Abstract
Aims
Contemporary 2nd-generation thin-strut drug-eluting stents (DES) are considered standard of care for revascularization of patients undergoing percutaneous coronary intervention. A previous meta-analysis of 10 randomized controlled trials (RCTs) with 11 658 patients demonstrated a 16% reduction in the 1-year risk of target lesion failure (TLF) with ultrathin-strut DES compared with conventional 2nd-generation thin-strut DES. Whether this benefit is sustained longer term is not known, and newer trial data may inform these relative outcomes. We therefore sought to perform an updated systematic review and meta-analysis of RCTs comparing clinical outcomes with ultrathin-strut DES (≤70 µm strut thickness) with conventional 2nd-generation thin-strut DES.
Methods and results
We performed a random-effects meta-analysis of all RCTs comparing ultrathin-strut DES to conventional 2nd-generation thin-strut DES. The pre-specified primary endpoint was long-term TLF, a composite of cardiac death, myocardial infarction (MI), or clinically driven target lesion revascularization (CD-TLR). Secondary endpoints included the components of TLF, stent thrombosis (ST), and all-cause death. There were 16 eligible trials in which 20 701 patients were randomized. The weighted mean follow-up duration was 2.5 years. Ultrathin-strut DES were associated with a 15% reduction in long-term TLF compared with conventional 2nd-generation thin-strut DES [relative risk (RR) 0.85, 95% confidence interval (CI) 0.76–0.96, P = 0.008] driven by a 25% reduction in CD-TLR (RR 0.75, 95% CI 0.62–0.92, P = 0.005). There were no significant differences between stent types in the risks of MI, ST, cardiac death, or all-cause mortality.
Conclusions
At a mean follow-up of 2.5 years, ultrathin-strut DES reduced the risk of TLF, driven by less CD-TLR compared with conventional 2nd-generation thin-strut DES, with similar risks of MI, ST, cardiac death, and all-cause mortality.
Keywords: Coronary artery disease, Drug-eluting stents, Meta-analysis, Percutaneous coronary intervention, Ultrathin-strut
Graphical Abstract
Summary of pooled estimates for key clinical endpoints at latest follow-up. Results from a random-effects meta-analysis of 16 trials in which 20 701 patients were randomized to ultrathin-strut drug-eluting stents (≤70 µm strut thickness) compared with conventional 2nd-generation thin-strut drug-eluting stents. The weighted mean follow-up duration was 2.5 years.
Introduction
Contemporary 2nd-generation drug-eluting stents (DES) are considered standard of care for revascularization of patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) and have improved safety and effectiveness compared with 1st-generation DES platforms.1 , 2 These clinical advances have arisen from optimization of anti-proliferative agents,3 the use of more biocompatible polymers,4 and a reduction in stent strut thickness with use of more malleable metal alloys.5 Despite these improvements, conventional 2nd-generation thin-strut DES are not event-free and remain associated with an ongoing risk of adverse clinical events beyond the 1st year of implantation.6
Ultrathin-strut DES (≤70 μm) with biodegradable polymers were developed to further improve outcomes after PCI by reducing vessel injury and late polymer-induced inflammation and promoting more rapid endothelization. A previous meta-analysis demonstrated that ultrathin-strut DES were associated with a significant reduction in the 1-year risk of target lesion failure (TLF) compared with conventional 2nd-generation thin-strut DES.7 However, since this report, longer-term follow-up of prior studies has been reported and additional relevant trials have been completed. We therefore performed an updated systematic review and meta-analysis of randomized controlled trials (RCTs) comparing clinical outcomes between ultrathin-strut and conventional 2nd-generation thin-strut DES.
Methods
This analysis was prospectively registered at the PROSPERO international prospective register of systematic reviews (CRD42020220738) and was conducted in accordance with published guidance.8
Search strategy
We performed a systematic search of the MEDLINE, Cochrane Central Register of Controlled Trials, and Embase databases from December 2010 through March 2021 for all RCTs comparing ultrathin-strut DES to conventional 2nd-generation thin-strut DES for the treatment of CAD. Our search strings are shown in the Supplementary material online, Appendix Table S1. We manually searched the bibliographies of selected studies and meta-analyses to identify further eligible studies. Abstracts were reviewed for suitability, and articles were accordingly retrieved. Conference abstracts were also searched for relevant studies. Two independent authors performed the search and literature screening (Y.A. and A.N.), with disputes resolved by consensus following discussion with a 3rd author (M.V.M.).
Inclusion criteria
Only RCTs were included. Trials were eligible if they reported clinical outcome data following randomization to ultrathin-strut DES vs. conventional 2nd-generation thin-strut DES with all forms of CAD. Ultrathin-strut stents were defined as those with strut thickness ≤70 μm. Conventional 2nd-generation thin-strut DES were defined as all DES with strut thickness >70 μm, excluding 1st-generation Cypher and Taxus DES.
Endpoints
The pre-specified primary endpoint was TLF, defined as a composite of cardiac death, target-vessel myocardial infarction (TV-MI) or clinically driven target lesion revascularization (CD-TLR), at the latest follow-up reported. The TLF composite was only assessed if it was reported; i.e. if the composite TLF rate was not provided in a study, summing of its individual components to provide a value for TLF was not performed. Secondary pre-specified endpoints included target vessel failure [TVF; the composite of cardiac death, TV-MI or clinically driven target vessel revascularization (CD-TVR)], the individual components of TLF and TVF, as well as all myocardial infarction (MI), definite/probable and definite stent thrombosis (ST) by Academic Research Consortium criteria,9 any revascularization, all-cause mortality, and non-cardiac death. If not specifically reported, non-cardiac death was calculated as the difference between all-cause mortality and cardiac death. The pre-specified definitions of TLF and TVF used in each trial are summarized in Supplementary material online, Appendix Table S2. In some cases, there were slight deviations from the standard TLF and TVF definitions, in which case the trial-specific definition was used.
Data extraction and analysis
Two authors (Y.A. and A.N.) independently abstracted the data from included trials in duplicate, verified by a 3rd author (M.V.M.). Included studies were assessed using the Cochrane Risk of Bias tool.10 Publication bias was assessed using a funnel plot.
All outcomes were assessed by intention-to-treat. Random-effects meta-analyses were performed using the restricted maximum likelihood estimator. All outcomes were assessed as relative risks (RRs) at the time of latest follow-up available for each trial. Additional analyses were performed to assess early events (≤1 year) and late events (>1 year) whenever such data were available. We used the I 2 statistic to assess heterogeneity.11 Sensitivity analyses were performed with a fixed-effect model, using hazard ratios (HRs) as the outcome measure when reported. We performed additional sensitivity analyses using incidence rate ratios (IRRs) as the outcome measure, and a further sensitivity analysis looking only at trials included in the prior 2018 meta-analysis. We also performed a Jackknife sensitivity analysis excluding each trial in turn for the primary endpoint. We performed sensitivity analyses looking at the types of ultrathin-strut and control stents. Pre-specified subgroup analyses for the primary endpoint were performed according to age, sex, diabetes, chronic kidney disease, presentation with acute coronary syndromes or ST-elevation MI, small vessels, long lesions, in-stent restenosis lesions, and multivessel disease. Interactions between subgroups were assessed with meta-regression using a mixed-effects model, with the subgroup characteristic as a moderator and the individual trial as a random effect. A moderating effect of the length of follow-up was assessed using a mixed-effects meta-analytical model with a random effect for each individual study, as well as tests for interaction between results at 1 year and beyond 1 year. We also performed regression tests for the type of stent used in both the ultrathin DES and control DES arms, the anti-proliferative drug used in the control DES arm, and the delta strut thickness between the two arms.
