Abstract
BACKGROUND
Rifapentine-based regimens have potent antimycobacterial activity that may allow for a shorter course in patients with drug-susceptible pulmonary tuberculosis.
METHODS
In an open-label, phase 3, randomized, controlled trial involving persons with newly diagnosed pulmonary tuberculosis from 13 countries, we compared two 4-month rifapentine-based regimens with a standard 6-month regimen consisting of rifampin, isoniazid, pyrazinamide, and ethambutol (control) using a noninferiority margin of 6.6 percentage points. In one 4-month regimen, rifampin was replaced with rifapentine; in the other, rifampin was replaced with rifapentine and ethambutol with moxifloxacin. The primary efficacy outcome was survival free of tuberculosis at 12 months.
RESULTS
Among 2516 participants who had undergone randomization, 2343 had a culture positive for Mycobacterium tuberculosis that was not resistant to isoniazid, rifampin, or fluoroquinolones (microbiologically eligible population; 768 in the control group, 791 in the rifapentine–moxifloxacin group, and 784 in the rifapentine group), of whom 194 were coinfected with human immunodeficiency virus and 1703 had cavitation on chest radiography. A total of 2234 participants could be assessed for the primary outcome (assessable population; 726 in the control group, 756 in the rifapentine–moxifloxacin group, and 752 in the rifapentine group). Rifapentine with moxifloxacin was noninferior to the control in the microbiologically eligible population (15.5% vs. 14.6% had an unfavorable outcome; difference, 1.0 percentage point; 95% confidence interval [CI], −2.6 to 4.5) and in the assessable population (11.6% vs. 9.6%; difference, 2.0 percentage points; 95% CI, −1.1 to 5.1). Noninferiority was shown in the secondary and sensitivity analyses. Rifapentine without moxifloxacin was not shown to be noninferior to the control in either population (17.7% vs. 14.6% with an unfavorable outcome in the microbiologically eligible population; difference, 3.0 percentage points [95% CI, −0.6 to 6.6]; and 14.2% vs. 9.6% in the assessable population; difference, 4.4 percentage points [95% CI, 1.2 to 7.7]). Adverse events of grade 3 or higher occurred during the on-treatment period in 19.3% of participants in the control group, 18.8% in the rifapentine–moxifloxacin group, and 14.3% in the rifapentine group.
CONCLUSIONS
The efficacy of a 4-month rifapentine-based regimen containing moxifloxacin was noninferior to the standard 6-month regimen in the treatment of tuberculosis. (Funded by the Centers for Disease Control and Prevention and others; Study 31/A5349 ClinicalTrials.gov number, NCT02410772.)
Highly potent treatment regimens in patients with tuberculosis could allow the treatment course to be shortened to less than the currently required 6 months, thereby facilitating improved adherence and potentially reducing adverse drug effects and costs.1–3 As an antimicrobial class, rifamycins are a cornerstone of antituberculosis treatment because of their ability to sterilize lesions and provide a durable cure.4 Results of preclinical models of tuberculosis and phase 2 clinical trials show a relationship between exposure to rifamycins and reduction in bacillary burden.5–7 Increasing exposure to rifamycins may be a promising strategy to shorten the course of tuberculosis treatment.
Rifapentine is a cyclopentyl derivative of rifampin, the most widely used rifamycin worldwide. Rifapentine has activity against Mycobacterium tuberculosis, and its longer half-life makes the drug an attractive option for increasing the duration of exposure to rifamycins while maintaining the once-daily dosing schedule that facilitates the completion of treatment.8 Moxifloxacin is a fluoroquinolone with activity against M. tuberculosis.9–12 The addition of moxifloxacin to other first-line antibiotics against tuberculosis, including rifampin, accelerates sputum-culture conversion to negative status early in the course of treatment but is insufficient to shorten therapy to 4 months.13–18 Combination treatment that includes both rifapentine and moxifloxacin was shown to be active in the murine model of tuberculosis and provided a stable cure after 3 months of treatment.19,20 In phase 2 clinical trials, no obvious safety concerns were noted with the use of rifapentine during the first 8 weeks of combination chemotherapy for pulmonary tuberculosis, and increasing the pharmacokinetic exposure to rifapentine was shown to be associated with sputum sterilization at the time of completion of the intensive phase.21,22
We conducted a phase 3 clinical trial to determine whether treatment regimens that included rifapentine, at a once-daily dose of 1200 mg, with or without moxifloxacin, at a once-daily dose of 400 mg, can provide a durable cure in participants with drug-susceptible pulmonary tuberculosis in 4 months, as compared with the standard 6-month regimen. Safety measures, including premature discontinuation of the assigned regimen for a reason other than microbiologic ineligibility, were also assessed and compared.
METHODS
TRIAL DESIGN AND OVERSIGHT
The Tuberculosis Trials Consortium Study 31/AIDS Clinical Trials Group A5349 (Study 31/A5349) was an international, multicenter, randomized, open-label, phase 3, noninferiority trial conducted at sites of the Centers for Disease Control and Prevention (CDC) Tuberculosis Trials Consortium and the National Institutes of Health AIDS Clinical Trials Group. Full details of the design and implementation of the trial have been published previously23 and are provided in the protocol, available with the full text of this article at NEJM.org. The trial protocol was approved by the institutional review board at the CDC. An institutional review board or ethics committee at each participating trial site reviewed and approved the protocol and informed consent documents, or a trial site relied formally on the approval from the CDC. All the participants provided written informed consent.
Members of the protocol team from the Tuberculosis Trials Consortium and the AIDS Clinical Trials Group designed and implemented the trial and collected and analyzed the data. The protocol team included some of the authors. The first draft of the manuscript was written by the first and corresponding authors. No one who was not an author contributed to the writing of the manuscript. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. Sanofi provided rifapentine, rifampin, moxifloxacin, and all companion drugs, and a company representative participated on the protocol team. The commercial interests of Sanofi did not influence the trial design; the collection, analysis, or interpretation of the data; the preparation of the manuscript; or the decision to submit the manuscript for publication. The trial was conducted in accordance with the principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines, and applicable regulatory requirements.
PARTICIPANTS
The participants were 12 years of age or older and had newly diagnosed pulmonary tuberculosis that was confirmed on culture to be susceptible to isoniazid, rifampin, and fluoroquinolones. Participants were required to have at least one sputum specimen that was positive for acid-fast bacilli on smear microscopy or positive for M. tuberculosis on a rapid nucleic acid amplification test (Xpert MTB/RIF, Cepheid), with a semiquantitative result of medium or high, which approximately matches the criteria for a positive smear.24,25 Persons with human immunodeficiency virus (HIV) infection were required to have a CD4 T-cell count of at least 100 cells per cubic millimeter and were enrolled to evaluate drug–drug interactions between rifapentine, 1200 mg once daily, and efavirenz.23 Full details of the criteria for eligibility are provided in the protocol and the Supplementary Appendix, available at NEJM.org.
