Abstract
Background
Globally, pneumonia is the leading cause of death among children. Few data exist regarding the effect of Haemophilus influenzae type b (Hib) vaccine and 13-valent pneumococcal conjugate vaccine (PCV-13) on the burden of childhood pneumonia in African settings.
Methods
We collected data on children aged 1 to 59 months at 3 hospitals in Botswana. Hib vaccine and PCV-13 were introduced in Botswana in November 2010 and July 2012, respectively. We compared pneumonia hospitalizations and deaths prevaccine (January 2009 to October 2010) with postvaccine (January 2013 to December 2017) using seasonally adjusted, interrupted time-series analyses.
Results
We identified 6943 pneumonia hospitalizations and 201 pneumonia deaths. In the prevaccine period, pneumonia hospitalizations and deaths increased by 24% (rate, 1.24; 95% CI, .94–1.64) and 59% (rate, 1.59; 95% CI, .87–2.90) per year, respectively. Vaccine introduction was associated with a 48% (95% CI, 29–62%) decrease in the number of pneumonia hospitalizations and a 50% (95% CI, 1–75%) decrease in the number of pneumonia deaths between the end of the prevaccine period (October 2010) and the beginning of the postvaccine period (January 2013). During the postvaccine period, pneumonia hospitalizations and deaths declined by 6% (rate, .94; 95% CI, .89–.99) and 22% (rate, .78; 95% CI, .67–.92) per year, respectively.
Conclusions
Pneumonia hospitalizations and deaths among children declined sharply following introduction of Hib vaccine and PCV-13 in Botswana. This effect was sustained for more than 5 years after vaccine introduction, supporting the long-term effectiveness of these vaccines in preventing childhood pneumonia in Botswana.
Keywords: immunization, sub-Saharan Africa, mortality, pediatric, bacterial pneumonia
This study evaluates the impact of Hib and pneumococcal conjugate vaccines on childhood pneumonia in Botswana. Using multicenter data, we demonstrate a marked decline in hospitalizations and deaths that was sustained for more than 5 years after vaccine introduction.
Pneumonia is the leading infectious cause of death among children [1, 2]. Pneumonia mortality is particularly high in sub-Saharan Africa, where nearly 500 000 child pneumonia deaths occur each year [1, 3, 4]. Historically, Streptococcus pneumoniae (pneumococcus) and Haemophilus influenzae type b (Hib) were the 2 most frequent bacterial causes of pneumonia among African children [5]. However, the use of effective conjugate vaccines against these pathogens in Africa increased substantially over the past decades, largely driven by support from the Global Alliance for Vaccines and Immunizations (GAVI) [6]. As of 2017, all 47 World Health Organization African Region countries had introduced a Hib vaccine and 39 of 47 (83%) countries had introduced a pneumococcal conjugate vaccine to their routine immunization program [6]. Although Hib vaccines dramatically reduce the incidence of invasive Hib disease [7], increases in the proportion of invasive disease caused by non–type b H. influenzae have been reported in the post-Hib vaccine era [8, 9]. Similarly, pneumococcal conjugate vaccines effectively prevent invasive disease caused by vaccine serotypes, but the emergence of nonvaccine serotypes has the potential to compromise the long-term benefit of these vaccines over time [10, 11]. At a time when a number of African countries are anticipated to transition out of GAVI support in the near future, there is an urgent need for data on the long-term effectiveness of these vaccines on the burden of childhood pneumonia in African countries.
In this study, we sought to determine the impact of introduction of Hib vaccine and 13-valent pneumococcal conjugate vaccine (PCV-13) on pneumonia hospitalizations and deaths over a 9-year period in Botswana.
