Abstract
Background
Infections with hookworms affect about half a billion people worldwide. Recommended therapy includes 400 mg of albendazole, which is moderately efficacious. Higher doses have been rarely assessed.
Methods
A randomized, controlled dose-finding trial was conducted in Côte d’Ivoire with the aim of recruiting 120 preschool-aged children (PSAC), 200 school-aged children (SAC), and 200 adults. Eligible PSAC were randomized 1:1:1 to 200 mg, 400 mg, or 600 mg of albendazole; the other age groups were randomized 1:1:1:1:1 to placebo or 200 mg, 400 mg, 600 mg, or 800 mg. The primary outcome was cure rates (CRs) assessed 14–21 days post-treatment by quadruplicate Kato-Katz thick smears. Hyperbolic Emax models were used to determine dose-response.
Results
38 PSAC, 133 SAC, and 196 adults were enrolled. In adults, predicted CRs increased with ascending doses of albendazole, with a CR of 74.9% (95% confidence interval [CI], 55.6%–87.7%) in the 800-mg arm. Observed CRs increased with ascending doses of albendazole reaching a maximum of 94.1% (95% CI, 80.3%–99.3%). In SAC, the predicted dose-response curve increased marginally, with CRs ranging from 64.0% in the 200-mg arm to 76.0% in the 800-mg arm. Sample size in PSAC was considered too small to derive meaningful conclusions. 10.7% and 5.1% of participants reported any adverse event at 3 hours and 24 hours post-treatment, respectively.
Conclusions
A single 800-mg albendazole dose provides higher efficacy against hookworm and is well tolerated in adults and should be considered for community-based strategies targeting adults. For PSAC and SAC, current recommendations suffice.
Clinical Trials Registration
Keywords: hookworm, Côte d’Ivoire, albendazole, soil-transmitted helminthiasis, drug safety
A higher dose of albendazole in adults for the treatment of hookworm infection provides increased cure rates with high tolerability, whereas an increase in dose does not provide additional benefit in children.
Necator americanus or Ancylostoma duodenale, two types of hookworm, affect approximately 500 million individuals worldwide [1, 2]. As a soil-transmitted helminth (STH), hookworm is most common in tropical and subtropical settings where larvae are able to survive in moist, warm soil.
Hookworm accounted for approximately 845 000 disability-adjusted life-years lost in 2017, with an estimated economic loss of more than $100 billion [3, 4]. The hookworm can cause skin irritation when it enters through the skin, acute lung inflammation and difficulty breathing when larvae migrate through the pulmonary system, and gastrointestinal issues such as blood loss, diarrhea, and abdominal pain [5, 6]. Morbidity is highly correlated with infection intensity, and more serious infections can cause anemia from blood loss [3, 7].
Albendazole is one of two benzimidazoles currently on the World Health Organization (WHO) Model List of Essential Medicines as a anthelmintic against intestinal STHs [8]. Developed in 1975 and licensed less than a decade later for human use, albendazole is part of current global control strategies [9, 10]. The mainstay of control is based on targeted preventative chemotherapy (PC) in preschool-aged children (PSAC), school-aged children (SAC), and women of reproductive age (WRA) given annually/biannually using either 400 mg of albendazole or 500 mg of mebendazole [10]. In 2012, the WHO published targets for 75% of coverage with PC in PSAC and SAC in all endemic countries and regular treatment of about 600 million PSAC and SAC who are in need of treatment by 2020 [11]. In 2018, additional targets were developed with the aim of STH control by 2030, including achieving and maintaining elimination in PSAC and SAC and expanding control programs to WRA [12].
Though PC with benzimidazoles has been successfully used as a cornerstone of control programs, efficacy against hookworm remains moderate (cure rates [CRs] of 79.5% for 400 mg albendazole and 32.5% for 500 mg of mebendazole) [13]. Several studies have shown that multiple doses of albendazole over an increased number of days have higher efficacy; however, this strategy could prove difficult during mass drug administration (MDA) campaigns [14–18]. A single dose that is greater than 400 mg may be more efficacious and effective at curing hookworm infection, though an optimal dose has not been identified, and limited evidence on the efficacy and safety for PSAC and adults is available. The novel aim of this trial is to characterize the dose-response relationship for the efficacy and safety of albendazole against hookworm in PSAC, SAC, and adults.
METHODS
Study Design, Area, and Population
A phase 2, parallel, randomized, controlled trial was conducted in 21 villages surrounding the town of Rubino in southeastern Côte d’Ivoire from 5 January 2019 to 17 April 2019. PSAC aged 2–5 years, SAC aged 6–12 years, and adults aged ≥21 years were invited to participate in the trial.
