Abstract
Background
Knowledge on tuberculosis (TB) infection epidemiology in women of reproductive age living in TB-endemic areas is limited. We used a composite definition of TB infection in a cohort of pregnant women recruited in an Ethiopian city as a model for TB exposure patterns, and to identify factors associated with TB infection.
Methods
Women seeking antenatal care at public health facilities underwent structured interviews, physical examination, and QuantiFERON-TB Gold-Plus (QFT) testing. Women with symptoms compatible with TB disease, and all human immunodeficiency virus (HIV)–positive women, were investigated for active TB by sputum bacteriological testing. TB infection (TB+) was defined as either positive QFT (≥ 0.35 IU/mL), self-reported previous active TB, or current active TB. Associations between TB infection and clinical, demographic, and socioeconomic characteristics were tested in multiple logistic regression analysis.
Results
Among 1834 participants, 679 (37.0%) met criteria for TB+ (80 [4.4%] previous active TB, 5 [0.3%] current active TB, and 594 [32.4%] QFT-positive without previous or current active TB). Age (annual adjusted odds ratio [AOR], 1.069 [95% confidence interval {CI}, 1.045–1.093]) and HIV infection (AOR, 1.43 [95% CI, 1.033–1.988]) were independently associated with TB+. The relationship with increasing age was only observed in HIV-negative women, and translated to an estimated annual risk of TB infection of 2.1% in HIV-negative women.
Conclusions
TB infection in women of reproductive age in Ethiopia was independently associated with HIV infection and increasing age, suggesting exposure to contagious TB and continuous acquisition of TB infection in this population.
Keywords: tuberculosis, QuantiFERON, HIV, Ethiopia, pregnancy
In this cross-sectional study of pregnant women in Ethiopia, 679 of 1834 (37.0%) had tuberculosis infection, which was associated with HIV infection and increasing age (estimated at an annual risk of 2.1% among HIV-negative women), suggesting continuous exposure to contagious tuberculosis.
(See the Invited Commentary by Beckerman on pages 211–2.)
In 2018, 10.0 million new cases of active tuberculosis (TB) occurred worldwide, causing 1.45 million deaths, of which 251 000 were among people living with human immunodeficiency virus (HIV), making Mycobacterium tuberculosis (Mtb) the single infectious agent responsible for the highest numbers of deaths globally [1]. Furthermore, 1.7 billion persons are estimated to have latent tuberculosis infection (LTBI) [1]. Notification rates for active TB are higher for men than women. While notification rates are biased by care-seeking behavior and access to healthcare, undiagnosed active TB is also more common in men, with a global prevalence-to-notification ratio of 2.6 for men and 1.6 for women [2]. However, the sex ratio for active TB varies considerably in different age spans, with a less prominent male predominance in early adulthood [3, 4]. Importantly, increasing proportions of cases are reported in women in sub-Saharan Africa [5]. Whereas HIV coinfection is one reason for this “feminization” of the TB epidemic [4], other factors may also be involved [6]. The rate of progression to active TB is highest during the first year after infection [7, 8], but is also related to various conditions that affect the immune control of LTBI [9, 10]. While experimental studies indicate that biological differences, including sex hormone profiles, may protect females [6], the physiological immune modulation during pregnancy could explain the relatively increased incidence of active TB in women of fertile age [11].
Whereas most studies on LTBI epidemiology have shown that prevalence is linked to male sex and increasing age, findings are discordant with regard to the impact of other conditions, such as socioeconomic status and HIV infection [12–17]. Knowledge on LTBI prevalence and associated factors is necessary for a better understanding of TB transmission in communities.
We aimed to study TB exposure patterns in women of reproductive age living in a TB-endemic area. For this purpose, we used a composite measure of either previous active TB, current active TB, or LTBI. This outcome was investigated in a cohort of pregnant women in Ethiopia, with analysis of clinical, demographic, and socioeconomic characteristics associated with TB infection.
MATERIALS AND METHODS
Study Setting and Participants
This study was based on a prospective cohort of women of fertile age (ClinicalTrials.gov identifier: NCT03305991), conducted at 3 antenatal care (ANC) clinics in Adama, a city with around 300 000 inhabitants in central Ethiopia. From November 2015 to February 2018, pregnant women were recruited during their first ANC visit for the current pregnancy, after providing informed consent. Repeated pregnancies were not considered. The national TB program [18] recommends screening for active TB for all pregnant women. Neither testing nor treatment for LTBI is recommended in connection to pregnancy. For HIV-positive individuals (irrespective of sex), isoniazid preventive therapy (IPT) for 6 months is recommended after exclusion of active TB.
Study Procedure
Interviews covering socioeconomic and demographic characteristics, as well as medical and obstetric history, were performed using standardized questionnaires, along with physical examination. HIV serostatus was determined with rapid tests, with testing for CD4+ T-cell count (CD4) and HIV viral load (VL) from HIV-positive participants. Bacteriological TB investigations (smear microscopy, MTB/RIF GeneXpert, and liquid culture) were performed on 2 spontaneously expectorated morning sputum samples for participants with symptoms or signs suggestive of active TB, and for all HIV-positive participants, irrespective of clinical presentation. Participants diagnosed with active TB were linked to TB clinics for management according to national guidelines [18].
