Abstract
Background
Pneumonia is a common, serious illness in the elderly, with a poorly characterized long-term impact on health-related quality of life (HRQoL). The Japanese Goto Epidemiology Study is a prospective, active, population-based surveillance study of adults with X-ray/CT scan–confirmed community-onset pneumonia, assessing the HRQoL outcome quality-adjusted life-years (QALYs). We report QALY scores and losses among a subset of participants in this study.
Methods
QALYs were derived from responses to the Japanese version of the EuroQol-5D-5L health-state classification instrument at days 0, 7, 15, 30, 90, 180, and 365 after pneumonia diagnosis from participants enrolled from June 2017 to May 2018. We used patients as their own controls, calculating comparison QALYs by extrapolating EuroQol-5D-5L scores for day −30, accounting for mortality and changes in scores with age.
Results
Of 405 participants, 85% were aged ≥65 years, 58% were male, and 69% were hospitalized for clinically and radiologically confirmed pneumonia. Compliance with interviews by patients or proxies was 100%. Adjusted EuroQol-5D-5L scores were 0.759, 0.561, 0.702, and 0.689 at days −30, 0 (diagnosis), 180, and 365, respectively. Average scores at all time points remained below the average day −30 scores (P ≤ .001). Pneumonia resulted in a 1-year adjusted loss of 0.13 QALYs (~47.5 quality-adjusted days) (P < .001).
Conclusions
Substantial QALY losses were observed among Japanese adults following pneumonia diagnosis, and scores had not returned to prediagnosis levels at 1 year postdiagnosis. QALY scores and cumulative losses were comparable to those in US adults with chronic heart failure, stroke, or renal failure.
Keywords: pneumococcal pneumonia, quality of life, quality-adjusted life years
This prospective study evaluated quality-adjusted life-year (QALY) scores and losses due to pneumonia among adults in Japan. The average 1-year QALY loss was 0.13 (P < .001), which is larger than many evaluations of pneumonia-prevention programs assume.
Pneumonia is a major cause of morbidity and mortality among older adults worldwide, including Japan [1]. Incidence and case fatality rates of pneumonia increase with age, being higher among individuals with chronic comorbidities [2]. In Japan, the annual incidence of community-onset pneumonia (COP) has been estimated at 1690 per 100 000 population aged 15 years or older, with estimates for adults 65–74, 75–84, and 65 years and older at 2460, 5290, and 7930 per 100 000 persons, respectively [1]. Approximately 1.9 million COP episodes in adults occur every year, 69% of them in adults aged 65 years or older [1]. With approximately 120 000 deaths per year, pneumonia is the third leading cause of death, only surpassed by malignancies and heart disease [3].
While the clinical characteristics of pneumonia are well known, there has been less study of associated health-related quality of life (HRQoL) impacts. For cost-effectiveness analyses, HRQoL is usually measured in terms of quality-adjusted life-years (QALYs). This combination of length of survival and life quality is used to support estimation of the burden of illness and evaluation of the potential value of interventions [4, 5]. For example, substantial pneumonia burden in adults arises from vaccine-preventable Streptococcus pneumoniae [1, 6, 7], which imposes major morbidity, mortality, and health costs [8–10].
Dozens of cost-effectiveness analyses of adult pneumococcal vaccination programs have been published, yet the data underlying QALY estimates are generally weak. To address this knowledge gap, we assessed QALY scores and QALY decrements for 1 year following pneumonia diagnosis in adults as part of the Goto Island, Japan, Epidemiology Study.
METHODS
Goto Epidemiology Study
The Goto Epidemiology Study is an ongoing prospective, active-surveillance, population-based study of adults with COP in Goto City, Japan, that began in December 2015. Goto City, located in the southwest part of Japan, has approximately 40 000 inhabitants, 85% of them aged 18 years or older.
As defined in Japan, COP includes patients with symptoms and signs of pneumonia with an onset outside a hospital setting, and includes both community-acquired pneumonia (CAP) and healthcare-associated pneumonia (HCAP) [11, 12]. According to the guidelines by the American Thoracic Society and the Infectious Diseases Society of America, HCAP is defined as pneumonia that occurs among patients who were hospitalized for 2 days or more in the prior 90 days, resided in a nursing home or extended care institution, received infusion therapy (including antimicrobial drugs), received long-term dialysis (including hemodialysis and peritoneal dialysis) within 30 days of entering the study, or had wound healing at home [11]. The Goto study enrolled all consenting adult patients with chest X-ray or computed tomography (CT) scan confirmation of pneumonia who sought medical care at either a hospital or clinic in Goto City [13, 14].
The study’s main goals were to estimate all-cause, pneumococcal, and serotype-specific pneumococcal pneumonia incidence and antimicrobial susceptibility. The study did not have an intervention component and all participants received standard-of-care treatment.