METHODS
Study Setting
This was a retrospective study conducted from January to March 2019 at 3 hospitals in Botswana: Nyangabgwe Referral Hospital in Francistown, Princess Marina Hospital in Gaborone, and Letsholathebe II Hospital in Maun. Nyangabgwe Referral Hospital and Princess Marina Hospital are Botswana’s 2 tertiary medical centers and are located in northeastern and southern Botswana, respectively, while Letsholathebe II Hospital is a district hospital in northwestern Botswana. These hospitals serve the 3 most populous regions in Botswana, which together account for over 70% of the country’s urban population [12]. Botswana’s Expanded Programme on Immunization is fully funded by the government [13, 14]. Hib vaccine, administered as a component of a pentavalent vaccine (diphtheria, tetanus, pertussis, hepatitis B, and H. influenzae type b conjugate vaccine; Serum Institute of India Ltd, Pune, India), was introduced in November 2010 and PCV-13 (Prevnar-13; Pfizer, Inc, New York, NY), was introduced in July 2012. Both vaccines are administered at 2, 3, and 4 months of age and were introduced without a catch-up campaign. Complete vaccine series coverage rates for Hib vaccine and PCV-13 are estimated at 82–95% and 81–95%, respectively [13]. This study was approved by the Health Research and Development Committee (Ministry of Health, Botswana), ethics committees at each hospital site, and institutional review boards at the University of Botswana, the Children’s Hospital of Philadelphia, the University of Pennsylvania, and Duke University.
Study Population
We recorded hospitalizations of children 1 to 59 months of age with a discharge diagnosis of pneumonia between January 2009 and December 2017. Due to the difficulty in distinguishing between lower respiratory tract diagnoses in a setting with limited diagnostic capabilities, we defined pneumonia as a discharge diagnosis of “pneumonia,” “lower respiratory infection,” “respiratory tract infection,” “lower respiratory tract infection,” “bronchitis,” “bronchiolitis,” “PCP,” “aspiration pneumonia,” “bronchopneumonia,” or “pulmonary TB.” If the discharge diagnosis was missing, we used the admission diagnosis.
To assess for changes in the catchment populations served by the study hospitals over time, we recorded the number of hospitalizations for ingestion among children 1 to 59 months of age between January 2009 and December 2017. We chose ingestions as a comparator group because this indication for hospitalization was not anticipated to be affected by vaccine introduction and the incidence of ingestions was previously shown to be stable in a study conducted at these hospitals between 2009 and 2014 [15]. We recorded hospitalizations with a discharge diagnosis of drug or toxin ingestion, intoxication, poisoning, chemical pneumonitis, drug overdose, or foreign body ingestion [15]. We excluded hospitalizations for food poisoning or food intoxication, foreign body (in the ear, eye, or nose), and traditional medicine ingestion.
Data Collection
We abstracted data from written admission and ward registers at each of the 3 hospital sites. Study data were collected and managed using REDCap electronic data-capture tools [16]. We recorded data on demographics and, when available, information on human immunodeficiency virus (HIV) exposure and vaccination status. We recorded if the child was discharged to home, died, or was transferred to the hospital intensive care unit or to another hospital.
Statistical Analysis
We calculated the number of pneumonia hospitalizations and the number of pneumonia deaths for each calendar month at each hospital site. We excluded the 6-month periods following the introduction of each vaccine to allow for vaccine rollout and the development of immunity. In addition, the short time period between introduction of Hib vaccine and PCV-13 in Botswana precluded evaluation of the individual effect of these vaccines. Thus, we considered the prevaccine period to be from January 2009 to October 2010 and the postvaccine period (after introduction of both vaccines) to be from January 2013 to December 2017. We used a seasonally adjusted, interrupted time-series analysis using negative binomial regression to evaluate the effect of introduction of these vaccines on child pneumonia hospitalizations and deaths [17, 18]. Models included time in months, vaccine period (prevaccine or postvaccine), and the interaction between time in months and the vaccine period as independent variables. We assumed that the numbers of pneumonia hospitalizations and deaths at the 3 hospital sites were independent. To adjust for seasonal trends in pneumonia hospitalizations and deaths, we employed harmonic terms that specified 2 pairs of sine and cosine functions within a period of 12 months [19]. We assessed autocorrelation by examining the residuals, autocorrelation functions, and partial autocorrelation functions. For the outcome of pneumonia deaths, we excluded children who were transferred to the intensive care unit or another hospital, for whom mortality data were unavailable. However, we conducted a sensitivity analysis wherein children who were transferred to the intensive care unit or another hospital were classified as having died. Complete data were available for 235 of 246 site-months, including 56 of 66 (85%) site-months in the prevaccine period and 179 of 180 (99%) site-months in the postvaccine period. We assumed these missing data to be missing at random and excluded these data from analyses. We used negative binomial regression to evaluate for a trend in the number of ingestion hospitalizations during the 9-year study period. We performed statistical analyses using R version 3.6.1 (R Core Team, Vienna, Austria).