QuantiFERON-TB Gold-Plus (QFT) was used for LTBI testing. Venous blood was collected in lithium heparin tubes, with transfer to the 4 test tubes containing TB1 or TB2 Mtb antigen mixtures, negative and positive controls, respectively, at the study laboratory within 8 hours of venipuncture, followed by mixing and incubation for 16–24 hours at 37°C. Supernatants were stored at −20°C after centrifugation. Interferon-γ enzyme-linked immunosorbent assay (ELISA) was performed in batches according to the manufacturer’s recommendations. For indeterminate QFT results, the ELISA was repeated on the same samples.
Study Design
The primary outcome of this cross-sectional study was TB infection (TB+), defined as a composite of either current active TB (bacteriologically confirmed or clinically diagnosed according to national guidelines), self-reported previous treatment for active TB, or LTBI. Absence of all of these criteria was defined as TB negative (TB−).
LTBI was defined as a positive QFT result, using a cutoff of nil-corrected TB1- or TB2-stimulated interferon gamma (IFN-γ) ≥ 0.35 IU/mL, without past or current active TB. Since immunosuppression can reduce IFN-γ secretion after Mtb antigen incubation [19–21], we also separately analyzed the proportion and characteristics of women with IFN-γ levels < 0.35 IU/mL but ≥ 0.20 IU/mL in at least 1 antigen formulation (referred to as low borderline range [22]).
Predictive models for TB+ in the whole study population were constructed. In addition, a model was constructed for TB+ in the subset of HIV-positive women. A descriptive analysis comparing study participants with QFT IFN-γ levels ≥ 0.35 IU/mL and 0.20–0.34 IU/mL, respectively, was also performed.
Based on previous reports of characteristics associated with active or latent TB [9, 16, 17, 23–25], self-reported contact with active TB, demographic and socioeconomic factors including housing conditions, and factors related to host immunity (HIV-infection, nutritional status, and gestational age) were included in the analyses.
Statistical Analysis
Parsimonious predictive models for TB+ were generated for analysis of each of the study objectives. The χ 2 test and Fisher exact test were used to test univariate associations for categorical variables. Subsequently, logistic regression models were constructed by stepwise forward selection, entering variables with univariate P values < .10 sequentially. Likelihood ratio tests were performed after entry of new variables to evaluate the gained additional fitness. If the fitness was significantly improved (using P < .05 as threshold), the variable remained in the model. Subsequently, interaction terms between the remaining variables were considered, based on plausibility and contribution to the model fitness. For interaction terms based on numerical variables, reference points were changed to reflect the population median of the respective variable.
Due to the possibility of false-positive significant associations because of multiple tests, Bonferroni-adjusted levels of significance for each model were calculated.
Quantile regression analysis was performed to explore the dynamics of the relationship between TB1-stimulated IFN-γ secretion and variables associated with TB+ in prior analyses [26]. All statistical analysis was performed using R, version 3.5.1. Quantile regression analysis was performed using the quantreg R package.
Ethical Considerations
This study was performed in accordance with the Helsinki Declaration, and was approved by the ethical review committee of Lund University, Sweden, and the national ethical review board of the Ministry of Science and Technology, Addis Ababa, Ethiopia. Individual informed consent was obtained prior to enrollment, with the assistance of proxies for illiterate participants. QFT results were not communicated to healthcare providers since testing for LTBI is not part of routine care in Ethiopia.
RESULTS
Study Participants
Among 2088 cohort participants, 254 (12.2%) were excluded due to missing QFT results. The median age of the 1834 included women was 25 years (interquartile range [IQR], 22–28 years), the median gestational age was 18 weeks (IQR, 14–21 weeks), and 170 (9.3%) were living with HIV (Table 1). HIV-positive individuals had higher median age, a lower proportion were married, and more women reported previous pregnancies (Supplementary Table 1). The characteristics of participants excluded due to missing QFT results were similar to those included (Supplementary Table 2).
Table 1.
Characteristics of Pregnant Women Stratified by Tuberculosis Infection Status
| Characteristic | Total | TB− | TB+ | P Value | |||
|---|---|---|---|---|---|---|---|
| Total | 1834 | 1155 | 679 | ||||
| Previous active TB | 80 | (4.4) | … | … | 80 | (11.8) | |
| Current active TB | 5 | (0.27) | … | … | 5 | (0.7) | |
| Age, y | < .001 | ||||||
| ≤ 20 | 350 | (19.1) | 255 | (22.1) | 95 | (14.0) | |
| 21–25 | 725 | (39.5) | 490 | (42.4) | 235 | (34.7) | |
| 26–30 | 610 | (33.3) | 334 | (28.9) | 276 | (40.7) | |