Study Sample for QALY Score/QALY Assessment
The HRQoL analysis was conducted within a subset of the Goto Epidemiology Study. To be included in the HRQoL analysis, participants had to enroll in the Goto Epidemiology Study between 1 June 2017 (HRQoL study initiation) and 13 May 2018 and provide written informed consent. Participants’ demographic characteristics and selected underlying comorbid conditions were documented. At-risk conditions included asthma, chronic obstructive pulmonary disease (COPD), congestive heart failure, chronic heart disease, autoimmune diseases (unless under systemic long-term steroid use or on biologics in which case the patient was considered high risk), and liver disease. High-risk conditions included the following: end-stage renal disease, organ transplant, immunodeficiency, immunosuppressive drug therapy, cancer (for solid tumors, current or recent history of active treatment), generalized malignancy, and splenectomy.
Participants were followed for 1 year after enrollment. Using simulation, we identified a required sample size of 360 patients; we increased this to 405 patients to account for either attrition or death.
QALY Score Instruments
Participants were administered the Japanese Versions of the EuroQol-5D-5L (EQ-5D-5L), the prespecified primary QALY score instrument for the study, the EQ-5D visual analog scale, and the Short Form-36 version 2 health survey (SF-36v2) [15, 16]. Responses from the latter instrument were used to derive the Short Form-6 Dimension health state classification instrument (SF-6D), an alternative QALY score [17]. Both the EQ-5D-5L and SF-6D are validated preference-weighted HRQoL instruments [8, 17–20]. This analysis reports the primary outcome for the study, EQ-5D-5L QALY scores and the QALYs derived from these scores.
The EQ-5D-5L is a 5-domain preference-weighted HRQoL instrument that addresses mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each domain is rated on a 1 to 5 scale, where 1 equals best functioning (eg, “no problems”) and 5 equals worst functioning (eg, “extreme problems”). QALY scores were derived from these ratings by use of the published Japanese scoring rule [21].
Periodic Administration of Instruments
QALY scores were calculated for study days 0, 7, 15, 30, 90, 180, and 365 after diagnosis. During the day 0 interview participants were asked to assess both their current health and, via recall, their health 30 days before pneumonia diagnosis (referred to as day −30).
The EQ-5D-5L was administered via structured interview by Japanese interviewers who received training on the administration of HRQoL instruments in general as well as training on the 3 instruments used in the study. The study aimed at enrolling a broad range of patients regarding severity and wanted to minimize missing responses. To facilitate the inclusion of severe patients and their potential for missing responses during critical phases of the disease, if the participant was unavailable or unable to respond proxies were asked to respond instead. As per the study protocol, when a proxy was selected, priority was given to the primary caregiver, who was identified on enrollment in the study, followed by other proxies who were knowledgeable about the participant. The interviews were conducted either in person or by telephone.
QALYs
QALYs represent the area under the survival-weighted QALY score curve [22]. Points on the curve represent the product of the QALY scores and survival probabilities at each time point. The area under this curve was calculated by use of the trapezoidal method, which weights the height of the curve at the different measurement time points by the length of time that is represented by the time points [22]. Two sets of QALY estimates were made, one using unadjusted and a second using adjusted estimates of scores and survival probabilities.
Analysis
We calculated unadjusted means of the QALY scores at days 0, 7, 15, 30, 90, 180, and 365 and estimated adjusted means for these same intervals by use of a multivariable generalized linear model that accounted for multiple responses from the same participants. Ordinary least-squares regression was used to test if the day 0, 7, 15, 30, 90, 180, and 365 scores remained significantly below the initial day −30. Unadjusted survival probabilities were estimated by use of a Kaplan-Meier estimator. Adjusted survival probabilities were estimated by use of a multivariable parametric failure time model.
Participants with pneumonia served as their own controls by extrapolation of their recalled QALY scores for day −30 (assessed at day 0) to days 0, 7, 15, 30, 90, 180, and 365. First, we adjusted these scores to account for naturally occurring age- and gender-specific changes in scores calculated from Japanese EQ-5D-5L QALY score norms [23]. We estimated the unadjusted mean of these scores and used a GLM to estimate the adjusted mean. Average adjusted QALY score curves were created for patients both when they had pneumonia and when they served as their own control. Second, survival probabilities were derived by use of age- and gender-specific Japanese national mortality rates [24]. The mortality rates were adjusted upwards using an assumed relative risk of 2 to account for patients who developed pneumonia having a greater likelihood of comorbidity than the general population from whom the national mortality rates were derived [1]. Average adjusted survival curves were created for patients both when they had pneumonia and when they served as their own control. We evaluated the impact of this assumption by sensitivity analysis. Finally, survival-weighted QALY score curves were also created for patients both when they had pneumonia and when they served as their own control.
QALYs lost were calculated as the difference between the areas under the survival-weighted QALY score curves for persons with pneumonia and for the same persons when used as their own controls. Standard errors, P values, and 95% confidence intervals (CIs) for the QALY estimates were estimated by use of a nonparametric bootstrap.
We also assessed if 1-year QALY losses due to pneumonia differed between patients with COP who had outpatient evaluation only and those requiring hospitalization for their initial COP treatment.
In sensitivity analysis, we evaluated the effects of the adjustment for naturally occurring changes (typically reductions) in scores with age (range, no change in scores to a doubling of the rate of change) and Japanese national mortality rates (range, equal to the national mortality rates to a quadrupling of the mortality rates).
The Institutional Review Board of Nagasaki University approved the study. Participants provided separate informed consents for the Goto Epidemiology and the HRQoL studies.
