Abstract
Slit ventricle syndrome (SVS) is a well-known complication of long-standing shunts. Patients develop intermittent severe headache, vomiting with other symptoms of increased intra-cranial pressure. Brain computed tomography (CT) usually reveals slit-like ventricles with nearly obstructed proximal catheters. Treatment for SVS usually involves upgrading the shunt valve pressure setting. Currently, many patients carry programmable shunts and pressure setting can be adjusted noninvasively. However, when the programmable valve pressure setting is upgraded, some patients with SVS experience worsened symptoms. This is caused by the time gap between ICP increase and real ventricular expansion (and freeing proximal catheter) after shunt upgrading. Therefore, it is important to control a patient’s symptoms during the transition period. We report our experience in controlling ICP in a patient with SVS using external ventricular drainage.
INTRODUCTION
A ventriculoperitoneal shunt (VPS) is a common surgical treatment for infantile hydrocephalus. However, patients who undergo VPS in early childhood often face various complications in their lifetimes. Among the various complications, overdrainage of cerebrospinal fluid (CSF) can produce various symptoms, frequently with slit-like ventricles on computed tomography (CT). In 1982, Rekate et al. defined this syndrome as a slit ventricle syndrome (SVS) and posited the following symptom triad: a headache lasting for 10–90 min, small and slit ventricles in imaging studies and a slow refilling of the valve [1]. While a large proportion of patients with VPS show slit ventricles after a long time, only a small number of patients exhibit SVS symptoms [2]. No treatment is required when only small ventricles are seen without symptoms, but when symptoms appear, patients habitually visit the emergency room (ER). Common treatment of SVS involves upgrading shunt pressure setting to increase the opening pressure of the shunt [3, 4]. However, some patients cannot endure the short transition period for upgrading shunt pressure levels. This situation is quite challenging because conventional treatment for SVS cannot be instigated.
We present a patient with SVS where alternative method was used to overcome transition problems, and we discuss the usefulness of this method according to pathophysiology.
CASE REPORT
A 15-year-old female had a history of communicating hydrocephalus diagnosed at 5 months of age. She underwent VPS surgery (STRATA® programmable valve Medtronic, Dublin, Ireland) at 6 months of age. The initial valve level was set at 1.0. After surgery, her head circumference was kept within normal ranges. Two years later, follow-up brain CT showed a collapsed ventricle, but she had no symptoms. After 7 years, the ventricle was still collapsed, and the shunt pressure setting was upgraded gradually to 1.5. In the 10th year after VP shunt insertion, she developed headache and vomiting. Brain CT showed collapsed ventricles. After mannitol administration, her headache was improved. A shunt function test with 99mTc-DTPA revealed good patency of shunt system.
Fifteen years after shunt surgery, she began to visit ER with headache and vomiting. Her shunt pressure setting was upgraded in every visit but the pressure adjustment did not improve her symptoms, and the pressure was lowered from 2.5 to 1.5. Two months later, she returned to the ER with headache. Brain CT still showed slit-like ventricles with more collapsed right ventricle where the proximal catheter was located (Fig. 1A). Her headache did not improve with analgesics or mannitol administration. We tried to upgrade pressure levels to 2.0, but multiple attempts failed because she could not endure headache aggravation whenever the pressure level was upgraded. The CT scan taken after the pressure level upgrade to 2.0 showed no change in the collapsed right lateral ventricle (Fig. 1B). To overcome the resistance of stiff ventricular walls, an external ventricular drain (EVD) was inserted into the left lateral ventricle for temporary relief of ICP, with simultaneous upgrading of shunt pressure level to 2.5. The EVD opening pressure was high (22 mmHg). She had intermittent headache, but her symptom decreased gradually for 2 days after EVD insertion. On the 3rd day, follow-up brain CT showed a slightly expanded right lateral ventricle, and the patient stopped complaining of headache (Fig. 1C and D). The EVD was maintained for 2 more days and removed (Fig. 2). The patient was discharged while maintaining the shunt pressure level at 2.5. She is still doing well without headache after 15 months.
Figure 1 .
