Abstract
Background
Although Staphylococcus aureus and gram-negative bacterial bloodstream infections (SAB/GNB) cause substantial morbidity, little is known regarding patient perceptions’ of their impact on quality of life (QOL). Guidance for assessing QOL and disease-specific measures are lacking. We conducted a descriptive qualitative study to gain an in-depth understanding of patients’ experiences with SAB/GNB and concept elicitation phase to inform a patient-reported QOL outcome measure.
Methods
We conducted prospective one-time, in-depth, semi-structured, individual, qualitative telephone interviews 6– 8 weeks following bloodstream infection with either SAB or GNB. Patients were enrolled in an institutional registry (tertiary academic medical center) for SAB or GNB. Interviews were audio-recorded, transcribed, and coded. Directed content analysis identified a priori and emergent themes. Theme matrix techniques were used to facilitate analysis and presentation.
Results
Interviews were completed with 30 patients with SAB and 31 patients with GNB. Most patients were at or near the end of intravenous antibiotic treatment when interviewed. We identified 3 primary high-level concepts: impact on QOL domains, time as a critical index, and sources of variability across patients. Across both types of bloodstream infection, the QOL domains most impacted were physical and functional, which was particularly evident among patients with SAB.
Conclusions
SAB/GNB impact QOL among survivors. In particular, SAB had major impacts on multiple QOL domains. A combination of existing, generic measures that are purposefully selected and disease-specific items, if necessary, could best capture these impacts. Engaging patients as stakeholders and obtaining their feedback is crucial to conducting patient-centered clinical trials and providing patient-centered care.
Keywords: bacterial bloodstream infections, quality of life, measure development, patient-reported outcomes
SAB and GNB impact quality of life among survivors. Existing, generic measures that are purposefully selected and disease-specific items, if necessary, could best capture these impacts. Engaging patients as stakeholders is crucial to conducting patient-centered clinical trials and providing patient-centered care.
Staphylococcus aureus and gram-negative bacterial bloodstream infections (SAB and GNB, respectively) cause substantial morbidity and mortality [1, 2]. Successful treatment often requires extended intravenous antibiotic therapy, invasive procedures to obtain source control, prolonged hospital stays, and considerable recovery time. These treatments and the infection may both adversely affect patients’ quality of life (QOL) [3–5]. Understanding the patients’ experience with bloodstream infection is crucial for assessing their clinical impact and for measuring patient-centered outcomes in trials designed to evaluate new treatment strategies.
To date, little is known about patients’ experiences, from their perspective, with SAB and GNB. Guidance for assessing QOL among patients with SAB/GNB and disease-specific QOL measures are lacking. Limited quantitative research suggests that community-acquired bacteremia has a prolonged negative effect on functional status and health-related QOL [6]; however, existing QOL measures tend to be general and/or specific to other diseases.
We conducted a descriptive qualitative study to gain an in-depth understanding of patients’ experiences with SAB or GNB. The results of this qualitative study and concept elicitation will be used to inform development/selection of a QOL measure(s) to be further refined, validated, and tested in future work.
METHODS
Study Design
One-time, in-depth, semi-structured, individual, qualitative telephone interviews lasting approximately 60 minutes were conducted 6–8 weeks following culture-confirmed bloodstream infection. This time frame was selected because most patients were expected to have completed antibiotic treatment at this point. The Duke University Health System Institutional Review Board approved this study prior to data collection.
Participants and Setting
Using stratified purposeful sampling, we prospectively enrolled adults from the Bloodstream Infection Biorepository (BSIB), an ongoing prospective cohort study at 1 tertiary academic medical center of patients with a culture-confirmed diagnosis of SAB or GNB who agreed to be contacted regarding future studies. Work began with patients with SAB and expanded to patients with GNB with overlapping recruitment and enrollment. Consecutive patients with SAB or GNB were eligible for enrollment into the BSIB if they were older than 18 years of age, had an absolute neutrophil count ≥1 × 109/L, were hospitalized, had not been previously enrolled into the BSIB, had signs or symptoms of infection, had monomicrobial bacteremia, and provided written informed consent. Patients consenting for the BSIB who also agreed to be contacted for future studies were introduced to this study during their hospital stay via an additional written informed consent document. Designated research staff completed a contact form with a preferred phone number and other detailed contact information to maximize the potential of reaching the participant. Participants were excluded from this study if their preferred language was not English, they were receiving comfort care, had no telephone access or were concerned about usage of telephone, unable or unwilling to provide informed consent, previously enrolled, or had mixed gram-positive and gram-negative bacteremia at time of enrollment (if polymicrobial bacteremia was diagnosed after enrollment, the patient remained eligible and was retained).
A priori, our target sample size for the number of completed interviews was 30 patients with SAB and 30 patients with GNB, as this sample size was expected to be sufficient to reach thematic saturation. Prior research suggests that a median of 16 in-depth interviews is needed to reach 90% saturation [7]. However, 5 factors impacting rate of saturation have also been proposed: degree of instrument structure, sample homogeneity, study topic complexity, study purpose, and analyst categorization style [8]. A sample size of 30 patients per group provided flexibility and greater assurance of reaching thematic saturation given the semi-structured nature of the interview guide, possible sample heterogeneity with regard to important demographic and infection characteristics, and moderate complexity of the study topic.