RANDOMIZATION AND TREATMENT
The participants were randomly assigned in a 1:1:1 ratio to one of three regimens with the use of a central Web-based system and the “big stick” design by Soares and Wu,26 with a maximum allowable imbalance of two (Fig. S1 in the Supplementary Appendix). Randomization was stratified according to trial site, presence of cavitation on chest radiography at baseline, and HIV status. The control regimen involved 8 weeks of once-daily rifampin, isoniazid, pyrazinamide, and ethambutol followed by 18 weeks of once-daily rifampin and isoniazid.1,2 The rifapentine regimen involved 8 weeks of once-daily rifapentine, isoniazid, pyrazinamide, and ethambutol followed by 9 weeks of once-daily rifapentine and isoniazid. The rifapentine–moxifloxacin regimen involved 8 weeks of once-daily rifapentine, isoniazid, pyrazinamide, and moxifloxacin followed by 9 weeks of once-daily rifapentine, isoniazid, and moxifloxacin. Rifapentine was administered at a daily dose of 1200 mg, and moxifloxacin at a daily dose of 400 mg.7,22 Other drugs were administered at standard doses adjusted for body weight (Table S1).1 Because food affects the absorption of rifapentine and rifampin differently, rifapentine was administered within 1 hour after ingesting food, and rifampin was administered on an empty stomach.1,22,27,28 The medications in each regimen were administered 7 days per week, including at least 5 days of in-person directly observed therapy per week.
TRIAL PROCEDURES
The participants were monitored according to the schedule provided in Table S2. Sputum specimens were collected for mycobacterial cultures and blood samples for complete blood counts and biochemical analyses. Two sputum specimens were collected at all scheduled visits at or after week 17. Mycobacteriologic procedures across the trial-site laboratories were harmonized according to 20 key elements (Table S16).23 Smear microscopy and mycobacterial culture on liquid media (Mycobacteria Growth Indicator Tubes [MGIT] System, Becton Dickinson) and solid media were performed at designated trialsite laboratories. Phenotypic testing of drug susceptibility to at least isoniazid, rifampin, and fluoroquinolones was performed on the M. tuberculosis isolates obtained at baseline and on the first of any M. tuberculosis isolates obtained at or after week 17. Whole-genome sequencing was used to compare the M. tuberculosis isolate obtained from a participant at baseline with any isolate obtained at or after week 17.29 The microbiologists who handled the sputum specimens and the clinical trial operations team at the Data and Coordinating Center were unaware of the treatment-group assignments and trial week.
TRIAL OUTCOMES
The primary efficacy outcome was survival free of tuberculosis at 12 months after randomization (see the Supplementary Appendix).30 The total duration of follow-up was 18 months. A secondary efficacy outcome analysis that considers survival free of tuberculosis at 18 months has not yet been performed. The status with respect to the primary outcome (favorable, unfavorable, or not assessable) was determined for each participant. Favorable status was assigned if a participant met all the following criteria: was alive and free of tuberculosis at 12 months after randomization; did not meet the criteria for unfavorable or not-assessable status; and had either an M. tuberculosis–negative result on the sputum culture at month 12 or, at month 12, was unable to produce sputum or produced sputum that was contaminated but without evidence of M. tuberculosis. Unfavorable status was assigned if a participant had M. tuberculosis–positive cultures from two sputum specimens obtained at or after week 17 without an intervening negative culture, died or was withdrawn from the trial or lost to followup during the treatment period, had an M. tuberculosis–positive culture when last seen, died from tuberculosis during the post-treatment follow-up, or received additional treatment for tuberculosis. Status was not assessable if a participant did not already have an unfavorable outcome and met any one of the following criteria: did not attend the month 12 visit but had a negative culture when last seen, had a change in treatment because of pregnancy, died from a cause unrelated to tuberculosis during the follow-up period, received additional treatment for tuberculosis after exogenous reinfection was identified on whole-genome sequencing, or died from a violent cause or had an accidental death during the treatment period. Stable conversion of sputum cultures to negative was defined as two negative cultures without an intervening positive culture.
The primary safety outcome was an adverse event of grade 3 or higher with an onset during the on-treatment period (defined as the period during which the trial medications were administered and up to 14 days after the last dose). Adverse events were graded by the site investigators according to National Cancer Institute Common Terminology Criteria for Adverse Events.31 Premature discontinuation was recorded when an assigned regimen was discontinued prematurely for a reason other than microbiologic ineligibility. The trial was reviewed annually by a data and safety monitoring board.
ANALYSIS POPULATIONS
The primary analysis was performed in the microbiologically eligible and the assessable analysis populations. The microbiologically eligible population included all the participants except those who had no evidence of M. tuberculosis–positive cultures, who had tuberculosis that was resistant to isoniazid, rifampin, or fluoroquinolones, or who were enrolled in violation of the eligibility criteria; the participants with an outcome status that was not assessable were reclassified as having had an unfavorable outcome. The assessable population included the participants in the microbiologically eligible population who met the criteria for favorable or unfavorable status with respect to the primary outcome. Secondary analysis populations included the per-protocol 95% and per-protocol 75% populations, in which participants who did not complete 95% or 75% of treatment doses, respectively, were excluded unless the reason for inadequate treatment was death or bacteriologic treatment failure, and the intention-to-treat analysis population, which included all participants who had undergone randomization. A total of 15 sensitivity analyses were prespecified in the statistical analysis plan, available with the protocol.
STATISTICAL ANALYSIS
Assuming that 15% of the participants who could be assessed would have an unfavorable outcome, that an additional 12% would be excluded from the microbiologically eligible population, and that a further 12% would have an outcome status that could not be assessed, we estimated that a sample size of 2500 participants would provide the trial with 90% power (in the assessable population) and 80% power (in the microbiologically eligible population) to test the primary hypotheses that the 4-month rifapentine–moxifloxacin regimen or the 4-month rifapentine regimen would be noninferior to the 6-month standard control regimen, with a non-inferiority margin of 6.6 percentage points and a two-sided type I error rate of 5%.16,32,33 In the primary efficacy analysis, we calculated the absolute between-group difference, with the 95% confidence interval, in the percentage of participants who had a favorable outcome, with adjustment for cavitation on chest radiography and HIV status using Cochran–Mantel–Haenszel weights.34 Noninferiority was shown if the upper boundary of the 95% confidence interval around the difference was 6.6 percentage points or less in both the microbiologically eligible and the assessable analysis populations. To account for multiplicity, a hierarchical ordering of hypotheses was prespecified in the protocol — the rifapentine–moxifloxacin group was compared with the control group first, and if noninferiority was demonstrated, the rifapentine group was compared with the control group.