| 31–35 | 114 | (6.2) | 57 | (4.9) | 57 | (8.4) | |
| ≥ 36 | 34 | (1.9) | 19 | (1.6) | 15 | (2.2) | |
| NA | 1 | (0.1) | … | … | … | … | |
| Hemoglobin, g/dL, mean (SD) | 12.37 | (1.2) | 12.39 | (1.22) | 12.35 | (1.17) | .61 |
| Marital status | .16a | ||||||
| Married | 1757 | (95.8) | 1102 | (95.6) | 655 | (96.6) | |
| Single | 56 | (3.1) | 35 | (3.0) | 21 | (3.1) | |
| Divorced | 15 | (0.8) | 13 | (1.1) | 2 | (0.3) | |
| Widowed | 3 | (0.2) | 3 | (0.3) | 0 | (0) | |
| NA | 3 | (0.2) | … | … | … | … | |
| Education | .017 | ||||||
| Higher education | 201 | (11.0) | 117 | (10.1) | 84 | (12.4) | |
| 6–12 grades | 1038 | (56.6) | 669 | (57.9) | 369 | (54.4) | |
| < 6 grades | 360 | (19.6) | 239 | (20.7) | 121 | (17.8) | |
| Illiterate | 234 | (12.8) | 130 | (11.3) | 104 | (15.3) | |
| NA | 1 | (0.1) | … | … | … | … | |
| Family size | |||||||
| < 4 | 1348 | (73.5) | 883 | (77.1) | 465 | (68.7) | < .001 |
| 4–6 | 401 | (21.9) | 221 | (19.3) | 180 | (26.6) | |
| > 6 | 74 | (4.0) | 42 | (3.7) | 32 | (4.7) | |
| NA | 11 | (0.6) | … | … | … | … | |
| One room | 995 | (54.3) | 658 | (57.2) | 337 | (49.9) | .003 |
| NA | 8 | (0.4) | … | … | … | … | |
| No solid fuel combustion for cooking | 441 | (24.0) | 264 | (23.0) | 177 | (26.3) | .12 |
| NA | 12 | (0.7) | … | … | … | … | |
| No electricity | 76 | (4.1) | 47 | (4.1) | 29 | (4.3) | .90 |
| NA | 13 | (0.7) | … | … | … | … | |
| Occupation | .044 | ||||||
| Housewife | 1165 | (63.5) | 747 | (64.8) | 418 | (61.8) | |
| Employed | 234 | (12.8) | 141 | (12.2) | 93 | (13.8) | |
| Self-employed | 148 | (8.1) | 97 | (8.4) | 51 | (7.5) | |
| Daily laborer | 219 | (11.9) | 122 | (10.6) | 97 | (14.3) | |
| Student | 39 | (2.1) | 31 | (2.7) | 8 | (1.2) | |
| Unemployed | 23 | (1.3) | 14 | (1.2) | 9 | (1.3) | |
| NA | 6 | (0.3) | … | … | … | … | |
| Previous pregnancies | < .001 | ||||||
| 0 | 671 | (36.6) | 465 | (41.6) | 206 | (31.0) | |
| 1 | 641 | (35.0) | 400 | (35.8) | 241 | (36.3) | |
| 2 | 293 | (16.0) | 155 | (13.9) | 138 | (20.8) | |
| > 2 | 176 | (9.6) | 97 | (8.7) | 79 | (11.9) | |
| NA | 53 | (2.9) | … | … | … | … | |
| Gestational age, wk | .36 | ||||||
| < 14 | 301 | (16.4) | 178 | (19.6) | 123 | (22.7) | |
| 14–27 | 1070 | (58.3) | 681 | (74.8) | 389 | (71.6) | |
| > 27 | 82 | (4.5) | 51 | (5.6) | 31 | (5.7) | |
| NA | 381 | (20.8) | … | … | … | … | |
| MUAC < 23 cm | 398 | (21.7) | 259 | (22.8) | 139 | (20.9) | .38 |
| NA | 30 | (1.6) | … | … | … | … | |
| HIV-positive | 170 | (9.3) | 86 | (7.4) | 84 | (12.4) | .001 |
Data are presented as no. (%) unless otherwise indicated. Table shows characteristics of study participants with evidence of TB infection, either current or previous active TB or latent TB using the conventional cutoff (interferon-γ ≥ 0.35 IU/mL) after stimulation with TB1 or TB2 Mycobacterium tuberculosis antigen mixtures. The Total column reports total percentages, whereas the others report valid percentages. Univariate comparisons were made using t test for normally distributed variables (hemoglobin) and χ 2 test or Fisher exact test for categorical variables. Bonferroni-adjusted level of significance: 0.0038.
Abbreviations: HIV, human immunodeficiency virus; MUAC, mid-upper arm circumference; NA, not available; SD, standard deviation; TB, tuberculosis.
aFisher exact test was used.
Prevalence and Associated Factors of TB Infection
According to the study definition, 679 of 1834 women were TB+ (37.0%); 594 (32.4%) had LTBI, 80 (4.4%) reported previous treatment for active TB and 5 (0.3%) had prevalent active TB, of whom 3 were HIV-positive. Two of 3 HIV-positive women with active TB reported no symptoms suggestive of TB. Sixty-nine participants with negative QFT-result had IFN-γ levels 0.20–0.34 IU/mL. In 25 (1.4%) study participants, initial QFT results were indeterminate, but after repeat testing, QFT status could be determined in all these cases.
In univariate analysis, TB+ was associated with higher median age compared to TB−. In addition, TB+ was associated with HIV infection, greater number of previous pregnancies, greater number of household members, and 1-room household residence (Table 1).
In multivariate analysis, age (annual adjusted odds ratio [AOR], 1.069 [95% confidence interval {CI}, 1.045–1.093]) and HIV infection (AOR, 1.43 [95% CI, 1.033–1.988]) were independently associated with TB+ (Table 2).
Table 2.
Logistic Regression Analysis for Tuberculosis Infection
| Characteristic | AOR | 95% CI | P Value | |
|---|---|---|---|---|
| HIV-status | ||||
| HIV-negative | Ref | Ref | Ref | |
| HIV-positive | 1.43 | 1.03 | 1.99 | .031 |
| Age, y | 1.069 | 1.045 | 1.093 | < .0001 |
Multivariate logistic regression model for tuberculosis (TB) infection, defined as latent TB infection or previous or current active TB infection. The model was constructed in a stepwise forward selection method, with variables with univariate P < .10 eligible for inclusion and likelihood ratio test used to determine the contributed model fitness. Bonferroni-adjusted level of significance: 0.0038.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus.