Ethical Considerations
Ethical permission from the Comité National d’Éthique des Sciences de la Vie et de la Santé in Côte d’Ivoire was issued on 3 July 2018, while the Northwestern and Central Switzerland Ethics Committee provided ethical clearance on 20 July 2018. Written informed consent was obtained from adult participants and from the parents/guardians of participating children after they had attended a community-wide information meeting. Additionally, SAC provided written consent.
Eligibility Criteria
Participants were eligible for the trial if they were willing to provide informed consent and two stool samples. Only participants with hookworm infection intensity of ≥24 eggs per gram (EPG) of stool were included. Those with acute or uncontrolled systemic illness (eg, severe anemia defined as hemoglobin <8.0 g/dL, infection, clinical malaria with fever) as assessed by a medical doctor, those who had received anthelmintics within the previous four weeks, those with allergies to benzimidazoles, and women who were pregnant or breastfeeding were excluded.
Randomization and Treatment
A computer-generated, stratified block randomization code was generated by the trial statistician who was not involved in participation recruitment, treatment administration, or outcome assessment. The randomization list with varying blocks of three and six for PSAC or five and ten for SAC and adults was stratified by baseline hookworm infection intensity (either light or moderate/heavy) per WHO guidelines [19]. After a lengthy period of recruitment, it was decided to eliminate the placebo group for the PSAC cohort. PSAC were randomized 1:1:1 to albendazole at 200 mg, 400 mg, or 600 mg. SAC and adults were randomized 1:1:1:1:1 to albendazole at 200 mg, 400 mg, 600 mg, or 800 mg or placebo. Outcome assessors were blinded to treatment allocation. Participants were potentially aware of the treatment arm that they were assigned due to the different number of tablets provided by unblinded study investigators.
Field and Laboratory Procedures
After a census in each community was conducted, children and adults in the appropriate age groups were asked to provide two stool samples. From each sample, duplicate Kato-Katz thick smears (each 41.7 mg) were prepared and examined for hookworm, Trichuris trichiura, and Ascaris lumbricoides eggs by two independent, trained technicians using light microscopes [20]. A third technician conducted an independent quality control for 10% of the slides. Results were considered correct if no difference in presence/absence of each helminth or egg counts were ±10 eggs for counts ≤100 eggs or ±20% for counts >100 eggs (for each species) [21]. Slides with incorrect results were re-read until a consensus was met.
Participants were invited for a clinical examination where individuals were physically examined, questioned for clinical symptoms, and underwent a series of rapid tests for malaria and pregnancy. Additionally, individuals were asked to provide a venous blood sample to assess complete blood count and hepatic/renal function.
Active adverse event (AE) reporting was conducted 3 hours, 24 hours, and 14–21 days post-treatment. At 14–21 days post-treatment, enrolled participants were asked to provide two stool samples for quadruplicate Kato-Katz smears, which were collected and analyzed as described above. At the end of the trial, all individuals who provided any stool sample and remained positive for any STH infection were treated with 400 mg of albendazole per WHO recommendations [10].
Outcome Measures
CR against hookworm infection at 14–21 days post-treatment was the primary outcome. Secondary outcomes were egg reduction rates (ERRs) against hookworm, CRs and ERRs against other STHs, and drug safety.
Sample Size
Multiple simulations were conducted to justify the required sample size. Assumed CRs against hookworm for placebo and albendazole at 200 mg, 400 mg, 600 mg, and 800 mg were 2.5%, 30%, 50%, 70%, and 80%, respectively, while loss to follow-up was estimated at 10%. Simulations indicated that 40 participants per arm would be sufficient to predict the dose-response curve with a median precision, defined as one-half the length of the corresponding confidence band of approximately 10 percentage points [22].
Statistical Analyses
Data were collected on paper forms by trained personnel and entered twice into a database (Microsoft Access 2010, Wallisellen, Switzerland). Data were cross-checked using Beyond Compare 4 (Scooter Software, Madison, WI), and discrepancies were corrected using the original data. Analysis was performed using Stata, version 15 (StataCorp, College Station, TX) and R software, version 3.5.1 (www.r-project.org).
An analysis set of all randomized participants who provided any follow-up data was used to perform an available-case analysis. CRs were calculated as the percentage of egg-positive participants at baseline who become egg-negative post-treatment. EPG was calculated using the mean egg counts from the quadruplicate Kato-Katz thick smears and multiplying by a factor of 24. ERRs were calculated using geometric mean egg counts with the following formula:
The 95% confidence intervals (CIs) for ERR point estimates were calculated using a bootstrap resampling method with 5000 replicates. ERRs were not calculated for infections of T. trichiura and A. lumbricoides because too few infections were observed. To predict dose-response curves in terms of CRs and ERRs, hyperbolic Emax models were fitted using the R software DoseFinding package.
