Abstract
Background
Bictegravir (B)/emtricitabine (F)/tenofovir alafenamide (TAF) is guideline-recommended treatment for human immunodeficiency virus type 1 (HIV-1). We evaluated whether people receiving dolutegravir (DTG) plus F/TAF or F/TDF (tenofovir disoproxil fumarate) with viral suppression can switch to B/F/TAF without compromising safety or efficacy, regardless of preexisting nucleoside reverse transcriptase inhibitor (NRTI) resistance.
Methods
In this multicenter, randomized, double-blinded, active-controlled, noninferiority trial, we enrolled adults who were virologically suppressed for ≥6 months before screening (with documented/suspected NRTI resistance) or ≥3 months before screening (with no documented/suspected NRTI resistance) on DTG plus either F/TDF or F/TAF. We randomly assigned (1:1) participants to switch to B/F/TAF or DTG + F/TAF once daily for 48 weeks, each with matching placebo. The primary endpoint was proportion of participants with plasma HIV-1 RNA ≥50 copies/mL at week 48 (snapshot algorithm); the prespecified noninferiority margin was 4%.
Results
Five hundred sixty-seven adults were randomized; 565 were treated (284 B/F/TAF, 281 DTG + F/TAF). At week 48, B/F/TAF was noninferior to DTG + F/TAF, as 0.4% (1/284) vs 1.1% (3/281) had HIV-1 RNA ≥50 copies/mL (difference, −0.7% [95.001% confidence interval {CI}, −2.8% to 1.0%]). There were no significant differences in efficacy among participants with suspected or confirmed prior NRTI resistance (n = 138). No participant had treatment-emergent drug resistance. Median weight change from baseline at week 48 was +1.3 kg (B/F/TAF) vs +1.1 kg (DTG + F/TAF) (P = .46). Weight change differed by baseline NRTIs (+2.2 kg [F/TDF] and +0.6 kg [F/TAF], P < .001), with no differences between B/F/TAF and DTG + F/TAF.
Conclusions
The single-tablet regimen B/F/TAF is a safe, effective option for people virologically suppressed on DTG plus either F/TDF or F/TAF, including in individuals with preexisting resistance to NRTIs.
Clinical Trials Registration
Keywords: HIV, INSTI, bictegravir, dolutegravir, tenofovir alafenamide
Switching to bictegravir (B)/emtricitabine (F)/tenofovir alafenamide (TAF) was noninferior to dolutegravir (DTG) plus F/TAF at week 48, with high rates of virologic suppression. B/F/TAF is a safe, effective option for people virologically suppressed on DTG + F/tenofovir disoproxil fumarate or F/TAF, including individuals with preexisting resistance to nucleoside reverse transcriptase inhibitors.
Current treatment guidelines recommend an integrase strand transfer inhibitor (INSTI) plus nucleoside reverse transcriptase inhibitors (NRTIs) as preferred initial therapy [1–3]. Among INSTIs, the agents dolutegravir (DTG) and bictegravir (BIC, B) have low incidence of treatment-emergent resistance. This higher resistance barrier is evident in results of prospective clinical trials, which demonstrate no incident resistance among those experiencing virologic failure if they received either DTG or BIC plus 2 NRTIs [4–7]. Based in part on these results, International AIDS Society (IAS)–USA guidelines only recommend BIC or DTG as INSTIs for initial therapy [3].
This resistance benefit translates into advantages in treatment-experienced individuals as well. In the DAWNING study of people with human immunodeficiency virus (HIV) failing first-line treatment with a nonnucleoside reverse transcriptase inhibitor (NNRTI) plus 2 NRTIs, the strategy of DTG plus at least 1 active NRTI was superior to lopinavir/ritonavir plus 1 active NRTI [8]. Many participants in this study had extensive NRTI resistance. In an earlier study, DTG was superior to raltegravir in treatment-experienced people failing treatment [9]; some of these participants also had extensive NRTI resistance but were still successfully treated with DTG plus NRTIs [10]. These results demonstrate that individuals who harbor some NRTI resistance can achieve and maintain viral suppression with DTG plus 2 NRTIs, even if not all NRTIs in the regimen are fully active.
Bictegravir is a novel INSTI coformulated with emtricitabine and tenofovir alafenamide (F/TAF) into a single-tablet treatment (B/F/TAF) for HIV type 1 (HIV-1). Previous studies demonstrated the safety and efficacy of switching to B/F/TAF among people with viral suppression and no history of NRTI resistance [11, 12]. In the present study, we sought to extend these findings to individuals with viral suppression currently receiving a multiple pill regimen of DTG plus either F/TDF (tenofovir disoproxil fumarate) or F/TAF, with particular interest in those harboring baseline resistance to NRTIs.
METHODS
Study Description and Population
Study GS-US-380–4030 is an ongoing, 48-week, randomized, double-blinded, multicenter, active-controlled, noninferiority phase 3 trial. Investigators enrolled adults (≥18 years) with HIV who were virologically suppressed (plasma HIV-1 RNA <50 copies/mL) on a stable once-daily antiretroviral regimen of DTG plus either F/TDF or F/TAF. Documented or suspected resistance to NRTIs was permitted. Participants required ≥6 months of virologic suppression if NRTI resistance was documented or suspected or ≥3 months with no documented or suspected NRTI resistance. Resistance to protease inhibitors (PIs) or NNRTIs was permitted. Documented resistance to INSTIs or confirmed virologic failure (2 consecutive HIV-1 RNA ≥50 copies/mL after achieving <50 copies/mL) while on an INSTI-containing regimen was not allowed. All participants had estimated glomerular filtration rate (eGFR) ≥30 mL/minute (Cockcroft-Gault). Individuals with chronic hepatitis C or B infection were permitted to enroll.