RESULTS
Patient Characteristics
Characteristics of the study population by vaccine period are shown in Table 1. The median (interquartile range [IQR]) age of children hospitalized with pneumonia was 10 (5–19) months, while the median (IQR) age of children who died of pneumonia was 6 (3–11) months. Data on HIV exposure status were missing from the majority of children during the prevaccine period. However, during the postvaccine period, nearly one-third of pneumonia hospitalizations and almost half of pneumonia deaths were among HIV-exposed children.
Table 1.
Characteristics of the Study Population by Vaccine Period
| Pneumonia Hospitalizations | Pneumonia Deaths | |||||||
|---|---|---|---|---|---|---|---|---|
| Prevaccine Period (n = 2954) |
Postvaccine Period (n = 3989) |
Prevaccine Period (n = 97) |
Postvaccine Period (n = 104) |
|||||
| n | % | n | % | n | % | n | % | |
| Hospital site | ||||||||
| Francistown | 1359 | 46 | 1835 | 46 | 34 | 35 | 46 | 44 |
| Gaborone | 1034 | 35 | 1316 | 33 | 39 | 40 | 34 | 33 |
| Maun | 561 | 19 | 838 | 21 | 24 | 25 | 24 | 23 |
| Age | ||||||||
| 1–11 m | 1568 | 53 | 2178 | 55 | 84 | 87 | 73 | 70 |
| 12–59 m | 1386 | 47 | 1811 | 45 | 13 | 13 | 31 | 30 |
| Sex | ||||||||
| Female | 1300 | 44 | 1755 | 44 | 48 | 49 | 52 | 50 |
| Male | 1654 | 56 | 2234 | 56 | 49 | 51 | 52 | 50 |
| HIV exposure status | ||||||||
| HIV-exposed | 561 | 19 | 1197 | 30 | 21 | 22 | 49 | 47 |
| HIV-unexposed | 620 | 21 | 2393 | 60 | 17 | 18 | 50 | 48 |
| Unknown | 1773 | 60 | 399 | 10 | 59 | 60 | 5 | 5 |
The prevaccine period corresponds to January 2009 to October 2010 and the postvaccine period (after introduction of Hib vaccine and PCV-13) corresponds to January 2013 to December 2017.
Abbreviations: Hib, Haemophilus influenzae type b; HIV, human immunodeficiency virus; PCV-13, 13-valent pneumococcal conjugate vaccine.
Pneumonia Hospitalizations
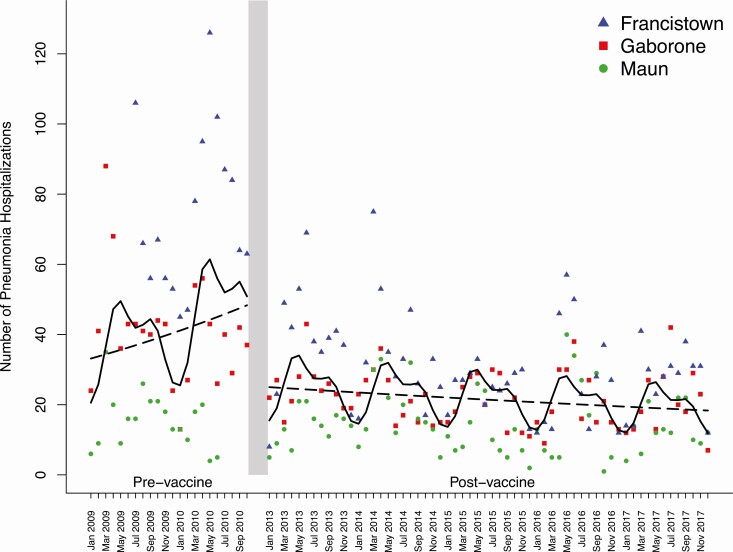
There were 6943 pneumonia hospitalizations during the study period, of which 3194 were at Nyangabgwe Referral Hospital in Francistown, 2350 were at Princess Marina Hospital in Gaborone, and 1399 were at Letsholathebe II Memorial Hospital in Maun. The annual mean number of pneumonia hospitalizations at all sites combined prior to vaccine introduction was 1290. During the prevaccine period, the number of pneumonia hospitalizations increased by 24% per year (rate, 1.24; 95% confidence interval [CI], .94–1.64) (Figure 1). Between the end of the prevaccine period (October 2010) and the start of the postvaccine period (January 2013), there was a 48% (95% CI, 29–62%) decrease in the number of pneumonia hospitalizations. During the postvaccine period, the annual mean number of pneumonia hospitalizations across all sites declined to 798. Moreover, the number of pneumonia hospitalizations continued to decrease by 6% per year (rate, .94; 95% CI, .89–.99) during the postvaccine period.