The number and proportion of AEs and participants reporting AEs were descriptively summarized before and after treatment.
RESULTS
Baseline Characteristics
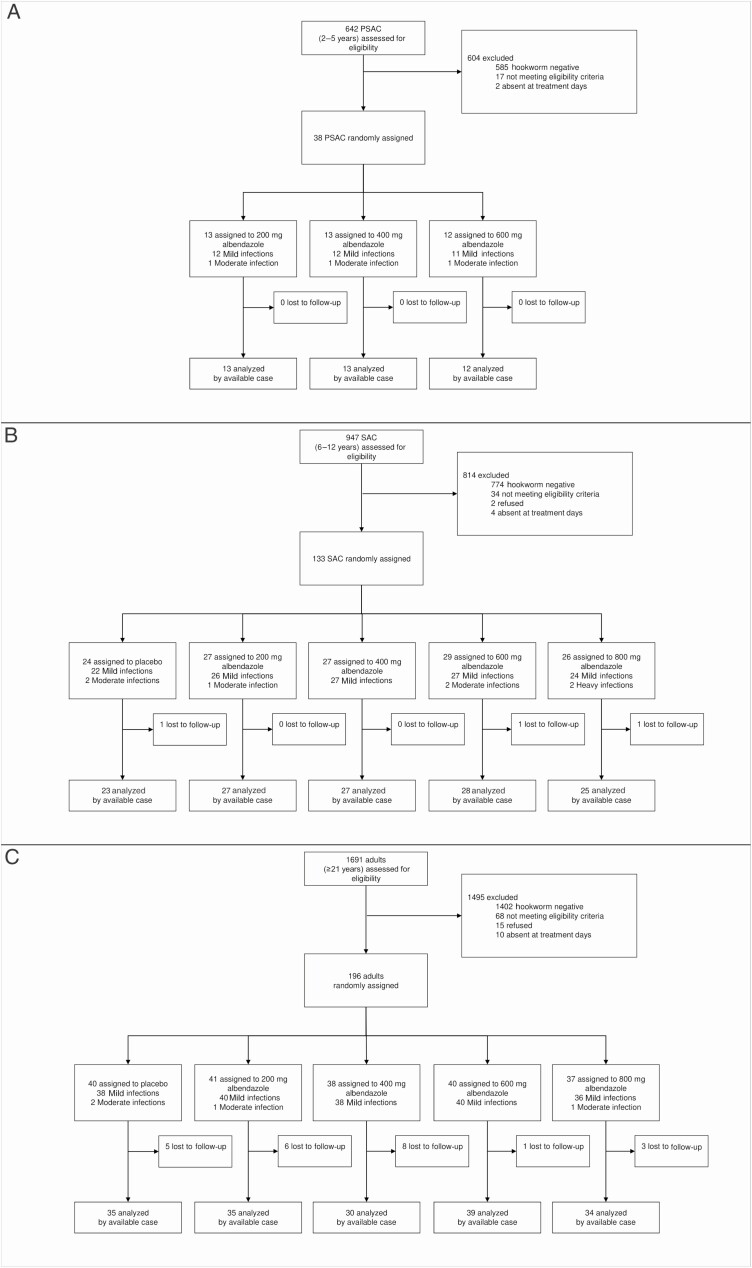
Figure 1 shows participant flow charts for each age cohort. A total of 642 PSAC, 947 SAC, and 1691 adults were screened for eligibility. Of those screened, 57 PSAC, 173 SAC, and 289 adults were positive for hookworm infection. Hence, despite exhaustive screening efforts, target sample sizes for PSAC (120) and SAC (200) were not reached. Based on eligibility criteria of ≥24 EPG, 50 PSAC, 164 SAC, and 256 adults were invited to participate in clinical examination. There were 12 PSAC, 31 SAC, and 60 adults who refused to participate, were excluded based on eligibility criteria on the day of treatment, or were absent.
Figure 1.
Trial flow charts. A, PSAC participant flow chart. B, SAC participant flow chart. C, Adults participant flow chart. Abbreviations: PSAC, preschool-aged children; SAC, preschool-aged children.
On treatment day, 38 PSAC, 133 SAC, and 196 adults were randomized to either three or five treatment arms (Figure 1). At 14–21 days post-treatment, 3 SAC (2.3%) and 23 adults (11.7%) did not provide any stool samples or were absent; all PSAC provided stool samples at follow-up.