RESULTS
A total of 405 participants were enrolled. The mean age of study participants was 77.9 years (SD, 14.3); 84.9% were aged 65 years or older, 41.5.% were female, 41.7% had HCAP, and 68.9% received hospital treatment for their initial pneumonia diagnosis (Table 1). Sixty-four percent of participants were at or high risk, including 21.5% with diabetes mellitus, 20.7% with COPD, 16.0% with congestive heart failure, and 15.3% with cancer.
Table 1.
Demographic and Clinical Characteristics of Patients With Pneumonia
| Characteristics | Value (N = 405) |
|---|---|
| Age ≥65 years, % | 84.9 |
| Age, mean (SD), years | 77.9 (14.3) |
| Female, % | 41.5 |
| Healthcare-acquired pneumonia, % | 41.7 |
| Pneumonia severity index score, mean (SD) | 110.5 (38.2) |
| Initial pneumonia episode treated in hospital, % | 68.9 |
| At or high risk, % | 64.2 |
| Selected comorbid conditions, % | |
| Chronic obstructive pulmonary disease | 20.7 |
| Diabetes mellitus | 21.5 |
| Congestive heart failure | 16.0 |
| Cancer | 15.3 |
| End-stage renal disease | 10.9 |
| Asthma | 10.4 |
| Liver disease | 10.4 |
The 405 study participants or their proxies provided responses for a total of 2959 time points for days −30, 0, 7, 15, 30, 90, 180, and 365. Participants provided 77.9% of these responses, followed by 11.0% from primary caregivers, and 11.1% from other proxies. Responses came mainly from face-to-face interviews (76.4%) and less frequently from telephone interviews (23.6%). All participants or their proxies completed all interviews if they were alive (ie, between patients and proxies, 100% compliance). A total of 291 (71.9%) participants or proxies completed the 1-year interview (ie, the remaining 28.1% of enrollees had died during the 1 year of follow-up after enrollment).
The average adjusted QALY score at day −30 was 0.759 (SE, .012; 95% CI, .735–.783). This declined to 0.561 (SE, .014; 95% CI, .534–.588) on diagnosis and increased to 0.689 (SE, .015; 95% CI, .660–.718) by day 365 (Table 2). None of the average scores at days 0, 7, 15, 30, 90, 180, and 365 had returned to the level of the average day −30 score (P ≤ .001 for all comparisons) (Table 2). Significant predictors of higher QALY scores included interviews further from the pneumonia diagnosis (eg, at days 90, 180, and 365 vs days 0, 7 and 15), higher day −30 EQ-5D-5L QALY scores, younger age, lower pneumonia severity index scores, and initial treatment in the outpatient setting (see Supplementary Tables 1 and 2, for coefficients and marginal effects of unit changes in the explanatory variables from the model predicting the scores, and Supplementary Tables 3 and 4, for coefficients and marginal effects for day −30 scores).
Table 2.
Unadjusted and Adjusted EQ-5D-5L QALY Scores Among Patients With Pneumonia Through Day 365 After Diagnosis
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Study Day | Mean Score | SD | n | Mean Score | SE | 95% Confidence Interval (CI) (Lower-Upper) |
| −30a | 0.759 | 0.259 | 405 | 0.759 | .012 | .735–.783 |
| 0b | 0.560 | 0.280 | 405 | 0.561 | .014 | .534–.588 |
| 7b | 0.629 | 0.293 | 398 | 0.622 | .015 | .593–.651 |
| 15b | 0.684 | 0.289 | 396 | 0.676 | .015 | .647–.705 |
| 30b | 0.674 | 0.290 | 385 | 0.665 | .014 | .638–.692 |
| 90b | 0.702 | 0.273 | 354 | 0.683 | .014 | .656–.710 |
| 180b | 0.730 | 0.258 | 325 | 0.702 | .014 | .675–.729 |
| 365b | 0.732 | 0.261 | 291 | 0.689 | .015 | .660–.718 |
Abbreviations: CL, confidence limit; EQ-5D-5L, EuroQol-5D-5L; QALY, quality-adjusted life-year.
aDay −30 scores were derived via recall during first (day 0) interview and are used as baseline QALY scores when QALYs are calculated for participants when they served as their own controls. Skewness and kurtosis tests indicate we cannot reject that the adjusted mean scores are distributed normally.
bDifferences between the day −30 score and the day 0, 7, 15, 30, 90, 180, and 365 scores are statistically significant, P < .0001, for each of the 7 unadjusted and adjusted contrasts.