Figure 1.
Pneumonia hospitalizations among children 1–59 months of age in Botswana. The monthly number of pneumonia hospitalizations by study site is shown during the prevaccine (January 2009 to October 2010) and postvaccine (January 2013 to December 2017) periods. Each point represents the number of pneumonia hospitalizations in a given month at an individual hospital site. Lines were fitted to demonstrate trends with marginal effects (dashed lines) and seasonal effects (solid lines). During the prevaccine period, the fitted lines correspond to a 2.4% increase per year in pneumonia hospitalizations. During the postvaccine period, the marginal fitted lines correspond to a 6.1% decrease per year in pneumonia hospitalizations. A 48.3% decrease in pneumonia hospitalizations was observed between the marginal fitted lines at the end of the prevaccine period and the start of the postvaccine period.
The magnitude of the effect of vaccine introduction on the number of pneumonia hospitalizations differed by child age (Table 2). Among children 1–11 months of age, vaccine introduction was associated with a 58% (95% CI, 39–70%) reduction in the number of pneumonia hospitalizations and a continued decline of 6% per year (rate, .94; 95% CI, .88–.999) during the postvaccine period. Among children older than 12 months of age, vaccine introduction was associated with a 30% (95% CI, 3–50%) reduction in the number of pneumonia hospitalizations and a continued decline of 6% per year (rate, .94; 95% CI, .89–.996) during the postvaccine period.
Table 2.
Effect of Vaccine Introduction on Pneumonia Hospitalizations and Deaths by Child Age
| Annual Rate of Change (95% CI) in the Number of Pneumonia Hospitalizations or Deaths: Prevaccine (January 2009–October 2010) | Absolute Change in the Number (95% CI) of Pneumonia Hospitalizations or Deaths: Vaccine Introduction (October 2010–January 2013) | Annual Rate of Change (95% CI) in the Number of Pneumonia Hospitalizations or Deaths: Postvaccine (January 2013–December 2017) | |
|---|---|---|---|
| Pneumonia hospitalizations | |||
| All ages | 1.24 (.94, 1.64) | −48.3% (−62.4%, −28.9%) | .94 (.89, .99) |
| 1–11 m | 1.39 (1.02, 1.91) | −57.5% (−70.2%, −39.3%) | .94 (.88, .999) |
| 12–59 m | 1.03 (.77, 1.37) | −29.9% (−49.6%, −2.7%) | .94 (.89, .996) |
| Pneumonia deaths | |||
| All ages | 1.59 (.87, 2.90) | −50.1% (−74.9%, −.8%) | .78 (.67, .92) |
| 1–11 m | 1.44 (.70, 2.99) | −51.8% (−79.2%, +11.9%) | .83 (.69, 1.01) |
| 12–59 m | 1.94 (.82, 4.61) | −49.5% (−80.5%, +30.9%) | .70 (.54, .90) |
The rates of change in the number of hospitalizations and deaths per year are presented for the pre- and postvaccine periods. Changes in the number of hospitalizations (or deaths) related to vaccine introduction were calculated as the difference between the number of pneumonia hospitalizations (or deaths) at the end of the prevaccine period and the start of the postvaccine period.
Abbreviation: CI, confidence interval.