Baseline demographic and parasitological characteristics of PSAC, SAC, and adults included are presented in Table 1. The proportions of females were lower in the 200-mg group in PSAC and higher in the 200-mg arm in SAC and adults. Other baseline characteristics were balanced. The majority of infections were of mild intensity (ranging from 92% to 100% across arms) in all three age cohorts; only 3 PSAC, 5 SAC, and 4 adults had moderate-intensity infections, and there were no high-intensity infections. Only 1 PSAC, 6 SAC, and 2 adults were coinfected with T. trichiura, and 2 PSAC, 3 SAC, and 1 adult were coinfected with A. lumbricoides.
Table 1.
Baseline Characteristics of Participants
| Preschool-aged Children | School-aged Children | Adults | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Albendazole, 200 mg (n = 13) | Albendazole, 400 mg (n = 13) | Albendazole, 600 mg (n = 12) | Placebo (n = 24) | Albendazole, 200 mg (n = 27) | Albendazole, 400 mg (n = 27) | Albendazole, 600 mg (n = 29) | Albendazole, 800 mg (n = 26) | Placebo (n = 40) | Albendazole, 200 mg (n = 41) | Albendazole, 400 mg (n = 38) | Albendazole, 600 mg (n = 40) | Albendazole, 800 mg (n = 37) |
| Age, mean (SD), y | 3.8 (1.2) | 3.8 (1.3) | 3.8 (1.1) | 9.1 (2.2) | 9.1 (1.9) | 9.8 (1.9) | 9.2 (2.2) | 9.4 (2.3) | 35.4 (10.3) | 39.8 (12.2) | 36.3 (12.2) | 38.2 (11.5) | 35.7 (12.0) |
| Females | 5 (38%) | 9 (69%) | 8 (67%) | 8 (33%) | 13 (48%) | 8 (30%) | 5 (17%) | 7 (27%) | 7 (18%) | 16 (39%)a | 7 (18%) | 7 (18%) | 9 (24%) |
| Weight, mean (SD), kg | 15.5 (3.6) | 13.8 (3.8) | 14.4 (2.9) | 26.0 (5.4) | 25.7 (5.5) | 27.7 (6.8) | 24.8 (7.0) | 27.9 (10.3) | 59.7 (9.9) | 61.3 (9.5) | 61.6 (9.3) | 62.7 (8.0)b | 59.7 (8.8) |
| Height, mean (SD), cm | 98.8 (12.3) | 98.1 (9.5) | 96.1 (9.5) | 132.6 (13.2) | 127.2 (12.7) | 129.8 (13.1) | 123.9 (18.2) | 137.5 (39.7) | 165.8 (10.7)a | 166.6 (10.7) | 168.1 (7.5) | 168.3 (10.0)a | 165.2 (9.3)a |
| Hookworm | |||||||||||||
| Median EPG (interquartile range) | 216 (150–618) | 126 (60–540) | 252 (111–423) | 183 (78–450) | 126 (72–336) | 138 (78–246) | 156 (84–528) | 153 (66–366) | 57 (96–318) | 126 (54–444) | 96 (54–264) | 189 (63–570) | 114 (84–216) |
| EPG geometric mean | 251.1 | 178.4 | 238.8 | 209.1 | 166.7 | 162.1 | 219.5 | 226.4 | 136.5 | 147.9 | 116.3 | 198.4 | 151.8 |
| Infection intensity | |||||||||||||
| Mild (1–1999 EPG) | 12 (92%) | 12 (92%) | 22 (92%) | 22 (92%) | 26 (96%) | 27 (100%) | 27 (93%) | 24 (92%) | 38 (95%) | 40 (98%) | 38 (100%) | 40 (100%) | 36 (97%) |
| Moderate (2000–3999 EPG) | 1 (8%) | 1 (8%) | 2 (8%) | 2 (8%) | 1 (4%) | 0 (0%) | 2 (7%) | 0 (0%) | 2 (5%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (3%) |
| Heavy (≥4000 EPG) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Trichuris trichiura | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 |
| Ascaris lumbricoides | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
Abbreviations: EPG, eggs per gram; SD, standard deviation.
aMissing data from 1 participant.
bMissing data from 2 participants.
