Abstract
Background
Quantifying the amount and diversity of antibiotic use in United States hospitals assists antibiotic stewardship efforts but is hampered by limited national surveillance. Our study aimed to address this knowledge gap by examining adult antibiotic use across 576 hospitals and nearly 12 million encounters in 2016–2017.
Methods
We conducted a retrospective study of patients aged ≥ 18 years discharged from hospitals in the Premier Healthcare Database between 1 January 2016 and 31 December 2017. Using daily antibiotic charge data, we mapped antibiotics to mutually exclusive classes and to spectrum of activity categories. We evaluated relationships between facility and case-mix characteristics and antibiotic use in negative binomial regression models.
Results
The study included 11 701 326 admissions, totaling 64 064 632 patient-days, across 576 hospitals. Overall, patients received antibiotics in 65% of hospitalizations, at a crude rate of 870 days of therapy (DOT) per 1000 patient-days. By class, use was highest among β-lactam/β-lactamase inhibitor combinations, third- and fourth-generation cephalosporins, and glycopeptides. Teaching hospitals averaged lower rates of total antibiotic use than nonteaching hospitals (834 vs 957 DOT per 1000 patient-days; P < .001). In adjusted models, teaching hospitals remained associated with lower use of third- and fourth-generation cephalosporins and antipseudomonal agents (adjusted incidence rate ratio [95% confidence interval], 0.92 [.86–.97] and 0.91 [.85–.98], respectively). Significant regional differences in total and class-specific antibiotic use also persisted in adjusted models.
Conclusions
Adult inpatient antibiotic use remains high, driven predominantly by broad-spectrum agents. Better understanding reasons for interhospital usage differences, including by region and teaching status, may inform efforts to reduce inappropriate antibiotic prescribing.
Keywords: antibiotic stewardship, antimicrobial use, surveillance, inpatient
Quantifying the volume and diversity of antibiotic use in United States hospitals assists antibiotic stewardship efforts but is hampered by limited national surveillance. Our study examined adult antibiotic use across 576 hospitals and nearly 12 million encounters in 2016–2017.
Antibiotic stewardship—prescribing antibiotics only when clinically appropriate, for the right amount of time, and with the right drug—is a critical tool for fighting antibiotic resistance in inpatient settings [1]. Understanding the volume and types of antibiotics used across United States (US) hospitals is an integral component of antibiotic stewardship efforts. These national data can reveal large-scale prescribing trends, as well as significant geographic or facility-level prescribing differences, that remain obscured with local or institution-specific data. They can also identify potential policy targets and provide historical benchmarks for evaluating longitudinal trends.
Limited national surveillance makes quantifying inpatient antibiotic use challenging. Academic studies have filled this informational void, and in recent years a number of important studies have examined US inpatient antibiotic prescribing [2–5]. Our research continues these efforts by presenting updated data captured from a larger number of hospitals in more granular detail than previous work. The objective of the current study is to provide a comprehensive examination of adult inpatient antibiotic usage, including its association with different geographic, facility, and case-mix characteristics, across 576 US hospitals and nearly 12 million adult encounters in 2016–2017.
METHODS
Study Setting and Population
Adult inpatient encounters and associated data were collected from hospitals in the Premier Healthcare Database (“Premier Database”). The Premier Database is an all-payer repository of claims and clinical data from > 870 million inpatient and outpatient US hospital encounters, across > 800 hospitals. It includes approximately 1 of every 4 annual inpatient admissions [6] (Supplementary Data). All adult encounters (age ≥ 18 years at admission) with discharge dates on or between 1 January 2016 and 31 December 2017 at hospitals that continuously submitted data during the 24-month study period were included. This study did not include personally identifiable information and was exempt from institutional review board review.
Collected Data
The Premier Database contains comprehensive facility and demographic information, as well as a date-stamped log of all billed therapeutic services and medications. For each admission, we extracted the following data from the Premier Database: (1) facility data (eg, US census geographic division [7], bed size, teaching status); (2) patient sociodemographic data; and (3) patient clinical data, including intensive care unit (ICU) days, and all International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes and the Medicare Severity Diagnosis-Related Group (MS-DRG) code associated with each encounter. Using MS-DRG codes, we calculated each hospital’s case-mix index, a weighting schema used by the Centers for Medicare and Medicaid Services (CMS) to adjust for resource intensity [8–10]. We mapped ICD-10 diagnosis codes to Elixhauser comorbidities using Agency for Healthcare Research and Quality (AHRQ) software [11]. Elixhauser comorbidities were summed to create an unweighted Elixhauser score. The Premier Database does not contain microbiology data; therefore, we used AHRQ bacterial infection diagnosis codes to ascertain whether a patient’s primary ICD-10 diagnosis was infection-related [12]. Inpatient days for encounters with a primary infection diagnosis were treated as “bacterial infection” patient-days (PD). To measure antibiotic utilization, we obtained daily antibiotic charge data for each encounter, including drug name(s), route(s) of administration, and service-day unit location.
Antibiotic Class and Spectrum of Activity Classifications
To design study outcomes, we surveyed the literature to identify commonly reported antibiotic classes [2–4, 13]. Two infectious disease physicians (A. D. H. and S. E. C.) reviewed the results and proposed additional antibiotic classes and spectrum of activity categories based upon clinical relevance. Where in joint agreement, these outcomes were also included. All decisions were completed prior to data analysis, and based upon final consensus, we mapped each antibiotic to 1 of 18 mutually exclusive antibiotic classes: aminoglycosides; β-lactam/β-lactamase inhibitor combinations; β-lactam/β-lactamase inhibitor combinations for multidrug-resistant gram-negative organisms; carbapenems; first- and second-generation cephalosporins; fluoroquinolones; glycopeptides; lincosamides; macrolides; metronidazole; monobactams; oxazolidinones; penicillins; polymyxins; sulfonamides; tetracyclines; third- and fourth-generation cephalosporins; and other antibacterial agents (Supplementary Appendix A). In addition, we mapped antibiotics to non–mutually exclusive categories based upon activity against Bacteroides fragilis, Pseudomonas species, methicillin-resistant Staphylococcus aureus (MRSA), and Clostridioides difficile (Supplementary Appendix A).
