Abstract
Spike-specific antibodies contribute significantly to the neutralising activity against SARS-CoV-2 and are important for the therapeutic effect of convalescent plasma. B.1.1.7 is a recently emerged variant of SARS-CoV-2 that has several mutations in the gene encoding for the spike-protein. To assess the potential effect these mutations could have on the neutralising efficacy of antibodies, we evaluated 96 serum samples from convalescent plasma donors collected before the first occurrence of B.1.1.7 and tested their neutralising effect on wild-type SARS-CoV-2 and B.1.1.7. We found that B.1.1.7 is more resistant to neutralisation by convalescent plasma from patients infected with wild-type SARS-CoV-2 with an overall decrease in neutralising activity of 47.7%. Thus, the neutralising effect of convalescent plasma should be determined against the major circulating virus clades whenever possible to ensure the best possible therapeutic effect.
Keywords: Sars-cov-2, Covid-19, B.1.1.7., Convalescent plasma, Neutralisation
COVID-19 continues to threaten global health. As there are still no strongly effective, specific antiviral treatment options available, convalescent plasma is being used worldwide to treat patients suffering from severe COVID-19. However, the emergence and rapid spread of virus variants such as the variant of concern (VOC) 202,012/01, commonly referred to as B.1.1.7, raises the question of possible differences in neutralising ability and thus effectiveness of using convalescent plasma derived from donors infected with wild-type SARS-CoV-2 in patients infected with B.1.1.7. We evaluated 96 serum samples from convalescent plasma donors collected between April and May 2020 before the first occurrence of B.1.1.7 and tested their neutralising effect on both wild-type SARS-CoV-2 and B.1.1.7.
Lineage B.1.1.7 is one of several variants thought to be of particular importance, mainly because recent data suggest that they are significantly more transmissible than wild type (WT) SARS-CoV-2. [1]. One of the key questions here is whether certain mutations could enable immune evasion, including escape from neutralising antibodies (NAbs) as previously described in vitro [2]. amongst others, B.1.1.7 has several mutations in the receptor-binding domain of the spike protein [3]. However, it is known that spike-specific antibodies contribute significantly to the neutralising activity and are thus important for the therapeutic effect of convalescent plasma [4]. To assess the neutralising efficacy of NAbs in convalescent plasma from patients infected with WT virus on B.1.1.7, we evaluated 96 anonymised serum samples collected between April and May 2020. All samples were from donors who had recently recovered from COVID-19 and donated convalescent plasma. All patients had to be hospitalized and required oxygen during the course of the infection, none required mechanical ventilation. Samples were taken four weeks after complete recovery from COVID-19 according to official guidelines of the German Federal Institute for Vaccines and Biomedicines [5]. All samples had a serological profile consistent with recent SARS-CoV-2 infection, i.e. SARS-CoV-2 specific IgA and/or IgG antibodies determined by a commercial ELISA (Euroimmun, Lübeck, Germany) (data not shown).
Micro-neutralisation test
WT SARS-CoV-2 (strain MUC IMB-1, clade B1) and B.1.1.7 (strain MUC IMB-B.1.1.7) neutralising antibody (NAb) titres were determined as previously described [6]. Briefly, heat-inactivated serum samples (duplicates), including positive and negative control samples, were serially diluted in 96-well tissue culture plates starting at 1:5 [7] to a maximum of 1:640. Virus stocks (50 TCID/50 µl) were prepared and stored at −80 °C. Virus was pre-incubated (1 h, 37 °C) with diluted serum samples before Vero E6 cells (1 × 104 cells/50 µl) were added to each well. After 72 h (37 °C), supernatants were discarded and wells were fixed (3% formalin/PBS) and stained (crystal violet, 0.1%). The NAbs titre corresponded to the reciprocal of the highest serum dilution showing complete inhibition of CPE. A virus re-titration was performed on every plate and exact titres were determined by retrograde calculation. Overall, the mean NAbs titres were 36 for WT virus and 14 for B.1.1.7.
