This editorial refers to ‘Impact of sex on the management and outcome of aortic stenosis patients’, by D. Bienjonetti-Boudreau et al. doi:10.1093/eurheartj/ehab242.
Over the past decades, it has become clear that aortic stenosis (AS) represents a disease condition that differs in pathophysiology, presentation, and response to treatment between women and men. Even after accounting for body size, women need less valvular calcium than men to develop severe AS,1 entailing sex-specific thresholds for computed tomography-derived aortic valve calcification used to identify severe AS.2 Additionally, women with AS display more valvular fibrosis, denser connective tissue, and more concentric hypertrophy for a similar stenosis severity than men.3 Aortic valve replacement (AVR) is the only treatment option for patients with symptomatic severe AS, but sex-specific data on outcomes remain inconclusive. At the present time, it appears that women display a higher operative and long-term mortality, longer post-operative stay, and a higher stroke rate4 , 5 after surgical AVR (SAVR) potentially attributable to the more advanced stage of the disease at referral. In transcatheter AVR (TAVR) cohorts, women exhibit a higher incidence of vascular and bleeding complications with a trend towards a survival benefit over men after TAVR.6 , 7 These findings and data from a meta-analysis8 support the notion that TAVR appears to be the preferred intervention over SAVR in women when AVR is required—a hypothesis that is currently being tested in the RHEIA trial (NCT04160130). In this multicentre all-comers trial, female patients with severe symptomatic AS eligible for both AVR strategies based on a Heart Team decision are randomized 1:1 to receive TAVR or SAVR. Taken together, the overall impact of sex on the course and outcomes of AS is still under investigation.
In this issue of the European Heart Journal, Bienjonetti-Boudreau and colleagues focused on sex-specific differences in AS and their impact on clinical presentation and management.9 The authors studied a large cohort of 3632 consecutive patients with at least mild to moderate AS and distinguished four haemodynamic patterns according to indexed aortic valve area (AVAi) and mean transvalvular gradient (i.e. non-severe AS, severe AS, discordant high-gradient AS, and discordant low-gradient AS). After inverse propensity weighting, female sex could be linked to increased mortality and less referral to aortic valve intervention for the entire study population. This association was primarily driven by excess mortality observed in patients with discordant low-gradient AS defined as an AVAi ≤0.6 cm2/m2 (≤0.55 cm2/m2 for patients with body mass index ≥30 kg/m2) and a mean transvalvular gradient <40 mmHg. Women with discordant low-gradient AS who underwent initial medical management were at higher risk of mortality than their male counterparts, whereas no significant sex differences were observed in patients with discordant low-gradient AS who underwent aortic valve intervention.
Who are those female patients with ‘discordant low-gradient AS’?
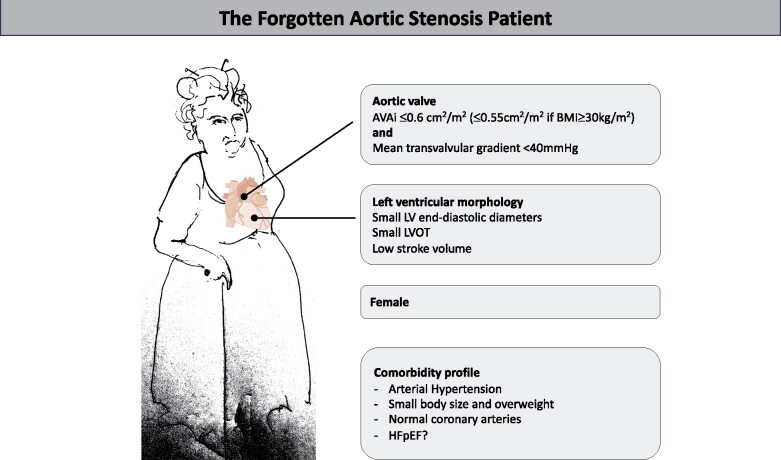
We have summarized the typical profile of these patients in the Graphical Abstract. Up to 50% of all patients with severe AS are known to have low-gradient AS, which is either: classical low-flow low-gradient AS (LF-LG AS) and related to significant left ventricular dysfunction and low left ventricular ejection fraction (LVEF); paradoxical LF-LG AS, where LVEF is preserved but flow is reduced; or normal-flow low-gradient (NF-LG) AS, where AVA is small combined with a low gradient, but normal flow. Because reduced LVEF was a priori excluded in the present study and aortic valve calcium scoring was not available, one might expect that a large proportion of those patients with discordant low-gradient AS may suffer from paradoxical LF-LG or NF-LG severe AS. As presented in table 3 of Bienjonetti-Boudreau et al., echocardiographically estimated stroke volume indices were low in the presence of a normal LVEF, and somewhat more reduced in females than in males (34.65 ± 6.76 vs. 35.42 ± 6.7 mL/m2). Because AS is a disease of both the aortic valve and left ventricle,10 a closer look has to be taken at the left ventricle of these patients. While cardiac amyloidosis does not seem to show a higher prevalence in females,11 other pathophysiological mechanisms may play a role. In a previous study by the same authors, it was shown that female sex is independently associated with a larger extracellular volume fraction and non-infarct pattern of late gadolinium enhancement, regardless of AS severity.12 In females with AS, the left ventricles are characterized by advanced diastolic dysfunction with higher E/eʹ, and diastolic heart failure or heart failure with preserved ejection fraction is more common in elderly female patients, possibly due to a higher prevalence of arterial hypertension.
Graphical Abstract.
Typical profile of a contemporary aortic stenosis patient with excess mortality.
Why were women with discordant low-gradient AS referred less for AVR?