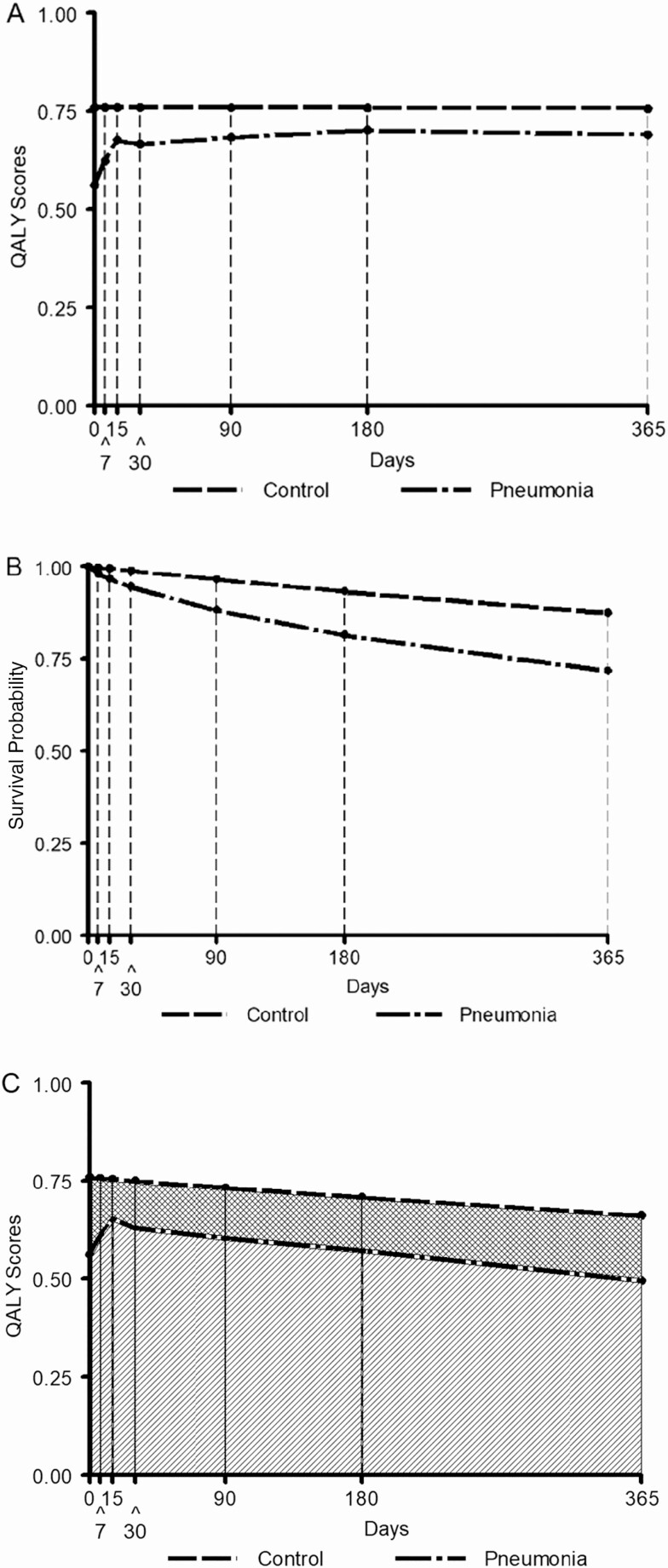
Figure 1 shows QALY curves among patients both when they had pneumonia and when they served as their own control (Figure 1A, adjusted average QALY score curves; Figure 1B, adjusted pneumonia survival curve; Figure 1C, survival-weighted QALY score curves). See Supplemental Tables 5 and 6 for calculation of unadjusted and adjusted QALYs.
Figure 1.
Construction of the QALY estimates for days 0 to 365. A, Average adjusted QALY score curves among patients both when they had pneumonia and when they served as their own control (ie, reflecting the naturally occurring gender-specific downward trajectory of QALY scores with aging [“natural decline”] had they not had pneumonia). B, Average adjusted survival curves among patients both when they had pneumonia and when they served as their own control. C, Average adjusted survival-weighted QALY score curves and areas under these curves among patients both when they had pneumonia and when they served as their own control. The hatched area in panel C represents QALYs experienced by patients both when they developed pneumonia and when they served as their own controls. The cross-hatched area represents QALYS lost due to pneumonia. Abbreviation: QALY, quality-adjusted life-year.
Significant predictors of elevated risks for death included higher pneumonia severity index scores, older age, the presence of cancer, and the presence of immunosuppressive disease. Hazard ratios for death are reported in Supplementary Table 7. See Supplementary Table 8 for details of follow-up time in the sample.
The average adjusted 365-day QALYs equaled 0.583 and 0.713 for participants with pneumonia and for participants when used as their own controls (compared to a maximum of 1 [365/365]). The difference was 0.13 QALYs (SE, .013; 95% CI, .105–.155; P < .001) equivalent to 47.5 (0.13 × 365) quality-adjusted days. The average adjusted QALYs through day 30 after diagnosis equaled 0.053 and 0.064 for participants with pneumonia and for participants when used as their own controls (compared to a maximum of 0.082 [30/365]), respectively (Table 3). The difference (ie, QALYs lost) between pneumonia and control QALYs was 0.011 (3.7 quality-adjusted days) (SE, .001; 95% CI, .009–.013; P < .001). The QALYs lost through days 90 and 180 were 0.03 and 0.06, respectively (both P < .001), equivalent to 11.0 and 22.0 quality-adjusted days.
Table 3.