A noninferiority margin of 6.6 percentage points was calculated to preserve more than 50% of the treatment effect of the control regimen and was considered to be an acceptable difference in efficacy, given the shorter treatment duration (see the Supplementary Appendix). The safety analysis population included all the participants who had undergone randomization and received at least one dose of the assigned treatment; the analysis of premature discontinuation of the assigned regimen was performed in the microbiologically eligible population. In the safety analyses, we calculated the absolute between-group differences, with 95% confidence intervals, with adjustment for baseline randomization factors. The time to an unfavorable outcome was calculated as the time from randomization to the event that caused the unfavorable outcome. For the time-to-event analyses, Cox regression was used to calculate a hazard ratio and 95% confidence interval stratified according to the randomization factors of HIV status and cavitation on chest radiography, and Schoenfeld residuals were used to test the proportional-hazards assumption. Apart from the primary efficacy analyses, between-group differences and confidence intervals were not adjusted for multiplicity and therefore cannot be used to infer treatment effects.
RESULTS
TRIAL POPULATION
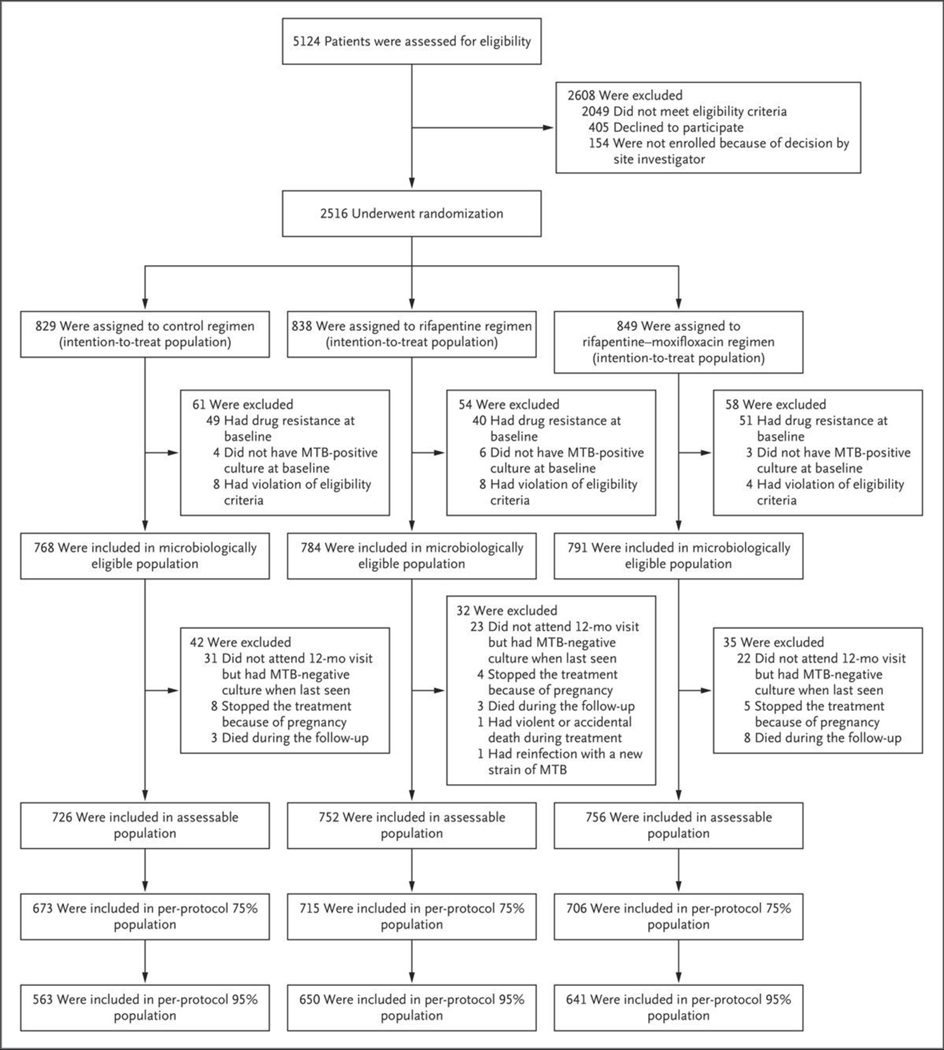
Between January 25, 2016, and October 30, 2018, a total of 5124 patients underwent screening at 34 trial sites in Brazil, China (Hong Kong), Haiti, India, Kenya, Malawi, Peru, South Africa, Thailand, Uganda, the United States, Vietnam, and Zimbabwe, and 2516 were randomly assigned to a treatment group. A total of 173 participants who had undergone randomization were excluded from the microbiologically eligible population, which comprised 2343 participants (768 were in the control group, 791 in the rifapentine–moxifloxacin group, and 784 in the rifapentine group) (Fig. 1). The assessable population comprised 2234 participants (726 were in the control group, 756 in the rifapentine–moxifloxacin group, and 752 in the rifapentine group). The baseline demographic and clinical characteristics of the participants were similar in the three treatment groups (Table 1). Among the participants in the microbiologically eligible population, 728 (94.8%) in the control group, 759 (96.0%) in the rifapentine–moxifloxacin group, and 754 (96.2%) in the rifapentine group were retained in the trial through the end of the 12-month follow-up or were known to have died during this period.
Figure 1. Screening, Randomization, and Follow-up.
MTB denotes Mycobacterium tuberculosis.
Table 1.
Characteristics of the Participants at Baseline in the Microbiologically Eligible Population.*
| Characteristic | Control (N = 768) | Rifapentine– Moxifloxacin (N = 791) |
Rifapentine (N = 784) | Total (N = 2343) |
|---|---|---|---|---|
| Male sex — no./total no. (%) | 544/768 (71) | 563/791 (71) | 563/784 (72) | 1670/2343 (71) |
| Median age (range) — yr | 30.9 (13.7–77.5) | 31.0 (14.6–72.5) | 31.0 (14.1–81.4) | 31.0 (13.7–81.4) |
| Age group — no./total no. (%) | ||||
| 12–17 yr | 19/768 (2) | 25/791 (3) | 19/784 (2) | 63/2343 (3) |
| 18–35 yr | 479/768 (62) | 486/791 (61) | 485/784 (62) | 1450/2343 (62) |
| >35 yr | 270/768 (35) | 280/791 (35) | 280/784 (36) | 830/2343 (35) |
| Race — no./total no. (%)† | ||||
| Asian | 86/765 (11) | 89/790 (11) | 93/783 (12) | 268/2338 (11) |
| Black | 553/765 (72) | 552/790 (70) | 571/783 (73) | 1676/2338 (72) |
| White | 15/765 (2) | 13/790 (2) | 8/783 (1) | 36/2338 (2) |
| Multiracial | 111/765 (15) | 136/790 (17) | 111/783 (14) | 358/2338 (15) |
| HIV positivity — no./total no. (%) | 64/768 (8) | 62/791 (8) | 68/784 (9) | 194/2343 (8) |
| Median CD4 count among those with HIV positivity (IQR) | 334 (249–485) | 346 (253–458) | 351 (221–437) | 344 (223–455) |
| Cavitation on chest radiography — no./total no. (%)‡ | ||||
| Absent | 206/768 (27) | 213/791 (27) | 206/784 (26) | 625/2343 (27) |
| <4 cm | 251/768 (33) | 277/791 (35) | 246/784 (31) | 774/2343 (33) |
| ≥4 cm | 307/768 (40) | 295/791 (37) | 327/784 (42) | 929/2343 (40) |
| Median body weight — kg | 52.0 | 53.0 | 53.3 | 53.1 |
| WHO smear grade — no./total no. (%)§¶ | ||||
| Negative | 21/766 (2.7) | 31/789 (3.9) | 36/782 (4.6) | 88/2337 (3.8) |
| Scanty or 1–9 acid-fast bacilli | 121/766 (15.8) | 147/789 (18.6) | 124/782 (15.9) | 392/2337 (16.8) |
| 1+ | 187/766 (24.4) | 168/789 (21.3) | 172/782 (22.0) | 527/2337 (22.6) |
| 2+ | 229/766 (29.9) | 228/789 (28.9) | 227/782 (29.0) | 684/2337 (29.3) |
| 3+ | 198/766 (25.8) | 209/789 (26.5) | 214/782 (27.4) | 621/2337 (26.6) |
| Positive smear, WHO scale not used§ | 10/766 (1.3) | 6/789 (0.8) | 9/782 (1.2) | 25/2337 (1.1) |
| Median body-mass index (range)‖ | 18.9 (12.8–45.2) | 19.0 (14.1–39.1) | 18.9 (13.4–35.4) | 18.9 (12.8–45.2) |
| Current smoker — no./total no. (%) | 196/768 (26) | 175/791 (22) | 200/784 (26) | 571/2343 (24) |
| Prior course of tuberculosis treatment — no./total no. (%) | 83/768 (11) | 97/791 (12) | 85/784 (11) | 265/2343 (11) |
The microbiologically eligible population included all the participants except those who had no evidence of Mycobacterium tuberculosis–positive cultures, who had tuberculosis that was resistant to isoniazid, rifampin, or fluoroquinolones, or who were enrolled in violation of the eligibility criteria. HIV denotes human immunodeficiency virus, and IQR interquartile range.