Procedures (Data Collection)
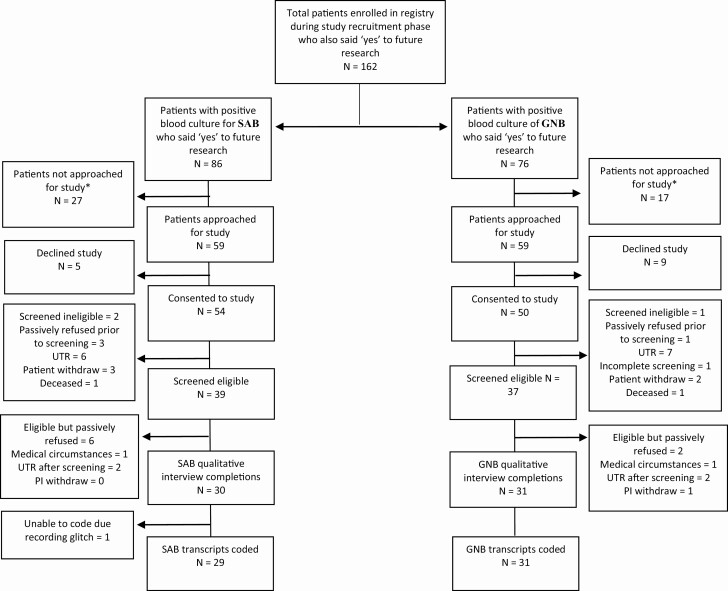
Participants were contacted via telephone 6–8 weeks following bloodstream infection to arrange a convenient day and time to complete the qualitative interview. During this phone call, research staff reconfirmed eligibility and administered a 6-item screener [9] to identify participants with cognitive impairment (see Supplementary Appendix 1). If an enrolled participant had 2 or more errors on the cognitive screener, he or she was no longer eligible to complete the interview. Also, to reconfirm eligibility, research staff reviewed the enrolled participant’s chart for potential conditions (ie, current health state, including acute psychiatric illness) that may have impacted his or her ability to complete the qualitative interview. See Figure 1 (project flow) for a description of study activities as well as their location and timing.
Figure 1.
Project flow diagram (621 call attempts). Abbreviations: GNB, gram-negative bacterial bloodstream infection; SAB, Staphylococcus aureus bloodstream infection.
Interviews were conducted by 2 of the authors from May 2017 to February 2018. The interview guide (see Supplementary Appendix 2) was based on a literature review of SAB/GNB and QOL studies. Of primary interest to the current study, patients were asked to describe and provide examples of how their bloodstream infection and its treatment impacted them in different areas of their life, including identifying which domains had been impacted the most and least. Interviews were audio-recorded and then transcribed. Participants were compensated $25 for completion of the interview.
Data Analysis
Directed content analysis was used, organizing the data in response to the a priori questions contained in the interview guide [10]. Four coders examined transcripts for common and emergent themes as well as a priori themes. Open coding was used to identify manifest and latent content, followed by axial coding to identify patterns between code categories [11]. Both first (eg, elemental and affective approaches) and second cycle coding processes were applied. A subset of 10 transcripts were coded by all coding team members (representing both qualitative and relevant clinical expertise) for codebook development and to establish intercoder reliability. The remaining transcripts were coded by either the primary (n = 37) or secondary (n = 13) coder under the supervision of the principal investigator. The primary coder all work of the secondary coder, while the work of the primary coder was reviewed by/with the principal investigator. The codebook, containing definitions and exemplars, was developed by the coding team and presented to the larger study team for feedback. The coding team maintained an audit trail of coding and analytic decisions. A systematic process of mutual consensus was used [12–15]. We also employed theme matrix techniques and salience (ie, what was important to patients and/or recurring) to facilitate data analysis and presentation [16]. For both SAB and GNB, quotes were organized in separate matrices with rows (QOL domains—cognitive, functional, emotional, physical, social) and columns (level of impact—major, minor, none) identifying critical dimensions. Qualitative data analysis software (ie, NVivo 12, QSR International) was used to manage the transcripts and assist with coding and analysis.
RESULTS
Interviews were completed with 30 patients with SAB and 31 patients with GNB (see Figure 2 for the participant flow diagram). The average interview time was 47.4 minutes. A recording error for 1 SAB interview precluded coding and transcription. Table 1 shows characteristics of interviewed patients and patients enrolled but not interviewed (SAB = 24, GNB = 19). Demographic characteristics between these 2 groups did not differ significantly. Overall, those with GNB were more likely to have come from home and also more likely to be discharged to home. Patients with SAB commonly had a skin or endovascular source, whereas those with GNB most commonly had a gastrointestinal or genitourinary source. Patients with SAB were more likely to have persistent bacteremia, metastatic sites of infection, and to require home health services after discharge. Patients with GNB had shorter hospital stays, received shorter courses of antibiotics, and required fewer procedures in the treatment of their infections.
Figure 2.
Participant flow diagram. Passive refusal indicates patients who were consented for the study but could not be reached for qualitative interview. Note: If the study team found that a participant’s current health state, including acute psychiatric illness, may have impacted his or her ability to complete the interview, the participant was no longer eligible to proceed with the qualitative interview. Determining eligibility was up to the discretion of the PI and/or clinical team. *These patients were not approached for this study because the majority were accompanied by a legally authorized representative who signed the Bloodstream Infection Biorepository consent on their behalf, rendering them unapproachable for this study due to exclusion criteria. Other reasons include co-occurrence of SAB and GNB, having already reached study recruitment goal, and being unable to consent due to pain. Reasons were based on 10 patients. Abbreviations: GNB, gram-negative bacterial bloodstream infection; PI, principal investigator; SAB, Staphylococcus aureus bloodstream infection; UTR, unable to reach.
Table 1.