Women of childbearing potential were required to have a negative serum pregnancy test and to use a protocol-defined acceptably effective form of contraception [13]. The protocol was amended and highly effective contraception with a failure rate of <1% per year was required after the reporting of potential increased risk for neural tube defects in infants born to mothers taking DTG [14–16].
This study was undertaken in accordance with the Declaration of Helsinki and was approved by central or site-specific review boards or ethics committees. All participants provided written informed consent.
Randomization and Masking
We randomly assigned (1:1) participants to B/F/TAF (50/200/25 mg) or to DTG (50 mg) plus F/TAF (200/25 mg). Participants also received placebo tablets; thus, all participants received a total of 3 tablets (active treatment[s] and placebo) once daily. A computer-generated allocation sequence (block size 4) was created by Bracket (San Francisco, California). Randomization was stratified by treatment NRTIs at screening (F/TAF vs F/TDF) and documented or suspected history of NRTI resistance [17]. We stratified participants with IAS-USA–defined NRTI resistance mutations in 3 tiered categories [17] (Table 1). Proviral DNA assays were conducted retrospectively for all participants with an available baseline sample (GenoSure Archive, Monogram Biosciences, South San Francisco, California) and resistance classifications used for subsequent on-study analyses incorporated all available data including investigator assessment, historical, and proviral genotypes.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | B/F/TAF (n = 284) | DTG + F/TAF (n = 281) |
|---|---|---|
| Age, y, median ( range) | 51 (22–79) | 50 (20–79) |
| Women | 39 (14%) | 41 (15%) |
| Race | ||
| White | 200 (71%) | 199 (72%) |
| Black | 68 (24%) | 61 (22%) |
| Other | 9 (3%) | 13 (5%) |
| Asian | 3 (1%) | 3 (1%) |
| Native Hawaiian or Pacific Islander | 2 (1%) | 1 (<1%) |
| Ethnicity | ||
| Hispanic or Latino | 61 (22%) | 49 (18%) |
| Region | ||
| United States | 216 (76%) | 215 (77%) |
| Ex–United States | 68 (24%) | 66 (23%) |
| HIV disease status | ||
| Asymptomatic | 240 (85%) | 227 (81%) |
| Symptomatic | 14 (5%) | 21 (7%) |
| AIDS | 30 (11%) | 33 (12%) |
| HIV risk factora | ||
| Homosexual sex | 189 (67%) | 191 (68%) |
| Heterosexual sex | 75 (26%) | 73 (26%) |
| Intravenous drug use | 15 (5%) | 15 (5%) |
| Unknown | 12 (4%) | 8 (3%) |
| Other | 2 (1%) | 1 (<1%) |
| Vertical transmission | 2 (1%) | 0 |
| Transfusion | 0 | 1 (<1%) |
| Number with HIV type 1 RNA | ||
| <50 copies/mL | 276 (97%) | 275 (98%) |
| ≥50 copies/mL | 8 (3%) | 6 (2%) |
| CD4 count, cells/μL, median (IQR) | 659 (486–885) | 642 (462–791) |
| CD4 cell count, cells/μL | ||
| <200 | 6 (2%) | 7 (2%) |
| 200–499 | 71 (25%) | 78 (28%) |
| ≥500 | 207 (73%) | 196 (70%) |
| CD4%, median (IQR) | 35 (29–41) | 34 (27–41) |
| Creatinine clearance by Cockcroft-Gault formula, mL/minute , median (IQR) | 97 (79–114) | 100 (83–124) |
| HIV/HBV coinfected | 13 (5%) | 7 (2%) |
| HIV/HCV coinfected | 1 (<1%) | 5 (2%) |
| BMI, kg/m2 , median (IQR) | 26 (24–31) | 27 (24–31) |
| NRTI resistance stratumb (by category) | ||
| 1. K65R/E/N or ≥3 TAMs | 16 (6%) | 14 (5%) |
| 2. Any other pattern of NRTI mutation | 55 (19%) | 53 (19%) |
| 3. No NRTI mutation | 213 (75%) | 214 (76%) |
| NRTI backbone stratum | ||
| F/TAF | 194 (68%) | 195 (69%) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; BMI, body mass index; DTG + F/TAF, dolutegravir plus emtricitabine/tenofovir alafenamide; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; NRTI, nucleoside reverse transcriptase inhibitor; TAM, thymidine analogue mutation.
aA participant may fit >1 HIV risk factor category; therefore, percentages may add to >100%.
bCategory 1 included high-level NRTI resistance defined as K65R/E/N or ≥3 TAMs, 1 of which was M41L or L210W (TAMs [M41L, D67N, K70R, L210W, T215F/Y, and K219Q/E/R/N]) or T69 insertions; category 2 included any other pattern of NRTI resistance including ≤2 TAMs, T69D, K70E/G/M/Q/S/T, L74I/V, V75A/S/M/T Y115F, Q151M, and M184V/I; category 3 was no NRTI resistance mutations. For participants who met criteria for >1 category, stratification was prioritized by category 1, then 2, then 3. Participants with suspected resistance were categorized based on the investigator’s review of their treatment history.
Procedures
We conducted postbaseline study visits at weeks 4, 8, 12, 24, 36, and 48, after which participants were eligible to receive open-label B/F/TAF in an extension phase. Laboratory tests included complete blood count, serum chemistry tests, fasting lipid parameters, CD4 counts, and plasma HIV-1 RNA level (Roche TaqMan 2.0; Roche Diagnostics, Rotkreuz, Switzerland). Protocol-defined resistance testing consisted of genotypic and phenotypic analysis of integrase, protease, and reverse transcriptase (Monogram Biosciences, South San Francisco, California) for any participant who had a confirmed plasma HIV-1 RNA ≥50 copies/mL with the confirmation plasma HIV-1 RNA ≥200 copies/mL or having plasma HIV-1 RNA ≥200 copies/mL at week 48, or at the last visit on study drug.
