was no information on actual compliance and antibiotic intake by patients. Patients with URTI were also not routinely tested for viral or bacterial infections as point-of-care testing has only been introduced to a limited extent in recent years.
In conclusion, waiting to treat URTI was associated with increased risk of infection-related complications. There was considerable variation in the risks of infection-related hospital admission, although the probability of delayed antibiotic prescribing was unrelated to these. There is an important need to better target delayed antibiotic prescribing to patients with URTI with moderate risks of complications and immediate antibiotic prescribing to those with higher risks. Further research on the cost-effectiveness of the most optimal threshold is needed to establish the treatment thresholds.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge all the data providers and general practices that made the anonymized data available for research. The protocol was approved by the Independent Scientific Advisory Committee for CPRD research (protocol number 16_153R3AMn2A) and SAIL’s Information Governance Protocol Review Panel (protocol number 0693).
Disclaimer. The interpretation and conclusions contained in this study are those of the authors alone, and not necessarily those of the SAIL, UK Medicines and Healthcare Products Regulatory Agency (MHRA), Northern Health Science Alliance (NHSA), National Health Service (NHS), or the Department of Health.
Financial support. Connected Health Cities is a Northern Health Science Alliance–led program funded by the Department of Health and delivered by a consortium of academic and NHS organizations across the north of England. This study is partly based on data from the Clinical Practice Research Datalink obtained under license from the MHRA. The data are provided by patients and collected by the NHS as part of their care and support. This study also used anonymized data held in the SAIL system, which is part of the national e-health records infrastructure for Wales.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Palin V, Mölter A, Belmonte M, et al. Antibiotic prescribing for common infections in UK general practice: variability and drivers. J Antimicrob Chemother 2019; 74:2440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petersen I, Hayward AC, SACAR Surveillance Subgroup . Antibacterial prescribing in primary care. J Antimicrob Chemother 2007; 60(Suppl. 1):i43–7. Available at: http://academic.oup.com/jac/article/60/suppl_1/i43/999396/Antibacterial-prescribing-in-primary-care. Accessed 2 June 2019. [DOI] [PubMed]
- 3. O’Neill J; on behalf of the Review on Antimicrobial Resistance. Tackling drug resistant-infection globally: final report and recommendations. Available at: https://amr-review.org/sites/default/files/160518_Finalpaper_withcover.pdf. Accessed 8 March 2018.
- 4.Royal College of General Practitioners. TARGET Antibiotic Toolkit. Available at: https://www.rcgp.org.uk/TARGETantibiotics. Accessed 10 February 2019.
- 5. National Institute of Health and Care Excellence. Overview | Respiratory tract infections (self-limiting): prescribing antibiotics | Guidance | NICE. Available at: https://www.nice.org.uk/guidance/cg69. Accessed 30 May 2019. [PubMed]
- 6. McCullough AR, Glasziou PP. Delayed antibiotic prescribing strategies—time to implement? JAMA Intern Med 2016; 176:29. Available at: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/jamainternmed.2015.7095. Accessed 15 March 2019. [DOI] [PubMed] [Google Scholar]
- 7. Spurling GK, Del Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst Rev 2017; 9:CD004417. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28881007. Accessed 29 May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Little P, Stuart B, Hobbs FDR, et al. Antibiotic prescription strategies for acute sore throat: a prospective observational cohort study. Lancet Infect Dis 2014; 14:213–9. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1473309913702949. Accessed 29 May 2019. [DOI] [PubMed] [Google Scholar]
- 9. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones KH, Ford DV, Jones C, et al. A case study of the Secure Anonymous Information Linkage (SAIL) gateway: a privacy-protecting remote access system for health-related research and evaluation. J Biomed Inform 2014; 50:196–204. Available at: http://www.j-biomed-inform.com/article/S1532046414000045/fulltext. Accessed 8 February 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ford DV, Jones KH, Verplancke J-P, et al. The SAIL databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res 2009; 9:157. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19732426. Accessed 29 August 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nowakowska M, Palin V, van Staa T. Adherence to antibiotic prescribing guidelines—rates and drivers of inappropriate prescribing in UK general practices. J Antimicrob Chemother 2019; 74:3371–8. [DOI] [PubMed] [Google Scholar]
- 13. Gulliford MC, Moore MV, Little P, et al. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ 2016; 354:i3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charlson M, Wells MT, Ullman R, King F, Shmukler C. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS One 2014; 9:e112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang B, de Vries F, Setakis E, van Staa T-P. The pattern of risk of myocardial infarction in patients taking asthma medication: a study with the General Practice Research Database. J Hypertens 2009; 27:1485–92. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19491706. Accessed 1 September 2013. [DOI] [PubMed] [Google Scholar]
- 16. Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract 2013; 67:407–11. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23574101. Accessed 6 April 2020. [DOI] [PubMed] [Google Scholar]
- 17. Borenstein M, Hedges L, Higgins J, Rothstein H Introduction to meta-analysis. Wiley, 2009. Available at: www.meta-analysis.com. Accessed 29 May 2019. [Google Scholar]
- 18. National Institute of Health and Care Excellence. Overview | Sore throat (acute): antimicrobial prescribing | guidance | NICE. Available at: https://www.nice.org.uk/guidance/ng84. Accessed 27 July 2019.
- 19. Little P, Stuart B, Hobbs FDR, et al. Predictors of suppurative complications for acute sore throat in primary care: prospective clinical cohort study. BMJ 2013; 347:f6867. Available at: http://www.bmj.com/cgi/doi/10.1136/bmj.f6867. Accessed 27 July 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spurling GK, Del Mar CB, Dooley L, Foxlee R. Delayed antibiotics for respiratory infections. Coc hrane Database Syst Rev 2007:CD004417. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17636757. Accessed 10 April 2019. [DOI] [PubMed] [Google Scholar]
- 21. Mistry C, Martin G, Palin V, et al. Development and validation of a multivariable prediction model for infection-related complications in patients with common infections in UK primary care. BMC Med. 2020; 18:118. Published online 2020 May 21. doi: 10.1186/s12916-020-01581-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de la Poza Abad M, Mas Dalmau G, Moreno Bakedano M, et al. Prescription strategies in acute uncomplicated respiratory infections. JAMA Intern Med 2016; 176:21. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26719947. Accessed 15 March 2019. [DOI] [PubMed] [Google Scholar]
- 23. Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997; 314:722–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9116551. Accessed 10 April 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Little P, Moore M, Kelly J, et al. Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: pragmatic, factorial, randomised controlled trial. BMJ 2014; 348:g1606. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24603565. Accessed 15 March 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryves R, Eyles C, Moore M, McDermott L, Little P, Leydon GM. Understanding the delayed prescribing of antibiotics for respiratory tract infection in primary care: a qualitative analysis. BMJ Open 2016; 6:e011882. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27864242. Accessed 29 May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Public Health Wales. Measles outbreak: data. Available at: http://www.wales.nhs.uk/sitesplus/888/page/66389. Accessed 24 July 2019.
- 27. Goldacre MJ, Maisonneuve JJ. Hospital admission rates for measles and mumps in England: historical perspective. Lancet 2013; 382:308–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.