Characteristics of Enrolled Patients with Staphylococcus aureus and Gram-negative Bloodstream Infections as a Function of Interview Status
| SAB (n = 54) | GNB (n = 50) | ||||
|---|---|---|---|---|---|
| Characteristics | All (SAB and GNB) (N = 104) | Interviewed (n = 30) | Not Interviewed (n = 24) | Interviewed (n = 31) | Not Interviewed (n = 19) |
| Age, mean (median), years | 61.1 (66) | 57.4 (57.5) | 57.3 (59) | 61.1 (66) | 44.4 (48) |
| Sex, n (%) | |||||
| Male | 59 (56.7) | 19 (63.3) | 14 (58.3) | 15 (48.4) | 11 (57.9) |
| Female | 45 (43.3) | 11 (36.7) | 10 (41.7) | 16 (51.6) | 8 (42.7) |
| Race, n (%) | |||||
| White | 67 (64.4) | 22 (73.3) | 13 (54.2) | 23 (74.2) | 9 (47.4) |
| African-American | 33 (31.7) | 8 (26.7) | 11 (45.8) | 7 (22.6) | 7 (36.8) |
| Asian | 3 (2.9) | 0 (0.0) | 0 (0.0) | 1 (3.2) | 2 (10.5) |
| Unknown | 1 (0.96) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) |
| Point of entry, n (%) | |||||
| Home | 77 (74.0) | 17 (56.7) | 14 (58.3) | 28 (90.3) | 18 (94.7) |
| Nursing home/rehabilitation facility | 5 (4.8) | 2 (6.7) | 3 (12.5) | 0 (0.0) | 0 (0.0) |
| Outside hospital | 21 (20.2) | 11 (36.7) | 6 (25.0) | 3 (9.7) | 1 (5.3) |
| Homeless | 1 (1.0) | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0 (0.0) |
| Underlying comorbidity, n (%) | |||||
| Neoplasma | 17 (16.4) | 1 (3.3) | 4 (16.7) | 8 (25.8) | 4 (21.1) |
| Diabetic | 35 (33.7) | 10 (33.3) | 11 (45.8) | 9 (29.0) | 5 (26.3) |
| Hemodialysis dependent | 9 (8.7) | 3 (10.0) | 3 (12.5) | 1 (3.2) | 2 (10.5) |
| HIV positive | 1 (0.96) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) |
| Transplant recipientb | 9 (8.7) | 4 (13.3) | 0 (0.0) | 3 (9.7) | 2 (10.5) |
| Injection drug usec | 6 (5.8) | 1 (3.3) | 2 (8.3) | 1 (3.2) | 2 (10.5) |
| Corticosteroid use (30-day) | 21 (20.2) | 7 (23.3) | 5 (20.8) | 5 (16.1) | 4 (21.1) |
| Surgery past 30 days | 19 (18.3) | 7 (23.3) | 5 (20.8) | 5 (16.1) | 2 (10.5) |
| Site of acquisition, n (%) | |||||
| Hospital-acquired | 11 (10.6) | 2 (6.7) | 2 (8.3) | 3 (9.7) | 4 (21.1) |
| HCA community-acquired | 54 (51.9) | 11 (36.7) | 15 (62.5) | 20 (64.5) | 8 (42.1) |
| Non-HCA community-acquired | 39 (37.5) | 17 (56.7) | 7 (29.1) | 8 (25.8) | 7 (36.8) |
| Source of bacteremia, n (%) | |||||
| Endovascular infection | 15 (14.4) | 5 (16.7) | 6 (25.0) | 2 (6.5) | 2 (10.5) |
| GI/GU infection | 25 (24.0) | 2 (6.7) | 0 (0.0) | 14 (45.1) | 9 (47.4) |
| Respiratory/lung | 1 (0.96) | 1 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Skin, soft tissue, joint/bone infection | 26 (25.0) | 12 (40.0) | 8 (33.3) | 3 (9.7) | 3 (15.8) |
| Unknown | 37 (35.6) | 10 (33.3) | 10 (41.7) | 12 (38.7) | 5 (26.3) |
| Persistent bacteremia, n (%) | 12 (11.5) | 9 (30.0) | 2 (8.3) | 0 (0.0) | 1 (5.3) |
| Metastatic infection, n (%) | 34 (32.7) | 13 (43.3) | 13 (54.2) | 6 (19.4) | 2 (10.5) |
| APACHE II, mean (SD) | 14.7 (4.6) | 15.4 (6.7) | 13.2 (6.3) | 16.4 (7.3) | 12.7 (5.3) |
| LOS categories, n (%) | |||||
| <9 days | 46 (44.2) | 11 (36.7) | 5 (20.8) | 20 (64.5) | 10 (52.6) |
| 9–14 days | 31 (29.8) | 11 (36.7) | 11 (45.8) | 6 (19.4) | 3 (15.8) |
| 15–20 days | 16 (15.4) | 6 (20.0) | 2 (8.3) | 5 (16.1) | 3 (15.8) |
| >20 days | 11 (10.6) | 2 (6.7) | 6 (25.0) | 0 (0.0) | 3 (15.8) |
| Number of days antibiotics used, mean (SD) | 32.1 (9.7) | 46.1 (35.7) | 41.7 (14.3) | 21.3 (17.9) | 20.0 (14.8) |
| Procedures used to treat the infection, n (%) | 55 (52.9) | 23 (76.7) | 18 (75.0) | 8 (25.8) | 6 (31.6) |
| Type of procedures used to treat the infection, n (%) | |||||
| Surgical removal of foreign device | 19 (18.3) | 8 (26.7) | 11 (45.8) | 0 (0.0) | 0 (0.0) |
| Surgical debridement | 15 (14.4) | 9 (30.0) | 5 (20.8) | 1 (3.2) | 0 (0.0) |
| Surgical insertion of foreign device | 27 (26.0) | 12 (40.0) | 8 (33.3) | 4 (12.9) | 3 (15.8) |
| Abscess drainage | 14 (14.3) | 4 (13.3) | 7 (29.2) | 2 (6.5) | 1 (5.3) |
| Line removal | 10 (9.6) | 3 (10.0) | 4 (16.70 | 2 (6.5) | 1 (5.3) |
| Other | 13 (13.3) | 7 (23.3) | 3 (12.5) | 1 (3.2) | 2 (10.5) |
| Discharge location | |||||
| Home or self-care | 46 (44.2) | 5 (16.7) | 7 (29.1) | 20 (64.5) | 14 (73.7) |
| Home with home health | 35 (33.7) | 20 (66.7) | 8 (33.3) | 6 (19.4) | 1 (5.3) |
| Home with hospice | 1 (0.96) | 0 (0.00) | 0 (0.0) | 1 (3.2) | 0 (0.0) |
| Skilled nursing facility/rehabilitation | 22 (21.2) | 5 (16.7) | 9 (37.5) | 4 (12.9) | 4 (21.1) |
| Outcome (90-day), n (%) | |||||
| Cure | 88 (84.6) | 27 (90.0) | 18 (75.0) | 28 (90.3) | 15 (78.9) |
| Recurrent GPC/GNB infection | 11 (10.58) | 3 (10.0) | 3 (12.5) | 2 (6.5) | 3 (15.8) |
| Death due to GPC/GNB infection | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Death due to other causes | 5 (4.8) | 0 (0.00) | 3 (12.5) | 1 (3.2)d | 1 (5.3) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; BMT, bone marrow transplant; GI/GU, gastrointestinal/genitourinary; GNB, gram-negative bloodstream infection; GPC, gram-positive cocci; HCA, healthcare-associated [17]; HIV, human immunodeficiency virus; LOS, length of stay; SAB, Staphylococcus aureus bloodstream infection.