Safety was assessed by physical examinations, laboratory tests, 12-lead electrocardiography, concomitant drugs, and recording of adverse events (AEs), which were coded using the Medical Dictionary for Regulatory Activities (MedDRA, version 21.1).
Statistical Analysis
We performed the primary analysis after all participants completed their week 48 study visit or had prematurely discontinued the study drug. The primary efficacy endpoint was the proportion of participants who had plasma HIV-1 RNA ≥50 copies/mL at week 48 as defined by the United States Food and Drug Administration (FDA) snapshot algorithm (Supplementary Statistical Methods) [18]. We assessed the primary efficacy endpoint using a 95% confidence interval (CI) for the difference in virologic failure rates (B/F/TAF minus DTG + F/TAF); the upper bound of the 95% CI was assessed using a prespecified noninferiority margin of 4%. All randomized participants who received ≥1 dose of study drug were included in the primary efficacy analysis. A secondary per-protocol analysis excluded participants who did not have a plasma HIV-1 RNA value in the week 48 analysis window due to reasons other than early study drug discontinuation because of lack of efficacy, low adherence (defined as adherence below the 2.5th percentile), and key entry criteria violations.
Assuming 2% of participants in each group would have HIV-1 RNA ≥50 copies/mL at week 48, a sample size of 520 would achieve ≥90% power to detect a noninferiority margin of 4% in difference between groups at a 1-sided α level of .025. One planned external independent data monitoring committee interim analysis was performed after all enrolled participants completed their week 12 study visit or prematurely discontinued study drugs. An α penalty of .000 01 was applied for the planned interim analysis. Therefore, the significance level for the 2-sided noninferiority test for the primary endpoint at week 48 was 0.049 99, corresponding to a 95.001% CI. We constructed the point estimate of treatment difference in percentage of participants with HIV-1 RNA ≥50 copies/mL at week 48 and the associated 2-sided 95.001% CI based on an unconditional exact method using 2 inverted 1-sided tests.
A key secondary efficacy endpoint was proportion of participants with plasma HIV-1 RNA <50 copies/mL at week 48 by FDA snapshot algorithm, analyzed similarly to the primary efficacy endpoint using the noninferiority margin of 10%. Treatment differences in changes from baseline in CD4+ cell count (other secondary efficacy endpoint) and CD4 percentage and 95% CIs were constructed with analysis of variance model, including treatment group as a fixed effect in the model. Subgroup analyses of the proportion of participants with plasma HIV-1 RNA <50 copies/mL at week 48 were conducted based on age, sex, race, geographic region, adherence rate, and baseline NRTI resistance. The week 48 efficacy endpoint was also analyzed with a plasma HIV-1 RNA cutoff of <20 copies/mL or target not detected by FDA snapshot algorithm, and the proportion of participants with plasma HIV-1 RNA <50 copies/mL at week 48 was also analyzed when imputing missing as failure (M = F) and missing as excluded (M = E).
We performed a post-hoc analysis identifying predictors associated with baseline NRTI resistance or with M184V/I mutation using in participants using intrinsic and HIV-specific baseline variables in a multivariate logistic regression model with stepwise selection significance level for entry α = .20 and significance level for stay α = .05 (Supplementary Statistical Methods).
We summarized baseline characteristics with descriptive statistics for all randomized participants who received ≥1 dose of study drug. Safety data are described using all data collected on or after study drug was first given up to either the data cut date or, for participants who discontinued treatment early, up to 30 days after the last dose of study drug. For categorical baseline data, P values were calculated from the Cochran-Mantel-Haenszel (CMH) test (general association statistic was used for nominal data, row mean scores differ statistic was used for ordinal data). For continuous data, P values were derived from the 2-sided Wilcoxon rank-sum test. The Fisher exact test was used to compare the difference between treatment groups in the rate of participants who took lipid-lowering agents at baseline and those who initiated lipid-lowering agents during the study.
We used SAS version 9.4 software (SAS Institute, Cary, North Carolina) for all analyses.
This study was conducted according to protocol without substantial deviations and is registered with ClinicalTrials.gov (identifier NCT03110380).
RESULTS
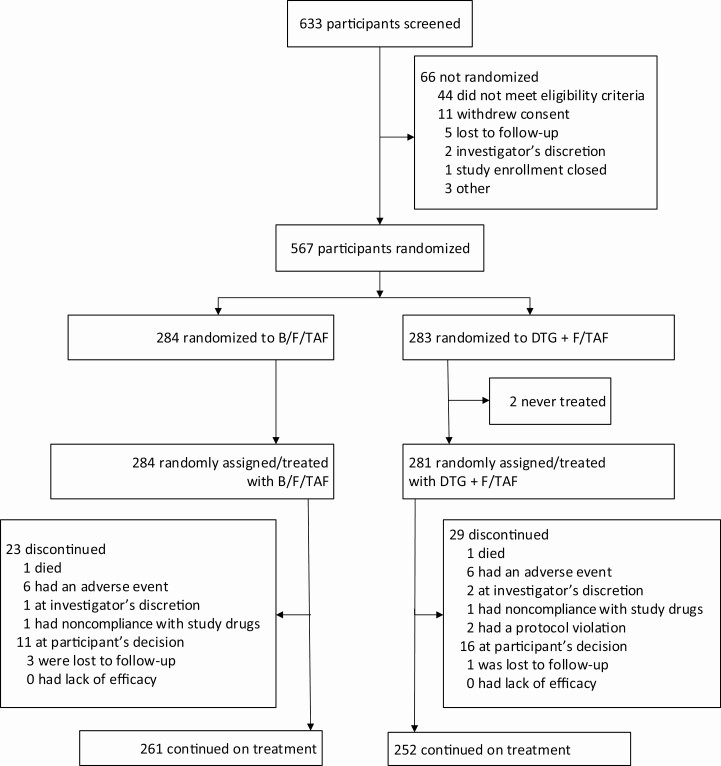
Between 12 June 2017 and 7 November 2017, 633 participants were screened, and 567 were randomized: 284 to B/F/TAF and 283 to DTG + F/TAF (Figure 1). Two participants randomized to DTG + F/TAF did not receive study drugs due to withdrawal of consent (n = 1) and protocol violation (n = 1). At baseline, 69% (389/565) of participants were taking F/TAF, and 31% (176/565) were taking F/TDF. Demographics and baseline characteristics were generally balanced between groups, with the exception of baseline CD4 cell counts, which were higher in the B/F/TAF group than in the DTG + F/TAF group (Table 1).