Mean values are expressed as mean ± SD unless otherwise stated. Statistical significance was set at P < 0.05. P-values are two-tailed and were not adjusted for multiplicity. The statistical programming environment R12 with the metaphor package13 was used for all statistical analyses.
Results
Sixteen trials14–41 randomizing 20 701 patients were eligible for inclusion in this meta-analysis (Supplementary material online, Appendix Figure S1); 10 884 patients were randomized to ultrathin-strut DES and 9817 to conventional 2nd-generation thin-strut DES. The weighted mean follow-up duration across all trials was 31.0 months. The longest follow-up duration was 5 years in three trials, 3 years in six trials, 2 years in three trials, 1 year in three trials, and 9 months in one trial. The ultrathin stents studied included Orsiro (12 trials), MiStent (2 trials), BioMime (1 trial), and Supraflex (1 trial). Control stents in these trials were Xience (10 trials), Resolute (3 trials), Nobori (1 trial), BioFreedom (1 trial), and Endeavor (1 trial). The characteristics of each of these stents are listed in Supplementary material online, Appendix Table S3.
The characteristics of the included trials are summarized in Supplementary material online, Appendix Table S4 and the risk of bias is shown in the Supplementary material online, Appendix Table S5. There was no evidence of publication bias (Supplementary material online, Appendix Figures S2–S6).
Target lesion failure
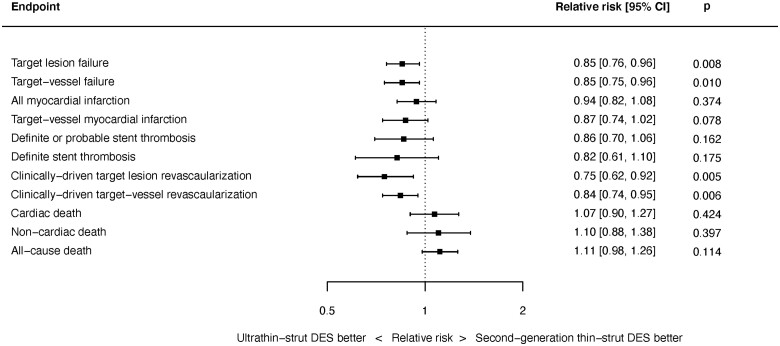
Target lesion failure outcomes were available from 14 studies with 20 115 randomized patients. As shown in Figure 1, at latest follow-up ultrathin-strut DES reduced the risk of TLF compared with conventional 2nd-generation thin-strut DES [RR 0.85, 95% confidence interval (CI) 0.76–0.96, P = 0.008]. There was mild heterogeneity present between studies (I 2 = 27.1%). Reduced risks of early (≤1 year) events (RR 0.84, 95% CI 0.74–0.95, P = 0.005, I 2 = 0.0%) as well as late (>1 year) events (RR 0.86, 95% CI 0.76–0.98, P = 0.019, I 2 = 32.9%) with ultrathin-strut DES compared with conventional 2nd-generation thin-strut DES were present (Supplementary material online, Appendix Figures S7 and S8). The RRs between the stent types for TLF were consistent before and after 1 year (P interaction = 0.501).
Figure 1.
Risk of target lesion failure at latest follow-up.
Target vessel failure
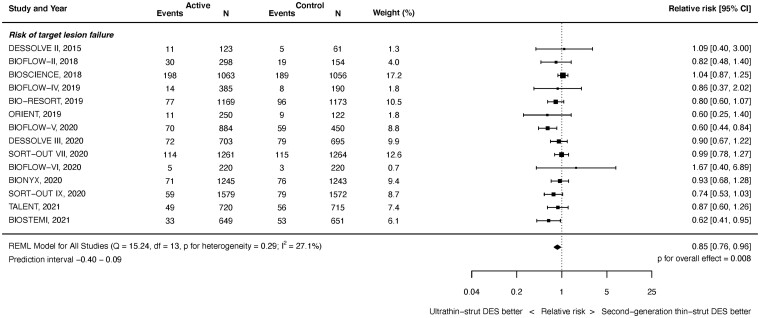
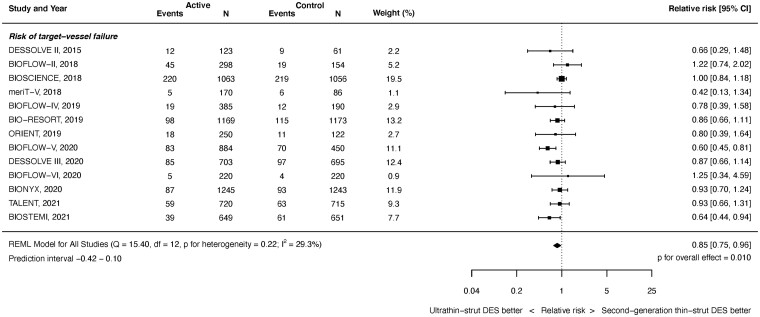
Target vessel failure outcomes were available from 13 studies with 14 695 randomized patients. As shown in Figure 2, at latest follow-up ultrathin-strut DES were associated with a reduced risk of TVF compared with conventional 2nd-generation thin-strut DES (RR 0.85, 95% CI 0.75–0.96, P = 0.010). There was moderate heterogeneity (I 2 = 29.3%). There was a reduced risk of early events (RR 0.88, 95% CI 0.77–1.00, P = 0.045, I 2 = 0.0%) and later events (RR 0.85, 95% CI 0.75–0.97, P = 0.017, I 2 = 36.1%) (Supplementary material online, Appendix Figures S9 and S10). The RRs between the stent types for TVF were consistent before and after 1 year (P interaction = 0.893).
Figure 2.
Risk of target vessel failure at latest follow-up.
Myocardial infarction
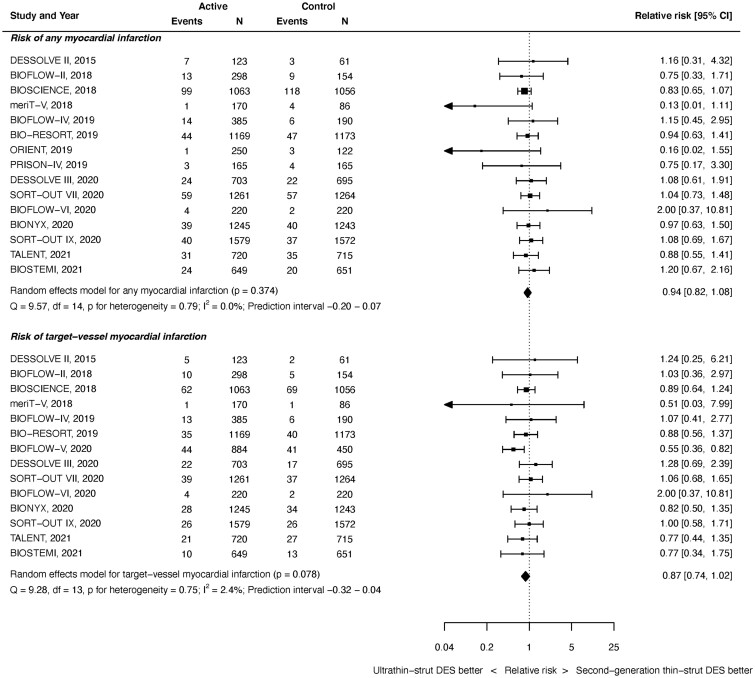
All MI outcomes were available from 15 studies with 19 367 randomized patients. As shown in Figure 3 top, at latest follow-up, there was no significant difference between ultrathin-strut DES and conventional thin-strut DES for the risk of any MI (RR 0.94, 95% CI 0.82–1.08, P = 0.374). There was no heterogeneity present (I 2 = 0.0%). Similarly, there were no significant differences in the risk of early or later MI events noted between groups (Supplementary material online, Appendix Figures S11 and S12). The RRs between the stent types for all MI were consistent before and after 1 year (P interaction = 0.732).