aNeoplasm: received therapy for neoplasm in the 30 days before bacterial infection.
bTransplant recipient: most solid-organ (if nonneutropenic), but may be BMT or stem cell in rare cases.
cInjection drug use: active or at any time as record in the medical record.
dPatient interviewed prior to death.
We identified 3 primary high-level concepts: impact on QOL domains, time as a critical index, and sources of variability across patients. These themes and subthemes contained therein are described in the following sections. Illustrative, supportive quotations are provided in matrix form in Tables 2 and 3, separately for SAB and GNB.
Table 2.
Illustrative Quotes: Staphylococcus aureus Bacteremia
| Quality of Life Domain | Major | Minor | None |
|---|---|---|---|
| Physical | • “I lost a lot of weight. I used to be almost 130 pounds. After the infection, I was 89 pounds. I lost really drastically. It was bam and I was really tiny—and weak.” Female, age 20 [CONCEPTS 1, 2, 3] • “Well, I guess it varied over time but right now it’s physical... I just don’t have the strength—I don’t have strength in my—I mean, I can walk around but I get fatigued. So I’m cutting back on all physical activities.” Male, age 71 [CONCEPTS 1, 2] |
• “As far as the effects, I just have to be much more careful whenever I reach for spices or items that would be above shoulder level, when I’m cooking, whenever I’m getting things out of the refrigerator, when I’m showering. I have to use a brush on a stick in order to reach areas that I used to reach with my hands and a washcloth. It’s just a matter of making slight adaptations to the physical limitations.” Male, age 60 [CONCEPTS 1, 3] • “Well, it never did really impact me all that much. I can still walk, and do everything I ever did. So, it was really not that big an impact. Those times I had an I.V. in my arm, and just had to work around that.” Male, age 77 [CONCEPTS 1, 3] |
• “Physically? I haven’t had any complications or anything with it.” Female, age 47 [CONCEPT 1] |
| Functional | • “And it was irritating. It was a pain because it interrupted our life, really. We had to plan everything around it—our bed schedule, our sleeping schedule.” Female, age 75 [CONCEPTS 1, 2] • “So, personally, my living conditions have been put on hold as well, I guess. So, I’ve got a lot to do later— about September or so… My ability to function, I guess, would be affected the most. My ability to function in my normal life.” Female, age 56 [CONCEPTS 1, 2] |
• “If I wanted to go somewhere, we would just simply take the meds with us and administer them wherever we had to be.” Male, age 47 [CONCEPTS 1, 3] • “Well, actually, even though I’ve had the PICC line and the bag, I wasn’t allowed to drive, but that didn’t keep me from being able to ride with people, and go out to eat, or go to the grocery store, go shopping. It didn’t limit that, and I think that was good.” Female, age 65 [CONCEPTS 1, 3] |
• “Going forward, it’s kind of like taking aspirin in the morning, the antibiotic pills.” Male, age 78 [CONCEPTS 1, 2, 3] |
| Emotional | • “It was so emotionally bad. I would just cry at night. I was like, “Oh, my god. I’m never getting better. This is it for me.” Female, age 20 [CONCEPTS 1, 2, 3] • “It’s hard to sit in a chair all day and maybe not feel depressed or thinking why am I not up walking more? Why am I not able to do more? Why am I not able to control things better? So, life for me for a long time was either a chair or a bed. And that was a hard way go for a very active person.” Male, age 47 [CONCEPTS 1, 2, 3] |
• “ ...it’s kind of scary sometimes. And anytime you’re going through any type of sickness, you’re gonna worry about it. So, the impact wasn’t too bad, but it was there.” Male, age 59 [CONCEPTS 1, 3] • “But just emotionally, probably, just a little bit. But it’s kind of who I normally am.” Male, age 57 [CONCEPTS 1, 3] • “Not really difficult, just kind of a little overwhelming. I didn’t want anything to happen to it [referring to PICC line] while I was at home. When I’m at the hospital, they’ve got nurses running around all over the place. But for me, to be sitting at home, and I’ve got this central line that they’ve got me having access to, sometimes it’s a little overwhelming because I was scared that something was going to happen to it while I was at home and then I would have to go back in, or do whatever. I wanted to make sure I was taken care of the best I could, so I wouldn’t have any added complications… Mentally, and emotionally, it showed me that it’s okay to be a little apprehensive. It’s okay to be a little overwhelmed with what’s going on with my body, or have an infection I’ve never had in my life.” Female, age 47 [CONCEPTS 1, 3] |
• “I don’t really think it has. I mean I’ve not had any emotional outbreaks or anything worrying about it. Like I said, having been through it before made it a lot easier to say, “Okay, well, we’re dealing with it again. We just do what we gotta do and move on.” So, I don’t think it’s had a real emotional impact on me.” Male, age 58 [CONCEPTS 1, 3] • “It has not impacted me at all. I’m good, and positive, and always have been.” Male, age 77 [CONCEPTS 1, 3] |