Figure 1.
Study profile through week 48. Abbreviations: B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; DTG + F/TAF, dolutegravir plus emtricitabine/tenofovir alafenamide.
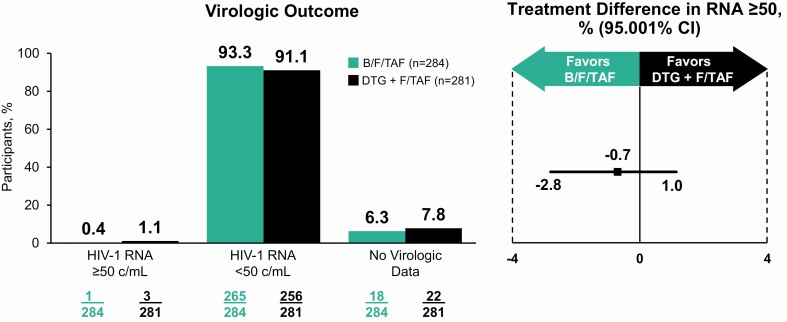
Switching to B/F/TAF was noninferior to DTG + F/TAF for the primary outcome of proportion of participants with plasma HIV-1 RNA ≥50 copies/mL at week 48 as defined by the FDA snapshot algorithm (0.4% [1 of 284 participants] vs 1.1% [3 of 281]; difference, −0.7% [95.001% CI, −2.8% to 1.0%]) (Table 2, Figure 2). Results from the per-protocol analysis confirmed those of the primary outcome in full analysis set. No participant in either group with baseline NRTI resistance had HIV-1 RNA ≥50 copies/mL at week 48 or their last visit.
Table 2.
Virologic Outcomes at Week 48
| Outcome | B/F/TAF (n = 284) | DTG + F/TAF (n = 281) | B/F/TAF vs DTG + F/TAF |
|---|---|---|---|
| Difference in Percentages (95.001% CI)a | |||
| HIV-1 RNA <50 copies/mL | 265 (93.3%) | 256 (91.1%) | 2.2% (−2.3% to 6.8%) |
| HIV-1 RNA ≥50 copies/mL | 1 (0.4%) | 3 (1.1%) | −0.7% (−2.8% to 1.0%) |
| HIV-1 RNA ≥50 copies/mL | 1 (0.4%) | 1 (0.4%) | … |
| Discontinued due to lack of efficacy | 0 | 0 | … |
| Discontinued study drug due to AE/death and last available HIV-1 RNA ≥50 copies/mL | 0 | 0 | … |
| Discontinued due to other reasonsb and last available HIV-1 RNA ≥50 copies/mL | 0 | 2 (0.7%) | … |
| No virologic data | 18 (6.3%) | 22 (7.8%) | … |
| Discontinued due to AE/death and last available HIV-1 RNA <50 copies/mL | 6 (2.1%) | 6 (2.1%) | … |
| Discontinued due to other reasonsb and last available HIV-1 RNA <50 copies/mL | 12 (4.2%) | 15 (5.3%) | … |
| Missing data but on study drug | 0 | 1 (0.4%) | … |
| HIV-1 RNA <50 copies/mL by per-protocol snapshot analysis | 259/259 (100%) | 237/237 (100%) | NA |
| HIV-1 RNA <50 copies/mL by missing = failurec | 266/284 (93.7%) | 260/281 (92.5%) | 1.1% (−3.2% to 5.5%) |
| HIV-1 RNA <50 copies/mL by missing = excludedc | 266/269 (98.9%) | 260/261 (99.6%) | −0.7% (−2.9% to 1.2%) |
| HIV-1 RNA <20 copies/mL | 257/284 (90.5%) | 241/281 (85.8%) | 4.7% (−.7% to 10.3%) |
Data are presented as no. (%) unless otherwise indicated. Virology outcomes are based on snapshot algorithm unless otherwise specified. The week 48 window is between days 295 and 378 (inclusive). Per-protocol analysis excluded patients in the full analysis set who were off study drug at week 48 or had low adherence, that is, adherence ≤2.5th percentile among those in the study.
Abbreviations: AE, adverse event; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; CI, confidence interval; DTG + F/TAF, dolutegravir plus emtricitabine/tenofovir alafenamide; HIV-1, human immunodeficiency virus type 1; NA, not applicable.
aThe differences in percentages of subjects between treatment groups and their 95.001% CIs (for HIV-1 RNA <50 copies/mL or HIV-1 RNA ≥50 by US Food and Drug Administration snapshot algorithm), or otherwise 95% CI, were calculated based on an unconditional exact method using 2 inverted 1-sided tests.
bOther reasons include subjects who discontinued study drug due to the investigator’s discretion, subject decision, loss to follow-up, noncompliance with study drug, protocol violation, pregnancy, and study terminated by sponsor.
cDifferences in percentages, and 95% CI, were based on a dichotomized response: HIV-1 RNA <50 copies/mL vs HIV-1 RNA ≥50 copies/mL or missing for the missing = failure approach and HIV-1 RNA <50 copies/mL vs HIV-1 RNA ≥50 copies/mL for the missing = excluded approach.