Unadjusted and Adjusted QALY Estimates and QALY Losses Among Patients With Pneumonia Through Day 365 After Diagnosis
| Study Day | Control QALYs | Pneumonia QALYs | QALY Differencesa | SEb | P | 95% Confidence Interval (Lower-Upper) |
|---|---|---|---|---|---|---|
| Unadjusted results | ||||||
| 30 | 0.064 | 0.054 | 0.010 | 0.001 | <.001 | .008–.012 |
| 90 | 0.187 | 0.158 | 0.030 | 0.003 | <.001 | .024–.035 |
| 180 | 0.369 | 0.306 | 0.063 | 0.006 | <.001 | .051–.075 |
| 365 | 0.729 | 0.587 | 0.142 | 0.01 | <.110 | .116–.169 |
| Adjusted results | ||||||
| 30 | 0.064 | 0.053 | 0.011 | 0.001 | <.001 | .009–.013 |
| 90 | 0.186 | 0.156 | 0.030 | 0.003 | <.001 | .025–.036 |
| 180 | 0.365 | 0.304 | 0.060 | 0.006 | <.001 | .049–.072 |
| 365 | 0.713 | 0.583 | 0.130 | 0.013 | <.001 | .105–.155 |
Abbreviations: EQ-5D-5L, EuroQol-5D-5L; QALY, quality-adjusted life-year.
aDifferences due to rounding.
bUncertainty estimates for QALYs calculated when participants served as their own controls. Patients include sampling uncertainty for the day −30 scores and for the age and gender distribution in the sample (and thus indirectly on the mortality rates). They do not include sampling uncertainty that stems from the measurement of the mortality rates themselves or in the measurements that went into the calculation of the natural decline in ED-5D-5L scores with aging.
The average adjusted 1-year QALY losses due to pneumonia differed significantly between patients with COP who received outpatient services only and those requiring hospitalization for their initial COP treatment (0.091 and 0.147, respectively; QALY losses difference, 0.056; SE, 0.015; 95% CI, .028–.884; P < .001).
Within the ranges analyzed, our sensitivity analyses suggested that our assumptions about the relative risk (RR) for mortality and the natural decline of QALY scores with age for participants when used as their own controls had little impact on our results (Table 4). Varying the RR for the age- and gender-specific mortality rates between 1 (the same mortality rates as in the Japanese population) and 4 (4 times the mortality rates in the Japanese population) yielded estimates of average adjusted QALY losses through day 365 between 0.094 (RR = 4) and 0.129 (RR = 1).
Table 4.
Sensitivity Analyses, Relative Risk for Mortality, and Natural Decline in QALY Scores With Aging Among Patients When They Served as Their Own Controls
| Analysis | QALY Differences | QALY Differences, SE | P | 95% Confidence Interval (Lower-Upper) |
|---|---|---|---|---|
| Unadjusted results | ||||
| Baseline 30-day QALYs | 0.0101 | 0.0008 | <.001 | .0085–.0118 |
| Mortality relative risk = 1 | 0.0102 | 0.0008 | <.001 | .0086–.0119 |
| Mortality relative risk = 4 | 0.0100 | 0.0008 | <.001 | .0084–.0115 |
| No natural decline | 0.0102 | 0.0008 | <.001 | .0086–.0118 |
| Doubled natural decline | 0.0101 | 0.0008 | <.001 | .0085–.0117 |
| Baseline 90-day QALYs | 0.0296 | 0.0027 | <.001 | .0244–.0349 |
| Mortality relative risk = 1 | 0.0305 | 0.0027 | <.001 | .0252–.0357 |
| Mortality relative risk = 4 | 0.0279 | 0.0027 | <.001 | .0226–.0332 |
| No natural decline | 0.0298 | 0.0026 | <.001 | .0247–.0349 |
| Doubled natural decline | 0.0294 | 0.0027 | <.001 | .0241–.0347 |
| Baseline 180-day QALYs | 0.0631 | 0.0050 | <.001 | .0533–.0728 |
| Mortality relative risk = 1 | 0.0665 | 0.0051 | <.001 | .0565–.0764 |
| Mortality relative risk = 4 | 0.0564 | 0.0052 | <.001 | .0462–.0665 |
| No natural decline | 0.0637 | 0.0050 | <.001 | .0540–.0734 |
| Doubled natural decline | 0.0624 | 0.0051 | <.001 | .0524–.0724 |
| Baseline 365-day QALYs | 0.1424 | 0.0095 | <.001 | .1237–.1611 |
| Mortality relative risk = 1 | 0.1561 | 0.0099 | <.001 | .1366–.1756 |
| Mortality relative risk = 4 | 0.1161 | 0.0102 | <.001 | .0961–.1361 |
| No natural decline | 0.1447 | 0.0099 | <.001 | .1254–.1640 |
| Doubled natural decline | 0.1401 | 0.0098 | <.001 | .1210–.1592 |
| Adjusted results | ||||
| Baseline 30-day QALYs | 0.0108 | 0.0009 | <.001 | .0091–.0125 |
| Mortality relative risk = 1 | 0.0110 | 0.0009 | <.001 | .0093–.0127 |
| Mortality relative risk = 4 | 0.0105 | 0.0009 | <.001 | .0088–.0125 |
| No natural decline | 0.0109 | 0.0008 | <.001 | .0092–.0125 |
| Doubled natural decline | 0.0108 | 0.0009 | <.001 | .0091–.0125 |
| Baseline 90-day QALYs | 0.0303 | 0.0027 | <.001 | .0250–.0356 |
| Mortality relative risk = 1 | 0.0317 | 0.0027 | <.001 | .0264–.0371 |
| Mortality relative risk = 4 | 0.0275 | 0.0027 | <.001 | .0223–.0328 |
| No natural decline | 0.0305 | 0.0026 | <.001 | .0253–.0356 |
| Doubled natural decline | 0.0301 | 0.0027 | <.001 | .0248–.0355 |
| Baseline 180-day QALYs | 0.0605 | 0.0056 | <.001 | .0494–.0715 |
| Mortality relative risk = 1 | 0.0660 | 0.0057 | <.001 | .0547–.0772 |
| Mortality relative risk = 4 | 0.0502 | 0.0057 | <.001 | .0389–.0614 |
| No natural decline | 0.0610 | 0.0055 | <.001 | .0501–.0718 |
| Doubled natural decline | 0.0599 | 0.0058 | <.001 | .0485–.0713 |
| Baseline 365-day QALYs | 0.1302 | 0.0124 | <.001 | .1060–.1545 |
| Mortality relative risk = 1 | 0.1512 | 0.0126 | <.001 | .1266–.1758 |
| Mortality relative risk = 4 | 0.0935 | 0.0128 | <.001 | .0684–.1186 |
| No natural decline | 0.1319 | 0.0122 | <.001 | .1081–.1558 |
| Doubled natural decline | 0.1285 | 0.0128 | <.001 | .1034–.1536 |
Abbreviation: QALY, quality-adjusted life-year.