Pneumonia Deaths
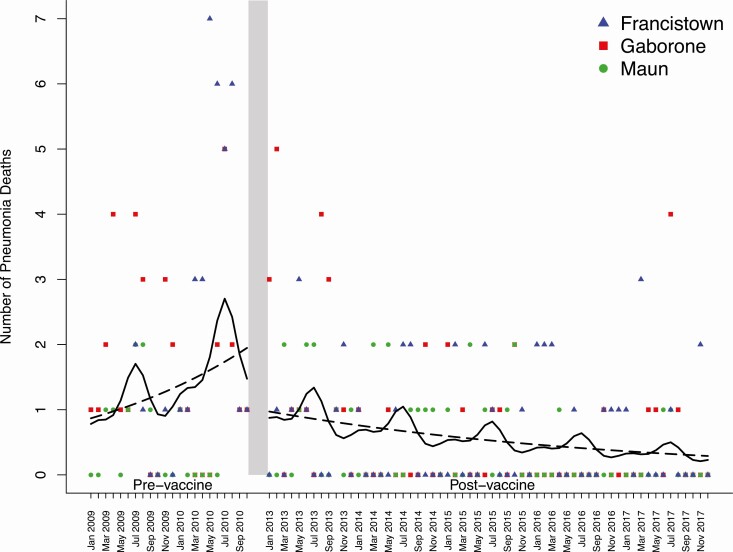
There were 201 pneumonia deaths recorded during the study period, of which 80 were in Francistown, 73 were in Gaborone, and 48 were in Maun. The annual mean number of pneumonia deaths at all hospital sites combined prior to vaccine introduction was 35. During the prevaccine period, the number of pneumonia deaths increased by 59% per year (rate, 1.59; 95% CI, .87–2.90) (Figure 2). Between the end of the prevaccine period (October 2010) and the start of the postvaccine period (January 2013), there was a 50% (95% CI, 1–75%) decrease in the number of pneumonia deaths. During the postvaccine period, the annual mean number of pneumonia deaths declined to 21. In addition, there was a sustained decrease in the number of pneumonia deaths of 22% per year (rate, .78; 95% CI, .67–.92). Three hundred eighty-six (6%) children were transferred from the pediatric ward to the intensive care unit or another hospital during the study period. Our findings did not substantively differ in a sensitivity analysis in which we classified these children as in-hospital deaths (Supplementary Table 1). The effect of vaccine introduction on pneumonia deaths by age is shown in Table 2.
Figure 2.
Pneumonia deaths among children 1–59 months of age in Botswana. The monthly number of pneumonia deaths by study site is shown during the prevaccine (January 2009 to October 2010) and postvaccine (January 2013 to December 2017) periods. Each point represents the number of pneumonia deaths in a given month at an individual hospital site. Lines were fitted to demonstrate trends with marginal effects (dashed lines) and seasonal effects (solid lines). During the prevaccine period, the fitted lines correspond to a 58.7% increase per year in pneumonia deaths. During the postvaccine period, the fitted lines correspond to a 21.8% decrease per year in pneumonia deaths. A 50.1% decrease in pneumonia deaths was observed between the marginal fitted lines at the end of the prevaccine period and the start of the postvaccine period.
Ingestion Hospitalizations
There were 1538 ingestion hospitalizations during the study period, of which 848 were in Francistown, 458 were in Gaborone, and 232 were in Maun. The median (IQR) age of children hospitalized for ingestion was 2.1 (1.7, 3.0) years. The number of ingestion admissions did not change over the 9-year study period (relative rate per year, 1.019; 95% CI, .987–1.052).
Discussion
This multicenter study is the first to evaluate the impact of Hib vaccine and PCV-13 on the burden of childhood pneumonia in Botswana. We demonstrate that the introduction of these vaccines was associated with a marked decrease in the numbers of pneumonia hospitalizations and deaths among children 1 to 59 months of age. This effect was sustained during a 5-year period after vaccine introduction, providing evidence for the long-term effectiveness of these vaccines for the prevention of childhood pneumonia in sub-Saharan Africa.
We found that sequential introduction of Hib vaccine and PCV-13 was associated with a 48% reduction in pneumonia hospitalizations and a 50% reduction in pneumonia deaths among children in Botswana. These findings are broadly consistent with those of prior studies that evaluated the short-term impact of Hib and pneumococcal conjugate vaccines on childhood pneumonia. A systematic review reported that Hib conjugate vaccines prevent 4–6% of cases of clinical pneumonia and 18% of cases of radiologically confirmed pneumonia among children in low- and middle-income countries [20]. However, substantial variability in the effect of Hib vaccines on childhood pneumonia has been reported across studies. For instance, administration of 3 doses of Hib conjugate vaccine was estimated to have a vaccine efficacy of 62–70% for radiologically confirmed pneumonia among children in Pakistan, while Hib vaccine did not reduce pneumonia incidence among children in Indonesia [21, 22]. The available data suggest that pneumococcal conjugate vaccines prevent a substantial proportion of childhood pneumonia cases, with vaccine efficacies being higher for radiologically confirmed cases. In The Gambia, a randomized controlled trial of 9-valent pneumococcal conjugate vaccine (PCV-9) demonstrated a vaccine efficacy of 37% for radiological pneumonia [23]. Similarly, in South Africa, PCV-9 resulted in a 17% reduction in alveolar consolidation among 39 836 children enrolled in a double-blind, placebo-controlled trial [24]. In a hospital surveillance study in Kenya, 10-valent pneumococcal conjugate vaccine was associated with 27% and 48% reductions in hospitalizations for clinically defined pneumonia and radiologically confirmed pneumonia, respectively [25]. In a surveillance study conducted in 2 districts in Malawi, PCV-13 was associated with a 47% decline in cases of hypoxemic pneumonia and a 36% decline in pneumonia deaths among children [26]. Finally, in a surveillance study conducted at a single hospital in Zimbabwe, PCV-13 reduced severe outpatient acute respiratory infections among children by 30% [27].