Efficacy
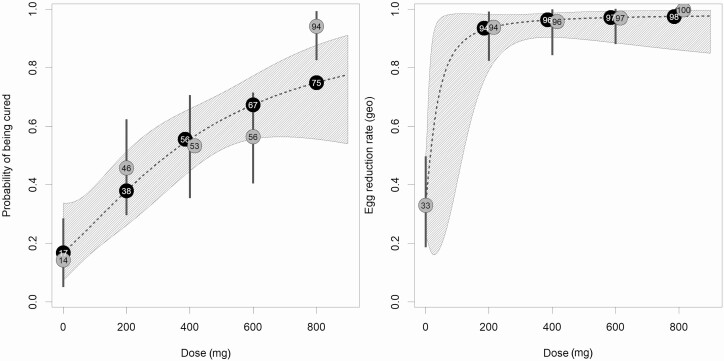
For adults, CRs and ERRs of each arm against hookworm infection are presented in Table 2; results for PSAC and SAC can be found in Supplementary Table 1. The Emax model predicted a maximum CR of 97.2% (placebo adjusted, 80.4%) and a half maximal-effect dose (ED50) on logit scale at 724 mg in the adult cohort (314 mg on probability scale). The dose-response curve showed no plateau within the observed dose range. The predicted CRs were 16.8% (95% CI, 7.4%–33.8%), 37.9% (95% CI, 26.2%–51.3%), 55.6% (95% CI, 44.7%–66.0%), 67.3% (95% CI, 55.6%–77.2%), and 74.9% (95% CI, 55.6%–87.7%) for placebo, 200 mg, 400 mg, 600 mg, and 800 mg, respectively (Figure 2). Likewise, observed CRs increased with ascending doses of albendazole and reached a maximum of 94.1% (95% CI, 80.3%–99.3%). In terms of ERRs, the Emax predicted ERR at the first investigated dose of 200 mg was 93.5% (95% CI, 79.1%–98.2%) and increased to 97.5% (95% CI, 86.0%–99.6%) in the 800-mg albendazole arm (Figure 2). Observed and predicted ERRs differed only slightly across all treatment arms.
Table 2.
Observed and Predicted Cure Rates and Egg Reduction Rates Against Hookworm of Adults at 3 Weeks Follow-up
| Result | Placebo | Albendazole, 200 mg | Albendazole, 400 mg | Albendazole, 600 mg | Albendazole, 800 mg |
|---|---|---|---|---|---|
| Positive before treatment | 35 | 35 | 30 | 39 | 34 |
| Cured after treatment | 5 | 16 | 16 | 22 | 32 |
| Observed CR (95% CI) | 14.3 (4.8 to 30.3) | 45.7 (28.8 to 63.4) | 53.3 (34.3 to 71.7) | 56.4 (39.6 to 72.2) | 94.1 (80.3 to 99.3) |
| Predicted CR (95% CI) | 16.8 (7.4 to 33.8) | 37.9 (26.2 to 51.3) | 55.6 (44.7 to 66.0) | 67.3 (55.6 to 77.2) | 74.9 (55.6 to 87.7) |
| EPG geometric mean | |||||
| Baseline | 135.9 | 132.3 | 118.6 | 204.1 | 139.6 |
| 3 weeks follow-up | 91.1 | 8.2 | 5.0 | 6.2 | 0.2 |
| Observed ERR (95% CI) | 33.0 (−27.1 to 67.3) | 93.8 (87.0 to 97.3) | 95.8 (90.2 to 98.3) | 97.0 (92.5 to 98.9) | 99.8 (99.5 to 100.0) |
| Predicted ERR (95% CI) | 33.0 (19.6 to 49.9) | 93.5 (79.1 to 98.2) | 96.4 (90.4 to 98.7) | 97.1 (88.4 to 99.3) | 97.5 (86.0 to 99.6) |
| EPG arithmetic mean | |||||
| Baseline | 348.3 | 325.0 | 242.0 | 414.9 | 275.6 |
| 3 weeks follow-up | 478.6 | 63.9 | 32.2 | 69.5 | 2.1 |
| Observed ERR (95% CI) | −37.4 (−137.1 to 44.6) | 80.3 (58.1 to 91.8) | 86.7 (75.3 to 94.0) | 83.3 (68.0 to 92.7) | 99.2 (97.6 to 100.0) |
Abbreviations: CI, confidence interval; CR, cure rate; EPG, eggs per gram; ERR, egg reduction rate.
Figure 2.
Cure rates (CRs) and egg reduction rates (ERRs) of albendazole in adults predicted by the hyperbolic Emax model. Dotted lines represent the dose-response curve, and the hatched area corresponds to the 95% confidence band. White numbers present the predicted CRs and ERRs for the investigated doses. Gray circles with black numbers represent the observed dose group CRs, and the gray vertical lines correspond to the 95% confidence intervals. Abbreviation: geo, geometric.