Figure 2.
Virologic outcome at week 48. Abbreviations: B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; c/mL, copies per milliliter; CI, confidence interval; DTG + F/TAF, dolutegravir plus emtricitabine/tenofovir alafenamide; HIV-1 human immunodeficiency virus type 1.
Secondary outcomes also supported the primary efficacy outcome. The proportion of participants with plasma HIV-1 RNA <50 copies/mL at week 48 by the FDA snapshot algorithm was 93.3% (265/284) B/F/TAF and 91.1% (256/281) DTG + F/TAF (difference, 2.2% [95.001% CI, −2.3% to 6.8%]) (Table 2). Efficacy was similar across prespecified subgroups (Table 3) and testing for homogeneity found no significant interactions between treatment and subgroup. Results from the M = F and M = E analyses for the proportion of participants with plasma HIV-1 RNA <50 copies/mL were consistent with the secondary endpoint (Table 2). Using the plasma HIV-1 RNA threshold of <20 copies/mL, the proportion of participants virologically suppressed at week 48 (FDA snapshot algorithm) was 90.5% (257/284) B/F/TAF and 85.1% (241/281) DTG + F/TAF (difference, 4.7% [95% CI, −.7% to 10.3%]). The proportion of participants with any preexisting NRTI resistance and with the M184V/I mutation who had HIV-1 RNA <50 copies/mL, <20 copies/mL, or target not detected were consistent with the overall study results (Table 3). There were no clinically significant changes from baseline in CD4 cell count or percentage at week 48 (Supplementary Appendix Table 1). In separate multivariate models identifying predictors associated with baseline NRTI resistance mutations and with the M184V/I mutations, the time since antiretroviral therapy (ART) start, prior use of a PI, PI resistance, NNRTI resistance, and black race were identified to be independent predictors of any NRTI resistance mutation and of the M184V/I mutation (Supplementary Appendix Table 2).
Table 3.
Treatment Differences in Virologic Outcomes at Week 48, by Subgroup
| Characteristic | B/F/TAF (n = 284), No. (%) | DTG + F/TAF (n = 281), No. (%) | Difference in Percentages (95% CI) |
|---|---|---|---|
| HIV-1 RNA <50 copies per mL | |||
| Overall | 265 (93.3%) | 256 (91.1%) | 2.2% (−2.3% to 6.8%) |
| Age | |||
| <50 y | 118/127 (92.9%) | 120/130 (92.3%) | 0.6% (−6.3% to 7.6%) |
| ≥50 y | 147/157 (93.6%) | 136/151 (90.1%) | 3.6% (−2.7% to 10.3%) |
| Sex | |||
| Male | 230/245 (93.9%) | 220/240 (91.7%) | 2.2% (−2.5% to 7.1%) |
| Female | 35/39 (89.7%) | 36/41 (87.8%) | 1.9% (−13.6% to 18.1%) |
| Race | |||
| Black | 62/68 (91.2%) | 56/61 (91.8%) | −0.6% (−11.3% to 10.3%) |
| Not black | 201/214 (93.9%) | 197/217 (90.8%) | 3.1% (−2.0% to 8.5%) |
| Region | |||
| United States | 202/216 (93.5%) | 197/215 (91.6%) | 1.9% (−3.3% to 7.1%) |
| Ex–United States | 63/68 (92.6%) | 59/66 (89.4%) | 3.3% (−7.3% to 14.5%) |
| Study drug adherence | |||
| <95% | 51/59 (86.4%) | 60/66 (90.9%) | −4.5% (−17.0% to 7.2%) |
| ≥95% | 214/225 (95.1%) | 196/213 (92.0%) | 3.1% (−1.6% to 8.1%) |
| Baseline NRTI resistance | |||
| No NRTI mutation | 199/213 (93.4%) | 191/214 (89.3%) | 4.2% (−1.3% to 9.9%) |
| Any NRTI mutation | 66/71 (93.0%) | 65/67 (97.0%) | −4.1% (−13.2% to 4.5%) |
| Baseline M184V/I resistance | |||
| No M184V/I | 223/237 (94.1%) | 224/247 (90.7%) | 3.4% (−1.5% to 8.4%) |
| M184V/I | 42//47 (89.4%) | 32/34 (94.1%) | −4.8% (−18.2% to 9.9%) |
| HIV-1 RNA <20 copies per mL | |||
| Overall | 257 (90.5%) | 241 (85.8%) | 4.7% (−.7% to 10.3%) |
| Baseline NRTI resistance | |||
| No NRTI mutation | 194/213 (91.1%) | 182/214 (85.0%) | 6.0% (−.2% to 12.4%) |
| Any NRTI mutation | 63/71 (88.7%) | 59/67 (88.1%) | 0.7% (−10.7% to 12.3%) |
| Baseline M184V/I mutation | |||
| No M184V/I | 216/237 (91.1%) | 212/247 (85.8%) | 5.3% (−.5% to 11.2%) |
| M184V/I | 41/47 (87.2%) | 29/34 (85.3%) | 1.9% (−13.8% to 19.6%) |
| Undetectable HIV-1 RNA | |||
| Overall | 182 (64.1%) | 170 (60.5%) | 3.6% (−4.5% to 11.6%) |
| Baseline NRTI resistance | |||
| No NRTI mutation | 133/213 (62.4%) | 129/214 (60.3%) | 2.2% (−7.1% to 11.4%) |
| Any NRTI mutation | 49/71 (69.0%) | 41/67 (61.2%) | 7.8% (−8.4% to 23.8%) |
| Baseline M184V/I mutation | |||
| No M184V/I | 153/237 (64.6%) | 153/247 (61.9%) | 2.6% (−6.0% to 11.3%) |
| M184V/I | 29/47 (61.7%) | 17/34 (50.0%) | 11.7% (−10.5% to 33.6%) |
Virology outcomes are based on snapshot algorithm. For race, subjects who reported “not permitted” were excluded from the percentage. Study drug adherence subgroup analyses are based on the adherence up to week 48 visit for active study drug; only subjects who returned at least 1 bottle and had calculable drug adherence were included.