Comparison of NAbs titres against WT and B.1.1.7
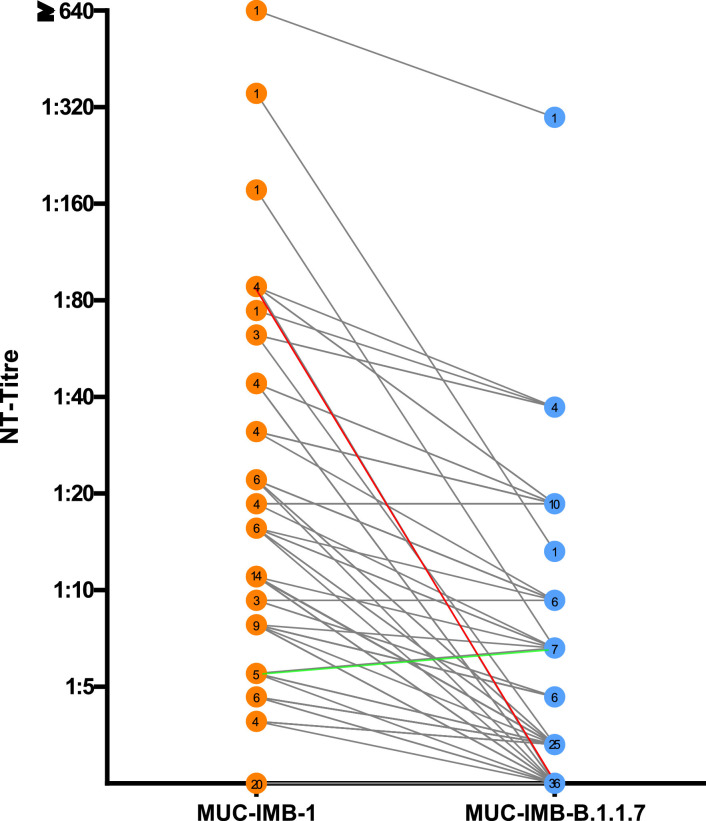
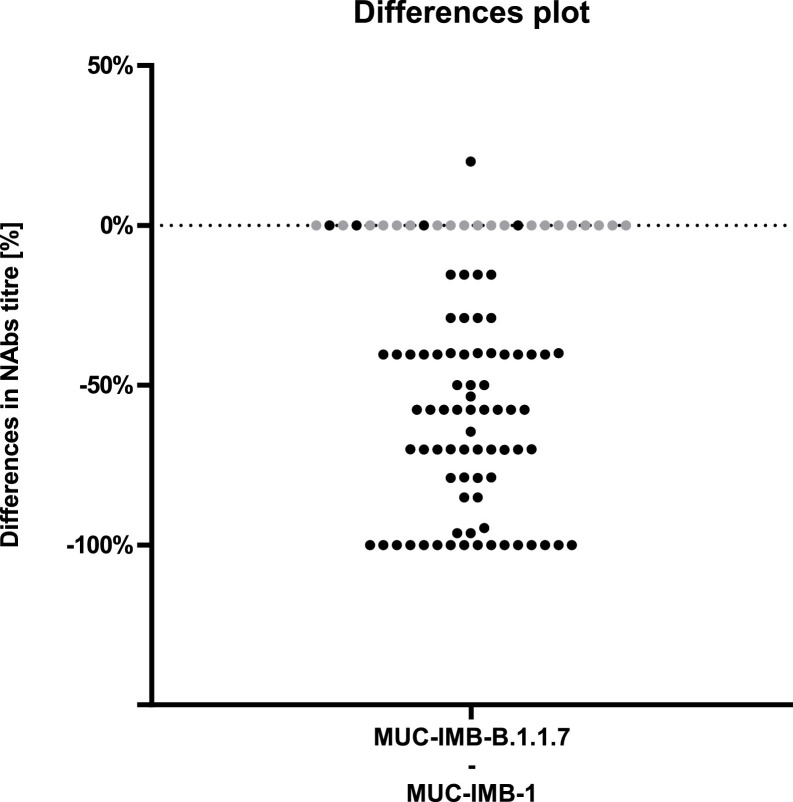
Direct comparison of NAbs titres showed that the majority (60/76) of serum samples retained their ability to neutralise when tested against B.1.1.7. However, titres against B.1.1.7 were generally lower compared to titres against WT virus (Fig. 1 ). Overall, the ability to neutralise was reduced in all but four samples, where titres remained the same. A total of 16 serum samples showed no neutralising effect when tested against B.1.1.7, although they were able to further neutralise WT virus in parallel control tests. No change in titre was observed four samples. Additionally, all 20 samples that were initially negative for NAbs against WT virus also remained negative when tested against B.1.1.7. Thus, only four samples were able to fully retain their neutralising ability. Only one sample increased its neutralising ability by 20% when tested against B.1.1.7. The mean loss of neutralising activity against B.1.1.7 was 47.7% compared to WT virus, but we observed a wide range of titre changes (Fig. 2 ). The largest decrease was observed in a serum that showed an initial titre of 88 against WT SARS-CoV-2 but no neutralising activity against B.1.1.7 (Fig. 1, red). Overall, the decrease in NAbs titres against B.1.1.7 compared to WT virus was statistically significant (p<0.0001 by Wilcoxon) (Fig. 2). Taken together, these results suggest that B.1.1.7 has the ability to escape NAbs in some, but not all, convalescent plasmas of early COVID-19 patients.