In SAC, observed CRs and ERRs against hookworm showed a less pronounced dose-response effect compared with adults. The increase in terms of CR after 400 mg was small. Observed CRs ranged from 63.0% (95% CI, 42.4%–80.6%) in the 200-mg arm to 76.0% (95% CI, 54.9%–90.6%) in the 800-mg arm. The observed CR in the placebo group was 43.5% (95% CI, 23.2%–65.5%). Geometric ERRs were high in all arms. The number of eligible PSAC was far below the anticipated sample size, and results are only descriptively analyzed. Observed CRs were 69.2%, 61.5%, and 83.3% and geometric mean–based ERRs were 99.0%, 96.2%, and 99.6% for 200 mg, 400 mg, and 600 mg of albendazole, respectively.
Supplementary Table 2 presents the proportions of participants cured of hookworm infection within each treatment arm by sex.
There were very few coinfections with T. trichiura and/or A. lumbricoides (9 and 5 participants, respectively). Only two participants remained coinfected with mild infection at three weeks post-treatment, and one participant with trichuriasis was lost to follow-up.
Safety
At baseline, 38 PSAC, 130 SAC, and 173 adults were questioned for symptoms. Among these, 34 participants (10.0%) reported a total of 43 mild symptoms at baseline, the majority of which were reported by adults. The most common reported symptoms at baseline were abdominal pain (9.2%), headache (6.4%), and itching (4.7%) in adults.
At three hours post-treatment, 34 PSAC, 118 SAC, and 137 adults were interviewed. From these, 2 PSAC, 11 SAC, and 18 adults reported at least one AE (10.7%). The most common AEs reported at 3 hours follow-up were abdominal pain (10.1% of SAC and 5.8% of adults) and headache (10.0% in adults, 5.9% in PSAC, and 2.2% in SAC).
A total of 28 PSAC, 89 SAC, and 120 adults were interviewed at 24 hours follow-up; 12 reported experiencing any AE (5.1%). Abdominal pain was the most common reported AE at the 24-hour examination, reported by 2.2% of SAC and 5.0% of adults.
The number of AEs reported and number of participants reporting any AE by each treatment arm can be found in Table 3. A more detailed report of symptom-specific AEs can be found in Supplementary Table 3.
Table 3.
Number of Adverse Events and Number of Participants Reporting Adverse Events
| Preschool-aged Children | School-aged Children | Adults | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | Number of | Albendazole, 200 mg | Albendazole, 400 mg | Albendazole, 600 mg | Total | Placebo | Albendazole, 200 mg | Albendazole, 400 mg | Albendazole, 600 mg | Albendazole, 800 mg | Total | Placebo | Albendazole, 200 mg | Albendazole, 400 mg | Albendazole, 600 mg | Albendazole, 800 mg | Total |
| Before treatment | Symptoms | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 3 | 6 | 10 | 11 | 5 | 7 | 39 |
| Participants | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 3 | 5 | 8 | 8 | 4 | 5 | 30 | |
| 3 hours after treatment | Adverse events | 1 | 0 | 1 | 2 | 2 | 3 | 2 | 1 | 3 | 11 | 2 | 4 | 5 | 4 | 5 | 20 |
| Participants | 1 | 0 | 1 | 2 | 2 | 3 | 2 | 1 | 3 | 11 | 2 | 4 | 5 | 3 | 4 | 18 | |
| 24 hours after treatment | Adverse events | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 3 | 1 | 2 | 2 | 5 | 1 | 11 |
| Participants | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 3 | 1 | 2 | 2 | 3 | 1 | 9 | |
DISCUSSION
Currently, there are several drugs approved by the WHO for the treatment/control of STH infections, though albendazole and mebendazole are the most common due to their ease of administration (single dose regardless of weight) and low cost [8, 23]. Albendazole, being more efficacious than mebendazole against hookworm, is a cornerstone of PC programs in both moderate/high and low STH transmission settings; however, the standard dose of 400 mg varies in efficacy [13]. Factors such as infection intensity, coinfection with other STHs, and species type have all been proposed as determinants of the variability of albendazole efficacy [13, 15, 23–25].
The results of this trial confirm that at a recommended 400-mg dose, albendazole is moderately efficacious against hookworm infection in PSAC, SAC, and adults (CRs of 61.5%, 74.1%, and 53.3%, respectively). In a recent systematic review, a comparable CR of 400 mg of albendazole against hookworm was found to be 70.5% (95% CI, 54.5%–82.6%) in trials conducted between 2000 and 2016 [13]. A published review of the literature until 1999 showed that efficacy of a single dose of 400 mg of albendazole was 77.7%, specifically 91.8% against A. duodenale and 75.0% against N. americanus [15]. In the same review, the efficacy of 200 mg of albendazole was found to be significantly lower (70.0%) in older children and adults, which is clearly confirmed in this trial for adults [15].