Race was reported by the trial participants; information about race was not available for 5 participants.
Cavity size refers to the aggregate diameter of all cavities.
Sputum smears were not available for 6 participants.
Sputum smears were graded according to the World Health Organization (WHO) classification, which provides different criteria for each grade depending on the type of microscopy and magnification used at the site laboratory.35
The body-mass index is the weight in kilograms divided by the square of the height in meters.
PRIMARY OUTCOME
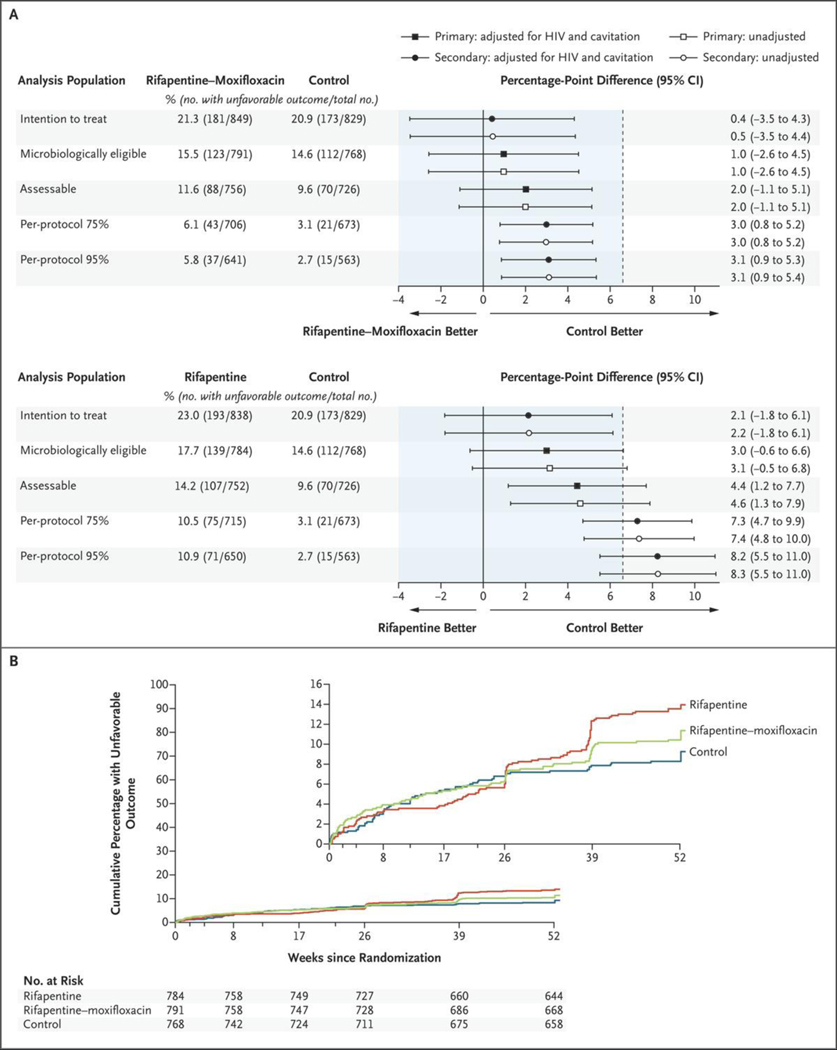
In the comparison between the rifapentine–moxifloxacin group and the control group, non-inferiority was confirmed in both analysis populations. In the microbiologically eligible population, an unfavorable outcome occurred in 15.5% of the participants in the rifapentine–moxifloxacin group and in 14.6% of those in the control group, for an adjusted absolute difference of 1.0 percentage points (95% confidence interval [CI], −2.6 to 4.5). The corresponding values in the assessable population were 11.6% and 9.6%, for an adjusted absolute difference of 2.0 percentage points (95% CI, −1.1 to 5.1) (Table 2 and Fig. 2A). The rifapentine regimen was not shown to be noninferior to the control regimen in either analysis population (adjusted absolute differences of 3.0 percentage points [95% CI, −0.6 to 6.6] in the microbiologically eligible population and 4.4 percentage points [95% CI, 1.2 to 7.7] in the assessable population). The time from randomization to an unfavorable outcome among the participants in the assessable population is shown in Figure 2B, with censoring of the data from the participants who could not be assessed. The results in the per-protocol and intention-to-treat populations and the results of further sensitivity analyses of the primary efficacy outcome were consistent with the findings in the microbiologically eligible and the assessable analysis populations (Fig. 2A, Tables S3 and S4, and Figs. S2 and S3).
Table 2.