Figure 3.
Risk of myocardial infarction at latest follow-up.
Target-vessel MI outcomes were available from 14 studies with 19 999 randomized patients. As shown in Figure 3 bottom, there was no significant difference between stent types for the risk of TV-MI at time of latest follow-up (RR 0.87, 95% CI 0.74–1.02, P = 0.078). There was mild heterogeneity (I 2 = 2.4%). Similarly, there were no significant differences in the risks of early or later TV-MI noted between groups (Supplementary material online, Appendix Figures S13 and S14). The RRs between the stent types for TV-MI were consistent before and after 1 year (P interaction = 0.933).
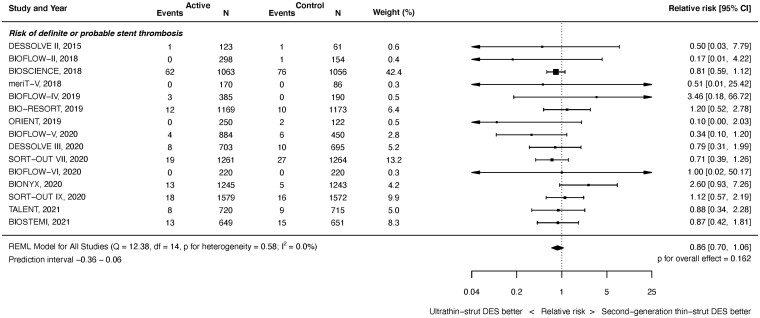
Stent thrombosis
Definite or probable ST outcomes were available from 15 studies with 20 371 randomized patients. As shown in Figure 4, at latest follow-up, there was no significant difference between ultrathin-strut DES and conventional 2nd-generation thin-strut DES for the risk of definite or probable ST (RR 0.86, 95% CI 0.70–1.06, P = 0.162). There was no heterogeneity (I 2 = 0.0%). Similarly, there were no significant differences in the risk of early or later ST events between stent types (Supplementary material online, Appendix Figures S15 and S16). The RRs between the stent types for definite or probable ST were consistent before and after 1 year (P interaction = 0.795). Nor were there significant differences between stent types in the risk of definite ST at any time period (Supplementary material online, Appendix Figures S17 and S19).
Figure 4.
Risk of definite or probable stent thrombosis at latest follow-up.
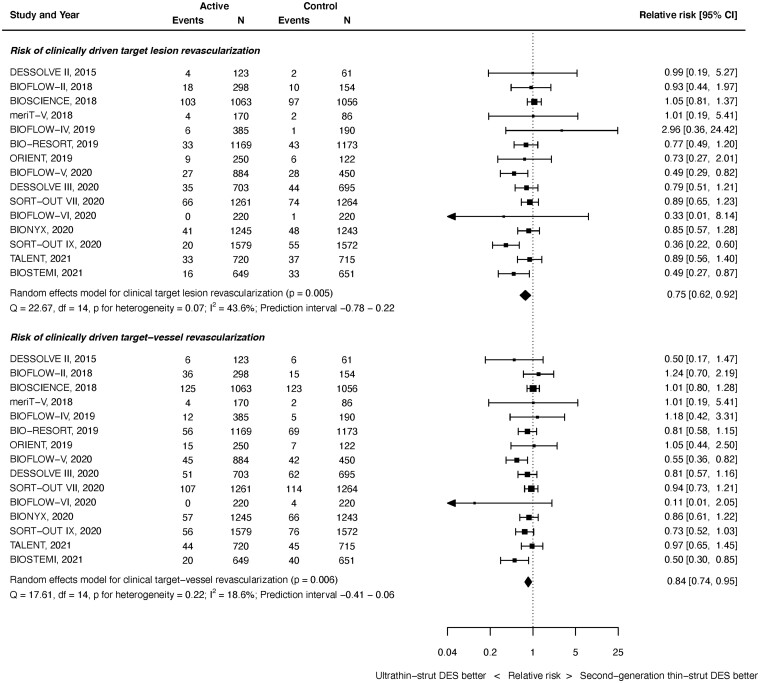
Repeat revascularization
Clinically driven TLR outcomes were available from 15 studies with 20 371 randomized patients. As shown in Figure 5 top panel, at latest follow-up, ultrathin-strut DES were associated with a reduced risk of CD-TLR compared with conventional 2nd-generation thin-strut DES (RR 0.75, 95% CI 0.62–0.92, P = 0.005). There was moderate heterogeneity (I 2 = 43.6%). The reduction in early events did not reach statistical significance (RR 0.78, 95% CI 0.61–1.02, P = 0.068, I 2 = 41.4%) whereas the reduction in later events did (RR 0.82, 95% CI 0.70–0.96, P = 0.013, I 2 = 20.8%) (Supplementary material online, Appendix Figures S20 and S21). However, the RRs between the stent types for CD-TLR were consistent before and after 1 year (P interaction = 0.660).
Figure 5.
Risk of clinically driven revascularization at latest follow-up.
Clinically driven target vessel revascularization outcomes were available from 15 studies with 20 371 randomized patients. As shown in Figure 5 bottom, at latest follow-up, ultrathin-strut DES were associated with a reduced risk of CD-TVR compared with conventional 2nd-generation thin-strut DES (RR 0.84, 95% CI 0.74–0.95, P = 0.006). There was mild heterogeneity (I 2 = 18.6%). A reduced risk of early events (RR 0.84, 95% CI 0.71–0.99, P = 0.040, I 2 = 15.0%) and later events (RR 0.85, 95% CI 0.74–0.97, P = 0.019, I 2 = 26.3%) were present (Supplementary material online, Appendix Figures S22 and S23). The RRs between stent types for CD-TVR were consistent before and after 1 year (P interaction = 0.891).
There were no significant differences between stent types for all TLR, all TVR, and all repeat revascularization at any timepoint (Supplementary material online, Appendix Figures S24–S32).