Abbreviations: B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; CI, confidence interval; DTG + F/TAF, dolutegravir plus emtricitabine/tenofovir alafenamide; HIV-1, human immunodeficiency virus type 1; NRTI, nucleoside reverse transcriptase inhibitor.
Three participants met protocol-defined criteria for resistance testing; all were in the DTG group. No emergent virologic resistance was detected to any component of their regimen.
Both treatments were well tolerated through median duration of exposure of 58.6 (interquartile range [IQR], 53.1–63.3) weeks for the B/F/TAF group and 58.3 (IQR, 52.3–63.3) weeks for the DTG + F/TAF group. Most AEs were mild or moderate in severity. Table 4 shows AEs reported by 5% or more of participants in either group. AEs leading to study drug discontinuation occurred in 2% of participants in each group (6/284 B/F/TAF, 6/281 DTG + F/TAF). Study drug–related AEs were reported for 41 participants (14%) on B/F/TAF and 28 (10%) on DTG + F/TAF; events were primarily mild or moderate in severity. Most common drug-related AEs were diarrhea and headache; each was reported in 1% on B/F/TAF and 2% on DTG + F/TAF. One participant in each group died during the study. In the B/F/TAF group, 1 individual died of cardiopulmonary arrest, assessed as not related to study drug by the investigator. In the DTG + F/TAF group, 1 participant died of suspected myocardial infarction, assessed as related to the study drug by the investigator. No pregnancies were reported during the study.
Table 4.
Adverse Events Through Week 48
| Adverse Event | B/F/TAF (n = 284) | DTG + F/TAF (n = 281) |
|---|---|---|
| Any AE | 236 (83%) | 243 (86%) |
| Grade 3 or 4 AE | 23(8%) | 13(5%) |
| Serious AEb | 30 (11%) | 19 (7%) |
| Study drug–related AE | 41 (14%) | 28 (10%) |
| Study drug–related serious AE | 0 | 1 (<1%) |
| Any AE leading to study drug discontinuationa | 6 (2%) | 6 (2%) |
| Death | 1 (<1%) | 1 (<1%) |
| AE ≥5% in either arm | ||
| Nasopharyngitis | 32 (11%) | 28 (10%) |
| Diarrhea | 23 (8%) | 32 (11%) |
| Upper respiratory tract infection | 20 (7%) | 30 (11%) |
| Headache | 13 (5%) | 23 (8%) |
| Arthralgia | 16 (6%) | 17 (6%) |
| Influenza | 16 (6%) | 14 (5%) |
| Fatigue | 21 (7%) | 8 (3%) |
| Insomnia | 18 (6%) | 11 (4%) |
| Back pain | 15 (5%) | 11 (4%) |
| Bronchitis | 14 (5%) | 11 (4%) |
| Pain in extremity | 12 (4%) | 11 (4%) |
| Cough | 6 (2%) | 16 (6%) |
Data are presented as no. (%).
Abbreviations: AE, adverse event; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; DTG + F/TAF, dolutegravir plus emtricitabine/tenofovir alafenamide.
aAEs leading to study drug discontinuation in the bictegravir group included fatigue (n = 1), device-related infection (n = 1), dyspepsia (n = 1), fatigue, nightmare, and hyperhidrosis (n = 1), abnormal dreams (n = 1), and agitation (n = 1); all events were considered by the investigator to be related to study drug except device-related infection. AEs leading to study drug discontinuation in the dolutegravir group included insomnia, fatigue, headache, nausea, and anxiety (n = 1), generalized rash (n = 1), maculopapular rash and conjunctival hyperemia (n = 1), asthenia and flatulence (n = 1), yolk sac tumor (site unspecified) (n = 1), and abnormal dreams and sleep disorder (n = 1); all events were considered by the investigator to be related to study drug except anxiety, generalized rash, and yolk sac tumor (site unspecified).
bSerious AEs in the bictegravir group included diverticulitis (n = 2) and biliary dyskinesia, bronchospasm, cardiorespiratory arrest, cellulitis, chest pain, cholecystitis, chronic obstructive pulmonary disease, clavicle fracture, concussion, coronary artery disease, device-related infection, drug abuse, dyspnea, foreign body in gastrointestinal tract, gastroenteritis, infectious diarrhea, large intestine perforation, ligament rupture, lumbar spinal stenosis, malignant lung neoplasm, overdose, perirectal abscess, pneumonia, pneumonitis, pneumothorax, postprocedural infection, pyelonephritis, rebound psychosis, rhabdomyolysis, seizure, sinus tachycardia, tendon rupture, and ventricular tachycardia (n = 1 each). Serious AEs in the dolutegravir group included abdominal pain, angina pectoris, ankle fracture, appendicitis, increased blood glucose, cholecystitis, acute cholecystitis, diverticulitis, dyspnea, inguinal hernia, lisfranc fracture, myocardial infarction, osteonecrosis, pyelonephritis, subdural hemorrhage, vertebral artery dissection, viral upper respiratory tract infection, vomiting, and yolk sac tumor site unspecified (n = 1 each).