Primary Efficacy Analysis in the Microbiologically Eligible and the Assessable Populations.*
| Outcome | Microbiologically Eligible Population | Assessable Population | ||||||
|---|---|---|---|---|---|---|---|---|
| Control (N = 768) | Rifapentine– Moxifloxacin (N = 791) | Rifapentine (N = 784) | Total (N = 2343) | Control (N = 726) | Rifapentine– Moxifloxacin (N = 756) | Rifapentine (N = 752) | Total (N = 2234) | |
| Favorable | ||||||||
| Participants with outcome — no. (%) | 656 (85.4) | 668 (84.5) | 645 (82.3) | 1969 (84.0) | 656 (90.4) | 668 (88.4) | 645 (85.8) | 1969 (88.1) |
| Adjusted difference from control — percentage points (95% CI) | NA | 1.0 (−2.6 to 4.5) | 3.0 (−0.6 to 6.6) | NA | NA | 2.0 (−1.1 to 5.1) | 4.4 (1.2 to 7.7) | NA |
| Participant had negative culture at month 12 — no. (%) | 643 (83.7) | 656 (82.9) | 636 (81.1) | 1935 (82.6) | 643 (88.6) | 656 (86.8) | 636 (84.6) | 1935 (86.6) |
| Participant was seen at month 12 but no sputum was produced or cultures were contaminated but without evidence of M. tuberculosis — no. (%) | 13 (1.7) | 12 (1.5) | 9 (1.1) | 34 (1.5) | 13 (1.8) | 12 (1.6) | 9 (1.2) | 34 (1.5) |
| Unfavorable | ||||||||
| Participants with outcome — no. (%) | 112 (14.6) | 123 (15.5) | 139 (17.7) | 374 (16.0) | 70 (9.6) | 88 (11.6) | 107 (14.2) | 265 (11.9) |
| Outcome related to tuberculosis — no. (%) | 24 (3.1) | 45 (5.7) | 75 (9.6) | 144 (6.1) | 24 (3.3) | 45 (6.0) | 75 (10.0) | 144 (6.4) |
| Two consecutive positive cultures at or after week 17† | 11 (1.4) | 34 (4.3) | 63 (8.0) | 108 (4.6) | 11 (1.5) | 34 (4.5) | 63 (8.4) | 108 (4.8) |
| Participant not seen at month 12 but had positive culture when last seen | 11 (1.4) | 3 (0.4) | 4 (0.5) | 18 (0.8) | 11 (1.5) | 3 (0.4) | 4 (0.5) | 18 (0.8) |
| Clinical diagnosis of tuberculosis recurrence and treatment restarted | 2 (0.3) | 8 (1.0) | 8 (1.0) | 18 (0.8) | 2 (0.3) | 8 (1.1) | 8 (1.1) | 18 (0.8) |
| Outcome not related to tuberculosis — no. (%) | 46 (6.0) | 43 (5.4) | 32 (4.1) | 121 (5.2) | 46 (6.3) | 43 (5.7) | 32 (4.3) | 121 (5.4) |
| Consent withdrawn during treatment period with no adverse event reported | 14 (1.8) | 15 (1.9) | 11 (1.4) | 40 (1.7) | 14 (1.9) | 15 (2.0) | 11 (1.5) | 40 (1.8) |
| Change in treatment because of adverse event | 8 (1.0) | 16 (2.0) | 9 (1.1) | 33 (1.4) | 8 (1.1) | 16 (2.1) | 9 (1.2) | 33 (1.5) |
| Death during treatment period | 7 (0.9) | 3 (0.4) | 3 (0.4) | 13 (0.6) | 7 (1.0) | 3 (0.4) | 3 (0.4) | 13 (0.6) |
| Loss to follow-up during treatment period | 8 (1.0) | 2 (0.3) | 2 (0.3) | 12 (0.5) | 8 (1.1) | 2 (0.3) | 2 (0.3) | 12 (0.5) |
| Consent withdrawn during treatment period after occurrence of adverse event | 2 (0.3) | 3 (0.4) | 3 (0.4) | 8 (0.3) | 2 (0.3) | 3 (0.4) | 3 (0.4) | 8 (0.4) |
| Treatment changed or restarted for other reasons | 7 (0.9) | 4 (0.5) | 4 (0.5) | 15 (0.6) | 7 (1.0) | 4 (0.5) | 4 (0.5) | 15 (0.7) |
| Not assessable | ||||||||
| Participants with outcome — no. (%) | 42 (5.5) | 32 (4.0) | 35 (4.5) | 109 (4.7) | NA | NA | NA | NA |
| Participant not seen at month 12 but had negative culture when last seen | 31 (4.0) | 22 (2.8) | 23 (2.9) | 76 (3.2) | NA | NA | NA | NA |
| Treatment discontinued because of pregnancy | 8 (1.0) | 5 (0.6) | 4 (0.5) | 17 (0.7) | NA | NA | NA | NA |
| Death unrelated to tuberculosis during follow-up | 3 (0.4) | 8 (1.0) | 3 (0.4) | 14 (0.6) | NA | NA | NA | NA |
| Violent or accidental death during treatment period | 0 | 0 | 1 (0.1) | 1 (<0.1) | NA | NA | NA | NA |
| Exogenous reinfection confirmed on WGS | 0 | 0 | 1 (0.1) | 1 (<0.1) | NA | NA | NA | NA |
The assessable population included the participants in the microbiologically eligible population who met the criteria for favorable or unfavorable status with respect to the primary outcome. NA denotes not applicable, and WGS whole-genome sequencing.
A mong the participants who had a microbiologically unfavorable outcome, one in the rifapentine–moxifloxacin group had an isolate of recurrent Mycobacterium tuberculosis that showed phenotypic evidence of resistance to isoniazid plus rifampin but was susceptible to isoniazid and rifampin on line-probe molecular testing (WGS results were not available) and three in the rifapentine group had isolates of recurrent M. tuberculosis that showed evidence of resistance to isoniazid plus rifampin (WGS results were not available).
Figure 2. Primary and Secondary Efficacy Analyses and Time to an Unfavorable Outcome.
Panel A shows the results of the primary efficacy analysis in the microbiologically eligible and the assessable analysis populations and of the secondary analyses in the intention-to-treat and the per-protocol analysis populations (top, rifapentine–moxifloxacin regimen vs. control regimen; bottom, rifapentine regimen vs. control regimen). The noninferiority margin of 6.6 percentage points is designated by the dashed vertical line. Participants were classified as having an unfavorable outcome if they had M. tuberculosis–positive cultures from two sputum specimens obtained at or after week 17 without an intervening negative culture, died or were withdrawn from the trial or lost to follow-up during the treatment period, had an M. tuberculosis–positive culture when last seen, died from tuberculosis during the post-treatment follow-up, or received additional treatment for tuberculosis. The microbiologically eligible analysis population included all the participants except those who had no evidence of M. tuberculosis–positive cultures, who had tuberculosis that was resistant to isoniazid, rifampin, or fluoroquinolones, or who were enrolled in violation of the eligibility criteria; the participants with an outcome status that was not assessable were reclassified as having had an unfavorable outcome. The assessable analysis population included the participants in the microbiologically eligible population except those whose outcome status had been reclassified from not assessable to unfavorable. The per-protocol 75% and per-protocol 95% analysis populations included the participants in the assessable population, except those who did not complete 75% or 95% of the assigned treatment doses, respectively, unless the reason for inadequate treatment was death or bacteriologic treatment failure. Panel B shows the results for the time to unfavorable outcome for the microbiologically eligible population. Data were censored for the participants who could not be assessed. HIV denotes human immunodeficiency virus. The inset shows the same data on an expanded y axis.