| Cognitive | • “You gotta understand, I was not in the right frame of mind at all during this. It was a fairly traumatizing event. What I’m telling you today is as accurate as I can possibly recall.” Male, age 45 [CONCEPTS 1, 2] | • “A lot of things I deal with head-on but not so much as it made me sad or depressed or anything. It’s sorta just frustrating me and pushing me to try and do better at some things I guess I can say—Not much of a mental struggle with it. It hasn’t really impacted my mental much at all. It’s just only really frustrated me some because of the things I can’t do versus the little things that I can do. So, I guess that’s where I’m at as far as my mentality.” Male, age 42 [CONCEPTS 1, 3] | • “I don’t know if it really impacted me at all. Like I said, it’s just another setback.” Male, age 50 [CONCEPTS 1, 3] |
|
• “It’s definitely made me more aware of when my body isn’t responding the way it usually would in a way, so I’m definitely more aware if something is off with my body, like medically or if something just doesn’t seem right, things like that. It’s definitely made me far more aware than what I already was. So, I’m more eager to get answers sooner instead of waiting around and putting it off because I don’t wanna pay the copay, or drive to the doctors, or something… Lasting impact, definitely being more aware of what my body is telling me and to take is seriously. I mean, your body is supposed to function at a certain level for a reason, and if you feel like it’s not, something could be wrong.” Female, age 30 [CONCEPTS 1, 2, 3] |
• “I really didn’t think much about it, just hoping they can get rid of it before it got worse.” Female, age 73 [CONCEPT 1] |
||
| Social | • “I think most impact would have been social, just, like I said, because it took so much time during the day to make sure you got the treatments done and do everything carefully to avoid any infections or anything through the PICC line. I think that had the most impact.” Male, age 58 [CONCEPTS 1, 2] | • “As I progress and feel better, I think my strategies are better. I feel I’ve gone to church twice; I’ve gone out to dinner once. I’m trying to get out and do a little more than I was not comfortable doing when I first got home.” Male, age 47 [CONCEPTS 1, 2, 3] • “I’m president of a local organization and I’ve not been able to do my job with that except by phone or email and that kind of thing. But everybody’s been good about helping out on that, so that’s good.” Female, age 56 [CONCEPT 1] |
• “It actually didn’t impact me at all because I was always very open when a friend, or a family member, or a coworker had a question about my PICC line, or my medication, or see how I felt. I’m not afraid to answer questions, so it didn’t really, I guess, bother me in terms of socially. So, it wasn’t that bad.” Female, age 30 [CONCEPTS 1, 3] |
The quality of life domains are both overlapping and distinct. These 26 selected quotes represent exemplars from 19 different participants with 5 of these with 2 quotations and 1 with 3 quotations
Abbreviation: PICC line, peripherally inserted central catheter.
Table 3.
Illustrative Quotes: Gram-negative Bacteremia
| Quality of Life Domain | Major | Minor | None |
|---|---|---|---|
| Physical | • “Even though you don’t have the infection, it just leaves your body weak. So, that’s what I’m dealing with. That’s the worst part of it is just drained.” Female, age 68 [CONCEPT 1] • “As time goes on, it, hopefully, will get better. But right now, physical has been. It just sort of kicked my butt, so to speak.” Female, age 68 [CONCEPTS 1, 2] • “I’d say physical would be the most. Like I said, I wear out pretty quickly compared to normal. And I know I can start lifting a little bit heavier stuff, but I also know I have to take it easy on that. After years of being a trainer and helping people recover from their injuries, I know it all isn’t done in a day.” Male, age 60 [CONCEPTS 1, 2, 3] |
• “Nothing other than being a little tired now and then. Excuse me, other than being tired.” Male, age 70 [CONCEPT 1] • “But it hasn’t had that much impact except the physical. I just don’t have the get-up-and-go.” Female, age 69 [CONCEPT 1] • “I’d say, I’m feeling more tired than I normally do, but it doesn’t keep me from doing things.” Male, age 86 [CONCEPTS 1, 3] |
• “Besides the scar on my knee, hopefully nothing. So, hopefully I’ll just look down at that knee in the years to come and be like, ‘Oh, yeah, and one time I had this infection’.” Female, age 46 [CONCEPTS 1, 2, 3] • “Long-term, none that I can tell... I’ve resumed back to normal activity.” Male, age 69 [CONCEPTS 1, 2, 3] |
| Functional | • “The most, functioning... I would like to be able to move on my own; nobody have to help me and hold me or whatever they have to do for me, help me with the bathroom situation.” Female, age 64 [CONCEPT 1] • “So, those things are frustrating, you know, having that PICC line in, physically. And then of course, because of my knee, you know, that was really irritating, because home with a walker and it’s hard to move around. So, that had a huge impact on my life… And then you come home, and you have a walker and you need help with everything. Yeah, you know, it changes your life.” Female, age 46 [CONCEPTS 1, 2] |