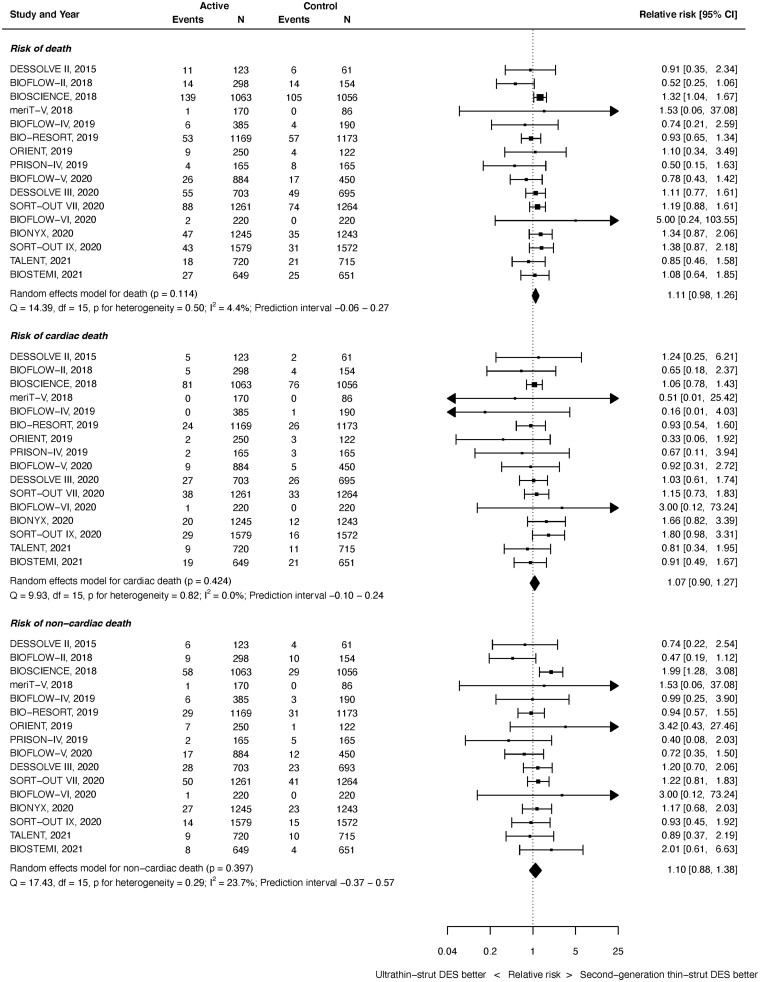
Mortality
All-cause death outcomes were available from 16 studies with 20 701 randomized patients. As shown in Figure 6 top panel, there was no significant difference between ultrathin-strut DES and conventional 2nd-generation thin-strut DES for the risk of death (RR 1.11, 95% CI 0.98–1.26, P = 0.114). There was minimal heterogeneity noted (I 2 = 4.4%). The difference in deaths between the devices was statistically significant in the early (≤1 year) period (RR 1.25, 95% CI 1.04–1.51, P = 0.020, I 2 = 0.0%), but not in the later (>1 year) period (RR 1.08, 95% CI 0.94–1.24, P = 0.300, I 2 = 12.1%) (Supplementary material online, Appendix Figures S33 and S34). However, the RRs between stent types for all-cause death were not significantly different before and after 1 year (P interaction = 0.309).
Figure 6.
Risk of death at latest follow-up.
Cardiac death outcomes were available from 16 studies with 20 701 patients. As shown in Figure 6 middle, at latest follow-up, there was no significant difference between ultrathin-strut DES and conventional thin-strut DES for the risk of cardiac death (RR 1.07, 95% CI 0.90–1.27, P = 0.424). There was no heterogeneity (I 2 = 0.0%). Similarly, there were no differences between groups for the risks of early or later cardiac death (Supplementary material online, Appendix Figures S35 and S36). The RRs between stent types for cardiac death were consistent before and after 1 year (P interaction = 0.599).
Non-cardiac death outcomes were available from 16 studies with 20 701 patients. As shown in Figure 6 bottom, at latest follow-up, there was no significant difference between ultrathin-strut DES and conventional thin-strut DES for the risk of non-cardiac death (RR 1.10, 95% CI 0.88–1.38, P = 0.397). There was mild heterogeneity (I 2 = 23.7%). The difference in non-cardiac deaths between the devices was statistically significant in the early (≤1 year) period (RR 1.39, 95% CI 1.03–1.88, P = 0.029, I 2 = 0.0%), but not in the later (>1 year) period (RR 1.10, 95% CI 0.86–1.42, P = 0.441, I 2 = 33.5%) (Supplementary material online, Appendix Figures S37 and S38). However, the RRs between stent types for all-cause death were not significantly different before and after 1 year (P interaction = 0.195).
Subgroup and stent-type analyses
There were no significant interactions between stent type and any of the subgroups tested on the risk of TLF at latest follow-up (Supplementary material online, Appendix Table S6). Similarly, there was no evidence that the type of ultrathin-strut DES or 2nd-generation thin-strut DES had a moderating effect on the risk of any of the clinical outcome measures (Supplementary material online, Appendix Table S7). There was no evidence that the delta strut thickness between the arms or the anti-proliferative drug-type on the stents had a moderating effect on the risk of any of the clinical outcomes (Supplementary material online, Tables S8 and S9). There was also no evidence of a moderating effect of follow-up duration on any clinical outcomes (Supplementary material online, Table S10).
Sensitivity analyses
The results of the random-effects meta-analyses were consistent when assessed by fixed effect (Supplementary material online, Appendix Figures S39–S49). Fewer trials reported outcomes as HRs; the results are shown in Supplementary material online, Appendix Figures S50–S56. Results were consistent when assessed by IRRs (Supplementary material online, Appendix Figures S57–S66), although the reduction in TV-MI reached statistical significance (IRR 0.83, 95% CI 0.69–0.99, P = 0.043, I 2 = 18.1%, Supplementary material online, Figure S60). The primary outcome of TLF at latest follow-up remained significantly lower with ultrathin-strut DES compared with conventional 2nd-generation thin-strut DES after removing each individual trial one-by-one, except after removing BIOFLOW V (RR 0.92, 95% CI 0.83–1.01, P = 0.08) (Supplementary material online, Appendix Table S11). Sensitivity analyses of only trials in the prior Bangalore meta-analysis7 are shown in Supplementary material online, Appendix Figures S67–S75.
Discussion
The present systematic review and meta-analysis of 16 trials enrolling 20 701 patients is to our knowledge, the largest study to date examining outcomes after PCI with ultrathin-strut DES compared with conventional 2nd-generation thin-strut DES (which still represent the most widely used stents in the USA). The principal findings of this study (as summarized in the Graphical abstract) are (i) at a mean follow-up of 2.5 years, ultrathin-strut DES were associated with reduced risks of TLF and TVF compared with conventional 2nd-generation thin-strut DES; and (ii) there were no significant differences in the rates of cardiac death, MI, or ST between stent types, although CD-TLR and CD-TVR occurred less frequently with ultrathin-strut DES.
Outcomes with contemporary 2nd-generation thin-strut DES (most of which have strut thicknesses between 80 and 100 μm) are excellent and have not been improved upon by various iterative designs including bioresorbable polymer-based DES,42 polymer-free DES,43 or bioresorbable scaffolds.44 In contrast, ultrathin-strut stents (strut thickness ≤70 μm) have potential advantages in terms of deliverability, are less likely to disturb flow in side-branches, and may promote more rapid endothelialization. Bangalore and colleagues7 previously reported a meta-analysis of ultrathin-strut DES vs. conventional 2nd-generation thin-strut DES in 10 trials with 11 658 randomized patients, reporting lower 1-year rates of TLF and MI. However, the benefits were modest (e.g. 16% reduction in TLF) and of borderline statistical significance.