Statistical Methods
Descriptive statistics for patient and hospital characteristics were calculated using mean (standard deviation [SD]), median (range or interquartile range [IQR]), or frequency count (percentage). Consistent with other studies and Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN) antimicrobial use surveillance, we used total inpatient days of therapy (DOT) as the primary study outcome [2, 3, 14, 15]. For each admission, DOT sums were calculated for total antibiotic use, and separately for each antibiotic class and spectrum of activity category. If a patient received 2 different antibiotics on the same service day, these events qualified as 2 DOT [2, 14, 15]. To provide standardized summary measures, values were aggregated across the cohort and reported as rates per 1000 PD and as dichotomized outcomes (percentage of encounters with antibiotic use, yes/no). To facilitate comparisons with prior studies, we also calculated hospital mean total usage rates (rather than a single crude rate across all facilities), and rates stratified by teaching status and patient age.
For statistical models, patient-level characteristics were transformed into hospital-level case-mix variables (eg, hospital mean patient age) and DOT and PD were summed for each hospital. Relationships between DOT and hospital, case-mix, and geographic variables were evaluated at the facility level in univariable negative binomial regression models. We used an offset equal to the natural log of PD per hospital and summarized results with incidence rate ratios (IRRs) and 95% confidence intervals (CIs). Variables with P values < .10 were evaluated in multivariable negative binomial models; biologically plausible interaction terms were retained if at least 1 strata’s P value was < .10 and at least 1 other strata’s P value was ≥ .10, or if effect estimates across strata differed by > 10% in models including only the main and interaction effects. All tests were 2-tailed, and P values ≤ .05 were used for statistical significance testing. Analyses were performed using SAS version 9.4 (SAS Institute) and Stata 15.0 (StataCorp).
RESULTS
During the 2-year study period spanning calendar years 2016 and 2017, there were 11 701 326 unique adult inpatient encounters, totaling 64 064 632 PD, across 576 US hospitals (Supplementary Figure 1). Hospital and patient characteristics are presented in Table 1 and Supplementary Table 1. Twenty-eight percent (28%) of hospitals were located in the South Atlantic US census division, followed by the East North Central (18%) and the Pacific (12%). Seventy-five percent (75%) of hospitals were urban, and 30% had academic teaching status (Table 1). Patients had a median age of 62 (IQR, 42–75) years, 59% were female, and the median length of hospital stay was 4 (IQR, 3–6) service days (Supplementary Table 1).
Table 1.
Description of Facility, Case-mix, and Geographic Characteristics Among Adult Inpatient Encounters in the Premier Healthcare Database, United States, 2016–2017
| Characteristic | Hospitals (N = 576) |
|---|---|
| Total No. of encounters | 11 701 326 |
| No. of encounters by year | |
| 2016 | 5 834 810 |
| 2017 | 5 866 516 |
| Total patient-days | 64 064 632 |
| Facility fixed characteristics | |
| Urbana | 432 (75) |
| Teaching | 170 (30) |
| Bed size | |
| 0–99 | 126 (22) |
| 100–199 | 143 (25) |
| 200–299 | 102 (18) |
| 300–399 | 82 (14) |
| 400–499 | 41 (7) |
| ≥ 500 | 82 (14) |
| Facility case-mix characteristics | |
| Patient-days, median (IQR) | 80 318 (33 301–157 535) |
| Average patient age, y, median (IQR) | 59 (57–63) |
| Percentage of patient-days in ICUs, median (IQR) | 11 (6–14) |
| Percentage of bacterial infection patient-days, median (IQR)b | 24 (21–27) |
| Average Elixhauser comorbidity index score, mean (SD)c | 3.2 (0.59) |
| Case-mix index, median (IQR) | 1.46 (1.32–1.64) |
| Facility geographical characteristics | |
| US census region and divisiond | |
| Northeast | |
| Mid-Atlantic | 63 (11) |
| New England | 13 (2) |
| South | |
| East South Central | 37 (6) |
| West South Central | 62 (11) |
| South Atlantic | 161 (28) |
| Midwest | |
| West North Central | 46 (8) |
| East North Central | 101 (18) |
| West | |
| Mountain | 25 (4) |
| Pacific | 68 (12) |
Data are presented as no. (%) unless otherwise indicated. Percentages for mutually exclusive subcategories may exceed 100% due to rounding.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; SD, standard deviation; US, United States.
aDesignation provided by Premier, based upon American Hospital Association Annual Survey response.
bAgency for Healthcare Research and Quality (AHRQ) infection diagnosis codes were used to ascertain whether a patient’s primary International Classification of Diseases, Tenth Revision diagnosis was infection-related (AHRQ, 2019). Inpatient-days for encounters with a primary infection diagnosis were treated as “bacterial infection” patient-days.
cElixhauser comorbidity classifications were modified to include primary diagnoses, in addition to secondary diagnoses. Patient Elixhauser scores were calculated as an unweighted sum (1 point per comorbidity), and average scores were calculated for each facility.
dUS census divisions comprise 4 US census regions: Northeast (Middle Atlantic, New England); South (South Atlantic, East South Central, West South Central); Midwest (East North Central, West North Central); and West (Mountain, Pacific). States in each US census division are as follows: New England Division: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont; Middle Atlantic Division: New Jersey, New York, Pennsylvania; East North Central Division: Illinois, Indiana, Michigan, Ohio, Wisconsin; West North Central Division: Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, South Dakota; South Atlantic Division: Delaware, District of Columbia, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia; East South Central Division: Alabama, Kentucky, Mississippi, Tennessee; West South Central Division: Arkansas, Louisiana, Oklahoma, Texas; Mountain Division: Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, Wyoming; Pacific Division: Alaska, California, Hawaii, Oregon, Washington.