Incidence of grade 3 or 4 laboratory abnormalities was comparable between groups: 16% (46/284) B/F/TAF and 13% (37/280) DTG + F/TAF (Supplementary Appendix Table 3). At week 48, median changes from baseline in eGFR were similar between groups (0.6 [IQR, −7.9 to 7.3] mL/minute vs −0.9 [IQR, −7.7 to 5.4] mL/minute; P = .38). There were no discontinuations due to renal AEs and no cases of proximal renal tubulopathy in either arm. Changes from baseline in fasting lipids were similar between groups at week 48; a similar proportion of participants in each group were taking lipid-lowering medication at baseline and initiated lipid-lowering agents during the study (Supplementary Appendix Table 4). The median weight at baseline was 81 (IQR, 71–93) kg in the B/F/TAF group and 82 (IQR, 73–95) kg in the DTG + F/TAF group, corresponding to a median body mass index of 26 (IQR, 24–31) and 27 (IQR, 24–31) in the B/F/TAF and DTG + F/TAF groups, respectively. The median weight change from baseline at week 48 was +1.3 (IQR, −1.3 to +3.6) kg for B/F/TAF and +1.1 (IQR, −1.6 to +3.7) kg for DTG + F/TAF (P = .46). Change in weight from baseline at week 48 differed significantly based on NRTIs at baseline, with median weight change of +2.2 (IQR, −0.4 to +4.6) kg in participants on F/TDF and +0.6 (IQR, −1.7 to +3.1) kg in participants on F/TAF at baseline (P < .001), with no differences between the B/F/TAF and DTG+F/TAF groups overall or by baseline NRTI.
DISCUSSION
In this randomized, double-blind trial, we demonstrate that people with viral suppression on DTG plus either F/TAF or F/TDF can safely switch to the single-tablet regimen B/F/TAF. Viral suppression was maintained regardless of whether participants had resistance to NRTIs or a prior history of treatment failure. These results strongly suggest that the resistance barrier of BIC is high, as has been shown in vitro [19, 20].
Most studies of switch therapy in HIV limit enrollment to people with no documented history of treatment failure or resistance [21, 22]. While providing evidence about safety and tolerability of the test regimen, these studies provide limited information about its resistance barrier. Furthermore, studies that include only people without prior treatment failure preferentially select those with favorable adherence characteristics, biasing the results toward treatment success.
By contrast, our study permitted prior history of treatment failure or any resistance other than to INSTIs, provided the participant demonstrated at least 6 months of viral suppression on DTG plus F/TDF or F/TAF. At baseline, approximately 25% had NRTI resistance due to either prior virologic failure or treatment with nonsuppressive single or dual NRTI-based therapies. We accounted for this baseline resistance by stratifying at randomization by historical genotypes (when available) or investigator assessment of suspected resistance. We then conducted the final analysis based on this information plus proviral DNA resistance testing on most participants. Regardless of baseline resistance status, we found results similar to those of the overall study, with the BIC group displaying comparable viral suppression to the DTG group. Furthermore, only 3 participants (all in the DTG arm) met criteria for resistance testing; no treatment-emergent resistance was detected.
We further assessed independent predictors of NRTI resistance in these treatment-experienced individuals. Independent risk factors included longer time since starting ART (10% per year), prior PI-containing regimen, black race, and PI or NNRTI resistance. These data may help clinicians understand which virologically suppressed individuals are likely to harbor NRTI resistance. In such individuals, switches to regimens with lower resistance barriers should be made with caution.
Earlier studies in people without history of treatment failure or resistance showed that switching from coformulated DTG, abacavir, and lamivudine or a boosted PI to B/F/TAF yielded noninferior rates of viral suppression [11, 12]. The present study extends the population eligible for changing to B/F/TAF. Such a switch might be of particular interest in older adults or those with cardiovascular risk factors, as both abacavir and some boosted PI-containing regimens have been associated with an elevated risk of cardiovascular events [23–26].
Both regimens were well tolerated, with only 2% discontinuing treatment due to AEs. There were no discontinuations due to renal AEs, and there was no incident proximal renal tubulopathy. Two deaths occurred during the study (1 in each arm). One participant’s death on the DTG + F/TAF arm was due to a suspected myocardial infarction reported by the investigator as related to study drug, though limited information was available to assess the cause of death. Weight gain greater than comparator regimens was observed in studies of DTG, BIC, and TAF [27, 28]. While weight changes were similar between the B/F/TAF and DTG + F/TAF arms in this study, participants who at baseline were receiving TDF gained more weight than those on TAF. Similarly, more weight gain occurs with 2-drug regimens containing DTG and lamivudine than with DTG + F/TDF [29], and data from people taking TDF as part of HIV preexposure prophylaxis (PrEP) point to a relative weight suppressive effect of TDF when compared to placebo or to TAF-containing PrEP [30–32]. While a mechanism remains unclear, together these data suggest a relative weight-inhibiting effect of TDF that was eliminated when participants switched to a study regimen.
In summary, our study demonstrated that people with HIV who are virologically suppressed on DTG plus F/TAF or F/TDF can safely switch to the simpler single-tablet regimen of B/F/TAF. By including participants regardless of treatment or resistance history other than INSTI resistance, these data continue to support the high resistance barrier of both BIC and DTG when they are combined with 2 NRTIs, and the safety of switching to B/F/TAF in individuals with viral suppression.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the individuals who participated in this trial and their partners and families; the principal investigators (see Supplementary Appendix) and their staff; the Gilead study staff; and Anna Kido (Gilead) for providing editorial assistance.
Financial support. This work was supported by Gilead Sciences.