The present study is distinct from the Bangalore meta-analysis in several ways. Nearly twice as many patients were included in the present study (with six additional trials included) and with mean follow-up duration of 2.5 years rather than 1 year, affording a substantially greater number of events for more study power. Furthermore, to examine the time-relatedness between stent types, outcomes were categorized as occurring before or after the 1st year from stent implantation. We also performed detailed analyses by subgroup and stent type and have included other detailed sensitivity analyses that had not been performed previously.
The present study has confirmed a modest 15% long-term RR reduction of TLF with ultrathin-strut DES compared with conventional 2nd-generation thin-strut DES, with consistent reductions in risk before and after 1 year following stent implantation. Although the strength of evidence for the reduction in long-term TLF with ultrathin-strut DES (P = 0.008) is improved compared with the Bangalore report,7 the 95% CI was still wide, consistent with a reduction in TLF ranging from 4% to 24%. The composite endpoint of TVF at latest follow-up was also reduced by 15% with ultrathin-strut DES, with similar magnitude of risk reductions before and after 1 year.
The reductions in TLF and TVF with ultrathin-strut DES were driven by relative 25% and 16% reductions in CD-TLR and CD-TVR, respectively, favouring ultrathin-strut DES both before and after 1 year. In contrast, there were no significant differences between stent types in the risk of MI. These findings vary from those from the prior meta-analysis by Bangalore and colleagues7 in which the reduction in TLF between stent types was driven by a lower risk of MI with no difference in repeat revascularization. Mechanistically, thicker strut dimensions increase vascular injury, flow separation, and stagnation, thereby modulating thrombogenicity and neointimal hyperplasia.45 Increasing strut thickness is also associated with delayed or impaired endothelialization (in part related to these flow disturbances45), which may also promote increased neointimal formation.46 The independent impact of strut thickness on angiographic neointimal hyperplasia and clinical restenosis after bare-metal stents was previously demonstrated in the ISAR-STEREO trials.5 , 47 Despite the smaller amount of neointimal hyperplasia and lower CD-TLR rates after 2nd-generation thin-strut DES compared with 1st-generation DES or bare-metal stents, the present report confirms that further reducing strut thickness to <70 μm has a favourable effect on freedom from repeat revascularization. As the present outcomes were consistent across subgroups, the absolute benefit of ultrathin-strut DES would be expected to be greatest in patients (e.g. diabetics) and lesions (e.g. small vessels, diffuse disease) at high risk for restenosis.
In the present study, there was no significant difference in the risk of MI between stent types, either at latest follow-up or before or after 1 year. This was true for TV-MI as well as any MI. However, the point estimates favoured ultrathin-strut DES, and a small difference in MI between stent types cannot be excluded. Similarly, the difference in ST between stent types did not reach statistical significance, although given the point estimate again favouring ultrathin-strut DES (RR 0.87), a small reduction in ST might have emerged had more events accrued.
Thus, ultrathin-strut DES were associated with early and late reductions in CD-TLR with numerically fewer MI and ST events. Nonetheless, ultrathin-strut DES were associated with a non-significant 11% increase in the risk of all-cause mortality compared with conventional 2nd-generation thin-strut DES, with minimal heterogeneity between trials. Given the numerically lower rates of ST, TV-MI, any MI, and CD-TLR with ultrathin-strut DES (all of which have been associated with reductions in subsequent mortality after stent implantation),48–52 the mechanism(s) underlying a plausible increase in all-cause death is uncertain, especially as the difference was driven by greater non-cardiac mortality occurring within 1 year after implantation. Considering individual trials, all-cause death was significantly increased with the ultrathin-strut Orsiro stent in the BIOSCIENCE trial at 5-year follow-up18 and with the ultrathin-strut Supraflex stent in the TALENT trial at 1-year follow-up,28 but not at 2-year follow-up.40 In the BIOSCIENCE trial, the excess in all-cause mortality was driven by greater non-cardiac deaths, specifically with more patients dying from cancer in the Orsiro arm. The 1-year mortality difference observed in the TALENT trial was believed to be a chance finding related to a lower-than-expected all-cause death rate in the control (Xience) stent arm (0.6%), a hypothesis that appears to be confirmed with the 2-year results. Nevertheless, the present analysis demonstrates numerically greater all-cause mortality with ultrathin-strut DES with directional associations of increased cardiac and non-cardiac mortality. The upper limit of the 95% CI for all-cause mortality was 0.98, and the number of events required to shift the P-value to beyond the threshold for statistical significance is estimated to possibly only be 4 (although this number itself should be interpreted with caution as not all trials included had 1:1 randomization between arms, which is a prerequisite for the calculation of the fragility index, or reverse fragility index). Longer-term follow-up from the present trials, and ideally additional randomized studies, are necessary to clarify this uncertainty.
Twelve of the 16 trials included in our analysis used the 60 µm cobalt-chromium bioabsorbable-polymer-based sirolimus-eluting Orsiro stent as the ultrathin-strut DES, and 10 of the trials used the 81 µm cobalt-chromium durable polymer-based everolimus-eluting Xience stent as the thin-strut DES control. Interaction tests for both the ultrathin and the control stent type were negative for all outcomes, suggesting that the strut thickness rather than specific stent type drove the observed differences in outcomes. Nor were significant interactions for the primary outcome of TLF demonstrated between the stent type and any of the subgroups tested. However, these analyses should be interpreted with caution as not all trials provided detailed subgroup data, introducing selection bias and increasing the likelihood of type II error.
Limitations
This was a study-level meta-analysis, and as such is limited by the scope and shortcomings of each individual trial. Inter-study variability in the definitions of MI, TLF, and TVF were present in a few of the trials, which may have added some imprecision to our results, although statistical heterogeneity was generally low for most analyses. A pooled individual patient data analysis of these studies would prove useful in enabling more granular subgroup analyses, multivariable analysis to reduce variability from observed differences, and affording a structure to examine the temporal relationships in outcomes with greater accuracy. Second, HRs are often considered the most appropriate method for analysing time-to-event data, but most trials did not report their outcomes using this metric. The secondary analyses using this methodology, while included for completeness, are thus of limited utility. Instead, we assessed the primary outcome using RRs from individual event counts provided by the trials. Such effect sizes may be influenced by the follow-up time points. To address this (and recognizing the typical differential in risk after stent implantation beyond 1 year), we provided analyses for early (≤1 year) and later (>1 year) events when reported, for which the P-values for interaction were non-significant. However, we were not able to landmark events at 1 year to specifically evaluate outcomes in the late period. To further address variable follow-up duration, we assessed the impact of follow-up duration on clinical outcomes as a regression analysis, with no evidence of a significant moderating effect on any clinical outcomes. We also performed additional sensitivity analysis including IRRs, which were consistent with our primary analyses.
A variety of stent types were used in both the ultrathin group and the control group, although the most common ultrathin-strut stent was Orsiro and the most common control stent was Xience. We tested for an effect of stent type within both the ultrathin-strut group and the control group, and the statistical tests for interaction were non-significant in both groups. The Orsiro stent has thicker struts for larger stent diameters (≥3.5 mm). However, the median reference vessel diameters or maximum implanted stent diameters did not exceed 3.5 mm in any of the included trials, and in the majority, the mean reference vessel diameters were below 3.0 mm with standard deviations of 0.4–0.5 mm. We therefore believe that the use of 3.5 mm Orsiro stents would have been in a small minority of patients, likely under 10% of the total included patients. Individual patient data could help to clarify this further.