Antibiotic Usage
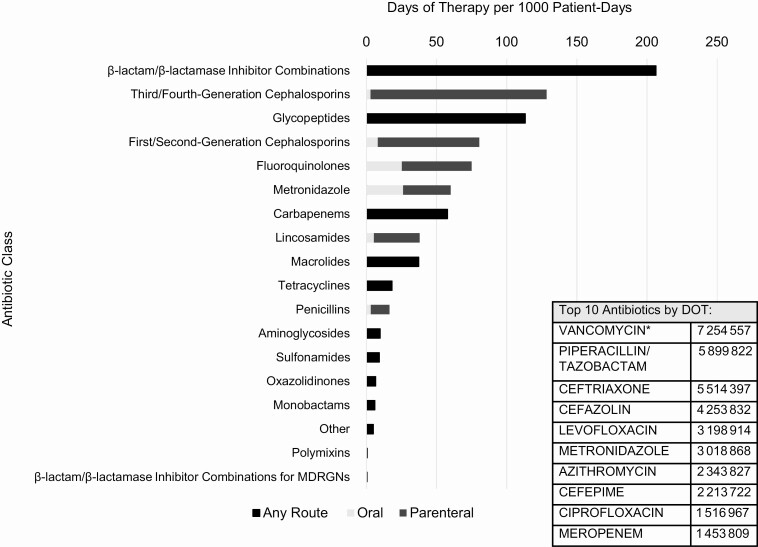
Overall, 65% of patients received at least 1 antibiotic while hospitalized (Table 2). The crude rate of total antibiotic use across all encounters was 870 DOT per 1000 PD. By antibiotic class, β-lactam/β-lactamase inhibitor combinations had the highest usage rate (206 DOT/1000 PD), driven predominantly by piperacillin-tazobactam administration (Figure 1). The remaining top 5 antibiotic classes by usage rate were third- and fourth-generation cephalosporins (128 DOT/1000 PD; 98% parenteral), glycopeptides (113 DOT/1000 PD; 99% of use attributable to vancomycin), first- and second-generation cephalosporins (81 DOT/1000 PD; 90% parenteral), and fluoroquinolones (75 DOT/1000 PD; 66% parenteral) (Figure 1 and Table 2). When dichotomizing use as present or absent per encounter, these antibiotic classes remained the most common, but their rankings changed (eg, first- and second-generation cephalosporins became the most common, used in 24% of all encounters) (Table 2).
Table 2.
Antibiotic Use Across 11 701 326 Adult Inpatient Encounters, United States, 2016–2017
| Antibiotic | DOT per 1000 PD | Percentage of Encounters With ≥ 1 DOT (N = 11 701 326) |
|---|---|---|
| Antibiotic class | ||
| All (total) | 869.5 | 64.9 |
| Aminoglycosides | 9.8 | 3.1 |
| β-lactam/β-lactamase inhibitor combinations | 206.4 | 14.5 |
| β-lactam/β-lactamase inhibitor combinations for MDRGNs | 0.5 | 0.03 |
| Carbapenems | 57.8 | 3.9 |
| First- and second-generation cephalosporins | 80.5 | 23.6 |
| Fluoroquinolones | 75.1 | 13.3 |
| Glycopeptides | 113.3 | 19.2 |
| Lincosamides | 38.0 | 4.1 |
| Macrolides | 37.3 | 6.9 |
| Metronidazole | 60.1 | 6.5 |
| Monobactams | 6.0 | 1.0 |
| Oxazolidinones | 6.7 | 0.8 |
| Penicillins | 16.5 | 4.1 |
| Polymyxins | 0.6 | 0.04 |
| Sulfonamides | 9.2 | 1.5 |
| Tetracyclines | 18.3 | 2.6 |
| Third- and fourth-generation cephalosporins | 128.5 | 19.7 |
| Other | 4.9 | 0.6 |
| Spectrum of activity category | ||
| Anti–Bacteroides fragilisa | 220.0 | 25.3 |
| Anti–Clostridioides difficilea | 23.2 | 2.9 |
| Anti-MRSAa | 161.0 | 23.9 |
| Anti–Pseudomonas sppa | 244.8 | 28.5 |
Abbreviations: DOT, days of therapy; MDRGN, multidrug-resistant gram-negative organism; MRSA, methicillin-resistant Staphylococcus aureus; PD, patient-days.
aSee Supplementary Appendix A for lists of agents in each spectrum of activity category.
Figure 1.
Inpatient antibiotic days of therapy (DOT) per 1000 patient-days, by antibiotic class and administration route for selected agents. *Vancomycin routes of administration, by DOT: parenteral, 6 555 702; oral, 627 706; miscellaneous, 71 149. “Miscellaneous” routes of administration could not be further delineated. Abbreviations: DOT, days of therapy; MDRGN, multidrug-resistant gram-negative organism.
We also categorized and measured antibiotic use by spectrum of activity. Agents with antipseudomonal activity had the highest usage (245 DOT/1000 PD and used in 29% of encounters), followed by agents active against B. fragilis (220 DOT/1000 PD and used in 25% of encounters), MRSA (161 DOT/1000 PD and used in 24% of encounters), and C. difficile (23 DOT/1000 PD and used in 3% of encounters) (Table 2).
Additional Metrics of Total Antibiotic Use to Facilitate Cross-study Comparisons
Stratifying by patient age, within categories of 18–44, 45–64, 65–84, and ≥ 85 years, rates of total antibiotic use were 682, 943, 913, and 889 DOT per 1000 PD, respectively. The hospital mean rate of total antibiotic use was 921 DOT/1000 PD (SD, 208). Stratifying by teaching status, the mean rate of total use in teaching and nonteaching hospitals was 834 (SD, 200) and 957 (SD, 200) DOT per 1000 PD, respectively (P < .001) (Supplementary Figure 2).