Remarkably, a higher dose of albendazole has increased efficacy, with a single dose of 800 mg of albendazole having a CR of 94.1% in adults. In PSAC and SAC, efficacy of albendazole remained moderate in all intervention arms; moreover, a slight increase in efficacy was seen in PSAC with ascending doses, though results should be interpreted carefully due to the small number of PSAC in each arm. No well-controlled trials were found specifically for PSAC or SAC; however, CRs for 200 mg, 600 mg, and 800 mg that were reported in a systematic review were within the ranges reported in this trial for the respective doses [15].
The greatest limitation of this trial was the poor recruitment of children. Only 24% and 67% of needed PSAC and SAC, respectively, were recruited into the trial. Hence, as mentioned before, it was difficult to identify the optimal dose of albendazole in these cohorts. The generalizability of the results are also limited to settings where infection intensity and coinfection with other STHs are low; evidence suggests that albendazole’s efficacy is affected by these factors [13, 23]. Since the diagnostic method used did not differentiate between the two common hookworm species, efficacy results may not be applicable to settings where A. duodenale is the predominant species, as N. americanus is most prevalent in sub-Saharan Africa [6, 16, 26, 27].
Current recommendations of a single dose of a benzimidazole administered annually or biannually to SAC may not be enough to eliminate helminthiasis. Recent models have shown that PC that targets only SAC will not eliminate hookworm infections and that to interrupt hookworm transmission, community-based PC that also targets adults who are the main reservoir of hookworm transmission, which was confirmed in this trial, is needed [25, 28, 29]. Community-based MDA was shown to reduce the infection rate of hookworm by 91% in a study carried out from 2012 to 2013 in the Republic of Congo [30]. A recent clinical trial conducted in Kenya showed community-based treatment strategies to be more effective than school-based treatment in reducing hookworm prevalence; moreover, the costs were equitable [31]. Currently, a series of trials are being conducted in one Asian and two African settings to evaluate the feasibility of hookworm and other STH transmission interruption with community-based MDA [32].
Community-based MDA aligns with the 2030 targets of the NTD Roadmap, which plans to expand control programs to WRA, who are at risk of iron-deficiency anemia [12, 23]. Evidence from this trial can have a direct impact on the program strategies for the control and elimination of hookworm infection. Given the remarkable higher efficacy observed, increasing the dose of albendazole for adults targeted by community-based MDA may aid in the interruption of transmission.
The estimated cost of one albendazole tablet is $0.018 with an additional 10% for overhead costs [33]. Recent estimates have shown that the average per-person cost of annual treatment at the community level is $0.68, which could be reduced during scale-up to $0.33 if coverage remains high [31]. These estimates are within the reported range of costs ($0.33–$0.70) for treating an infected individual reported by the WHO in 2017 [10]. Integrating an additional albendazole tablet into program strategies for treatment of adults would not drive costs outside of current ranges, though more evidence on the cost-effectiveness of two tablets of 400 mg albendazole for adults is needed.
In conclusion, this trial provides direct evidence on the optimal dose of albendazole for treatment of hookworm infection in low-transmission settings. Though sample sizes in SAC and PSAC were lower than anticipated, our findings support use of the currently recommended albendazole doses since increasing doses revealed no benefit in these age groups. However, we observed a considerably higher efficacy with administration of 800 mg of albendazole in the adult cohort. Strategies to integrate an 800-mg dose of albendazole to adults during community-based MDA should therefore be explored.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. P., J. T. C., J. H., and J. K. designed the study. C. P., J. T. C., D. H., and J. K. performed the study. C. P., J. H., and J. K. analyzed and interpreted the data. C. P. and J. K. wrote the initial draft of the manuscript. J. T. C., D. H., and J. H. revised, read, and approved the final version of the manuscript.