The range of the QALY losses related to assuming no natural decline in QALY scores or twice the natural decline had even smaller effects on our estimates of QALY losses. Average 1-year QALY loses ranged from 0.129 (doubled natural decline) to 0.132 (no natural decline).
DISCUSSION
This is the first prospective study measuring QALY scores and losses among Japanese adult patients with clinically and radiographically confirmed pneumonia. The results of this study represent the strongest data available for the evaluation of pneumonia-prevention strategies in Japan. Future policy decisions for pneumococcal vaccination in adults may therefore use our study’s estimates to model the benefit of alternative vaccination strategies.
On average, adjusted QALY scores fell from 0.759 at day −30 before diagnosis to 0.561 at diagnosis and increased to 0.689 by day 365 after diagnosis. Estimated losses in adjusted QALYs were 0.011, 0.030, 0.060, and 0.130 QALYs (equivalent to 3.7, 11.0, 22.0, and 47.5 quality-adjusted days) through days 30, 90, 180, and 365, respectively. They represented between 16% (0.156/0.186) and 18% (0.0583/0.713) reductions of the QALYs projected for participants when they were used as their own controls. Differences in mortality also continued to increase throughout the 365 days. Thus, it is likely that greater losses would have been observed had there been longer follow-up.
For comparison purposes, a Japanese study reported QALY score losses of 0.06 for stroke and renal disease and of 0.13 for musculoskeletal disease among Japanese patients [25]. The 1-year QALY losses observed in our study of Japanese adult patients with CAP were similar to those for US adults for heart failure, stroke, or chronic renal failure [26]. One-year QALY losses were significantly larger among patients with COP requiring hospitalization for their initial COP treatment than those who received outpatient care only.
QALYs are a function of the quality and length of survival. As expected, lower QALY scores after pneumonia diagnosis were associated with lower QALY scores at day −30, interviews closer to the pneumonia diagnosis, higher pneumonia severity index scores, older age, and hospital treatment for the initial diagnosis of pneumonia. Similarly, lower survival probabilities were associated with older age, pre-existing diagnoses of either cancer or immunosuppressive disease, and higher pneumonia severity index scores. Therefore, pre-existing underlying heath status is an important determinant of QALYs after pneumonia.
Our analysis and the one by Mangen et al [27] are the first 2 long-term prospective longitudinal studies of postpneumonia QALY scores in the literature, and ours is the first in Japan. Other studies have reported cross-sectional or longitudinal assessments of quality of life but did not assess QALYs [24, 28–33]. One study used the preference-weighted EQ-5D 3-level instrument to assess QALY scores 1 year after diagnosis, but given there was no baseline assessment this study was unable to assess QALYs or QALY losses [34]. Another study used the time-tradeoff method among parents and healthy members of the community to assess QALY losses for moderate and severe pneumonia among children [35].
There were a number of difference between Mangen et al’s [27] and our study: country, source of patients, treatment setting, period of follow-up, and preference score administration. Additionally, Mangen et al used for controls a separate population without pneumonia versus the self-control design we used. Nevertheless, both studies reported a 1-year QALY loss of 0.13, although Mangen et al’s 0.13 arose from QALY estimates of 0.81 for subjects without disease versus a 0.68 QALY for patients with suspected pneumonia, whereas ours arose from QALY estimates of 0.71 and 0.58, respectively.
Estimates of QALY scores and QALY loses are central to the evaluation of the cost-effectiveness of pneumococcal vaccination. None of the cost-effectiveness studies used pneumonia-specific scores, but rather have generally used scores based on perceived health status associated with limited activities of daily living [36]. In general, the QALY losses assumed by these and 2 key US studies [37, 38] are much smaller than the estimates calculated in the study by Mangen et al and in our study.