Relationship Between Antibiotic Use and Hospital, Case-mix, and Geographic Characteristics
Based upon the preceding results and clinical importance, we selected 7 antibiotic groups to evaluate for usage differences by hospital characteristics, including geographic and case-mix distributions: all antibiotics (total), β-lactam/β-lactamase inhibitor combinations, third- and fourth-generation cephalosporins, glycopeptides, carbapenems, antipseudomonal agents, and anti-MRSA agents. Evaluated variables are listed in Table 3. In univariable analyses, many characteristics were associated with antibiotic use across some or all antibiotic groups. At an α level of .10, teaching hospitals were associated with lower use across every antibiotic category (IRR range, 0.80–0.95). Only 1 variable, a hospital’s percentage of bacterial infection PD, was significantly associated with antibiotic use at an α level of P < .05 for all antibiotic groups (IRR range, where each unit increase represents a 10 percentage-point increase in bacterial infection patient-days, 1.26–1.50; all P values < .001). A facility’s percentage of ICU patient-days was not associated with total antibiotic use (IRR, 1.001 [95% CI, .998–1.003]; P = .51), but was associated with higher use of β-lactam/β-lactamase inhibitor combinations, glycopeptides, carbapenems, and antipseudomonal and anti-MRSA agents (IRR range, 1.004–1.011; all P values < .03). Unadjusted use also varied by geographic location and antibiotic category (Figure 2 and Supplementary Figure 3).
Table 3.
Association Between Hospital, Case-mix, and Geographic Characteristics and Antibiotic Days of Therapy in Adjusted Models
| Characteristic | Antibiotic Category (n = 576) | ||||||
|---|---|---|---|---|---|---|---|
| All | BL/BLIs | 3rd/4th-Generation Cephalosporins | Glycopeptides | Carbapenems | Anti-PSA Agents | Anti-MRSA Agents | |
| Facility characteristic | |||||||
| Teaching hospital | 0.99 (.95–1.02) | 0.97 (.89–1.06) | 0.92 (.85–.98)* | 0.98 (.92–1.04) | 1.03 (.90–1.17) | 0.91 (.86–.97)* | 1.04 (.99–1.09) |
| Urban | 0.99 (.96–1.03) | NA | 0.93 (.87–.99)* | NA | NA | 0.97 (.92–1.03) | NA |
| Bed sizea | 1.00 (.99–1.01) | NA | 0.99 (.97–1.02) | NA | NA | 1.03 (1.01–1.04)* | NA |
| US census division | |||||||
| East North Central | 0.98 (.94–1.03) | 0.96 (.86–1.06) | 1.01 (.93–1.10) | 1.03 (.96–1.10) | 1.03 (.88–1.21) | 0.98 (.92–1.05) | 0.95 (.90–1.01) |
| East South Central | 0.99 (.93–1.06) | 0.94 (.81–1.10) | 0.97 (.86–1.09) | 0.97 (.88–1.08) | 1.28 (1.02–1.62)* | 0.96 (.87–1.07) | 1.03 (.95–1.12) |
| Middle Atlantic | 0.82 (.77–.86)* | 0.74 (.65–.84)* | 0.93 (.84–1.03) | 0.95 (.87–1.03) | 0.81 (.67–.99)* | 0.82 (.75–.89)* | 0.82 (.76–.88)* |
| Mountain | 0.97 (.90–1.05) | 1.07 (.88–1.29) | 0.90 (.77–1.04) | 0.92 (.81–1.05) | 0.48 (.36–.64)* | 0.89 (.79–1.01) | 0.91 (.82–1.01) |
| New England | 0.85 (.77–.93)* | 0.68 (.54–.86)* | 1.10 (.91–1.32) | 0.96 (.82–1.13) | 0.41 (.29–.59)* | 0.69 (.59–.81)* | 0.92 (.80–1.05) |
| Pacific | 0.94 (.89–.98) | 0.86 (.77–.97)* | 1.06 (.97–1.16) | 0.91 (.83–.98)* | 0.80 (.67–.97)* | 0.82 (.76–.89)* | 0.90 (.84–.96)* |
| West North Central | 0.98 (.93–1.04) | 0.90 (.78–1.03) | 1.00 (.89–1.11) | 0.97 (.88–1.07) | 1.04 (.84–1.29) | 0.99 (.90–1.09) | 0.93 (.86–.99)* |
| West South Central | 1.04 (.99–1.10) | 0.98 (.86–1.10) | 0.98 (.89–1.08) | 0.99 (.91–1.07) | 1.64 (1.36–1.98)* | 1.07 (.98–1.16) | 0.99 (.92–1.06) |
| South Atlantic | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Percentage of PD with a bacterial infection diagnosisb | 1.33 (1.29–1.38)* | 1.47 (1.36–1.59)* | 1.49 (1.40–1.59)* | 1.45 (1.37–1.53)* | 1.66 (1.47–1.88)* | 1.51 (1.43–1.59)* | 1.36 (1.30–1.41)* |
| Percentage of PD in ICUs | NA | 1.009 (1.004–1.013)* | NA | 1.003 (1.000–1.007)* | 1.006 (.998–1.013) | 1.005 (1.002–1.008)* | 1.002 (.999–1.004) |
| Mean hospital patient age, yc | |||||||
| < 50 | 0.94 (.79–1.11) | 1.32 (.85–2.06) | 1.22 (.86–1.74) | 1.65 (1.21–2.24)* | 1.15 (.76–1.75) | 1.18 (.89–1.57) | 1.13 (.90–1.43) |
| 50 to < 60 | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 60 to < 70 | 1.00 (.97–1.04) | 0.96 (.88–1.04) | 0.95 (.89–1.01) | 0.97 (.92–1.03) | 0.95 (.84–1.07) | 1.00 (.94–1.05) | 0.96 (.91–1.00) |