• “I think it went very well. It was easy, wasn’t difficult. It was a bother after a bit, knowing that you had to be someplace at 12-hour intervals, and clean a work area, and get yourself organized, and all that, and then—but other than that—I mean, we knew what the process was, and it wasn’t difficult.” Male, age 70 [CONCEPTS 1, 2, 3] • “Yeah, actually, I didn’t feel much from it. Just a little bit of nausea and some side effects from the pill I take. But the symptoms will become better just after a couple of hours after taking the pill, so it doesn’t limit my activities too much.” Male, age 20 [CONCEPTS 1, 2] |
• “There hasn’t been anything, I don’t think... I feel the same way I did. I still work, still pour concrete, thank God for that, you know?” Male, age 51 [CONCEPTS 1, 3] |
| Social | • “I have been away from people and places and things that normally I probably would when I was using, so I am no longer doing those things right there because I do not want to end up back in this situation again.” Male, age 47 [CONCEPTS 1, 3] | • “I know as I recover I’m going to be able to feel more social again.” Male, age 60 [CONCEPTS 1, 2, 3] • “During most of the month that I was on IV, I avoided social contact unless it was one on one—no restaurants, no gatherings, no, nothing because of fear of being contaminated with something else—flu, for instance. So, yeah, from that perspective, it was limiting, but bearable.” GNB097, male, age 70 [CONCEPTS 1, 2, 3] |
• “It may come up in a conversation with a family member or a friend or something and I’ll, you know, give the old blow by blow rundown of all that happened to me while I was in the hospital and I’ll mention the bloodstream infection... Yeah, no impact socially or no sense of, I don’t know, that I would be contagious, I might be scary to them or something. No, none of anything near or anything like that, no... I know that I’m not bothered socially by it.” Male, age 59 [CONCEPT 1] |
| Emotional | • “Because I did not want to get that shit again. I—it’s almost like—you don’t want to die but, Lord, you don’t care if you do. Really, you feel so bad that you just wanna—you just want it to quit.” Male, age 65 [CONCEPT 1, 3] | • “But I can’t say that I have been deeply depressed. I’ve tried real hard to not be ornery. And I’ve been able to control my feelings. They said I was not a bad patient. I didn’t say anything ugly to people or things like that that happens a lot of times, when people are real sick.” Female, age 68 [CONCEPTS 1, 3] • “I guess it’s a matter of ego and pride, but you get depressed about it at times about not being able to do stuff. As I get stronger I know that will go away. I’ll get back to normal life.” Male, age 60 [CONCEPTS 1, 2, 3] |
• “I don’t think I’ve had a severe emotional response to it. I mean, I’m thankful, and I appreciate the support I had, and that sort of thing. But it hasn’t changed the way we live or how we think.” Male, age 70 [CONCEPTS 1, 3] |
| Cognitive | • “Well evidently, mentally. Because I talked and said things I didn’t know I was saying. So, evidently, it did affect my mental capacity...Yeah. Because I would never have thought, and said, and done things like that.” Female, age 85 [CONCEPTS 1, 3] | • “…going through the illness while I was sick was the awareness of saying, ‘Wow, there are some things you don’t have control over and things will happen, but what you can control is you can control your attitude and you can control how to move forward with nutrition and balance of life and balance of how you do everything.” Female, age 58 [CONCEPTS 1, 2, 3] | • “But mentally, you know I’ve been fine. I accepted it very well. Kind of knew the routine. So, mentally it basically hasn’t changed anything that I do.” Male, age 56 [CONCEPT 1] |
The quality of life domains are both overlapping and distinct. These 24 selected quotes represent exemplars from 18 different participants with 2 of these with 2 quotations and 2 with 3 quotations.
Abbreviation: PICC line, peripherally inserted central catheter.
Impact on Quality of Life Domains
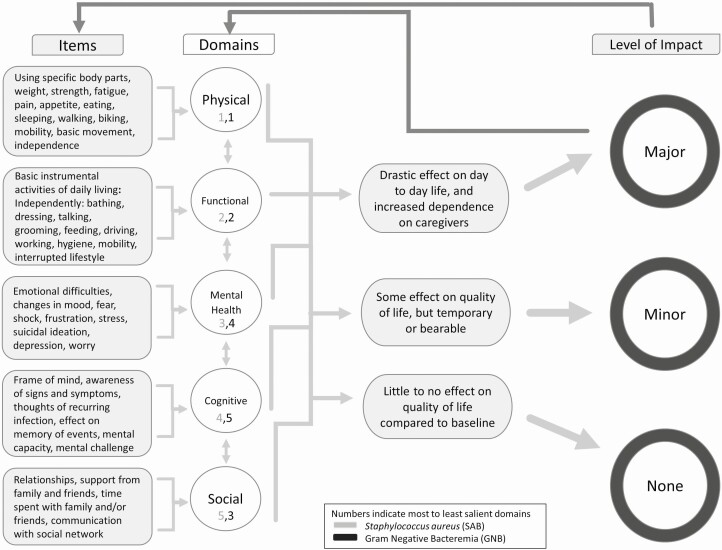
A range in the level of impact was evident with 3 levels that could be clearly differentiated: major, minor, and none, with operational definitions provided in Figure 3, which provides a map of the concepts elicited and their structure and relation, including specific items and domains.
Figure 3.
Concept map.
Across both types of bloodstream infection, the most salient QOL domains majorly impacted were physical and functional (also often co-occurring), which was particularly evident among SAB participants. Participants described weight loss, pain, fatigue, weakness, limited mobility and control, reduced strength and ability, and urinary and bowel incontinence (physical), both in the general sense and specific to particular body parts. Patients also spoke of disruptions and limitations to their daily activities (eg, work, errands), difficulties moving, eating, sleeping, and/or talking and loss of independence, requiring assistance/caregiving (functional).