Finally, to avoid bias from measured and unmeasured confounders, our study was limited to randomized trials, which by their nature included a selected cohort of patients, introducing concerns of generalizability. However, several of the individual studies were of an ‘all-comers’ design,18 , 19 , 28 , 30 , 33 , 34 , 38 and collectively, the 16 trials recruited a broad cross-section of patients including those with acute coronary syndrome and complex CAD.
Conclusions
In the present meta-analysis of 16 trials randomizing 20 701 patients and with a mean follow-up of 2.5 years, ultrathin-strut DES were associated with a modestly reduced long-term risk of TLF and TVF compared with conventional 2nd-generation thin-strut DES, the differences driven by lower rates of CD-TLR and CD-TVR. There were no significant differences in the risks of MI, ST, cardiac death, or all-cause mortality.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
Funding
M.V.M. was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to Columbia University Irving Medical Center (T32 HL007854). This research was funded in whole, or in part, by the Wellcome Trust [grant 212183/Z/18/Z awarded to J.PH]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Conflict of interest: Dr. Madhavan was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to Columbia University Irving Medical Center (T32 HL007854). Dr. Stone has received speaker honoraria from Cook and Terumo; has served as a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, and Matrizyme; and owns equity/options in Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, the MedFocus family of funds, and Valfix. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
Mahesh V Madhavan, Columbia University Irving Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA; Cardiovascular Research Foundation, New York, NY, USA.
James P Howard, National Heart and Lung Institute, Imperial College London, London, UK.
Azim Naqvi, Cardiovascular Research Foundation, New York, NY, USA.
Ori Ben-Yehuda, Cardiovascular Research Foundation, New York, NY, USA.
Bjorn Redfors, Cardiovascular Research Foundation, New York, NY, USA; Department of Cardiology, Sahlgrenska University Hospital, Gothenburg, Sweden.
Megha Prasad, Columbia University Irving Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Bahira Shahim, Cardiovascular Research Foundation, New York, NY, USA.
Martin B Leon, Columbia University Irving Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA; Cardiovascular Research Foundation, New York, NY, USA.
Sripal Bangalore, New York University School of Medicine, New York, NY, USA.
Gregg W Stone, Cardiovascular Research Foundation, New York, NY, USA; The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Yousif Ahmad, Smidt Heart Institute, Cedars Sinai Medical Center, San Vicente Boulevard, Los Angeles, CA 90048, USA.
References
- 1. Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ; SPIRIT IV Investigators. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med 2010;362:1663–1674. [DOI] [PubMed] [Google Scholar]
- 2. Bangalore S, Toklu B, Amoroso N, Fusaro M, Kumar S, Hannan EL, Faxon DP, Feit F. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ 2013;347:f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schömig A, Dibra A, Windecker S, Mehilli J, Suárez de Lezo J, Kaiser C, Park S-J, Goy J-J, Lee J-H, Di Lorenzo E, Wu J, Jüni P, Pfisterer ME, Meier B, Kastrati AA. Meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol 2007;50:1373–1380. [DOI] [PubMed] [Google Scholar]
- 4. Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich K-L, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011;123:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schühlen H, Neumann F-J, Fleckenstein M, Pfafferott C, Seyfarth M, Schömig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 2001;103:2816–2821. [DOI] [PubMed] [Google Scholar]
- 6. Madhavan MV, Kirtane AJ, Redfors B, Généreux P, Ben-Yehuda O, Palmerini T, Benedetto U, Biondi-Zoccai G, Smits PC, von BC, Mehran R, McAndrew T, Serruys PW, Leon MB, Pocock SJ, Stone GW. Stent-related adverse events >1 year after percutaneous coronary intervention. J Am Coll Cardiol 2020;75:590–604. [DOI] [PubMed] [Google Scholar]
- 7. Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease: meta-analysis of randomized trials. Circulation 2018;138:2216–2226. [DOI] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 9. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Es G-A, van Steg PG, Morel M, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 12.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.R-project.org/ (1 March 2021). [Google Scholar]
- 13. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software 36:1–48. [Google Scholar]
- 14. Saito S, Toelg R, Witzenbichler B, Haude M, Masotti M, Salmeron R, Witkowski A, Uematsu M, Takahashi A, Waksman R, Slagboom T. BIOFLOW-IV, a randomised, intercontinental, multicentre study to assess the safety and effectiveness of the Orsiro sirolimus-eluting stent in the treatment of subjects with de novo coronary artery lesions: primary outcome target vessel failure at 12 months. EuroIntervention 2019;15:e1006–e1013. [DOI] [PubMed] [Google Scholar]
- 15. Kandzari DE, Koolen JJ, Doros G, Garcia-Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE, Waksman R; BIOFLOW V Investigators. Ultrathin bioresorbable-polymer sirolimus-eluting stents versus thin durable-polymer everolimus-eluting stents for coronary revascularization: 3-year outcomes from the randomized BIOFLOW V trial. JACC Cardiovasc Interv 2020;13:1343–1353. [DOI] [PubMed] [Google Scholar]
- 16. Lefèvre T, Haude M, Neumann F-J, Stangl K, Skurk C, Slagboom T, Sabaté M, Goicolea J, Barragan P, Cook S, Macia J-C, Windecker S. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: 5-year outcomes of the randomized BIOFLOW-II trial. JACC Cardiovasc Interv 2018;11:995–1002. [DOI] [PubMed] [Google Scholar]
- 17. Buiten RA, Ploumen EH, Zocca P, Doggen CJM, Danse PW, Schotborgh CE, Scholte M, van Houwelingen KG, Stoel MG, Hartmann M, Tjon Joe Gin RM, Somi S, Linssen GCM, Kok MM, von Birgelen C. Very thin, or ultrathin strut biodegradable or durable polymer-coated drug-eluting stents: 3-year outcomes of BIO-RESORT. JACC Cardiovasc Interv 2019;12:1650–1660. [DOI] [PubMed] [Google Scholar]