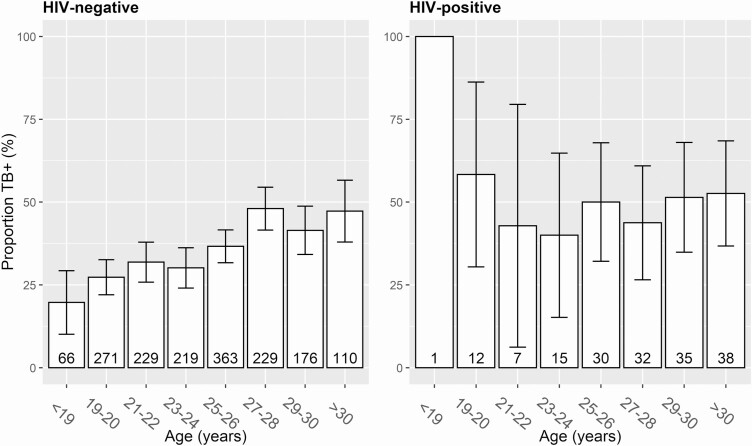
However, the association with age was different in HIV-positive and HIV-negative participants, ranging from 19.7% among women aged < 19 years to 45.6% among women > 26 years for HIV-negative participants, whereas no such association was observed in HIV-positive women (Figure 1). Consequently, an interaction term between age and HIV serostatus was added to the model (Table 3). In that model, age remained significantly associated among HIV-negative women (annual AOR, 1.079 [95% CI, 1.053–1.105]; P < .0001) but not in HIV-positive women (annual AOR, 0.998; not significant). Conversely, the association with HIV serostatus varied by age, but at the population median (25 years), HIV-positive women were more likely to be TB+ (AOR, 1.76 [95% CI, 1.21–2.54]; P = .0029). These findings remained significant using the Bonferroni-adjusted significance threshold (P < .0038). From this model, an annual risk of infection of 2.1% was derived for HIV-negative women. These results were similar in a sensitivity analysis excluding women with past and current active TB, although HIV serostatus was not associated with LTBI in this analysis (HIV negative: annual AOR, 1.081; HIV positive: annual AOR, 0.990; HIV at 25 years: AOR, 1.12; data not shown).
Figure 1.
Proportion of tuberculosis (TB) infection stratified by age and human immunodeficiency virus (HIV) serostatus. Bar chart depicting the distribution of TB+ across age categories in HIV-negative and HIV-positive study participants. TB+ was defined as past or present active TB and/or positive QuantiFERON-TB Gold-Plus using the recommended cutoff of 0.35 IU/mL. Whiskers represent 95% confidence intervals for the proportion; the group size is denoted at the bottom of each bar.
Table 3.
Logistic Regression Analysis for Tuberculosis Infection, model with interaction
| Characteristic | AOR | 95% CI | P Value | |
|---|---|---|---|---|
| HIV status, at age 25 y | ||||
| HIV-negative | Ref | Ref | Ref | |
| HIV-positive | 1.76 | 1.21 | 2.54 | .0029 |
| Age in HIV-negative women (years) | 1.079 | 1.053 | 1.105 | < .0001 |
| Age in HIV-positive women (years) | 0.998 | 0.931 | 1.069 | .028a |
Multivariate logistic regression model for tuberculosis infection (TB+), defined as latent TB infection, previous or current active TB infection. The model was constructed in a stepwise forward selection method, with variables with univariate P < .10 eligible for inclusion and likelihood ratio test used to determine the contributed model fitness. There was an interaction between age and HIV status; although the AOR in the HIV-negative group increased by 1.079 per living year, age did not affect the rate of TB+ in the HIV-positive group. Bonferroni-adjusted level of significance: 0.0038.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus.
a P values indicate the level of evidence that the age association was different in HIV-positive and HIV-negative individuals (ie, the interaction).
The 69 participants with QFT IFN-γ levels 0.20–0.34 IU/mL had similar characteristics as those with levels ≥ 0.35 IU/mL (Supplementary Table 3).
HIV-Specific Factors Associated With TB Infection
Among the 170 HIV-positive participants, 84 (49.4%) were TB+, compared to 595 of 1664 (35.7%) among HIV-negative women (P < .001). The proportion of previous and current active TB was significantly higher among HIV-positive women: 33 of 170 (19.4%; OR, 8.3; P < .0001) and 3 of 170 (1.8%; OR, 14.9; P = .0068), respectively. In univariate analysis, TB+ was associated with longer duration of antiretroviral therapy (ART), ART regimen, and VL > 1000 copies/mL (Table 4). The latter 2 factors remained associated with TB+ in multivariate analysis, although not reaching the Bonferroni-adjusted threshold of significance (P < .0031) (Table 5).
Table 4.