Fig. 1.
Direct comparison of NAbs titres against WT virus (orange) and B.1.1.7 (blue) shows a general trend towards a decrease in titre levels. While the majority of serum samples were still able to neutralise B.1.1.7, most samples show a decrease in NAbs titre levels. The greatest decrease was observed in one sample, which dropped from an initial titre of 80 against WT virus to no neutralising effect against B.1.1.7 (Fig. 1, red). 16 serum samples lost their neutralising effect completely against B.1.1.7. Only one sample showed a slight increase in neutralising efficacy when tested against B.1.1.7 (green). In total, 96 samples were tested. The number of samples with the same titre is shown in the respective circle.
Fig. 2.
Percentage differences in NAbs titres demonstrate an overall decrease in neutralising efficacy While the majority of samples showed a decrease in titre values, neutralising efficacy was completely abolished in 16 samples. Four samples retained their neutralising efficacy. Additionally, all 20 samples that were initially negative for NAbs against WT virus also remained negative when tested against B.1.1.7 (grey).
Correlation of the NAbs titres
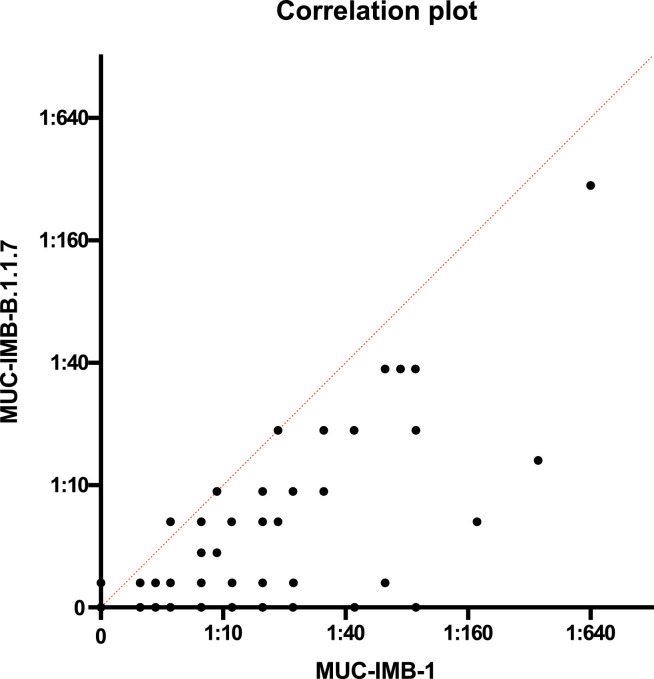
In addition, we examined the correlation of the NAbs titres. A weak correlation (r = 0.86) was observed between the NAb titre against WT SARS-CoV-2 and the titre against B.1.1.7 of the same serum sample (Fig. 3 ). However, as the titre changes were highly variable, no predictions could be made about the neutralising activity against B.1.1.7 based on the NAbs titre against WT virus.
Fig. 3.
A low-grade correlation was observed between the titres against WT virus and B.1.1.7. Despite the low degree correlation observed (r = 0.86, Pearson regression analysis), no prediction about the neutralising efficacy of a serum against B.1.1.7 can be extrapolated from the NAbs titre obtained against WT virus.
Discussion
Neutralising antibodies are a key component of the adaptive immune system that can be transferred as convalescent plasma to temporarily induce passive immunity [8]. The extent to which genetic variations in SARS-CoV-2 influence the effectiveness of convalescent plasma therapy is currently unknown [9].