Recent studies conducted in high-income countries suggest that the long-term population-level benefits of Hib and pneumococcal conjugate vaccines may be compromised by an increase in disease caused by nonvaccine serotypes, although the overall trend remains toward a reduction in disease burden. In Sweden, an increase in invasive disease caused by nontypeable and type f strains of H. influenzae was observed following introduction of Hib vaccine, although most of these infections were observed in adults [8]. Similar trends have been observed in the post–Hib vaccine eras in England and Wales [9], Australia [28], and Canada [29]. The incidence of invasive pneumococcal disease caused by nonvaccine serotypes doubled following sequential introduction of 7-valent pneumococcal conjugate vaccine and PCV-13 in England and Wales, although the majority of disease caused by replacement serotypes occurred in adults [10]. After introduction of PCV-13 in France, the emergence of nonvaccine pneumococcal serotypes, particularly serotype 24F, resulted in a 2.3% (95% CI, 1.1–3.6%) per month increase in the incidence of pneumococcal meningitis among children [11]. There are comparatively few data regarding serotype epidemiology following introduction of Hib and pneumococcal conjugate vaccines in low- and middle-income countries, and we did not directly evaluate for serotype replacement in this study. However, our findings indicate that any effect of serotype replacement on the burden of childhood pneumonia in Botswana was outweighed by the benefits of prevention of disease caused by vaccine serotypes for at least 5 years after vaccine introduction.
We found that the effect of Hib vaccine and PCV-13 on pneumonia incidence and mortality in Botswana was more pronounced among infants than among older children. There are several potential explanations for this observation. First, because these vaccines were introduced without catch-up campaigns in Botswana, vaccine coverage rates were likely lower among older children than among infants for much of the postvaccine period. Prior studies of pneumococcal conjugate vaccines introduced in sub-Saharan Africa without catch-up campaigns reported variable effects on the burden of pneumonia among these older children. In Senegal, the incidence of pneumonia hospitalizations among children 1–4 years of age did not change following PCV-13 introduction, while pneumonia hospitalizations declined by 38% (95% CI, 21–50%) in this age group following PCV-13 introduction in Zambia [30]. In addition, Botswana uses a 3 + 0 schedule (3 primary doses, no booster dose) for PCV-13. Prior studies suggest that vaccine effectiveness following the primary pneumococcal conjugate vaccine series declines as a result of waning immunity, an effect that can be mitigated through inclusion of a routine booster dose [31]. Finally, it is likely that the proportion of child pneumonia cases caused by Hib and S. pneumoniae differs by age in children. Previous studies indicate that the highest incidence of invasive infections caused by these pathogens prior to introduction of conjugate vaccines occurred during infancy [32–34]. Additionally, infants have higher pneumonia mortality than older children, suggesting that infants stand to benefit more from vaccination against common pneumonia pathogens [4].
Our study had several limitations. First, pneumonia diagnoses were not based on a standardized case definition or defined radiological criteria. Second, underreporting of deaths due to pneumonia was likely, given that we were unable to ascertain vital status for patients transferred to the intensive care unit or another hospital. However, this underreporting is unlikely to have changed significantly between the prevaccine and postvaccine periods and our findings were unchanged in sensitivity analyses in which we classified all children who were transferred as deaths. Third, we cannot exclude the possibility that the catchment populations for these hospital sites changed over time, although we did find that the number of hospitalizations for ingestion, a condition that is unlikely to be related to vaccination, was stable over the study period. We also cannot exclude the possibility that secular trends in pneumonia risk factors (eg, exposure to indoor air pollution) or pneumonia treatment strategies contributed to the observed declines in pneumonia hospitalizations and deaths. However, to our knowledge, there were no significant changes to clinic or hospital care of childhood pneumonia in Botswana over this time period. The lack of complete data on HIV-exposure status was an additional limitation that prevented us from evaluating for differences in vaccine effectiveness among HIV-exposed and HIV-unexposed children. Finally, the short time interval between introduction of Hib vaccine and PCV-13 in Botswana precluded analyses of the individual effects of these vaccines on the burden of childhood pneumonia.