- 18. Pilgrim T, Piccolo R, Heg D, Roffi M, Tüller D, Muller O, Moarof I, Siontis GCM, Cook S, Weilenmann D, Kaiser C, Cuculi F, Hunziker L, Eberli FR, Jüni P, Windecker S. Ultrathin-strut, biodegradable-polymer, sirolimus-eluting stents versus thin-strut, durable-polymer, everolimus-eluting stents for percutaneous coronary revascularisation: 5-year outcomes of the BIOSCIENCE randomised trial. Lancet 2018;392:737–746. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi K, Serruys PW, Kogame N, Buszman P, Lurz P, Jessurun GAJ, Koch KT, Troquay RPT, Hamer BJB, Oude OT, Milewski KP, Hofma SH, Wykrzykowska JJ, Onuma Y, Winter RJ, de Wijns W. Final 3-year outcomes of mistent biodegradable polymer crystalline sirolimus-eluting stent versus xience permanent polymer everolimus-eluting stent: insights from the DESSOLVE III all-comers randomized trial. Circ Cardiovasc Interv 2020;13:e008737. [DOI] [PubMed] [Google Scholar]
- 20. Kim S, Kang S, Lee JM, Chung W, Park JJ, Yoon C, Suh J, Cho Y, Doh J, Cho JM, Bae J, Youn T, Chae I. Three‐year clinical outcome of biodegradable hybrid polymer Orsiro sirolimus‐eluting stent and the durable biocompatible polymer Resolute Integrity zotarolimus‐eluting stent: a randomized controlled trial. Catheter Cardiovasc Interv 2020;96:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zivelonghi C, Agostoni P, Teeuwen K, van der Schaaf RJ, Henriques JPS, Vermeersch PHMJ, Bosschaert MAR, Kelder JC, Tijssen JGP, Suttorp MJ. 3-year clinical outcomes of the PRISON-IV trial: ultrathin struts versus conventional drug-eluting stents in total coronary occlusions. JACC Cardiovasc Interv 2019;12:1747–1749. [DOI] [PubMed] [Google Scholar]
- 22. Teeuwen K, van der SR, Adriaenssens T, Koolen JJ, Smits PC, Henriques JPS, Vermeersch PHMJ, Tjon Joe Gin RM, Schölzel BE, Kelder JC, Tijssen JGP, Agostoni P, Suttorp MJ. Randomized multicenter trial investigating angiographic outcomes of hybrid sirolimus-eluting stents with biodegradable polymer compared with everolimus-eluting stents with durable polymer in chronic total occlusions: the PRISON IV trial. JACC Cardiovasc Interv 2017;10:133–143. [DOI] [PubMed] [Google Scholar]
- 23. Ellert J, Maeng M, Raungaard B, Hansen KN, Kahlert J, Jensen SE, Bøtker HE, Hansen HS, Lassen JF, Christiansen EH, Jensen LO. Clinical outcomes three-year after revascularization with biodegradable polymer stents: ultrathin-strut sirolimus-eluting stent versus biolimus-eluting stent from the Scandinavian organization for randomized trials with clinical outcome VII trial. Coron Artery Dis 2020;31:485–492. [DOI] [PubMed] [Google Scholar]
- 24. Abizaid A, Kedev S, Kedhi E, Talwar S, Erglis A, Hlinomaz O, Masotti M, Fath-Ordoubadi F, Lemos PA, Milewski K, Botelho R, Costa R, Bangalore S. Randomised comparison of a biodegradable polymer ultra-thin sirolimus-eluting stent versus a durable polymer everolimus-eluting stent in patients with de novo native coronary artery lesions: the meriT-V trial. EuroIntervention 2018;14:e1207–e1214. [DOI] [PubMed] [Google Scholar]
- 25. Li C, Yang Y, Han Y, Song D, Xu J, Guan C, Gao R, Garcia-Garcia HM, Waksman R, Xu B. Comparison of the ultrathin strut, biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent in a chinese population: the randomized BIOFLOW VI trial. Clin Ther 2020;42:649–660.e9. [DOI] [PubMed] [Google Scholar]
- 26. Buiten RA, Ploumen EH, Zocca P, Doggen CJM, Jessurun GAJ, Schotborgh CE, Roguin A, Danse PW, Benit E, Aminian A, Houwelingen KG, van Schramm AR, Stoel MG, Somi S, Hartmann M, Linssen GCM, von Birgelen C. Thin composite-wire-strut zotarolimus-eluting stents versus ultrathin-strut sirolimus-eluting stents in BIONYX at 2 years. JACC Cardiovasc Interv 2020;13:1100–1109. [DOI] [PubMed] [Google Scholar]
- 27. Iglesias JF, Muller O, Heg D, Roffi M, Kurz DJ, Moarof I, Weilenmann D, Kaiser C, Tapponnier M, Stortecky S, Losdat S, Eeckhout E, Valgimigli M, Odutayo A, Zwahlen M, Jüni P, Windecker S, Pilgrim T. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial. Lancet 2019;394:1243–1253. [DOI] [PubMed] [Google Scholar]
- 28. Zaman A, Winter RJ, de Kogame N, Chang CC, Modolo R, Spitzer E, Tonino P, Hofma S, Zurakowski A, Smits PC, Prokopczuk J, Moreno R, Choudhury A, Petrov I, Cequier A, Kukreja N, Hoye A, Iniguez A, Ungi I, Serra A, Gil RJ, Walsh S, Tonev G, Mathur A, Merkely B, Colombo A, Ijsselmuiden S, Soliman O, Kaul U, Onuma Y, Serruys PW; TALENT Trial Investigators. Safety and efficacy of a sirolimus-eluting coronary stent with ultra-thin strut for treatment of atherosclerotic lesions (TALENT): a prospective multicentre randomised controlled trial. Lancet 2019;393:987–997. [DOI] [PubMed] [Google Scholar]
- 29. Serruys P, Zaman A, R de W, Kogame N, Modolo R, Tonino P, Hofma SH, Smits P, Prokopczuk J, Moreno R, Choudhury A, Petrov I, Cequier A, Kukreja N, Ungi I, Serra A, Gil R, Walsh S, Mathur A, Merkely B, Colombo A, Ijsselmuiden A, Soliman O, Onuma Y. A prospective multicenter randomized all-comers trial to assess the safety and effectiveness of the ultra-thin-strut sirolimus-eluting coronary stent supraflex: 2-year results of the TALENT trial [abstract]. J Am Coll Cardiol 2019;74:B41. [DOI] [PubMed] [Google Scholar]
- 30. Wijns W, Vrolix M, Verheye S, Schoors D, Slagboom T, Gosselink M, Benit E, Kandzari D, Donohoe D, Ormiston JA. Long-term clinical outcomes of a crystalline sirolimus-eluting coronary stent with a fully bioabsorbable polymer coating: five-year outcomes from the DESSOLVE I and II trials. EuroIntervention 2018;13:2147–2151. [DOI] [PubMed] [Google Scholar]
- 31. Wijns W, Vrolix M, Verheye S, Schoors D, Slagboom T, Gosselink M, Benit E, Donohoe D, Knape C, Attizzani GF, Lansky AJ, Ormiston J. Randomised study of a bioabsorbable polymer-coated sirolimus-eluting stent: results of the DESSOLVE II trial. EuroIntervention 2015;10:1383–1390. [DOI] [PubMed] [Google Scholar]
- 32. Windecker S, Haude M, Neumann F-J, Stangl K, Witzenbichler B, Slagboom T, Sabaté M, Goicolea J, Barragan P, Cook S, Piot C, Richardt G, Merkely B, Schneider H, Bilger J, Erne P, Waksman R, Zaugg S, Jüni P, Lefèvre T. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv 2015;8:e001441. [DOI] [PubMed] [Google Scholar]
- 33. Kandzari DE, Mauri L, Koolen JJ, Massaro JM, Doros G, Garcia-Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE, Waksman R; BIOFLOW V Investigators. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet 2017;390:1843–1852. [DOI] [PubMed] [Google Scholar]
- 34. von Birgelen C, Kok MM, van der Heijden LC, Danse PW, Schotborgh CE, Scholte M, Gin RMTJ, Somi S, van Houwelingen KG, Stoel MG, de Man FHAF, Louwerenburg J(H)W, Hartmann M, Zocca P, Linssen GCM, van der Palen J, Doggen CJM, Löwik MM. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet 2016;388:2607–2617. [DOI] [PubMed] [Google Scholar]