Given that our study was performed in Japan and used Japanese HRQoL instruments, our results may apply primarily to Japan. Outside of Japan, additional studies may well be conducted in other populations and countries. Nevertheless, until that time, Mangen et al’s [27] and our results are the best available data for informing current models, such as burden of illness studies and cost-effectiveness analyses in Japan and elsewhere, that require estimates of QALY losses after onset of pneumonia.
Our study had several limitations. First, as study enrollment was triggered by the diagnosis of pneumonia, we were unable to assess HRQoL before pneumonia onset. We instead used a recalled assessment at 30 days before hospitalization, which is supported by several studies that have found reasonable accuracy of recalled EQ-5D and SF-36 scores [39–42]. Second, we used adjusted Japanese lifetables for calculation of survival probabilities. These lifetables are reported by gender and age, but not by comorbidity, for the average population. However, within age and gender subgroups, those who develop pneumonia may have a different comorbidity burden than those who do not. Thus, the average survival probabilities for the general population may not be applicable to participants who developed pneumonia. We attempted to address this issue by assuming a 2-fold increased mortality risk in our population and varying this risk in sensitivity analysis between 1 (background mortality rate) and 4 times.
In conclusion, our study is 1 of only 2 studies that report longitudinal prospective data on QALY scores and associated QALYs lost due to pneumonia. We found a substantial 1-year loss following pneumonia diagnosis equivalent to 47.5 quality-adjusted days. By day 365, QALY scores had not yet returned to baseline levels and survival differences were increasing over time, suggesting that QALY scores may remain diminished and QALY losses may continue to increase beyond 1 year. We acknowledge that different settings may have varying QALY decrements, and thus that additional studies would be useful. Until this occurs, our results, combined with those of Mangen et al [27], provide the most robust results for assessing QALY decrements as part of a full public health evaluation of adult pneumonia-prevention strategies, such as pneumococcal and influenza vaccination programs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions . H. A. G. and J. A. S. drafted the manuscript, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors developed the study concept and design, critically reviewed drafts of the manuscript and approved the final version, and were involved in the acquisition, analysis, and/or interpretation of the data.
Acknowledgments. The authors acknowledge Professor Kiyoshi Ichihara (Yamaguchi University, Japan) for data management; Natsuko Komoto, Shiho Shimohara, Yuri Shinoda, Seiho Kubo, and Maki Koshikubi for administering the interviews with patients; and Shigeo Ura for the management of the collaboration across medical institutions in Goto City. Ann L. Davis, MPH, CMPP, an employee of Pfizer Inc, provided editorial support in an earlier version.
Financial support. This work was supported by Prizer Inc, through research contracts with Nagasaki University and the University of Pennsylvania (H. A. G.).
Potential conflicts of interest. J. A. S., E. G., B. D. G., L. J., R. E. I., and A. A. are Pfizer Inc employees and as such may hold company stock. H. A. G. has served as a paid consultant to and has received research support and travel support from Pfizer Inc. T. M. has received payment for research collaboration and support and speaking engagements from Pfizer Inc, as well as consulting fees and research support from Astellas, Merck Sharp & Dohme, and Toyama Chemical. K. H. has received payment for research collaboration and support from Pfizer Inc. S. K. has received payment for research collaboration and support and speaking engagements from Pfizer Inc, as well as consulting fees from Astellas, Merck Sharp & Dohme, and Toyama Chemical. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Morimoto K, Suzuki M, Ishifuji T, et al. ; Adult Pneumonia Study Group–Japan (APSG-J) . The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS One 2015; 10:e0122247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017; 65:1806–12. [DOI] [PubMed] [Google Scholar]
- 3. Ministry of Health, Labour, and Welfare. Handbook of Health and Welfare Statistics 2016 of Japan. Table 1–26. Number of deaths and death rates (per 100 000 population) by sex and causes (the condensed list of causes of death for Japan. 2017. Available at: https://www.mhlw.go.jp/english/database/db-hh/1-2.html. Accessed 6 June 2020.
- 4. Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press, 2016. [Google Scholar]
- 5. Neumann PJ, Cohen JT. QALYs in 2018-advantages and concerns. JAMA 2018; 319:2473–4. [DOI] [PubMed] [Google Scholar]
- 6. Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67:71–9. [DOI] [PubMed] [Google Scholar]
- 8. Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res 1993; 2:169–80. [DOI] [PubMed] [Google Scholar]
- 9. Huang SS, Johnson KM, Ray GT, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011; 29:3398–412. [DOI] [PubMed] [Google Scholar]
- 10.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416. [DOI] [PubMed] [Google Scholar]
- 12. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyazaki T, Hirano K, Kohno S, et al. Pneumonia incidence and pneumoccal serotype distribution in adults aged 18 years of older in Goto City, Japan. In: 28th European Congress of Clinical Microbiology and Infectious Diseases. Madrid, Spain; 21–24 April 2018.
- 14. Miyazaki T, Hirano K, Kohno S, et al. High pneumonia incidence in adults 18 years and older in Goto Island, Japan: early results from a population based prospective study. In: American Society of Microbiology (ASM) Microbe 2018. Atlanta, GA; 7–11 June 2018.