The relative salience of other QOL domains (social, mental health, cognitive) varied by type of bloodstream infection, although a more significant impact was evident qualitatively for SAB compared with GNB, regardless of domain and order of salience. For the social domain, participants who experienced major social impact from their bloodstream infection talked about feeling removed and isolated, given that social interactions and activities with others were limited, as well as time required to manage their bloodstream infection and associated treatment. For the mental health domain, changes in mood and a number of emotional difficulties were described ranging in severity from shock, frustration, stress, fear, and worry to depression and even suicidal ideation. For the cognitive domain, participants reflected on their frame of mind and mental capacity/functioning, including awareness/thoughts of the current and potentially recurring infection, memory and recall issues, and mental challenge.
Time as a Critical Index
Although not of primary or a priori interest, time emerged as a critical concept, which also has important measurement considerations, such as timing/frequency of assessments and recall period. Participants often referenced time and described the impact of their bloodstream infection on QOL varying over time. Time emerged both as a critical characteristic of the bloodstream infection itself and a defining feature of participant perceptions and reactions. Examples mentioned include duration of and time since infection and treatment as well as conceptualizations of “recovery,” including time and effort required to achieve this, if at all. Participant perceptions of time appeared to be related to the level of impact reported on QOL. Patients focused on the short-term and temporary nature of the bloodstream infection discussed limited impact of the infection on their QOL with few long-term consequences, minor inconveniences, and no to slight adaptations/accommodations made to fit their existing schedule. Alternatively, patients reporting more QOL impact felt treatment required a significant amount of time, was extremely disruptive, and limited their time available for other activities.
Sources of Variability Across Patients
As an extension of the above concept related to time, we noted additional sources of variability across patients. Participants often referenced their individual, prior/baseline state in evaluation of level of impact on QOL as well as the relative personal importance of various QOL domains. Knowledge of and/or prior experience with bloodstream infection was generally helpful in limiting the level of impact on QOL through routine-setting and expectation management, although concerns regarding persistent or recurrent infections were also evident. Furthermore, personality/coping style (in addition to infection characteristics) emerged as a contributor to the level of impact perceived.
DISCUSSION
This descriptive, qualitative project captured rich, in-depth data on patients’ perspectives and experiences with bacterial bloodstream infection, eliciting concepts to inform measurement of patient-reported quality of life. Our inclusion of both SAB and GNB made it possible to compare QOL patterns between different bacterial causes of bloodstream infection, insights currently lacking from the current bacteremia literature. Two primary conclusions arose from this work. First, SAB and GNB impact QOL among survivors, with SAB, in particular, having major impacts on multiple QOL domains. Overall, patients with SAB and GNB described similar experiences but with varying levels of severity and interference, highlighting that 1 measure(s) could be used for both bloodstream infection types if able to capture ranges in severity and interference (ie, on a continuum). A combination of existing, generic measures that are purposefully selected and newly constructed disease-specific items, if necessary, could best capture QOL among patients with bloodstream infection. Second, patients with bloodstream infection are impacted in ways that are not captured in standard “cure or failure” outcomes. This finding emphasizes that including patients as stakeholders and obtaining their feedback is crucial to conducting all aspects of patient-centered clinical trials, including the informed consent process, and providing patient-centered care.
Our study shows that even patients who were ultimately cured of their bloodstream infection experienced significant impacts across multiple QOL domains. Similar effects have been previously noted with severe sepsis [18] and endocarditis [19], 2 infectious conditions that overlap with the bloodstream infections included here. Our work confirms these observations and extends them to patients who are traditionally thought to have a better prognosis, including SAB from a skin source and GNB secondary to urinary tract infections. Even when it might appear to clinicians and investigators that the clinical course is uncomplicated and successful, patients perceive and report that their bloodstream infection and its treatment significantly impacted their QOL.
The presence of significant QOL effects among patients cured of their infections emphasizes the need for outcome measures that incorporate the patient experience. Patients who survived their bloodstream infection but were physically, emotionally, or socially unable to return to their prior level of functioning might be surprised to learn that their clinical outcomes would be considered successful by conventional clinical trial metrics. It is critical to integrate these perspectives when comparing management strategies, whether they are novel antibiotics, treatment algorithms, or other interventions. A clinical trial endpoint that includes QOL is more likely to capture outcomes that are meaningful to patients.
This study had a number of strengths, including a rigorous team-science approach to data collection and analysis using multiple interviewers and coders with both methodological and clinical expertise. In addition, both the set of patients and clinical scenarios presented were diverse. Finally, concept elicitation work, as conducted in this study, is the first crucial step in the measure development process and is recommended in Food and Drug Administration guidelines [20]. An immediate next step is aligning our findings with existing, validated QOL measures across different domains in order to select and prioritize measures and create any necessary additional items, all to be subsequently tested in cognitive interviews. Ultimately, measure(s) informed by and derived from this work will then be tested and validated within prospective trials, including those utilizing a Desirability of Outcome Ranking (DOOR) approach to create a rank-based outcome that incorporates QOL [21].
Our study had limitations. Only approximately 60% of the 100 enrolled individuals ultimately completed the interview 6–8 weeks after their bacteremia. It is possible that these patients experienced their bloodstream infection and its impact on their QOL differently than those who did not complete the interview. In addition, patients were recruited from 1 academic medical center/registry located in the southeastern United States and certain patients were excluded (eg, those with cognitive impairment or whose preferred language is not English), so the results may not represent the experiences of other patients, care settings, and geographic areas. Future measurement work in QOL among patients with SAB/GNB, as with other areas and patient groups, should address these limitations to generalizability when moving towards a more universally applicable patient-reported outcome measure. Finally, the timing limited our ability to understand QOL at patients’ preinfection baseline or at the time of acute bacteremia. Having serial measurements would have afforded more information, as would assessment of baseline QOL status, although at additional expense of cost and effort.