| ≥ 70 | 0.95 (.79–1.16) | 0.97 (.62–1.53) | 0.80 (.55–1.17) | 0.87 (.63–1.20) | 0.88 (.60–1.29) | 1.06 (.78–1.43) | 0.94 (.72–1.22) |
| Age-stratified effectsd | |||||||
| Hospital mean Elixhauser comorbidity index scoree | |||||||
| < 50 | 0.85 (.75–.98)* | 1.24 (.87–1.76) | 1.18 (.91–1.53) | 1.52 (1.20–1.92)* | f | 0.91 (.73–1.14) | 0.99 (.83–1.18) |
| 50 to < 60 | 0.95 (.91–.99)* | 0.96 (.86–1.08) | 1.04 (.96–1.13) | 0.95 (.88–1.02) | f | 0.91 (.85–.98)* | 0.99 (.93–1.05) |
| 60 to < 70 | 0.96 (.92–1.01) | 0.92 (.82–1.03) | 1.12 (1.03–1.23)* | 1.00 (.92–1.08) | f | 0.98 (.91–1.06) | 1.02 (.96–1.08) |
| ≥ 70 | 0.92 (.73–1.17) | 0.69 (.40–1.20) | 1.39 (.87–2.21) | 0.87 (.58–1.31) | f | 0.80 (.55–1.17) | 0.75 (.54–1.05) |
| Hospital CMI | |||||||
| < 50 | 1.89 (1.47–2.42)* | 2.36 (1.25–4.45)* | 2.16 (1.41–3.31)* | 3.71 (2.45–5.62)* | 6.10 (2.60–14.31)* | 2.63 (1.77–3.93)* | 2.97 (2.13–4.14)* |
| 50 to < 60 | 1.12 (1.02–1.22)* | 0.95 (.74–1.21) | 1.32 (1.15–1.51)* | 1.72 (1.49–2.00)* | 1.58 (1.07–2.33)* | 1.25 (1.10–1.42)* | 1.34 (1.20–1.51)* |
| 60 to < 70 | 1.11 (1.01–1.23)* | 1.09 (.86–1.39) | 1.09 (.90–1.32) | 1.71 (1.45–2.02)* | 1.66 (1.15–2.42)* | 0.99 (.84–1.17) | 1.37 (1.21–1.56)* |
| ≥ 70 | 1.25 (1.01–1.56)* | 1.66 (.96–2.87) | 1.15 (.78–1.68) | 1.94 (1.35–2.80)* | 2.03 (.88–4.72) | 1.47 (1.02–2.10)* | 1.63 (1.23–2.17)* |
Data are presented as adjusted incidence rate ratio (95% confidence interval). Associations were evaluated using multivariable negative binomial regression models with an offset equal to the natural log of total patient-days per facility. Included variables varied by antibiotic outcome.
Abbreviations: anti-MRSA, agents with targeted activity against methicillin-resistant Staphylococcus aureus; anti-PSA, agents with targeted activity against Pseudomonas spp; BL/BLI, β-lactam/β-lactamase inhibitor combination; CMI, case-mix index; ICU, intensive care unit; NA, variable was not included in a given model because in unadjusted analysis its P value was ≥ .10; PD, patient-days; US, United States.
aBed size was coded ordinally, categories 1–6. Each 1-unit increase represents an additional 100 beds, up to ≥ 500 (reference ≤ 99 beds).
bAgency for Healthcare Research and Quality (AHRQ) bacterial infection diagnosis codes were used to ascertain whether a patient’s primary International Classification of Diseases, Tenth Revision diagnosis was infection-related (AHRQ, 2019). Inpatient-days for encounters with a primary infection diagnosis were treated as “bacterial infection” patient-days. Each adjusted incidence rate ratio (aIRR) represents the average effect of a 10 percentage point increase in a hospital’s percentage of bacterial infection patient-days, holding other factors constant.
cHospital mean patient age was stratified into 4 categories to increase interpretability of interactions by age (mean Elixhauser score and CMI). aIRR estimates for each age strata reflect the adjusted effect of age, relative to the reference category of 50 to < 60 years, at the cohort’s mean Elixhauser score and mean CMI values (3.2 and 1.49, respectively).
dBiologically plausible interactions were retained in final multivariable models if at least 1 age strata’s P value was < .10 and at least 1 other strata’s P value was ≥ .10, or if effect estimates across age strata differed by > 10% in analyses including only the main and interaction effects. Each aIRR estimate reflects the adjusted average effect of a 1-unit increase in a given variable (CMI or mean Elixhauser comorbidity index score) in hospitals with this average patient age, holding other factors constant.
eElixhauser comorbidity categories were modified to include primary diagnoses, in addition to secondary diagnoses. Elixhauser scores represent unweighted Elixhauser comorbidity sums (1 point per comorbidity). An average patient Elixhauser score was calculated for each hospital.
fCarbapenems were the sole outcome where unadjusted analyses did not support an interaction between mean Elixhauser score and average patient age; thus, mean Elixhauser score was not age-stratified in the final adjusted model for carbapenem use. The aIRR for mean Elixhauser score in the adjusted carbapenem model was 0.83 (95% confidence interval, .73–.94; P = .004).
*Significant at an α level of P < .05 (and bolded).