- 35. Pilgrim T, Heg D, Roffi M, Tüller D, Muller O, Vuilliomenet A, Cook S, Weilenmann D, Kaiser C, Jamshidi P, Fahrni T, Moschovitis A, Noble S, Eberli FR, Wenaweser P, Jüni P, Windecker S. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet 2014;384:2111–2122. [DOI] [PubMed] [Google Scholar]
- 36. de Winter RJ, Katagiri Y, Asano T, Milewski KP, Lurz P, Buszman P, Jessurun GAJ, Koch KT, Troquay RPT, Hamer BJB, Ophuis TO, Wöhrle J, Wyderka R, Cayla G, Hofma SH, Levesque S, Żurakowski A, Fischer D, Kośmider M, Goube P, Arkenbout EK, Noutsias M, Ferrari MW, Onuma Y, Wijns W, Serruys PW. A sirolimus-eluting bioabsorbable polymer-coated stent (MiStent) versus an everolimus-eluting durable polymer stent (Xience) after percutaneous coronary intervention (DESSOLVE III): a randomised, single-blind, multicentre, non-inferiority, phase 3 trial. Lancet 2018;391:431–440. [DOI] [PubMed] [Google Scholar]
- 37. Kang S-H, Chung W-Y, Lee JM, Park J-J, Yoon C-H, Suh J-W, Cho Y-S, Doh J-H, Cho JM, Bae J-W, Youn T-J, Chae I-H. Angiographic outcomes of Orsiro biodegradable polymer sirolimus-eluting stents and resolute integrity durable polymer zotarolimus-eluting stents: results of the ORIENT trial. EuroIntervention 2017;12:1623–1631. [DOI] [PubMed] [Google Scholar]
- 38. Jensen LO, Maeng M, Raungaard B, Kahlert J, Ellert J, Jakobsen L, Villadsen AB, Veien KT, Kristensen SD, Ahlehoff O, Carstensen S, Christensen MK, Terkelsen CJ, Engstroem T, Hansen KN, Bøtker HE, Aaroe J, Thim T, Thuesen L, Freeman P, Aziz A, Eftekhari A, Junker A, Jensen SE, Lassen JF, Hansen HS, Christiansen EH, Thygesen K, Sørensen JT, Andersen HR; SORT OUT IX Study Group. Randomized comparison of the polymer-free biolimus-coated biofreedom stent with the ultrathin strut biodegradable polymer sirolimus-eluting orsiro stent in an all-comers population treated with percutaneous coronary intervention: the SORT OUT IX trial. Circulation 2020;141:2052–2063. [DOI] [PubMed] [Google Scholar]
- 39. Jensen LO, Thayssen P, Maeng M, Ravkilde J, Krusell LR, Raungaard B, Junker A, Terkelsen CJ, Veien KT, Villadsen AB, Kaltoft A, Tilsted H-H, Hansen KN, Aaroe J, Kristensen SD, Hansen HS, Jensen SE, Madsen M, Bøtker HE, Berencsi K, Lassen JF, Christiansen EH. Randomized comparison of a biodegradable polymer ultrathin strut sirolimus-eluting stent with a biodegradable polymer biolimus-eluting stent in patients treated with percutaneous coronary intervention: the SORT OUT VII trial. Circ Cardiovasc Interv 2016;9:e003610. [DOI] [PubMed] [Google Scholar]
- 40. Gao C, Kogame N, Sharif F, Smits PC, Tonino P, Hofma S, Moreno R, Choudhury A, Petrov I, Cequier A, Colombo A, Kaul U, Zaman A, Winter RJ, de Onuma Y, Serruys PW. Prospective multicenter randomized all-comers trial to assess the safety and effectiveness of the ultra-thin strut sirolimus-eluting coronary stent supraflex: two-year outcomes of the TALENT trial. Circ Cardiovasc Interv 2021;14:e010312. [DOI] [PubMed] [Google Scholar]
- 41. Pilgrim T, Muller O, Heg D, Roffi M, Kurz DJ, Moarof I, Weilenmann D, Kaiser C, Tapponnier M, Losdat S, Eeckhout E, Valgimigli M, Jüni P, Windecker S, Iglesias JF. Biodegradable- versus durable-polymer drug-eluting stents for STEMI: final 2-year outcomes of the BIOSTEMI trial. JACC Cardiovasc Interv 2021;14:639–648. [DOI] [PubMed] [Google Scholar]
- 42. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Smits PC, Kaiser C, D'Ascenzo F, Frati G, Mancone M, Genereux P, Stone GW. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2014;63:299–307. [DOI] [PubMed] [Google Scholar]
- 43. Nogic J, Thein P, Mirzaee S, Comella A, Soon K, Cameron JD, West NEJ, Brown AJ. Biodegradable-polymer versus polymer-free drug-eluting stents for the treatment of coronary artery disease. Cardiovasc Revasc Med 2019;20:865–870. [DOI] [PubMed] [Google Scholar]
- 44. Stone GW, Kimura T, Gao R, Kereiakes DJ, Ellis SG, Onuma Y, Chevalier B, Simonton C, Dressler O, Crowley A, Ali ZA, Serruys PW. Time-varying outcomes with the absorb bioresorbable vascular scaffold during 5-year follow-up: a systematic meta-analysis and individual patient data pooled study. JAMA Cardiol 2019;4:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waksman R, Lipinski MJ, Acampado E, Cheng Q, Adams L, Torii S, Gai J, Torguson R, Hellinga DM, Westman PC, Joner M, Zumstein P, Kolodgie FD, Virmani R. Comparison of acute thrombogenicity for metallic and polymeric bioabsorbable scaffolds: magmaris versus absorb in a porcine arteriovenous shunt model. Circ Cardiovasc Interv 2017;10:e004762. [DOI] [PubMed] [Google Scholar]
- 46. Garasic JM, Edelman ER, Squire JC, Seifert P, Williams MS, Rogers C. Stent and artery geometry determine intimal thickening independent of arterial injury. Circulation 2000;101:812–818. [DOI] [PubMed] [Google Scholar]
- 47. Pache J, Kastrati A, Mehilli J, Schühlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattelberger U, Schmitt C, Müller M, Dirschinger J, Schömig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol 2003;41:1283–1288. [DOI] [PubMed] [Google Scholar]
- 48. Brener Sorin J, Tarantini G, Leon MB, Serruys PW, Smits Pieter C, von Birgelen C, Aaron C, Ben-Yehuda O, Stone Gregg W. Cardiovascular and noncardiovascular death after percutaneous coronary intervention: insights from 32 882 patients enrolled in 21 randomized trials. Circ Cardiovasc Interv 2018;11:e006488. [DOI] [PubMed] [Google Scholar]
- 49. Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 2013;62:1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008;358:2218–2230. [DOI] [PubMed] [Google Scholar]
- 51. Giustino G, Serruys PW, Sabik JF, Mehran R, Maehara A, Puskas JD, Simonton CA, Lembo NJ, Kandzari DE, Morice M-C, Taggart DP, Gershlick AH, Ragosta M, Kron IL, Liu Y, Zhang Z, McAndrew T, Dressler O, Généreux P, Ben-Yehuda O, Pocock SJ, Kappetein AP, Stone GW. Mortality after repeat revascularization following PCI or coronary artery bypass grafting for left main disease: the EXCEL trial. JACC Cardiovasc Interv 2020;13:375–387. [DOI] [PubMed] [Google Scholar]
- 52. Palmerini T, Della Riva D, Biondi-Zoccai G, Leon MB, Serruys PW, Smits PC, Birgelen C. V, Ben-Yehuda O, Généreux P, Bruno AG, Jenkins P, Stone GW. Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention: an individual patient data pooled analysis of 21 randomized trials and 32,524 patients. JACC Cardiovasc Interv 2018;11:892–902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.