- 15. EuroQol Research Foundation. EQ-5D-5L. 2017. Available at: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Accessed 5 June 2018.
- 16. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30:473–83. [PubMed] [Google Scholar]
- 17. Petrou S, Hockley C. An investigation into the empirical validity of the EQ-5D and SF-6D based on hypothetical preferences in a general population. Health Econ 2005; 14:1169–89. [DOI] [PubMed] [Google Scholar]
- 18. Brazier JE, Fukuhara S, Roberts J, et al. Estimating a preference-based index from the Japanese SF-36. J Clin Epidemiol 2009; 62:1323–31. [DOI] [PubMed] [Google Scholar]
- 19. Brooks R. EuroQol: the current state of play. Health Policy 1996; 37:53–72. [DOI] [PubMed] [Google Scholar]
- 20. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikeda S, Shiroiwa T, Igarashi A, et al. Developing a Japanese version of the EQ-5D-5L value set. J Natl Inst Public Health 2015; 64:47–55. [Google Scholar]
- 22. Glick HA, Doshi JA, Sonnad SS, Polsky D. Assessing quality-adjusted life years. In: Economic Evaluation in Clinical Trials. New York: Oxford University Press, 2015: 63–95. [Google Scholar]
- 23. Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res 2016; 25:707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ministry of Health, Labour, and Welfare. Handbook of health and welfare statistics of Japan. Table 1–27 Number of deaths and death rates (per 100,000 population) by sex and age group, by year. 2017. Available at: https://www.mhlw.go.jp/english/database/db-hh/1-2.html. Accessed 3 June 2020.
- 25. Fujikawa A, Suzue T, Jitsunari F, Hirao T. Evaluation of health-related quality of life using EQ-5D in Takamatsu, Japan. Environ Health Prev Med 2011; 16:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006; 26:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mangen MJ, Huijts SM, Bonten MJ, de Wit GA. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis 2017; 17:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carratalà J, Fernández-Sabé N, Ortega L, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med 2005; 142:165–72. [DOI] [PubMed] [Google Scholar]
- 29. El Moussaoui R, Opmeer BC, de Borgie CA, et al. Long-term symptom recovery and health-related quality of life in patients with mild-to-moderate-severe community-acquired pneumonia. Chest 2006; 130:1165–72. [DOI] [PubMed] [Google Scholar]
- 30. Gleason PP, Kapoor WN, Stone RA, et al. Medical outcomes and antimicrobial costs with the use of the American Thoracic Society guidelines for outpatients with community-acquired pneumonia. JAMA 1997; 278:32–9. [PubMed] [Google Scholar]
- 31. Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA 2000; 283:749–55. [DOI] [PubMed] [Google Scholar]
- 32. Metlay JP, Fine MJ, Schulz R, et al. Measuring symptomatic and functional recovery in patients with community-acquired pneumonia. J Gen Intern Med 1997; 12:423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Torres A, Muir JF, Corris P, et al. Effectiveness of oral moxifloxacin in standard first-line therapy in community-acquired pneumonia. Eur Respir J 2003; 21:135–43. [DOI] [PubMed] [Google Scholar]
- 34. Honselmann KC, Buthut F, Heuwer B, et al. Long-term mortality and quality of life in intensive care patients treated for pneumonia and/or sepsis: Predictors of mortality and quality of life in patients with sepsis/pneumonia. J Crit Care 2015; 30:721–6. [DOI] [PubMed] [Google Scholar]
- 35. Prosser LA, Ray GT, O’Brien M, Kleinman K, Santoli J, Lieu TA. Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics 2004; 113:283–90. [DOI] [PubMed] [Google Scholar]
- 36. Erickson P, Wilson R, Shannon I. Years of healthy life. Healthy People 2000 Statistical Notes. US Centers for Disease Control and Prevention. 1995; 7:1–15. Available at: https://www.cdc.gov/nchs/data/statnt/statnt07.pdf. Accessed 3 June 2020. [DOI] [PubMed] [Google Scholar]
- 37. Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med 2016; 31:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stoecker C, Kobayashi M, Matanock A, Cho BH, Pilishvili T. Cost-effectiveness of continuing pneumococcal conjugate vaccination at age 65 in the context of indirect effects from the childhood immunization program. Vaccine 2020; 38:1770–7. [DOI] [PubMed] [Google Scholar]
- 39. Stull DE, Leidy NK, Parasuraman B, Chassany O. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin 2009; 25:929–42. [DOI] [PubMed] [Google Scholar]
- 40. Coyne KS, Soliman AM, Margolis MK, Thompson CL, Chwalisz K. Validation of the 4 week recall version of the Uterine Fibroid Symptom and Health-related Quality of Life (UFS-QOL) Questionnaire. Curr Med Res Opin 2017; 33:193–200. [DOI] [PubMed] [Google Scholar]
- 41. Kwong E, Black N. Retrospectively patient-reported pre-event health status showed strong association and agreement with contemporaneous reports. J Clin Epidemiol 2017; 81:22–32. [DOI] [PubMed] [Google Scholar]
- 42. Mendoza TR, Dueck AC, Bennett AV, et al. Evaluation of different recall periods for the US National Cancer Institute’s PRO-CTCAE. Clin Trials 2017; 14:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.