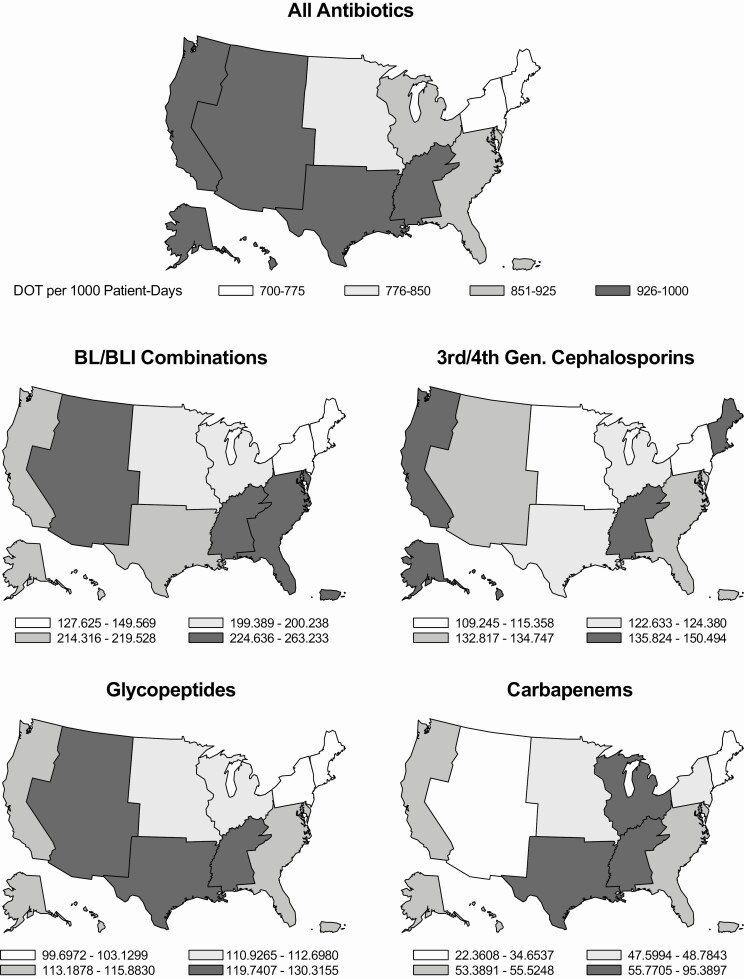
Figure 2.
Days of therapy per 1000 patient-days for selected antibiotic classes, by United States census division, 2016–2017. Abbreviations: BL/BLI, β-lactam/β-lactamase inhibitor; DOT, days of therapy.
Variables included in adjusted models varied by antibiotic outcome, based upon significance at a P value of < .10 in univariable analysis. In adjusted analyses, teaching status remained independently associated with lower use of third- and fourth-generation cephalosporins and antipseudomonal agents (adjusted IRRs, 0.91 [95% CI, .85–.98], P = .016 and 0.92 [95% CI, .86–.97], P = .002, respectively) (Table 3). For the other antibiotic outcomes, including total use, teaching status was not significant. Regional differences also persisted in adjusted models. Compared to the South Atlantic (chosen as the reference category because it had the largest representation in the cohort), rates of total antibiotic use were 6%, 15%, and 18% lower on average in the Pacific, New England, and the Middle Atlantic, respectively. Carbapenems reflected the most geographic variability, with significantly lower use in 4 divisions (Middle Atlantic, Mountain, New England, and the Pacific) and significantly higher use in the East South Central and the West South Central regions. Third- and fourth-generation cephalosporins reflected no significant geographic variability (Table 3).
DISCUSSION
To our knowledge, this is the largest study to date of US adult inpatient antibiotic use with respect to number of included facilities. Across 576 hospitals and nearly 12 million encounters, we found that antibiotics were used in 65% of adult hospitalizations, at a crude rate of 870 DOT for every 1000 PD. The most commonly used classes were β-lactam/β-lactamase inhibitor combinations, third- and fourth-generation cephalosporins, and glycopeptides. By spectrum of activity, the most commonly used antibiotics were antipseudomonal agents.
Total rates of antibiotic use in this 2016–2017 inpatient population were similar to some, but not all, previously published estimates. A 2011 survey of 70 academic hospitals (2009 data) found similar percentages of adult encounters receiving antibiotics, 63.7%, and a hospital mean usage rate of 840 DOT/1000 PD [3]. Our hospital mean rate was considerably higher—921 DOT/1000 PD—although when restricting to teaching hospitals the rates became similar (834 DOT/1000 PD). Another survey of approximately 300 hospitals by Baggs et al estimated a similar rate of antibiotic use in 2012 for patients aged 18–44 years, but its estimates for older patients were lower, particularly among those aged 45–64 years (850 vs 943 DOT per 1000 PD, respectively) [2]. Methodological differences limit direct comparisons, and differences in facility composition could explain some of these discrepancies. On balance though, because teaching hospitals were associated with lower use in both cohorts, our higher percentage of teaching hospitals (30% vs 23.2%) should have lowered our usage rates in comparison, not raised them. Thus, while we cannot conclusively determine that antibiotic use has increased since 2012, the data do not suggest that it has decreased—a finding paralleled in prior conclusions that US inpatient antibiotic use also did not significantly decrease between 2006 and 2012 [2].
Given well-publicized data that a high proportion of inpatient antibiotics are prescribed inappropriately [8, 9] and recent years’ increased emphasis on antibiotic stewardship [1, 10, 16], we were surprised that our total usage rate was similar to, and in some cases higher than, estimates from 5–8 years prior. Additional study results highlight at least 2 possible reasons for these findings. First, some evidence suggests that reductions in the use of certain agents are being offset by increases elsewhere. For example, the same study by Baggs et al found that fluoroquinolones were the most commonly used antibiotics in 2012, but their use had declined significantly since 2006, whereas gram-negative broad-spectrum agent use increased [2]. International settings, including England and Australia, have documented similarly stable or increased total antibiotic use despite reductions in certain classes such as fluoroquinolones, due to counterbalancing increases of other agents [17, 18]. Consistent with these trends, β-lactam/β-lactamase inhibitor combinations and third- and fourth-generation cephalosporins were the 2 highest-used classes in our 2016–2017 data, with fluoroquinolones dropping to fifth. The fluoroquinolone rate per 1000 PD (75 DOT) was also well below the 2012 estimate (117 DOT), even though the latter included pediatric patients [2]. Viewed in isolation, the reduction in fluoroquinolone use is encouraging [4, 19–23]. Globally, however, the relative increase in other broad-spectrum agents—and, at best, no apparent reduction in total antibiotic use—is concerning and underscores the importance of performing stewardship across all antibiotic classes, not just select broad-spectrum agents.