Potential conflicts of interest. P. E. S. has received a grant from Gilead to perform the current study, as well as grants and personal fees from Gilead and ViiV and personal fees from Janssen, Merck, and ViiV for participating in scientific advisory boards. J. K. R. reports speaker’s fees and advisory board fees from Gilead, Abbott, ViiV, AbbVie, Janssen, Theratechnologies, and Merck, and meeting expenses from Gilead and Abbott. A. F. L. has received grants from ViiV Healthcare Limited and Merck. D. W. reports grants and personal fees for research, speaker’s bureaus, and serving on advisory boards from Gilead Sciences and ViiV Healthcare. B. T. reports fees for consultation and to attend conferences from Gilead; consulting fees and research grants to his institution from Merck; and consulting fees from ViiV Healthcare, outside the submitted work. Y. Y. has been a board member receiving consultancy fees from AbbVie, BMS, Gilead, MSD, Johnson & Johnson, Pfizer, and ViiV Healthcare, outside the submitted work. H. L., R. A., S. E. C., D. M. B., and H. M. are employees of Gilead and hold stock interest in the company. A. R. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. European AIDS Clinical Society. Guidelines, version 9.1, October 2018. Available at: http://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf. Accessed 21 March 2020.
- 2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV.2019. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 21 March 2020.
- 3. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society–USA panel. JAMA 2018; 320:379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walmsley SL, Antela A, Clumeck N, et al. SINGLE Investigators . Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 5. Raffi F, Jaeger H, Quiros-Roldan E, et al. extended SPRING-2 Study Group . Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013; 13:927–35. [DOI] [PubMed] [Google Scholar]
- 6. Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017; 390:2063–72. [DOI] [PubMed] [Google Scholar]
- 7. Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390:2073–82. [DOI] [PubMed] [Google Scholar]
- 8. Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19:253–64. [DOI] [PubMed] [Google Scholar]
- 9. Cahn P, Pozniak AL, Mingrone H, et al. Extended SAILING Study Team . Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 10. Demarest J, Underwood M, St Clair M, Dorey D, Brown D, Zolopa A. Short communication: dolutegravir-based regimens are active in integrase strand transfer inhibitor-naive patients with nucleoside reverse transcriptase inhibitor resistance. AIDS Res Hum Retroviruses 2018; 34:343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV 2018; 5:e347–56. [DOI] [PubMed] [Google Scholar]
- 12. Molina JM, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV 2018; 5:e357–65. [DOI] [PubMed] [Google Scholar]
- 13. Clinical Trial Facilitation Group (CTFG). Recommendations related to contraception and pregnancy testing in clinical trials. 2014. Available at: http://www.hma.eu/fileadmin/dateien/Human_Medicines/01-About_HMA/Working_Groups/CTFG/2014_09_HMA_CTFG_Contraception.pdf. Accessed 21 March 2020.
- 14. World Health Organization. Potential safety issue affecting women living with HIV using dolutegravir at the time of conception. Geneva, Switzerland.2018. Available at: https://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf. Accessed 21 March 2020.
- 15. Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in transmission in the United States. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed 21 March 2020.
- 16. US Food and Drug Administration. FDA drug safety communication: FDA to evaluate potential risk of neural tube birth defects with HIV medicine dolutegravir (Juluca, Tivicay, Triumeq). Available at: https://www.fda.gov/Drugs/DrugSafety/ucm608112.htm. Accessed 21 March 2020.
- 17. Wensing AM, Calvez V, Günthard HF, et al. 2017 Update of the drug resistance mutations in HIV-1. Top Antivir Med 2016; 24:132–3. [PMC free article] [PubMed] [Google Scholar]
- 18. Smith F, Hammerstorm T, Soon G, et al. A meta-analysis to assess the FDA DAVP’s TLOVR algorithm in HIV submissions. Drug Inf J 2011; 45:291–300. [Google Scholar]
- 19. Tsiang M, Jones GS, Goldsmith J, et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother 2016; 60:7086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliveira M, Ibanescu RI, Anstett K, et al. Montreal Primary HIV (PHI) Cohort Study Group . Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology 2018; 15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Porter DP, Toma J, Tan Y, et al. Clinical outcomes of virologically-suppressed patients with pre-existing HIV-1 drug resistance mutations switching to rilpivirine/emtricitabine/tenofovir disoproxil fumarate in the SPIRIT study. HIV Clin Trials 2016; 17:29–37. [DOI] [PubMed] [Google Scholar]
- 22. Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, phase IIIb study. Antivir Ther 2017; 22:295–305. [DOI] [PubMed] [Google Scholar]
- 23. Elion RA, Althoff KN, Zhang J, et al. North American AIDS Cohort Collaboration on Research and Design of IeDEA . Recent abacavir use increases risk of type 1 and type 2 myocardial infarctions among adults with HIV. J Acquir Immune Defic Syndr 2018; 78:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sabin CA, Worm SW, Weber R, et al. D:A:D Study Group . Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008; 371:1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friis-Møller N, Reiss P, Sabin CA, et al. DAD Study Group . Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 26. Holmberg SD, Moorman AC, Williamson JM, et al. HIV Outpatient Study (HOPS) Investigators . Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet 2002; 360:1747–8. [DOI] [PubMed] [Google Scholar]
- 27. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gatell JM, Assoumou L, Moyle G, et al. NEAT022 Study Group . Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 2017; 31:2503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cahn P, Madero JS, Arribas JR, et al. GEMINI Study Team . Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393:143–55. [DOI] [PubMed] [Google Scholar]
- 30. Glidden DV, Mulligan K, McMahan V, et al. Metabolic effects of preexposure prophylaxis with coformulated tenofovir disoproxil fumarate and emtricitabine. Clin Infect Dis 2018; 67:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grant RM, Lama JR, Anderson PL, et al. iPrEx Study Team . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hare CB, Coll J, Ruane P, et al. The phase 3 DISCOVER study: daily F/TAF or F/TDF for HIV preexposure prophylaxis [abstract 104LB]. In: Conference of Retroviruses and Opportunistic Infections, Seattle, WA, March 4–7, 2019. Available at: http://www.croiconference.org/sessions/phase-3-discover-study-daily-ftaf-or-ftdf-hiv-preexposure-prophylaxis. Accessed 21 March 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.