SUBGROUP ANALYSES
No evidence was found of a difference in efficacy between the rifapentine–moxifloxacin group and the control group in any of the prespecified subgroup analyses (Fig. S4). In contrast, the difference in efficacy observed between the rifapentine group and the control group was smaller in certain subgroups, including female participants, participants without cavitation, participants with low-grade smear, and participants with a long time to positivity for M. tuberculosis growth in baseline liquid cultures (Fig. S5).
TIME TO CULTURE CONVERSION
The time to stable conversion of sputum cultures to negative was shorter in the 4-month regimen groups than that in the control group — the hazard ratios for stable culture conversion in the rifapentine–moxifloxacin group as compared with the control group were 1.4 (95% CI, 1.2 to 1.5) in liquid media and 1.3 (95% CI, 1.2 to 1.5) on solid media, and the hazard ratios in the rifapentine group as compared with the control group were 1.3 (95% CI, 1.2 to 1.4) in liquid media and 1.3 (95% CI, 1.2 to 1.4) on solid media (Tables S5 and S6 and Figs. S6 and S7). Among the participants in the microbiologically eligible population, culture conversion in liquid media occurred by 8 weeks (up to 70 days) in 63.4% of those in the control group, 78.5% of those in the rifapentine–moxifloxacin group, and 74.2% of those in the rifapentine group.
SAFETY
No evidence was found of a difference in the percentage of participants who had an adverse event of grade 3 or higher (the primary safety outcome) during the on-treatment period between the rifapentine–moxifloxacin group and the control group (18.8% [159 participants] vs. 19.3% [159]; adjusted difference, −0.6 percentage points; 95% CI, −4.3 to 3.2) (Table 3 and Table S7). The percentage of participants who had an adverse event of grade 3 or higher during the on-treatment period was lower in the rifapentine group (14.3% [119 participants]) than in the control group (adjusted difference, −5.1 percentage points; 95% CI, −8.7 to −1.5). All-cause mortality during the on-treatment period was similar across the treatment regimens (7 participants [0.8%] in the control group, 3 [0.4%] in the rifapentine–moxifloxacin group, and 4 [0.5%] in the rifapentine group) (Tables 3, Tables S8 through S10, and Fig. S8).
Table 3.
Safety and Premature Discontinuation of Assigned Regimen.*
| Variable | Control (N = 825) | Rifapentine–Moxifloxacin (N = 846) | Rifapentine (N = 835) | Total (N = 2506) |
|---|---|---|---|---|
| Primary safety outcome | ||||
| Grade 3 or higher adverse event — no. (%) | 159 (19.3) | 159 (18.8) | 119 (14.3) | 437 (17.4) |
| Percentage-point difference from control (95% CI)† | NA | −0.6 (−4.3 to 3.2) | −5.1 (−8.7 to −1.5) | NA |
| Secondary safety outcome | ||||
| Treatment-related grade 3 or higher adverse event — no. (%) | 81 (9.8) | 109 (12.9) | 64 (7.7) | 254 (10.1) |
| Percentage-point difference from control (95% CI)† | NA | 3.0 (−0.0 to 6.1) | −2.2 (−4.9 to 0.6) | NA |
| Other safety outcomes | ||||
| Any serious adverse event — no. (%) | 56 (6.8) | 37 (4.4) | 39 (4.7) | 132 (5.3) |
| Death — no. (%)‡ | 7 (0.8) | 3 (0.4) | 4 (0.5) | 14 (0.6) |
| Any adverse event resulting in discontinuation of assigned treatment — no. (%)§ | 7 (0.8) | 16 (1.9) | 11 (1.3) | 34 (1.4) |
| Any grade 3 or higher adverse event within 28 weeks after randomization | 159 (19.3) | 194 (22.9) | 138 (16.5) | 491 (19.6) |
| ALT or AST level ≥5×ULN — no. (%)¶ | 24 (2.9) | 16 (1.9) | 13 (1.6) | 53 (2.1) |
| ALT or AST level ≥10×ULN — no. (%) | 9 (1.1) | 4 (0.5) | 5 (0.6) | 18 (0.7) |
| Serum total bilirubin level ≥3×ULN — no. (%)‖ | 8 (1.0) | 28 (3.3) | 20 (2.4) | 56 (2.2) |
| Hy’s law criteria of ALT or AST level ≥3×ULN plus serum total bilirubin level ≥2×ULN — no. (%) | 7 (0.8) | 10 (1.2) | 8 (1.0) | 25 (1.0) |
| Premature discontinuation of assigned regimen in the microbiologically eligible population | ||||
| Discontinuation of assigned regimen for any reason — no./total no. (%) | 61/768 (7.9) | 55/791 (7.0) | 37/784 (4.7) | 153/2343 (6.5) |
| Percentage-point difference from control (95% CI)† | NA | −1.0 (−3.6 to 1.6) | −3.3 (−5.7 to −0.9) | NA |
The safety analysis population included all the participants who had undergone randomization and received at least one dose of the assigned treatment. Safety was assessed during the on-treatment period (the time during which the participants were receiving the trial medications and up to 14 days after the last dose), unless otherwise specified. Adverse events were graded by the site investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events.31 ALT denotes alanine aminotransferase, AST aspartate aminotransferase, and ULN upper limit of the normal range.
The analysis was adjusted for the stratification factors of presence of cavitation on baseline chest radiography at baseline and HIV status.
In the control group, death was due to paracoccidioides infection, sepsis, papillary thyroid cancer, central nervous system lesion, hemoptysis, or pulmonary embolism (in 1 participant each), and there was one unexplained death. In the rifapentine–moxifloxacin group, death was due to thrombotic thrombocytopenic purpura, congestive cardiac failure, or pulmonary tuberculosis (in 1 participant each). In the rifapentine group, death was due to alcohol poisoning, road traffic accident, or pulmonary embolism (in 1 participant each), and there was one unexplained death.
The assigned treatment was discontinued because of hepatitis (in 6 participants) or seizure (in 1 participant) in the control group; because of hepatitis (in 11 participants) or thrombocytopenia, QT prolongation, tendonitis, pruritus, or maculopapular rash (in 1 participant each) in the rifapentine–moxifloxacin group; and because of hepatitis (in 8 participants) or neutropenia, bacterial pneumonia, or drug reaction with eosinophilia and systemic symptoms (in 1 participant each) in the rifapentine group.
An ALT or AST level of at least 5×ULN corresponds with an adverse event of grade 3 or higher.
A total bilirubin level of at least 3×ULN corresponds with an adverse event of grade 3 or higher.