Characteristics Associated With Tuberculosis Infection in Human Immunodeficiency Virus–Positive Pregnant Women
| Characteristic | TB− | TB+ | P Value | ||
|---|---|---|---|---|---|
| Total | 86 | 84 | |||
| Previous active TB | NA | 33 | (39.3) | ||
| Current active TB | NA | 3 | (3.6) | ||
| QFT ≥ 0.35 IU/mL | NA | 62 | (75.6) | ||
| Age, y | .51a | ||||
| ≤ 20 | 5 | (5.8) | 8 | (9.5) | |
| 21–25 | 24 | (27.9) | 18 | (21.4) | |
| 26–30 | 39 | (45.3) | 38 | (45.2) | |
| 31–35 | 12 | (14.0) | 17 | (20.2) | |
| ≥ 36 | 6 | (7.0) | 3 | (3.6) | |
| Hemoglobin, g/dL, mean (SD) | 11.97 | (1.44) | 12.37 | (1.37) | .11 |
| Marital status | .23a | ||||
| Married | 74 | (86.0) | 79 | (94.0) | |
| Single | 9 | (10.5) | 3 | (3.6) | |
| Divorced | 2 | (2.3) | 2 | (2.4) | |
| Widowed | 1 | (1.2) | 0 | (0) | |
| Education | .061a | ||||
| Higher education | 2 | (2.3) | 5 | (6.0) | |
| 6–12 grades | 61 | (70.9) | 43 | (51.2) | |
| < 6 grades | 13 | (15.1) | 18 | (21.4) | |
| Illiterate | 10 | (11.6) | 18 | (21.4) | |
| Family size | |||||
| < 4 | 57 | (66.3) | 44 | (52.4) | .15 |
| 4–6 | 23 | (26.7) | 34 | (40.5) | |
| > 6 | 6 | (7.0) | 6 | (7.1) | |
| One room | 36 | (41.9) | 32 | (38.1) | .73 |
| No solid fuel combustion for cooking | 15 | (17.6) | 17 | (20.5) | .79 |
| No electricity | 5 | (5.8) | 6 | (7.2) | .95 |
| Occupation | .22a | ||||
| Housewife | 49 | (57.6) | 54 | (64.3) | |
| Employed | 14 | (16.5) | 5 | (6.0) | |
| Self-employed | 8 | (9.4) | 12 | (14.3) | |
| Daily laborer | 11 | (12.9) | 9 | (10.7) | |
| Student | 0 | (0) | 0 | (0) | |
| Unemployed | 3 | (3.5) | 4 | (4.8) | |
| No. of previous pregnancies | .47 | ||||
| 0 | 11 | (13.8) | 10 | (12.2) | |
| 1 | 34 | (42.5) | 29 | (35.4) | |
| 2 | 15 | (18.8) | 24 | (29.3) | |
| ≥ 3 | 20 | (25.0) | 19 | (23.2) | |
| Gestational age, wk | .36a | ||||
| < 14 | 16 | (25.4) | 19 | (27.5) | |
| 14–27 | 40 | (63.5) | 47 | (68.1) | |
| > 27 | 7 | (11.1) | 3 | (4.3) | |
| Wasted (MUAC < 23 cm) | 18 | (21.4) | 18 | (22.0) | 1 |
| ART duration, mo | .010a | ||||
| < 3 | 23 | (30.7) | 10 | (13.5) | |
| 3–11 | 5 | (6.7) | 1 | (1.4) | |
| 12–35 | 15 | (20.0) | 15 | (20.3) | |
| ≥ 36 | 32 | (42.7) | 48 | (64.9) | |
| NA | 11 | (12.8) | 10 | (11.9) | |
| ART regimen | .0085a | ||||
| ZDV + 3TC + NVP | 4 | (5.6) | 17 | (23.6) | |
| ZDV + 3TC + EFV | 2 | (2.8) | 3 | (4.2) | |
| TDF + 3TC + EFV | 55 | (77.5) | 46 | (63.9) | |
| TDF + 3TC + NVP | 10 | (14.1) | 5 | (6.9) | |
| Second line | 0 | (0) | 1 | (1.4) | |
| NA | 15 | (17.4) | 12 | (14.3) | |
| CD4 count, cells/µL | .17a | ||||
| < 350 | 12 | (32.4) | 4 | (16.0) | |
| 350–599 | 13 | (35.1) | 7 | (28.0) | |
| ≥ 600 | 12 | (32.4) | 14 | (56.0) | |
| NA | 49 | (57.0) | 59 | (70.0) | |
| Viral load, copies/mL | .007 | ||||
| < 1000 | 40 | (52.6) | 58 | (69.0) | |
| ≥ 1000 | 26 | (34.2) | 9 | (13.4) | |
| NA | 10 | (11.6) | 17 | (20.2) | |
Data are presented as no. (%) unless otherwise indicated. Characteristics of human immunodeficiency virus–positive study participants with evidence of TB infection, either current or previous active TB or latent TB, using the conventional cutoff (interferon-γ ≥ 0.35 IU/mL) after stimulation with either TB1 or TB2 antigen mixtures. Univariate comparisons were made using t test for normally distributed variables (hemoglobin) and using χ 2 test or Fisher exact test for categorical variables. Bonferroni-adjusted level of significance: 0.0031.
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; EFV, efavirenz; MUAC, mid-upper arm circumference; NA, not available; NVP, nevirapine; QFT, QuantiFERON-TB Gold-Plus; SD, standard deviation; TB, tuberculosis; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
a Fisher exact test.
Table 5.