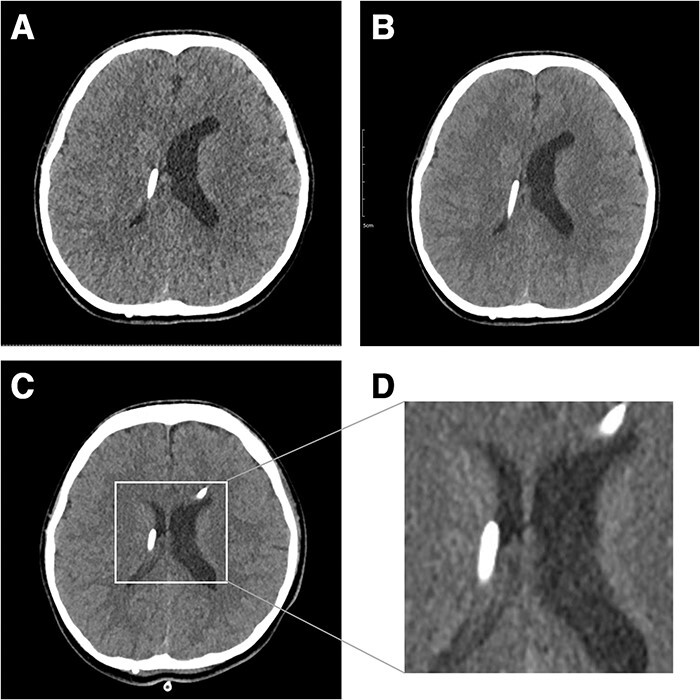
Fifteen years after VP shunt brain CT shows slit-like ventricle. In particular, the right ventricle collapsed where the proximal catheter was located (A). After a shunt pressure upgrade with mannitol administration, the patient’s symptoms worsened, and brain CT showed a collapsed ventricle (B). Three days after EVD insertion, the right ventricle was enlarged (C). Magnified image of enlarged ventricle (D).
Figure 2 .
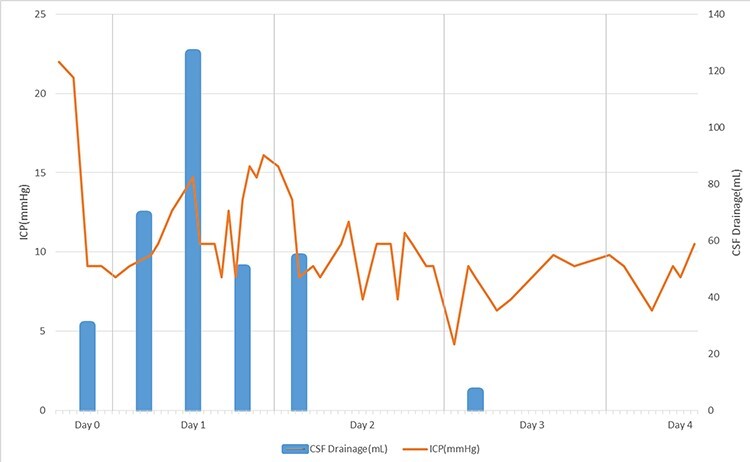
Changes of ICP after EVD insertion with the shunt upgrade (EVD setting is 22 mmHg, shunt valve upgrade 2.0–2.5). Changes in ICP have been shown to increase and decrease and gradually normalize. After one day of EVD insertion, CSF drainage also decreased with decreasing average ICP. On the second day, the patient’s symptom improved.