With regard to adverse events of interest (i.e., those known to be associated with a medication), the percentages of participants with grade 3 or higher alanine aminotransferase or aspartate aminotransferase levels were similar across the treatment regimens. A grade 3 or higher serum total bilirubin level was reported in a higher percentage of participants in the rifapentine-based regimen groups than in the control group (1.0% [8 participants] in the control group, 3.3% [28] in the rifapentine–moxifloxacin group, and 2.4% [20] in the rifapentine group), although 25 adverse events that met the Hy’s law criteria for drug-induced liver injury were distributed equally across the treatment groups (Table 3 and Fig. S9).36 Cardiac disorders of grade 3 or higher that occurred during the on-treatment period were reported in 3 participants (0.4%) in the rifapentine–moxifloxacin group — two events were considered by the site investigators as unlikely to be related to the trial regimen, and one event, reported as palpitations with borderline electrocardiographic QT prolongation, was considered to be related to the trial regimen (Table S11). No evidence was found of a difference in premature discontinuation between the rifapentine–moxifloxacin group and the control group (risk difference, −1.0 percentage points; 95% CI, −3.6 to 1.6). Premature discontinuation occurred less frequently with the rifapentine regimen than with the control regimen (−3.3 percentage points; 95% CI, −5.7 to −0.9).
DISCUSSION
In this phase 3 trial, the efficacy of the 4-month regimen containing rifapentine and moxifloxacin was noninferior to that of the standard 6-month regimen. Noninferiority of the rifapentine–moxifloxacin regimen to the control regimen was confirmed across analysis populations as well as in sensitivity and prespecified subgroup analyses. The efficacy of the 4-month regimen containing rifapentine without moxifloxacin did not meet the criteria for noninferiority.
The incidence of grade 3 or higher adverse events during the on-treatment period was similar in the rifapentine–moxifloxacin group and the control group and was slightly lower in the rifapentine group. We observed a higher incidence of hyperbilirubinemia in the rifapentine-based regimen groups, but there was no notable difference among the three treatment groups in the percentage of participants who had elevations in serum aminotransferase levels or who met the criteria of Hy’s law.36 Rifamycins can increase the serum bilirubin level through dose-dependent interference with bilirubin uptake that is typically subclinical and not associated with hepatocellular injury.37,38 Nevertheless, careful monitoring for hepatotoxicity should be performed during the course of the 4-month rifapentine-based regimens, given the theoretical increase in the risk of hepatotoxicity with increased exposure to a rifamycin, the difficulty of detecting signals of rare events in clinical trials, and the known risk of severe hepatitis associated with tuberculosis treatment regimens containing rifamycins, isoniazid, or pyrazinamide. There was no clinical evidence of increased risk of cardiotoxicity, although electrocardiographic monitoring was not a required component of the study.39
Antimicrobial activity, as assessed with the use of the intermediate marker of time to stable conversion of sputum cultures to negative, was greater with the experimental 4-month regimens than with the control regimen, a finding that was consistent with the result in a phase 2 trial.22 However, this marker differed little between the two rifapentine-based regimens, despite the difference in their ability to cure tuberculosis. This finding highlights the limitations of the use of sputum-culture conversion as a surrogate marker for cure and underscores both the importance of trials that use clinically relevant outcomes and the need for better markers of early response to treatment.
Our trial has several limitations. Placebos were not used, and therefore neither the participants nor the staff at the trial site were unaware of the treatment-group assignment. The rationale for this approach was twofold. First, food affects rifampin and rifapentine differently; therefore, the provision of treatment-specific guidance on whether to take a medication with or without food was needed to increase exposure to the rifamycin used in each trial regimen.8,27 Second, the use of placebos would have increased the number of daily pills to approximately 20, potentially affecting the rate of premature discontinuation. We minimized measurement bias by concealing both the treatment-group assignments and the trial week from the microbiologists handling sputum specimens and the team at the data coordinating center. The trial incorporated uniform visits and procedures regardless of treatment assignment and included a prespecified set of triggers and processes for evaluating participants who might not be responding well to treatment.23 Only the members of the data and safety monitoring board and the statisticians reporting to the data and safety monitoring board saw the aggregate data according to treatment group before the end of the trial. A total of 8% of the trial participants were coninfected with HIV, a fact that limits the power to compare regimens in this trial population.
Strengths of the trial include a high rate of retention of participants and the completeness of data from mycobacteriologic testing, both of which reflect the quality of trial implementation. In this noninferiority trial involving ambulatory participants, the validity of the results is supported by the finding that 96.9% of the participants who were assigned to the control regimen in the per-protocol 75% analysis population (a secondary analysis that was chosen to be comparable with the per-protocol analyses in recent trials) had a favorable outcome rate. This finding reflects the reported cure rates with the same regimen in trials conducted in the late 20th century, which involved mostly hospitalized patients.16,32,40 Other strengths are the inclusion of adolescents and adults from diverse populations in regions with varied burden of tuberculosis on four continents and the performance of microbiologic assays under rigorous quality management.
In considering the feasibility of using the rifapentine–moxifloxacin regimen in national tuberculosis programs, several issues are relevant. First, rapid drug-susceptibility testing to fluoroquinolones and isoniazid should be performed in addition to the widely available rapid molecular drug susceptibility testing for rifampin. This should be surmountable, because the genetic basis of M. tuberculosis resistance to isoniazid and fluoroquinolones is established, and rapid molecular tests are in advanced stages of clinical testing.41,42 Second, absorption of rifapentine in the gut is improved in the presence of high-fat foods.27 In our trial, the determination of the dose of rifapentine and the guidance to take the medication with any food were based on evidence and were pragmatically selected to achieve desirable pharmacokinetic exposures in a manner likely to be feasible in most settings.7,22 The trial incorporated a pharmacokinetic component that will allow a nuanced understanding of drug exposure–response relationships across populations. Finally, drug costs may be higher for the rifapentine–moxifloxacin regimen than for the current standard 6-month regimen, at least in the short term. Economic analyses will provide information about the extent to which incrementally higher drug costs are offset by a shorter regimen.
In this trial, a 4-month regimen that included rifapentine at a daily dose of 1200 mg and moxifloxacin at daily dose of 400 mg had an efficacy that was noninferior to that of the standard 6-month regimen across the primary, secondary, and sensitivity analysis populations.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC), the National Institute of Allergy and Infectious Diseases, or the Department of Health and Human Services.
Supported by the CDC; the Division of Tuberculosis Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (contracts 200-2009-32582, 200-2009-32593, 200-2009-32594, 200-2009-32589, 200-2009-32597, 200-200932598, 75D30119C06702, 75D30119C06701, 75D30119C06703, 75D30119C06222, 75D30119C06225, and 75D30119C06010); and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. Sanofi donated rifapentine and all other trial drugs, supported the shipment of the trial drugs to all sites, and provided funding support for pharmacokinetic testing and preparation of the final clinical study report.
Dr. Martinson reports receiving grant support, paid to his institution, from Pfizer; and Dr. Chaisson, receiving consulting fees from Sanofi. No other potential conflict of interest relevant to this article was reported.
We thank the trial participants who contributed their time to this trial and the local tuberculosis program staff who assisted in the clinical management of some trial participants; Phil LoBue, Carla Winston, and Jonathan Mermin for their continued support of the Tuberculosis Trials Consortium within the CDC; the staff at Westat and PPD for on-site monitoring; Charles M. Heilig for statistical support during the development of the protocol; William J. Burman for scientific contributions in the early development of the protocol; and Janet Anderson, Michael Chen, Cynthia Lee, Pei-Jean Feng, Courtney Fletcher, Richard Hafner, Jose M. Miro, Sachiko Miyahara, Kathleen Robergeau, and Lisa Wolf for contributions to the trial protocol.