Logistic Regression Analysis for Tuberculosis Infection in Women With Human Immunodeficiency Virus
| Regimen and Viral Load | AOR | (95% CI) | P Value |
|---|---|---|---|
| ART regimen | |||
| ZDV + 3TC + NVP | Ref | … | |
| ZDV + 3TC + EFV | 0.261 | (.027–2.50) | .24 |
| TDF + 3TC + EFV | 0.288 | (.086–.971) | .045 |
| TDF + 3TC + NVP | 0.125 | (.024–.640) | .013 |
| Viral load, copies/mL | |||
| < 1000 | Ref | … | |
| ≥ 1000 | 0.275 | (.098–.773) | .014 |
Multivariate logistic regression for tuberculosis infection (TB+) in women with human immunodeficiency virus using the conventional cutoff (0.35 IU/mL) for QuantiFERON-TB Gold-Plus. The model was constructed in a stepwise forward selection method, with variables with univariate P < .10 eligible for inclusion and likelihood ratio test used to determine the contributed model fitness. Bonferroni-adjusted level of significance: 0.0031.
Abbreviations: 3TC, lamivudine; AOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; EFV, efavirenz; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Quantile Regression Analysis of the Relationship Between TB Infection Status and Age
As increasing age in HIV-negative women was strongly associated with TB+ (Figure 1), we further explored this relationship using quantile regression, a semiparametric quantitative method, to determine whether these associations varied in different age ranges. Across the 20th to 80th age percentiles, TB+ was significantly associated with higher age compared to TB−. This difference ranged between 1 and 2 years (Supplementary Table 4).
DISCUSSION
In this cross-sectional study of 1834 women seeking antenatal care in Ethiopia, 37% met study criteria for TB infection. Among a large set of variables, the only factors associated with TB infection in multivariate analysis were age and HIV infection. In HIV-negative study participants, the absolute annual risk of acquiring TB infection was estimated at 2.1%.
Recently acquired TB infection is recognized as a major risk factor for progression to active TB [7]. The high rate of TB exposure among women of reproductive age is of particular concern, since TB is a leading cause of maternal mortality in low-income countries [27–30] and pregnancy has been linked to an elevated incidence of active TB [31, 32].
Although 20% of HIV-negative participants had acquired TB infection at the age of 18 years, the proportion of TB-infected women increased to 46% among those aged > 26 years, implying continued TB transmission in the communities where these women live. Some previous studies performed in endemic areas have found LTBI to be associated with increasing age [12, 14, 15, 33], being married, and higher socioeconomic status [15], while among South African adolescents, lower socioeconomic status, lower parental education, and crowding were associated with LTBI [33]. In our study, no differences in TB infection status were observed with regard to socioeconomic conditions, crowding, or indoor biomass combustion.
Apart from rising age, TB infection was also strongly associated with HIV infection. Interestingly, no association between TB infection and age was found among HIV-positive participants. Furthermore, the excess burden of TB infection in HIV-positive persons was due to a higher frequency of previous and current active TB, whereas the proportions with LTBI were similar with regard to HIV serostatus. This finding is in agreement with previous studies, showing no association between LTBI prevalence and HIV [15–17]. In our cohort, 9.3% of women were HIV positive, which is higher than the Ethiopian estimate of 1.4% for women of fertile age [34]. A majority of HIV-positive women had been diagnosed with HIV before the current pregnancy, and 110 (74%) were on ART since > 1 year.
Latent tuberculosis infection represents a reservoir for Mtb, and testing and treatment for LTBI is considered as a key component of TB control programs in low-endemic countries [35]. Although treatment of LTBI might be considered during pregnancy, this has mainly been studied in the context of HIV coinfection, and the efficacy of such treatment in areas with ongoing TB transmission for prevention of active TB is uncertain due to the high risk of reinfection. In settings such as the one in which this study was conducted, case-finding of individuals with contagious TB in the community, with adequate linkage to care and completion of treatment, should probably be prioritized for reduction of TB exposure in the community. The Global Tuberculosis Report 2019 estimated that almost one-third of active TB cases in Ethiopia are not diagnosed, demonstrating the need for improvement of existing TB control programs [1].
Early diagnosis and treatment of active TB, as well as prevention, is of special importance in pregnant women. Symptom-based screening for active TB is recommended as part of ANC in Ethiopia, but evidence is lacking on how such screening should be performed. As illustrated by the finding of 2 cases of active TB in HIV-positive women without TB-related symptoms, active TB during pregnancy can be asymptomatic or have atypical presentation [11].
Our findings highlight the need of improved TB control in connection to pregnancy. Since the incidence of active TB has been reported to be higher postpartum compared to during pregnancy [31, 32], assessment of women after delivery should also be considered. Further research is required in this field, including the role of diagnosis and treatment of LTBI.
To reflect the cumulative burden of TB in young women, we used a broad definition of TB infection, including both active and latent infection as well as self-reported history of previous treatment for active TB. For determination of LTBI status among women without active TB, we used the novel QFT assay. Similar to previous versions of QFT assays, this method is based on measurement of IFN-γ in plasma after whole-blood incubation with Mtb specific antigens. While initially considered as a binary test, the choice of a threshold level for definition of a positive reaction has been debated, especially since results close to the recommended cutoff level (0.35 IU/mL) are subject to variability on repeat testing [36]. We and other researchers have presented data suggesting that a lower threshold level (0.20 IU/mL) could be considered for identification of LTBI in persons with immunosuppressive conditions [37–39], to increase assay sensitivity in such individuals. In our cohort, 69 of 1749 women had IFN-γ levels in the low borderline range (0.20–0.34 IU/mL). The proportions of persons displaying this pattern did not differ with regard to HIV serostatus, nor for other characteristics.