Women with LF-LG AS in TOPAS had similar outcomes to men in the medically managed subset but markedly higher mortality in the subset of patients undergoing AVR (hazard ratio: 1.82; 95% confidence interval 1.08–3.13; P = 0.0248).13 Did these data impact treatment decisions? Of course, information is missing on whether more females rejected AVR as they were older and potentially frailer. Information is also missing on the degree of valvular calcification that helps determine the likelihood of severe AS in patients with discordant low-gradient AS.2
In summary, we should be alerted by those female patients with low-gradient AS depicted in the Graphical Abstract, and ensure that (i) severe AS is not missed because of lower computed tomography calcium thresholds and (ii) a decision for medical therapy is fully justified, for example by patient choice or frailty. Still, in a large consecutive series of patients from a world-leading centre of valvular heart disease care, female patients with AS had higher mortality. This is paradoxical because while the proportion of persons who survive to age 90 years has been increasing over the study period in both sexes, women have been living longer than men in every Westernized birth cohort.14
Conflict of interest: G.G. has no conflicts to declare. I.M.L. has relationships with drug companies including AOPOrphan Pharmaceuticals AG, Actelion-Janssen, MSD, United Therapeutics, Medtronic, Neutrolis, and Ferrer. In addition to being an investigator in trials involving these companies, relationships include consultancy service, research grants, and membership of scientific advisory boards.
Contributor Information
Georg Goliasch, Department of Internal Medicine II, Cardiology, Medical University of Vienna, Austria.
Irene M Lang, Department of Internal Medicine II, Cardiology, Medical University of Vienna, Austria.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Aggarwal SR, Clavel MA, Messika-Zeitoun D, Cueff C, Malouf J, Araoz PA, Mankad R, Michelena H, Vahanian A, Enriquez-Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging 2013;6:40–47. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 3. Fleury MA, Clavel MA. Sex and race differences in the pathophysiology, diagnosis, treatment, and outcomes of valvular heart diseases. Can J Cardiol 2021;doi: 10.1016/j.cjca.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 4. Barreto-Filho JA, Wang Y, Dodson JA, Desai MM, Sugeng L, Geirsson A, Krumholz HM. Trends in aortic valve replacement for elderly patients in the United States, 1999–2011. JAMA 2013;310:2078–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82–90. [DOI] [PubMed] [Google Scholar]
- 6. Kodali S, Williams MR, Doshi D, Hahn RT, Humphries KH, Nkomo VT, Cohen DJ, Douglas PS, Mack M, Xu K, Svensson L, Thourani VH, Tuzcu EM, Weissman NJ, Leon M, Kirtane AJ. Sex-specific differences at presentation and outcomes among patients undergoing transcatheter aortic valve replacement: a cohort study. Ann Intern Med 2016;164:377–384. [DOI] [PubMed] [Google Scholar]
- 7. Humphries KH, Toggweiler S, Rodés-Cabau J, Nombela-Franco L, Dumont E, Wood DA, Willson AB, Binder RK, Freeman M, Lee MK, Gao M, Izadnegahdar M, Ye J, Cheung A, Webb JG. Sex differences in mortality after transcatheter aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol 2012;60:882–886. [DOI] [PubMed] [Google Scholar]
- 8. Siontis GC, Praz F, Pilgrim T, Mavridis D, Verma S, Salanti G, Søndergaard L, Jüni P, Windecker S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J 2016;37:3503–3512. [DOI] [PubMed] [Google Scholar]
- 9. Bienjonetti-Boudreau D, Fleury MA, Voisine M, Paquin A, Chouinard I, Tailleur M, Duval R, Magnan PO, Beaudoin J, Salaun E, Clavel MA. Impact of sex on the management and outcome of aortic stenosis patients. Eur Heart J 2021;42:doi: 10.1093/eurheartj/ehab242. [DOI] [PubMed] [Google Scholar]
- 10. Ito S, Miranda WR, Nkomo VT, Connolly HM, Pislaru SV, Greason KL, Pellikka PA, Lewis BR, Oh JK. Reduced left ventricular ejection fraction in patients with aortic stenosis. J Am Coll Cardiol 2018;71:1313–1321. [DOI] [PubMed] [Google Scholar]
- 11. Nitsche C, Scully PR, Patel KP, Kammerlander AA, Koschutnik M, Dona C, Wollenweber T, Ahmed N, Thornton GD, Kelion AD, Sabharwal N, Newton JD, Ozkor M, Kennon S, Mullen M, Lloyd G, Fontana M, Hawkins PN, Pugliese F, Menezes LJ, Moon JC, Mascherbauer J, Treibel TA. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol 2021;77:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tastet L, Kwiecinski J, Pibarot P, Capoulade R, Everett RJ, Newby DE, Shen M, Guzzetti E, Arsenault M, Bédard É, Larose É, Beaudoin J, Dweck M, Clavel MA. Sex-related differences in the extent of myocardial fibrosis in patients with aortic valve stenosis. JACC Cardiovasc Imaging 2020;13:699–711. [DOI] [PubMed] [Google Scholar]
- 13. Bartko PE, Clavel MA, Annabi MS, Dahou A, Ristl R, Goliasch G, Baumgartner H, Hengstenberg C, Cavalcante JL, Burwash I, Enriquez-Sarano M, Bergler-Klein J, Rodes-Cabau J, Pibarot P, Mascherbauer J. Sex-related differences in low-gradient, low-ejection fraction aortic stenosis: results from the multicenter TOPAS Study. JACC Cardiovasc Imaging 2019;12:203–205. [DOI] [PubMed] [Google Scholar]
- 14. Newman AB, Murabito JM. The epidemiology of longevity and exceptional survival. Epidemiol Rev 2013;35:181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]