Footnotes
REFERENCES
- 1.Nahid P, Dorman SE, Alipanah N, et al. Executive summary: official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63: 853–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update. Geneva: World Health Organization, April 2017. (https://www.who.int/tb/publications/2017/dstb_guidance_2017/en/). [Google Scholar]
- 3.Abu-Raddad LJ, Sabatelli L, Achterberg JT, et al. Epidemiological benefits of more — effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A 2009; 106: 13980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnett LJ, Ken-Dror G, Koh GCKW, Davies GR. Comparing the efficacy of drug regimens for pulmonary tuberculosis: meta-analysis of endpoints in earlyphase clinical trials. Clin Infect Dis 2017; 65:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayaram R, Gaonkar S, Kaur P, et al. Pharmacokinetics–pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 2003;47: 2118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svensson EM, Svensson RJ, Te Brake LHM, et al. The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis 2018;67: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savic RM, Weiner M, MacKenzie WR, et al. Defining the optimal dose of rifapentine for pulmonary tuberculosis: exposure-response relations from two phase II clinical trials. Clin Pharmacol Ther 2017; 102: 321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet 2001; 40: 327–41. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez JC, Ruiz M, López M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against mycobacterium tuberculosis. Int J Antimicrob Agents 2002; 20: 464–7. [DOI] [PubMed] [Google Scholar]
- 10.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against mycobacterium tuberculosis. Antimicrob Agents Chemother 1998; 42:2066–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med 2004;169: 421–6. [DOI] [PubMed] [Google Scholar]
- 12.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med 2004; 170:1131–4. [DOI] [PubMed] [Google Scholar]
- 13.Burman WJ, Goldberg S, Johnson JL, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med 2006; 174:331–8. [DOI] [PubMed] [Google Scholar]
- 14.Conde MB, Efron A, Loredo C, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 2009;373: 1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rustomjee R, Lienhardt C, Kanyok T, et al. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008; 12: 128–38. [PubMed] [Google Scholar]
- 16.Gillespie SH, Crook AM, McHugh TD, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014; 371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorman SE, Johnson JL, Goldberg S, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med 2009; 180: 273–80. [DOI] [PubMed] [Google Scholar]
- 18.Conde MB, Mello FCQ, Duarte RS, et al. A phase 2 randomized trial of a rifapentine plus moxifloxacin-based regimen for treatment of pulmonary tuberculosis. PLoS One 2016;11(5): e0154778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal IM, Williams K, Tyagi S, et al. Weekly moxifloxacin and rifapentine is more active than the Denver regimen in murine tuberculosis. Am J Respir Crit Care Med 2005; 172: 1457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenthal IM, Zhang M, Almeida D, Grosset JH, Nuermberger EL. Isoniazid or moxifloxacin in rifapentine-based regimens for experimental tuberculosis? Am J Respir Crit Care Med 2008; 178: 989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenthal IM, Zhang M, Williams KN, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med 2007; 4(12): e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorman SE, Savic RM, Goldberg S, et al. Daily rifapentine for treatment of pulmonary tuberculosis: a randomized, dose-ranging trial. Am J Respir Crit Care Med 2015;191: 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorman SE, Nahid P, Kurbatova EV, et al. High-dose rifapentine with or without moxifloxacin for shortening treatment of pulmonary tuberculosis: study protocol for TBTC study 31/ACTG A5349 phase 3 clinical trial. Contemp Clin Trials 2020; 90:105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blakemore R, Nabeta P, Davidow AL, et al. A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. Am J Respir Crit Care Med 2011; 184: 1076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedrich SO, Venter A, Kayigire XA, Dawson R, Donald PR, Diacon AH. Suitability of Xpert MTB/RIF and genotype MTBDRplus for patient selection for a tuberculosis clinical trial. J Clin Microbiol 2011;49: 2827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares JF, Wu CFJ. Some restricted randomization rules in sequential designs. Commun Stat Theory Methods 1983; 12: 2017–34. [Google Scholar]
- 27.Zvada SP, Van Der Walt JS, Smith PJ, et al. Effects of four different meal types on the population pharmacokinetics of single-dose rifapentine in healthy male volunteers. Antimicrob Agents Chemother 2010;54: 3390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peloquin CA, Namdar R, Singleton MD, Nix DE. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 1999; 115: 12–8. [DOI] [PubMed] [Google Scholar]
- 29.Witney AA, Bateson ALE, Jindani A, et al. Use of whole-genome sequencing to distinguish relapse from reinfection in a completed tuberculosis clinical trial. BMC Med 2017; 15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunn AJ, Phillips PPJ, Mitchison DA. Timing of relapse in short-course chemotherapy trials for tuberculosis. Int J Tuberc Lung Dis 2010;14: 241–2. [PubMed] [Google Scholar]
- 31.The common terminology criteria for adverse events, version 4.03. Bethesda, MD: National Institutes of Health, June 14, 2010. (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf). [Google Scholar]
- 32.Jindani A, Harrison TS, Nunn AJ, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014;371: 1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merle CS, Fielding K, Sow OB, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med 2014;371: 1588–98. [DOI] [PubMed] [Google Scholar]
- 34.Mohamed K, Embleton A, Cuffe RL. Adjusting for covariates in non-inferiority studies with margins defined as risk differences. Pharm Stat 2011; 10: 461–6. [DOI] [PubMed] [Google Scholar]
- 35.Lumb R, Van Deun A, Bastian I, FitzGerald M. Laboratory diagnosis of tuberculosis by sputum microscopy: the handbook, global edition. Adelaide, Australia: SA: Pathology, 2013. (http://www.stoptb.org/wg/gli/assets/documents/TB%20MICROSCOPY%20HANDBOOK_FINAL.pdf). [Google Scholar]
- 36.Reuben A. Hy’s law. Hepatology 2004; 39:574–8. [DOI] [PubMed] [Google Scholar]
- 37.Capelle P, Dhumeaux D, Mora M, Feldmann G, Berthelot P. Effect of rifampicin on liver function in man. Gut 1972; 13: 36671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006; 174: 935–52. [DOI] [PubMed] [Google Scholar]
- 39.Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis 2005; 41: Suppl 2:S144–S157. [DOI] [PubMed] [Google Scholar]
- 40.Fox W. Whither short-course chemotherapy? Br J Dis Chest 1981; 75: 331–57. [DOI] [PubMed] [Google Scholar]
- 41.Koch A, Cox H, Mizrahi V. Drug-resistant tuberculosis: challenges and opportunities for diagnosis and treatment. Curr Opin Pharmacol 2018;42: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie YL, Chakravorty S, Armstrong DT, et al. Evaluation of a rapid molecular drug-susceptibility test for tuberculosis. N Engl J Med 2017; 377: 1043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.