To our knowledge, this study is the largest survey of TB infection among women of fertile age from an endemic setting using an IFN-γ release assay for LTBI detection. The study protocol included a large set of variables potentially associated with TB infection.
We acknowledge certain study limitations. This study was conducted in women seeking ANC at public health facilities, which we consider to be a representative population for women of reproductive age. However, certain categories of women in this age range were not included, especially nonpregnant women, but also women seeking ANC at private clinics or those who do not seek ANC during pregnancy. Three criteria were used to define TB infection. Compared to tuberculin skin test, which has previously been the reference method for LTBI diagnosis, QFT is considered to have higher specificity for Mtb infection, as well as greater sensitivity, especially in persons with immunosuppression. Yet, it has been suggested that false-negative QFT results might occur during pregnancy [19, 21]. As immunosuppression is most pronounced in the third trimester, and only 5.6% of our participants were tested during this period, we believe this is unlikely to bias our conclusions. The inclusion of 2 sets of Mtb antigen in the QFT assay could also improve sensitivity in immunocompromised individuals. We did not collect data on IPT for HIV-positive women, but it is possible that a majority of them had received such treatment according to Ethiopian guidelines previously. Although this intervention could have reduced rates of active TB in these individuals, we consider it unlikely that IPT has affected QFT results [40].
For detection of current active TB, bacteriological TB testing was performed on sputum samples. The number of diagnosed cases of active TB was relatively low. Although this testing strategy could have missed cases of extrapulmonary TB, only 2 additional TB cases were observed during subsequent follow-up. As a measure of cumulative TB exposure, we also recorded self-reported treatment for active TB. In the study setting, we were not able to check these reports, and recall bias is possible.
Simultaneous testing of many risk factors increases the risk for spurious associations. The association with ART regimen could represent such an example. To reduce the risk of false-positive associations, we used Bonferroni adjustment of the significance threshold following which only age and HIV serostatus remained significantly associated with TB infection.
Data for certain variables were missing; however, with the exception of CD4 count this proportion was low, and unlikely to bias the conclusions.
CONCLUSIONS
In this large cohort of Ethiopian women of reproductive age, 37% met criteria for TB infection, which was independently associated with HIV infection and increasing age. The absolute risk of acquiring TB infection was estimated at 2.1% per year, suggesting continuous exposure to contagious TB among young women living in Ethiopia.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. P. B. is the principal investigator and provided the funds for this project and initiated it together with N. W., T. T. B., S. H., M. J., and E. S. Data were collected by J. W., F. T., G. M., and M. K.; J. W. performed the statistical analysis and prepared the first draft of the manuscript. All authors have contributed to the intellectual content and have given their final approval for the version submitted for publication.
Acknowledgments. The authors extend their gratitude toward the women participating in this study, the healthcare staff at the study sites, and the Adama Lund University research team. The authors also thank the Armauer Hansen Research Institute, Addis Ababa, the Adama Regional Laboratory, and the Oromia Regional Health Bureau for their support and assistance.
Disclaimer. The QuantiFERON-TB Gold-Plus kits were provided by Qiagen through an unrestricted donation. Qiagen had no influence on study design, data collection or analysis, preparation of the manuscript, or decision to submit the manuscript for publication.
Financial support. This work was supported by the Swedish Heart-Lung Foundation (grant number 20170258 to P. B.); the Crafoord Foundation (grant number 20170537 to P. B.); governmental funding of clinical research within the National Health Services, Sweden (to J. W. and P. B.); Region Skåne research grants (to P. B.); the Alfred Österlund Foundation (to P. B.); and a private donation to Lund University, Sweden (to P. B.). Additional grants were obtained from the John and Hedda Forssman Foundation (to J. W.) and the Folke Nordbring Foundation (to J. W.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization. Global tuberculosis report 2019. Geneva, Switzerland: WHO,2019. [Google Scholar]
- 2. Horton KC, Macpherson P, Houben RMGJ, White G, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries : a systematic review and meta-analysis. PLoS Med 2016; 19. doi:10.1371/journal.pmed.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holmes CB, Hausler H, Nunn P. A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis 1998; 2:96–104. [PubMed] [Google Scholar]
- 4. Perumal R, Naidoo K, Padayatchi N. TB epidemiology: where are the young women? Know your tuberculosis epidemic, know your response. BMC Public Health 2018; 18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med 2009; 6:e1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hertz D, Schneider B. Sex differences in tuberculosis. Semin Immunopathol 2019; 41:225–37. [DOI] [PubMed] [Google Scholar]
- 7. Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ 2018; 362:k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013; 41:140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. 2013; 2013:828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med 2015; 372:2127–35. [DOI] [PubMed] [Google Scholar]