Future studies should pay particular attention to study procedures and consider best methods for reaching and enhancing engagement among patients with bloodstream infection once discharged from the hospital setting, particularly given various discharge locations including other than to home, such as skilled nursing facilities, as well as patients with various underlying comorbidities such as injection drug use.
In summary, SAB and GNB impact QOL among survivors of bloodstream infection. SAB, in particular, leads to major impacts on multiple QOL domains, especially physical and functional health. Engaging patients as stakeholders and obtaining their feedback are crucial to conducting patient-centered trials and providing patient-centered care, including for bloodstream infections. Trials that fail to incorporate the patient perspective via patient-centered outcomes may miss important differences and limit meaningful comparison between treatments. As demonstrated, time was a critical index and salient to patients. Time frames/periods that are important to patients may not be the same ones emphasized in clinical trials or by regulatory bodies. These results of this qualitative study will inform the development/selection of a QOL patient-reported outcome measure(s) to be further refined, tested, and utilized in both patient care and clinical trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily reflect the position or policy of Duke University, US Department of Veterans Affairs, or US government.
Financial support. This study was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award number UM1AI104681). This work was also supported by the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) (grant number CIN 13–410) at the Durham VA Health Care System.
Potential conflicts of interest. T. L. H. is a scientific advisory board member for Motif Bio and consultant for Basilea Pharmaceutica, Genentech, Motif Bio, The Medicines Company, and Theravance. S. B. D. is a consultant for Basilea Pharmaceutica and Genentech. H. B. B. has no direct conflicts of interest with the current work, but does report receiving grants to his home institutions from Sanofi, Otsuka, Novo Nordisk, Improved Patient Outcomes, Cover My Meds, Pharma Foundation, and Proteus as well as receiving consulting fees from Sanofi, Abbott, and Novartis. V. G. F. reports grant/research support to his institution from MedImmune, Cerexa/Forest/Actavis/Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Cubist/Merck, Medical Biosurfaces, Locus, Affinergy, Contrafect, Karius, Genentech, Regeneron, and Basilea, and has been a paid consultant for Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co, Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Cubist, Basilea, Affinergy, Janssen, xBiotech, Contrafect, Regeneron, Basilea, and Destiny. Membership: He also serves as the co-chair of the V710 vaccine scientific advisory committee at Merck, and reports receiving educational fees from Green Cross, Cubist, Cerexa, Durata, Theravance and Debiopharm. He has a patent pending in Sepsis diagnostics. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19:501–9. [DOI] [PubMed] [Google Scholar]
- 2. McNamara JF, Righi E, Wright H, Hartel GF, Harris PNA, Paterson DL. Long-term morbidity and mortality following bloodstream infection: a systematic literature review. J Infect 2018; 77:1–8. [DOI] [PubMed] [Google Scholar]
- 3. Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 2010; 362:1804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kreger BE, Craven DE, McCabe WR. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med 1980; 68:344–55. [DOI] [PubMed] [Google Scholar]
- 6. Dalager-Pedersen M, Thomsen RW, Schonheyder HC, Nielsen H. Functional status and quality of life after community-acquired bacteremia: a matched cohort study. Clin Microbiol Infect 2016; 22:78 e1–e8. [DOI] [PubMed] [Google Scholar]
- 7. Namey E, Guest G, McKenna K, Chen M. Evaluating bang for the buck: a cost-effectiveness comparison between individual interviews and focus groups based on thematic saturation levels. Am J Eval 2016; 37:425–40. [Google Scholar]
- 8. Guest G, Namey E, McKenna K. How many focus groups are enough? Building an evidence base for nonprobability sample sizes. Field Methods 2016; 29:3–22. [Google Scholar]
- 9. Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002; 40:771–81. [DOI] [PubMed] [Google Scholar]
- 10. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005; 15:1277–88. [DOI] [PubMed] [Google Scholar]
- 11. Richards L, Morse JM Readme first for a user’s guide to qualitative methods. 3rd ed. California: SAGE Publications, 2002. [Google Scholar]
- 12. Zade H, Drouhard M, Chinh B, Gan L, Aragon C. Conceptualizing disagreement in qualitative coding. In: Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems. Montreal, QC, Canada: Association for Computing Machinery, 21–26 April 2018:159. [Google Scholar]
- 13. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007; 42:1758–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golafshani N. Understanding reliability and validity in qualitative research. Qual Rep 2003; 8:597–607. [Google Scholar]
- 15. Lombard M, Snyder-Duch J, Bracken CC Practical resources for assessing and reporting intercoder reliability in content analysis research projects, 2010. Available at: http://matthewlombard.com/reliability/ [Google Scholar]
- 16. Miles MB, Huberman AM, Saldaña J Qualitative data analysis: a methods sourcebook. 4th ed. Thousand Oaks, CA: SAGE Publications, 2019. [Google Scholar]
- 17. Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 18. Yende S, Austin S, Rhodes A, et al. Long-term quality of life among survivors of severe sepsis: analyses of two international trials. Crit Care Med 2016; 44:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verhagen DW, Hermanides J, Korevaar JC, et al. Health-related quality of life and posttraumatic stress disorder among survivors of left-sided native valve endocarditis. Amsterdam, Netherlands: Clin Infect Dis 2009; 48:1559–65. [DOI] [PubMed] [Google Scholar]
- 20. US Food and Drug Administration. Patient-focused drug development guidance public workshop: methods to identify what is important to patients & select, develop or modify fit-for-purpose clinical outcomes assessments. Food and Drug Administration, 2018. [Google Scholar]
- 21. Evans SR, Rubin D, Follmann D, et al. Desirability of Outcome Ranking (DOOR) and Response Adjusted for Duration of Antibiotic Risk (RADAR). Clin Infect Dis 2015; 61:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.