In summary, this study highlights the declines in pneumonia hospitalizations and deaths that occurred in Botswana following introduction of Hib vaccine and PCV-13. The protective effect observed in the 5 years after vaccine introduction supports the long-term benefits of these vaccines despite the potential for emergence of non–type b H. influenzae and nonvaccine pneumococcal serotypes. This study contributes to a growing body of literature supporting the enormous child health benefits of these vaccines in sub-Saharan Africa and provides further evidence that increasing use of these vaccines is critical to reducing global child mortality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments . The authors thank the staff at Nyangabgwe Referral Hospital, Princess Marina Hospital, and Letsholathebe II Hospital who assisted with this project.
Disclaimer. The funding sources had no role in the study design, data collection, analysis, interpretation of data, writing of the report, or in the decision to submit the paper for publication.
Financial support. M. C. was supported by the Melissa Ketunuti Endowment, the Children’s Hospital of Philadelphia, and the American Academy of Pediatrics. C. K. C. received financial support from the National Institutes of Health (NIH) through the Duke Center for AIDS Research (grant number P30-AI064518). S. M. P. was supported by a VECD Global Health Fellowship, funded by the Office of AIDS Research (OAR) and the Fogarty International Center (FIC) of the NIH (grant number D43 TW009337). M. S. K. was supported by a National Institutes of Health Career Development Award (grant number K23-AI135090).
Potential conflicts of interest. C. K. C. reports advisory board fees from Sanofi, outside the submitted work. All other authors report no potential conflicts. The authors have no financial relationships relevant to this article to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNICEF. One is too many: Ending child deaths from pneumonia and diarrhoea. Available at: https://www.unicef.org/publications/index_93020.html. Accessed 26 November 2017.
- 2. McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 2019; 7:e47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Brien K. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 12:893–902. [DOI] [PubMed] [Google Scholar]
- 4. Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health 2018; 6:e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wall RA, Corrah PT, Mabey DC, Greenwood BM. The etiology of lobar pneumonia in the Gambia. Bull World Health Organ 1986; 64:553–8. [PMC free article] [PubMed] [Google Scholar]
- 6. Sambala EZ, Wiyeh AB, Ngcobo N, Machingaidze S, Wiysonge CS. New vaccine introductions in Africa before and during the decade of vaccines—are we making progress? Vaccine 2019; 37:3290–5. [DOI] [PubMed] [Google Scholar]
- 7. Bisgard KM, Kao A, Leake J, Strebel PM, Perkins BA, Wharton M. Haemophilus influenzae invasive disease in the United States, 1994–1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis. 1998; 4:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Resman F, Ristovski M, Ahl J, et al. Invasive disease caused by Haemophilus influenzae in Sweden 1997–2009; evidence of increasing incidence and clinical burden of non-type b strains. Clin Microbiol Infect 2011; 17:1638–45. [DOI] [PubMed] [Google Scholar]
- 9. Ladhani SN, Collins S, Vickers A, et al. Invasive Haemophilus influenzae serotype e and f disease, England and Wales. Emerg Infect Dis 2012; 18:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis 2018; 18:441–51. [DOI] [PubMed] [Google Scholar]
- 11. Ouldali N, Levy C, Varon E, et al. ; French Pediatric Meningitis Network . Incidence of paediatric pneumococcal meningitis and emergence of new serotypes: a time-series analysis of a 16-year French national survey. Lancet Infect Dis 2018; 18:983–91. [DOI] [PubMed] [Google Scholar]
- 12. Statistics Botswana. Population Census Atlas 2011: Botswana. Published May 27, 2016. Available at: http://www.statsbots.org.bw/population-census-atlas-2011-botswana. Accessed 3 May 2020. [Google Scholar]
- 13. Botswana: WHO and UNICEF estimates of immunization coverage: 2016 revision. Published online 2016. Available at: https://data.unicef.org/wp-content/uploads/country_profiles/Botswana/immunization_country_profiles/immunization_bwa.pdf. Accessed 24 March 2018.