DISCUSSION
SVS is not a single condition but a heterogeneous syndrome caused by several different mechanisms. Rekate defined five separate subtypes of SVS by monitoring ICP in patients with headache and small ventricles [5]. The prototype of SVS (corresponding to type 2 SVS in Rekate’s classification) is caused by intermittent proximal catheter obstruction by collapsed ventricular walls due to chronic overdrainage. With proximal obstruction, severe headache develop and persist until proximal catheter holes are reopened with slight ventriclular expansion. This process indicates that the pressure level of the valve is inadequate, and Rekate proposed exchanging the valve to a higher pressure level and incorporating an anti-siphon device [6]. Currently, with the widespread adoption of programmable shunt valves, upgrading the valve pressure is a standard management for patients with SVS. However, there are patients whose SVS symptoms cannot be resolved only by upgrading valve pressure level because they cannot endure severe headache caused by the sudden rise of ICP. In this situation, ventricular volume change does not follow pressure level upgrading simultaneously. Two hypotheses are suggested. First, according to the stiff ventricle theory, ventricular wall stiffness is caused by the formation of gliotic scar tissue following chronic CSF drainage which can interfere with expansion of the ventricle [7]. Another conceivable hypothesis is the theory of venous congestion and increased cerebral elastance. When ICP changes, brain volume is controlled by adjusting its fluid contents, which are closely related to cerebral venous outflow capacity. Venous distension occurs during cerebral hypotension due to shunt overdrainage, and when ICP increases, the draining vein collapses, and venous congestion worsens; then, the brain becomes rigid and incompressible [8]. Intermittent excessive pressure change in a ventricle caused by shunt overdrainage can lead to secondary reduction of brain compliance. As a result of increased brain stiffness, patients become very vulnerable to ICP change [4].
A shunt upgrade can increase ventricle size, but patient’s tolerance is important in the process and alleviation of ICP can help the transition period. EVD has advantages of monitoring ICP and controlling ICP by CSF drainage. In our patient, ICP increased intermittently and CSF drainage through EVD controlled the symptoms. Depending on the patient’s symptoms, an appropriate EVD setting can mitigate the symptoms that can occur after a shunt upgrade. EVD catheter insertion into a slit-like ventricle may be challenging but neuro-navigation system can definitely aid surgeons for the procedure.
CONCLUSION
For symptomatic SVS, a shunt upgrade is considered therapeutic. It is important to successfully control ICP during the transition period for shunt upgrading. Temporary CSF drainage can aid to controlling ICP in this clinical situation.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare that are relevant to the content of this article.
FUNDING
This study was supported by a research fund from the Seoul National University College of Medicine (to Phi JH; No. 800-20200460).
Contributor Information
Sang-Youl Yoon, Department of Neurosurgery, Kyungpook National University Chilgok Hospital, Kyungpook National University School of Medicine, Daegu, Republic of Korea.
Seung-Ki Kim, Division of Pediatric Neurosurgery, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea.
Ji Hoon Phi, Division of Pediatric Neurosurgery, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea.
References
- 1. Hyde-Rowan MD, Rekate HL, Nulsen FE. Reexpansion of previously collapsed ventricles: The slit ventricle syndrome. J Neurosurg 1982;56:536–9. [DOI] [PubMed] [Google Scholar]
- 2. Walker ML, Fried A, Petronio J. Diagnosis and treatment of the slit ventricle syndrome. Neurosurg Clin N Am 1993;4:707–14. [PubMed] [Google Scholar]
- 3. Olson S. The problematic slit ventricle syndrome: a review of the literature and proposed algorithm for treatment. Pediatr Neurosurg 2004;40:264–9. [DOI] [PubMed] [Google Scholar]
- 4. Chernov MF, Kamikawa S, Yamane F, Ishihara S, Hori T. Neurofiberscope-guided management of slit-ventricle syndrome due to shunt placement. J Neurosurg 2005;102:260–7. [DOI] [PubMed] [Google Scholar]
- 5. Rekate HL. Classification of slit-ventricle syndromes using intracranial pressure monitoring. Pediatr Neurosurg 1993;19:15–20. [DOI] [PubMed] [Google Scholar]
- 6. Rekate HL. Shunt-related headaches: the slit ventricle syndromes. Childs Nerv Syst 2008;24:423–30. [DOI] [PubMed] [Google Scholar]
- 7. Ros B, Iglesias S, Martín Á, Carrasco A, Ibáñez G, Arráez MA. Shunt overdrainage syndrome: review of the literature. Neurosurg Rev 2018;41:969–81. [DOI] [PubMed] [Google Scholar]
- 8. Benzel EC, Reeves JD, Kesterson L, Hadden TA. Slit ventricle syndrome in children: clinical presentation and treatment. Acta Neurochir 1992;117:7–14. [DOI] [PubMed] [Google Scholar]