Acknowledgments. The authors thank all participating children and adults from the 21 villages. Additional thanks to the field workers and the field/laboratory teams from the Centre Suisse de Recherches Scientifiques in Abidjan, Côte d’Ivoire, for their exemplary conduct in this trial.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant OPP1153928).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet 2018; 391:252–65. [DOI] [PubMed] [Google Scholar]
- 2. Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 2014; 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis 2016; 10:e0004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1859–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crompton DW. The public health importance of hookworm disease. Parasitology 2000; 121 Suppl:S39–50. [DOI] [PubMed] [Google Scholar]
- 6. Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med 2004; 351:799–807. [DOI] [PubMed] [Google Scholar]
- 7. Brooker S, Akhwale W, Pullan R, et al. Epidemiology of plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg 2007; 77:88–98. [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. WHO model list of essential medicines for children (21st list). Geneva: World Health Organization, 2019. [Google Scholar]
- 9. Dayan AD. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop 2003; 86:141–59. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. Geneva, Switzerland: World Health Organization, 2017. [PubMed] [Google Scholar]
- 11. World Health Organization. Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 12. World Health Organization. 2030 targets for soil-transmitted helminthiases control programmes. Geneva, Switzerland: World Health Organization, 2020. [Google Scholar]
- 13. Moser W, Schindler C, Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ 2017; 358:j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adegnika AA, Zinsou JF, Issifou S, et al. Randomized, controlled, assessor-blind clinical trial to assess the efficacy of single- versus repeated-dose albendazole to treat Ascaris lumbricoides, Trichuris trichiura, and hookworm infection. Antimicrob Agents Chemother 2014; 58:2535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology 2000; 121 Suppl:S113–32. [DOI] [PubMed] [Google Scholar]
- 16. Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers 2016; 2:16088. [DOI] [PubMed] [Google Scholar]
- 17. Steinmann P, Utzinger J, Du ZW, et al. Efficacy of single-dose and triple-dose albendazole and mebendazole against soil-transmitted helminths and Taenia spp.: a randomized controlled trial. PLoS One 2011; 6:e25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yap P, Du ZW, Wu FW, et al. Rapid re-infection with soil-transmitted helminths after triple-dose albendazole treatment of school-aged children in Yunnan, People’s Republic of China. Am J Trop Med Hyg 2013; 89:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Guidelines for evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Vitamin and Mineral Nutrition Information System. Geneva, Switzerland: World Health Organization, 1998. [Google Scholar]
- 20. Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 1972; 14:397–400. [PubMed] [Google Scholar]
- 21. Speich B, Ali SM, Ame SM, Albonico M, Utzinger J, Keiser J. Quality control in the diagnosis of Trichuris trichiura and Ascaris lumbricoides using the Kato-Katz technique: experience from three randomised controlled trials. Parasit Vectors 2015; 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klingenberg B. Proof of concept and dose estimation with binary responses under model uncertainty. Stat Med 2009; 28:274–92. [DOI] [PubMed] [Google Scholar]
- 23. Hotez PJ. “The Unholy Trinity”: the soil-transmitted helminth infections ascariasis, trichuriasis, and hookworm infection. In: Hotez PJ. Forgotten people forgotten diseases. Washington, DC: ASM Press, 2014:17–40. [Google Scholar]
- 24. Bennett A, Guyatt H. Reducing intestinal nematode infection: efficacy of albendazole and mebendazole. Parasitol Today 2000; 16:71–4. [DOI] [PubMed] [Google Scholar]
- 25. Haldeman MS, Nolan MS, Ng’habi KRN. Human hookworm infection: is effective control possible? A review of hookworm control efforts and future directions. Acta Trop 2020; 201:105214. [DOI] [PubMed] [Google Scholar]
- 26. Coulibaly JT, Hiroshige N, N’Gbesso YK, Hattendorf J, Keiser J. Efficacy and safety of ascending dosages of tribendimidine against hookworm infections in children: a randomized controlled trial. Clin Infect Dis 2018; 69:845–52. [DOI] [PubMed] [Google Scholar]
- 27. Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Buss P. Hookworm: “the great infection of mankind.” PLoS Med 2005; 2:e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Truscott JE, Turner HC, Farrell SH, Anderson RM. Soil-transmitted helminths: mathematical models of transmission, the impact of mass drug administration and transmission elimination criteria. Adv Parasitol 2016; 94:133–98. [DOI] [PubMed] [Google Scholar]
- 29. Turner HC, Truscott JE, Bettis AA, et al. An economic evaluation of expanding hookworm control strategies to target the whole community. Parasit Vectors 2015; 8:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pion SD, Chesnais CB, Bopda J, et al. The impact of two semiannual treatments with albendazole alone on lymphatic filariasis and soil-transmitted helminth infections: a community-based study in the Republic of Congo. Am J Trop Med Hyg 2015; 92:959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pullan RL, Halliday KE, Oswald WE, et al. Effects, equity, and cost of school-based and community-wide treatment strategies for soil-transmitted helminths in Kenya: a cluster-randomised controlled trial. Lancet 2019; 393:2039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ásbjörnsdóttir KH, Ajjampur SSR, Anderson RM, et al. ; DeWorm3 Trials Team . Assessing the feasibility of interrupting the transmission of soil-transmitted helminths through mass drug administration: the DeWorm3 cluster randomized trial protocol. PLoS Negl Trop Dis 2018; 12:e0006166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montresor A, Gabrielli AF, Diarra A, Engels D. Estimation of the cost of large-scale school deworming programmes with benzimidazoles. Trans R Soc Trop Med Hyg 2010; 104:129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.