- 14. World Health Organization. Analytical summary—immunization and vaccines development. Available at: http://www.aho.afro.who.int/profiles_information/index.php/Botswana:Analytical_summary_-_Immunization_and_vaccines_development.
- 15. Enane LA, Gastañaduy PA, Goldfarb DM, et al. Impact of rotavirus vaccination on hospitalizations and deaths from childhood gastroenteritis in Botswana. Clin Infect Dis 2016; 62(Suppl 2):S168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 18. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017; 46:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol 2013; 42:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Theodoratou E, Johnson S, Jhass A, et al. The effect of Haemophilus influenzae type b and pneumococcal conjugate vaccines on childhood pneumonia incidence, severe morbidity and mortality. Int J Epidemiol. 2010; 39(Suppl 1):i172–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gessner BD, Sutanto A, Linehan M, et al. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet 2005; 365:43–52. [DOI] [PubMed] [Google Scholar]
- 22. Khowaja AR, Mohiuddin S, Cohen AL, et al. ; Pakistan Hib Vaccine Study Group . Mortality and neurodevelopmental outcomes of acute bacterial meningitis in children aged <5 years in Pakistan. J Pediatr 2013; 163:S86–S91.e1. [DOI] [PubMed] [Google Scholar]
- 23. Cutts FT, Zaman SM, Enwere G, et al. ; Gambian Pneumococcal Vaccine Trial Group . Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365:1139–46. [DOI] [PubMed] [Google Scholar]
- 24. Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis 2005; 40:1511–8. [DOI] [PubMed] [Google Scholar]
- 25. Silaba M, Ooko M, Bottomley C, et al. Effect of 10-valent pneumococcal conjugate vaccine on the incidence of radiologically-confirmed pneumonia and clinically-defined pneumonia in Kenyan children: an interrupted time-series analysis. Lancet Glob Health 2019; 7:e337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCollum ED, Nambiar B, Deula R, et al. Impact of the 13-valent pneumococcal conjugate vaccine on clinical and hypoxemic childhood pneumonia over three years in central Malawi: an observational study. PLoS One 2017; 12:e0168209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dondo V, Mujuru H, Nathoo K, et al. Pneumococcal conjugate vaccine impact on meningitis and pneumonia among children aged <5 years—Zimbabwe, 2010-2016. Clin Infect Dis 2019; 69:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan Sai Cheong J, Smith H, Heney C, et al. Trends in the epidemiology of invasive Haemophilus influenzae disease in Queensland, Australia from 2000 to 2013: what is the impact of an increase in invasive non-typable H. influenzae (NTHi)? Epidemiol Infect 2015; 143:2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desai S, Jamieson FB, Patel SN, et al. The epidemiology of invasive Haemophilus influenzae non-serotype B disease in Ontario, Canada from 2004 to 2013. PLoS One 2015; 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faye PM, Sonko MA, Diop A, et al. Impact of 13-valent pneumococcal conjugate vaccine on meningitis and pneumonia hospitalizations in children aged <5 years in Senegal, 2010-2016. Clin Infect Dis 2019; 69:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jayasinghe S, Chiu C, Quinn H, Menzies R, Gilmour R, McIntyre P. Effectiveness of 7- and 13-valent pneumococcal conjugate vaccines in a schedule without a booster dose: a 10-year observational study. Clin Infect Dis 2018; 67:367–74. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization. Haemophilus influenzae type b (Hib) meningitis in the pre-vaccine era: a global review of incidence, age distributions, and case-fatality rates. Published online 2002. Available at: https://apps.who.int/iris/handle/10665/67572. Accessed 23 February 2020.
- 33. Zangwill KM, Vadheim CM, Vannier AM, Hemenway LS, Greenberg DP, Ward JI. Epidemiology of invasive pneumococcal disease in southern California: implications for the design and conduct of a pneumococcal conjugate vaccine efficacy trial. J Infect Dis 1996; 174:752–9. [DOI] [PubMed] [Google Scholar]
- 34. Butler JC, Breiman RF, Lipman HB, Hofmann J, Facklam RR. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: implications for development of a conjugate vaccine. J Infect Dis 1995; 171:885–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.