Second, it is possible that antibiotic stewardship programs (ASPs) are reducing total antibiotic use—but their uptake remains too limited across US facilities to drive national reductions. As noted previously, teaching hospitals averaged significantly less total antibiotic use (834 vs 957 DOT/1000 PD; P < .001). In adjusted analyses, teaching status also remained independently associated with lower use of third- and fourth-generation cephalosporins and antipseudomonal agents; nowhere was it associated with higher use. These findings are consistent with other studies [2, 24], and we hypothesize that teaching status may be a surrogate for well-established ASPs [10, 25–27], which are more common in teaching hospitals [28, 29]. As of 2015, however, only 48% of respondent US hospitals in an NHSN survey had implemented ASPs incorporating the “7 CDC Core Elements” [29]. Promisingly, ASP uptake is increasing [28–30], and CMS recently finalized regulations requiring ASPs in all acute care hospitals that participate in Medicare/Medicaid [31]. We hope that these regulatory changes will spur the development and refinement of ASPs, leading to further reductions in unnecessary antibiotic prescribing.
We documented significant regional differences in antibiotic use, even after controlling for many hospital and case-mix characteristics. For many antibiotic groups, adjusted usage rates were significantly lower in the Middle Atlantic, the Pacific, and New England, compared to the South Atlantic. For example, hospitals’ average use of antipseudomonal agents was 18%, 18%, and 31% lower in these respective divisions. The high use of antipseudomonal antibiotics in our study (245 DOT/1000 PD), coupled with intensifying concerns about multidrug-resistant Pseudomonas aeruginosa [32–35], make better understanding the reasons for these regional differences critical. We also compared antibiotic use to regional antimicrobial resistance using 2014 NHSN healthcare-acquired infection (HAI) data but only identified partial concordance. For example, while the East South Central had the highest proportion of MRSA isolates and the highest use of anti-MRSA agents, there were also notable discrepancies (eg, high use in the Mountain division despite low proportions of MRSA isolates) [36]. Interestingly, there was strong overlap between high inpatient usage regions and high outpatient usage regions using publicly available data from the same time period. In our cohort, by crude usage rate the East South Central and the West South Central regions were consistently in the “top 3,” including for total antibiotics, carbapenems, and antipseudomonal and anti-MRSA agents. Six of the 7 states with the highest rates of community antibiotic prescriptions in 2016 also fall in these regions [37]. These findings reinforce the importance of antibiotic stewardship at all points on the healthcare continuum, and further study may uncover shared reasons for high utilization in inpatient and outpatient settings.
Our study has several limitations. First, our study did not include pediatric patients. Continued exploration of antibiotic utilization in pediatric populations is an important area for future study. Second, because our cohort lacked microbiology data, we were unable to compare antibiotic usage and antimicrobial resistance directly. In the alternative, we cross-referenced usage to NHSN regional resistance data, but we recognize that causal inferences are limited by these ecologic comparisons. More directly correlating use and resistance would be important, including from other facility and non-HAI data sources. Third, although our database includes a large and diverse number of hospitals across the US, we did not explicitly weight our estimates to reflect national distributions, and some facility types may have been overrepresented. Nevertheless, our study population included nearly 25% of hospitals located in rural locations and/or possessing < 100 beds, and we reported stratified rates where informative (eg, by teaching status). Our adjusted models also controlled for many facility characteristics. Finally, our study used administrative claims data, which may have misclassified some information, although this is a recognized limitation of most similar surveys [2, 3, 38]. However, we would not expect any misclassification to be differential. Moreover, although a study of this size and geographic distribution would not have been feasible without a centralized repository of electronic claims data, these data did not include certain variables (eg, ASP presence, percentage of encounters with infectious disease consultations) that might further explain observed utilization differences. We hope that our research identifies important targets for follow-up study.
Overall, we found that in a large, diverse cohort of US hospitals, adult inpatients had high rates of antibiotic use, driven predominantly by broad-spectrum agents. Our results suggest that total antibiotic use has not decreased when compared to earlier studies. Teaching hospitals had lower rates of total antibiotic use, and lower use of third- and fourth-generation cephalosporins and antipseudomonal agents in adjusted models. Teaching status may be a surrogate for facilities that are more likely to have robust ASPs, but further testing of this hypothesis is needed. Even accounting for teaching status and many other facility characteristics, there remained significant regional differences in antibiotic use that geographic patterns of resistance do not appear to explain fully. Better understanding other reasons for these differences may inform efforts to optimize antibiotic use.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Premier for access to the database, discussion about data elements, and construction of the data pull/database that was used for the analysis, and for being contributors on the Agency for Healthcare Research and Quality (AHRQ) grant.
Financial support. This work was supported by funding from the AHRQ (grant number R01-HS026205 to A. D. H.).
Potential conflicts of interest. A. D. H. reports personal fees from Entasis and UpToDate, outside the submitted work. S. E. C. reports personal fees from Theravance, Basilea, and Novartis, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. The White House. National strategy for combating antibiotic-resistant bacteria.2014. Available at: https://www.cdc.gov/drugresistance/pdf/carb_national_strategy.pdf. Accessed 24 October 2019.
- 2. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176:1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53:1100–10. [DOI] [PubMed] [Google Scholar]
- 4. Tabak YP, Srinivasan A, Yu KC, et al. Hospital-level high-risk antibiotic use in relation to hospital-associated Clostridioides difficile infections: retrospective analysis of 2016–2017 data from US hospitals. Infect Control Hosp Epidemiol 2019; 40:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Premier Applied Sciences. Premier healthcare database: data that informs and performs.2018. Available at: https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf. Accessed 15 December 2019.
- 7. US Bureau of the Census. Map of the United States, showing census divisions and regions.1995. Available at: https://www.census.gov/prod/1/gen/95statab/preface.pdf. Accessed 15 December 2019.
- 8. Fridkin S, Baggs J, Fagan R, et al. Centers for Disease Control and Prevention . Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 9. Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003; 163:972–8. [DOI] [PubMed] [Google Scholar]
- 10. US Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs.2014. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed 15 December 2019.