Our results show that SARS-CoV-2 variant B.1.1.7 is more resistant than WT virus to neutralisation by convalescent plasma from patients infected between March and May 2020, before the emergence of B.1.1.7. Although a certain correlation between titres could be observed, no reliable prediction about a possible neutralising efficacy of a serum against B.1.1.7 can be derived from the NAbs titre obtained against WT virus.
Our findings are in contrast to the results of a recently published study by Planas et al., in which no significant reduction in neutralisation against B.1.1.7 was found [10]. On the other hand, there are several other studies reporting reduced neutralisation similar to our results [11], [12], [13]. These discrepancies might be attributed to the differences in virus strains serving as “wild-type” SARS-CoV-2 control viruses and should be investigated further.
Whether the potentially reduced susceptibility could indicate a higher risk of re-infection with new variants should be investigated in future studies. At the same time, it is important to continuously monitor the effectiveness of vaccines against emerging variants, as most currently licensed vaccines induce antibodies against the spike protein or parts thereof [8, 14].
The fact that one serum shows an increase in neutralising efficacy against B.1.1.7 may indicate that mutations in SARS-CoV-2 do not necessarily lead to limitations of the immune defence in every case. In individual cases, polyvalent antibody formation can apparently even lead to improvements in the neutralising ability against a mutation variant. It must be said, however, that the increase in neutralisation capacity observed by us was overall low in this single serum. Moreover, only a limited initial titre against WT virus was found.
Conclusion
The use of convalescent plasma in the treatment of patients with severe COVID-19 is undoubtedly an important tool in the fight against SARS-CoV-2 [15]. However, therapeutic success depends, amongst other things, on the quantity and specificity of NAbs. As new SARS-CoV-2 variants emerge, it is important to gather knowledge about the cross-reactivity of NAbs against different clades. Our results show that there is some cross-reactivity between NAbs generated against WT SARS-CoV-2 and B.1.1.7. Thus, the use of convalescent plasma in patients infected with B.1.1.7 may generally have a positive effect. However, we observed partly large differences in the neutralising activity. Therefore, the neutralising effect of convalescent plasma should be determined against the major circulating virus clades whenever possible and should be administered to patients infected with a clade that is efficiently neutralised by the plasma. In clinical situations where this is not always possible, our data suggest that convalescent plasma with high NAbs titres is more likely to maintain its neutralising effect albeit potential clade differences.
Funding statement
This study was funded by the Medical Biodefense Research Program of the Bundeswehr Medical Service.
Ethical statement
The study was carried out in-line with “The Code of Ethics of the World Medical Association (Declaration of Helsinki)”. The use of serum samples complied with the guidelines of the Central Ethics Committee of the German Medical Association (Dtsch Arztebl 2003; 100(23): A-1632). In accordance with these guidelines, the anonymized use of residual material from the samples sent to our laboratory for diagnostic purposes is permissible, provided that the patients have not decided against this procedure. Samples from patients who had decided against this procedure were excluded from the analyses.
CRediT authorship contribution statement
Katharina Müller: Methodology, Writing – original draft, Visualization, Writing – review & editing. Philipp Girl: Methodology, Writing – original draft, Visualization, Writing – review & editing. Andreas Giebl: Resources, Writing – review & editing. Heiner von Buttlar: Data curation, Writing – review & editing. Gerhard Dobler: Data curation, Writing – review & editing. Joachim J. Bugert: Data curation, Writing – review & editing. Stefanie Gruetzner: Conceptualization, Resources, Writing – review & editing. Roman Wölfel: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Leung K., Shum M.H., Leung G.M., Lam T.T., Wu J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Eurosurveillance. 2021;26 doi: 10.2807/1560-7917.ES.2020.26.1.2002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., Muecksch F., Rutkowska M., Hoffmann H.-.H., Michailidis E., Gaebler C., Agudelo M., Cho A., Wang Z., Gazumyan A., Cipolla M., Luchsinger L., Hillyer C.D., Caskey M., Robbiani D.F., Rice C.M., Nussenzweig M.C., Hatziioannou T., Bieniasz P.D. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees-Spear C., Muir L., Griffith S., Heaney J., Aldon Y., Snitselaar J., Thomas P., Graham C., Seow J., Lee N., Rosa A., Roustan C., Houlihan C., Sanders R., Gupta R., Cherepanov P., Stauss H., Nastouli E., Doores K., van Gils M., McCoy L. The impact of Spike mutations on SARS-CoV-2 neutralization. Immunology. 2021 doi: 10.1016/j.celrep.2021.108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu J., Peng P., Wang K., Fang L., Luo F., Jin A., Liu B., Tang N., Huang A. Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies. Cell. Mol. Immunol. 2021:1–3. doi: 10.1038/s41423-021-00648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul-Ehrlich-Institut News - Recommendation of the Paul-Ehrlich-Institut for the Collection and Manufacture of COVID-19 Convalescent Plasma. https://www.pei.de/EN/newsroom/hp-news/2020/200407-recommendation-pei-covid-19-convalescent-plasma.html;jsessionid=75FE2CC97FB1AC74E8529A023B965614.intranet241. Accessed 15 Mar 2021.