- 11. Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012; 55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kizza FN, List J, Nkwata AK, et al. Prevalence of latent tuberculosis infection and associated risk factors in an urban African setting. BMC Infect Dis 2015; 15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaiear N, Bourpoern J, Sawanyawisuth K, Sawanyawisuth K, Limpawattana P, Reechaipichitkul W. Age is associated with latent tuberculosis in nurses. Asian Pacific J Trop Dis 2016; 6:940–2. [Google Scholar]
- 14. Liu Y, Huang S, Jiang H, et al. The prevalence of latent tuberculosis infection in rural Jiangsu, China. Public Health 2017; 146:39–45. [DOI] [PubMed] [Google Scholar]
- 15. Ncayiyana JR, Bassett J, West N, et al. Prevalence of latent tuberculosis infection and predictive factors in an urban informal settlement in Johannesburg, South Africa: a cross-sectional study. BMC Infect Dis 2016; 16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boccia D, Hargreaves J, Ayles H, Fielding K, Simwinga M, Godfrey-Faussett P. Tuberculosis infection in Zambia: the association with relative wealth. Am J Trop Med Hyg 2009; 80:1004–11. [PMC free article] [PubMed] [Google Scholar]
- 17. Shanaube K, Hargreaves J, Fielding K, et al. Risk factors associated with positive QuantiFERON-TB Gold In-tube and tuberculin skin tests results in Zambia and South Africa. PLoS One 2011; 6:e18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ethiopian Ministry of Health. National guidelines for TB, DR-TB and leprosy in Ethiopia. Addis Ababa: Ministry of Health, Federal Democratic Republic of Ethiopia, 2017. [Google Scholar]
- 19. LaCourse SM, Cranmer LM, Matemo D, et al. Effect of pregnancy on interferon gamma release assay and tuberculin skin test detection of latent TB infection among HIV-infected women in a high burden setting. J Acquir Immune Defic Syndr 2017; 75:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edwards A, Gao Y, Allan RN, et al. Corticosteroids and in fnfliximab impair the performance of interferon-γ release assays used for diagnosis of latent tuberculosis. Thorax 2017; 72:946–49. [DOI] [PubMed] [Google Scholar]
- 21. Mathad JS, Bhosale R, Sangar V, et al. Pregnancy differentially impacts performance of latent tuberculosis diagnostics in a high-burden setting. PLoS One 2014; 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nemes E, Rozot V, Geldenhuys H, et al. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis infection. Am J Respir Crit Care Med 2017; 196:638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duarte R, Lönnroth K, Carvalho C, et al. Tuberculosis, social determinants and co-morbidities (including HIV). Pulmonology 2018; 24:115–9. [DOI] [PubMed] [Google Scholar]
- 24. Lin H, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. 2007; 4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Odone A, Crampin AC, Mwinuka V, et al. Association between socioeconomic position and tuberculosis in a large population-based study in rural Malawi. PLoS One 2013; 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koenker R, Chernozukov V, He X, Peng L.. Handbook of quantile regression. 1st edition. New York: Chapman and Hall/CRC, 2017. [Google Scholar]
- 27. Panchabhai T, Patil P, Shah D, Joshi A. An autopsy study of maternal mortality: a tertiary healthcare perspective. J Postgrad Med 2009; 55:8–11. [DOI] [PubMed] [Google Scholar]
- 28. Ahmed Y, Mwaba P, Chintu C, Grange JM, Ustianowski A, Zumla A. A study of maternal mortality at the University Teaching Hospital, Lusaka, Zambia: the emergence of tuberculosis as a major non-obstetric cause of maternal death. Int J Tuberc Lung Dis 1999; 3:675–80. [PubMed] [Google Scholar]
- 29. Getahun H, Sculier D, Sismanidis C, Grzemska M, Raviglione M. Prevention, diagnosis, and treatment of tuberculosis in children and mothers: evidence for action for maternal, neonatal, and child health services. J Infect Dis 2012; 205(Suppl 2):S216–27. [DOI] [PubMed] [Google Scholar]
- 30. Menéndez C, Romagosa C, Ismail MR, et al. An autopsy study of maternal mortality in Mozambique: the contribution of infectious diseases. PLoS Med 2008; 5:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 2012; 185:779–84. [DOI] [PubMed] [Google Scholar]
- 32. Jonsson J, Kühlmann-Berenzon S, Berggren I, Bruchfeld J. Increased risk of active tuberculosis during pregnancy and postpartum: a register-based cohort study in Sweden. Eur Respir J 2019:1901886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahomed H, Hawkridge T, Verver S, et al. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis 2011; 15:331–6. [PubMed] [Google Scholar]
- 34. Joint United Nations Programme on HIV/AIDS. AIDSinfo. Available at: http://aidsinfo.unaids.org/. Accessed 25 August 2019.
- 35. World Health Organization. The End TB Strategy. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 36. Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-TB Gold In-Tube assay in clinical practice. Am J Respir Crit Care Med 2013; 187:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. König Walles J, Tesfaye F, Jansson M, et al. Performance of QuantiFERON-TB Gold Plus for detection of latent tuberculosis infection in pregnant women living in a tuberculosis- and HIV-endemic setting. PLoS One 2018; 13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esmail H, Thienemann F, Oni T, Goliath R, Wilkinson KA, Wilkinson RJ. QuantiFERON conversion following tuberculin administration is common in HIV infection and relates to baseline response. BMC Infect Dis 2016; 16:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uzorka JW, Bossink AWJ, Franken WPJ, et al. Borderline QuantiFERON results and the distinction between specific responses and test variability. 2018; 111:102–8. [DOI] [PubMed] [Google Scholar]
- 40. Johnson JL, Geldenhuys H, Thiel BA, et al. Effect of isoniazid therapy for latent tb infection on QuantiFERON-TB Gold In-Tube responses in adults with positive tuberculin skin test results in a high TB incidence area. Chest 2014; 145:612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.