- 11. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 12. Agency for Healthcare Research and Quality. Patient safety indicators: appendix F (infection diagnosis codes).2019. Available at: https://www.qualityindicators.ahrq.gov/Downloads/Modules/PSI/V2019/TechSpecs/PSI_Appendix_F.pdf. Accessed 15 December 2019.
- 13. Fridkin SK, Steward CD, Edwards JR, et al. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis 1999; 29:245–52. [DOI] [PubMed] [Google Scholar]
- 14. Kazakova SV, Baggs J, McDonald LC, et al. Association between antibiotic use and hospital-onset Clostridioides difficile infection in U.S. acute care hospitals, 2006–2012: an ecologic analysis. Clin Infect Dis 2020; 70:11–18. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Antimicrobial use and resistance (AUR) module.2020. Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/11pscAURcurrent.pdf. Accessed 15 December 2019.
- 16. Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84:673–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooke J, Stephens P, Ashiru-Oredope D, Johnson AP, Livermore DM, Sharland M; Antimicrobial Stewardship Sub-Group of the Department of Health’s Advisory Committee for Antimicrobial Resistance and Healthcare Associated Infection . Antibacterial usage in English NHS hospitals as part of a national antimicrobial stewardship programme. Public Health 2014; 128:693–7. [DOI] [PubMed] [Google Scholar]
- 18. Australian Commission on Safety and Quality in Health Care. AURA 2019: third Australian report on antimicrobial use and resistance in human health. 2019. Available at: https://www.safetyandquality.gov.au/sites/default/files/2019-06/AURA-2019-Report.pdf. Accessed 30 January 2020. [DOI] [PubMed]
- 19. Liu X, Ma J, Huang L, et al. Fluoroquinolones increase the risk of serious arrhythmias: a systematic review and meta-analysis. Medicine (Baltimore) 2017; 96:e8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Etminan M, Sodhi M, Ganjizadeh-Zavareh S, Carleton B, Kezouh A, Brophy JM. Oral fluoroquinolones and risk of mitral and aortic regurgitation. J Am Coll Cardiol 2019; 74:1444–50. [DOI] [PubMed] [Google Scholar]
- 21. Morales D, Pacurariu A, Slattery J, Pinheiro L, McGettigan P, Kurz X. Association between peripheral neuropathy and exposure to oral fluoroquinolone or amoxicillin-clavulanate therapy. JAMA Neurol 2019; 76:827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Appaneal HJ, Caffrey AR, Beganovic M, Avramovic S, LaPlante KL. Predictors of Clostridioides difficile recurrence across a national cohort of veterans in outpatient, acute, and long-term care settings. Am J Health Syst Pharm 2019; 76:581–90. [DOI] [PubMed] [Google Scholar]
- 23. Dingle KE, Didelot X, Quan TP, et al. Modernising Medical Microbiology Informatics Group . Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017; 17:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haug JB, Berild D, Walberg M, Reikvam Å. Hospital- and patient-related factors associated with differences in hospital antibiotic use: analysis of national surveillance results. Antimicrob Resist Infect Control 2014; 3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nathwani D, Varghese D, Stephens J, Ansari W, Martin S, Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control 2019; 8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nhan D, Lentz EJM, Steinberg M, Bell CM, Morris AM. Structure of antimicrobial stewardship programs in leading US hospitals: findings of a nationwide survey. Open Forum Infect Dis 2019; 6:ofz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stenehjem E, Hersh AL, Buckel WR, et al. Impact of implementing antibiotic stewardship programs in 15 small hospitals: a cluster-randomized intervention. Clin Infect Dis 2018; 67:525–32. [DOI] [PubMed] [Google Scholar]
- 28. Pollack LA, van Santen KL, Weiner LM, Dudeck MA, Edwards JR, Srinivasan A. Antibiotic stewardship programs in U.S. acute care hospitals: findings from the 2014 National Healthcare Safety Network annual hospital survey. Clin Infect Dis 2016; 63:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Leary EN, van Santen KL, Webb AK, Pollock DA, Edwards JR, Srinivasan A. Uptake of antibiotic stewardship programs in US acute care hospitals: findings from the 2015 National Healthcare Safety Network annual hospital survey. Clin Infect Dis 2017; 65:1748–50. [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention. Antibiotic resistance and patient safety portal: changes over time in hospital antibiotic stewardship.2019. Available at: https://arpsp.cdc.gov/profile/geography/united-states. Accessed 15 November 2019.
- 31. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; regulatory provisions to promote program efficiency, transparency, and burden reduction; fire safety requirements for certain dialysis facilities; hospital and Critical Access Hospital (CAH) changes to promote innovation, flexibility, and improvement in patient care. 2019. Available at: https://www.federalregister.gov/documents/2019/09/30/2019-20736/medicare-and-medicaid-programs-regulatory-provisions-to-promote-program-efficiency-transparency-and. Accessed 28 October 2019. [Google Scholar]
- 32. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019.2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 15 December 2019.
- 33. Khan A, Tran TT, Rios R, et al. Extensively drug-resistant pseudomonas aeruginosa st309 harboring tandem Guiana extended spectrum β-lactamase enzymes: a newly emerging threat in the United States. Open Forum Infect Dis 2019; 6. Available at: https://academic.oup.com/ofid/article/6/7/ofz273/5512661. Accessed 19 November 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nathwani D, Raman G, Sulham K, Gavaghan M, Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2014; 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliver A, Mulet X, López-Causapé C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat 2015; 21–22:41–59. [DOI] [PubMed] [Google Scholar]
- 36. US Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) antibiotic resistance: map view (2014). Available at: https://gis.cdc.gov/grasp/PSA/MapView.html. Accessed 15 December 2019.
- 37. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, 2016. 2018. Available at: https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2016.html. Accessed 15 December 2019.
- 38. Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007; 44:664–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.