- 6.Haselmann V., Özçürümez M.K., Klawonn F., Ast V., Gerhards C., Eichner R., Costina V., Dobler G., Geilenkeuser W.-.J., Wölfel R., Neumaier M. Results of the first pilot external quality assessment (EQA) scheme for anti-SARS-CoV2-antibody testing. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-1183. [DOI] [PubMed] [Google Scholar]
- 7.Müller K., Girl P., von Buttlar H., Dobler G., Wölfel R. Comparison of two commercial surrogate ELISAs to detect a neutralising antibody response to SARS-CoV-2. J. Virol. Methods. 2021;292 doi: 10.1016/j.jviromet.2021.114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotkin S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., Wei Y., Atwal G.S., Murphy A.J., Stahl N., Yancopoulos G.D., Kyratsous C.A. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planas D., Bruel T., Grzelak L., Guivel-Benhassine F., Staropoli I., Porrot F., Planchais C., Buchrieser J., Rajah M.M., Bishop E., Albert M., Donati F., Behillil S., Enouf V., Maquart M., Gonzalez M., Sèze J.D., Péré H., Veyer D., Sève A., Simon-Lorière E., Fafi-Kremer S., Stefic K., Mouquet H., Hocqueloux L., Werf S van der, Prazuck T., Schwartz O. (2021) Sensitivity of infectious SARS-CoV-2 B1.1.7 and B.1.351 variants to neutralizing antibodies. bioRxiv 2021.02.12.430472. https://doi.org/10.1101/2021.02.12.430472.
- 11.Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Nutalai R., Tuekprakhon A., Wang B., Paesen G.C., Slon-Campos J., López-Camacho C., Hallis B., Coombes N., Bewley K., Charlton S., Walter T.S., Barnes E., Dunachie S.J., Skelly D., Lumley S.F., Baker N., Shaik I., Humphries H., Godwin K., Gent N., Sienkiewicz A., Dold C., Levin R., Dong T., Pollard A.J., Knight J.C., Klenerman P., Crook D., Lambe T., Clutterbuck E., Bibi S., Flaxman A., Bittaye M., Belij-Rammerstorfer S., Gilbert S., Hall D.R., Williams M.A., Paterson N.G., James W., Carroll M.W., Fry E.E., Mongkolsapaya J., Ren J., Stuart D.I., Screaton G.R. Reduced neutralization of SARS-CoV-2 B1.1.7 variant by convalescent and vaccine sera. Cell. 2021 doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen X., Tang H., McDanal C., Wagh K., Fischer W., Theiler J., Yoon H., Li D., Haynes B.F., Sanders K.O., Gnanakaran S., Hengartner N., Pajon R., Smith G., Glenn G.M., Korber B., Montefiori D.C. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021 doi: 10.1016/j.chom.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skelly D.T., Harding A.C., Gilbert-Jaramillo J., Knight M.L., Longet S., Brown A., Adele S., Adland E., Brown H., Tipton T., Stafford L., Johnson S.A., Amini A., Tan T.K., Schimanski L., Huang K.-.Y.A., Rijal P., Frater J., Goulder P., Conlon C.P., Jeffery K., Dold C., Pollard A.J., Townsend A.R., Klenerman P., Dunachie S.J., Barnes E., Carroll M.W., James W.S. (2021) Vaccine-induced immunity provides more robust heterotypic immunity than natural infection to emerging SARS-CoV-2 variants of concern. https://www.researchsquare.com. Accessed 18 Mar 2021.
- 14.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]