Abstract
Objective
To assess the associations between coronavirus disease 2019 (COVID-19) infection and thromboembolism including myocardial infarction (MI), ischemic stroke, deep vein thrombosis (DVT), and pulmonary embolism (PE).
Patients and Methods
A self-controlled case-series study was conducted covering the whole of Scotland’s general population. The study population comprised individuals with confirmed (positive test) COVID-19 and at least one thromboembolic event between March 2018 and October 2020. Their incidence rates during the risk interval (5 days before to 56 days after the positive test) and the control interval (the remaining periods) were compared intrapersonally.
Results
Across Scotland, 1449 individuals tested positive for COVID-19 and experienced a thromboembolic event. The risk of thromboembolism was significantly elevated over the whole risk period but highest in the 7 days following the positive test (incidence rate ratio, 12.01; 95% CI, 9.91 to 14.56) in all included individuals. The association was also present in individuals not originally hospitalized for COVID-19 (incidence rate ratio, 4.07; 95% CI, 2.83 to 5.85). Risk of MI, stroke, PE, and DVT were all significantly higher in the week following a positive test. The risk of PE and DVT was particularly high and remained significantly elevated even 56 days following the test.
Conclusion
Confirmed COVID-19 infection was associated with early elevations in risk with MI, ischemic stroke, and substantially stronger and prolonged elevations with DVT and PE both in hospital and community settings. Clinicians should consider thromboembolism, especially PE, among people with COVID-19 in the community.
Increasing evidence suggests a potential link between coronavirus disease 2019 (COVID-19) infection and thromboembolism, which could affect a range of organs resulting in myocardial infarction (MI), ischemic stroke, pulmonary embolism (PE), and deep vein thrombosis (DVT).
First indications of a potential link came from a case report that described PE in a patient infected with COVID-19 who had no relevant risk factors or past medical history.1 Subsequently, hospital-based case series supported the hypothesis, including ischemic stroke in five younger (33-49 years) patients who tested positive for COVID-19.2 A recent meta-analysis of 3487 COVID-19 patients from 30 studies produced a 26% pooled incidence of venous thromboembolism, but concluded that the existing evidence was low-quality and heterogeneous.3 Similar findings were reported by another meta-analysis focused on PE and DVT.4 Venous thromboembolism has now been recognized as a relatively common complication of COVID-19 and clinical guidelines recommend the use of pharmacological prophylaxis following risk assessment.5 However, clinical trials have provided heterogenous findings, potentially depending on the severity of COVID-19.6 , 7
The current evidence, however, is mainly based on crude incidence from hospitalized case series. Because hospitalized patients are a highly selected minority of those infected with COVID-19, these studies are unrepresentative and not generalizable to the general population.8 It is unknown whether people who are asymptomatic or who have mild COVID-19 symptoms (nonhospitalized) were also at a higher risk of thromboembolic events. Even in studies comparing thromboembolic risk between individuals with and without COVID-19,9 unobserved confounding is still a major concern. To address these limitations, we conducted a self-controlled case series (SCCS) study using a national, general population cohort. This method overcomes bias due to unobserved health conditions. Because an SCCS is conducted only among people with any thromboembolic events, we conducted a supplementary cohort analysis to verify the findings.
Patients and Methods
Data Sources
We undertook individual-level record linkage of five health databases covering the whole of Scotland (population, 5.5 million) between March 2018 and October 2020: The Community Health Index (CHI) register, Electronic Communication of Surveillance in Scotland (ECOSS), Rapid Preliminary Inpatient Data (RAPID), Scottish Morbidity Record 01 (SMR01), and death certificates.
The CHI register provides sociodemographic information (age, sex, area socioeconomic deprivation). Deprivation is measured using the Scottish Index of Multiple Deprivation (SIMD), derived from seven domains — income, education, health, employment, crime, housing, and access to services — and categorized into general population quintiles. The ECOSS collects laboratory data on infectious diseases, including test date and result. The RAPID collects real-time data on hospitalization, including dates of admission and discharge, and type of ward; and SMR01 records diseases using International Classification of Diseases 10th Revision (ICD-10) codes and procedures using Office of Population Censuses and Surveys version 4 (OPCS-4) codes. Death certificates provide the date and cause (using ICD-10) of all deaths, whether in hospital or the community. The CHI, a unique identifier, is used across all databases enabling exact matching. We extracted records covering March 1, 2018, to October 5, 2020, inclusive for all databases except the ECOSS COVID-19 test data which covered March 1, 2020, to October 5, 2020. The Scottish data were accessed through the Electronic Data Research and Innovation Service, Public Health Scotland, and have been used in several previous epidemiological studies.10 , 11 Approval for the study was provided by the Public Benefit and Privacy Panel for Health and Social Care (reference 2021–0064).
In the supplementary cohort analysis, all individuals with a positive COVID-19 test were included as the exposed group. For each exposed individual, 10 age-, sex-, and deprivation-matched individuals who did not have a test positive were included using probability density matching.
Outcomes
This study included five outcomes ascertained from SMR01 and death certificates: MI (ICD-10: I21), ischemic stroke (I63-64), PE (I26), and DVT (I80.1-80.9, I82.8, and I82.9), as well as thromboembolism (composite of all four). To test the specificity of any association between COVID-19 and thromboembolism, we also included a composite negative control outcome of elective surgery for hernia repair (OPCS-4 T19, T21-27), colonoscopy (OPCS-4 H22, H25, and H28), cataract surgery (OPCS-4 C71-75, C77, and C79), or hip/knee replacement (OPCS-4 W37-42, W93-95, and O18).
Statistical Analyses
The SCCS method was chosen to analyze the association between COVID-19 infection and outcomes (Supplementary Figure 1, available online at http://www.mayoclinicproceedings.org), in favor of a traditional cohort approach because of its ability to control for intrapersonal time-invariant confounders, and the United Kingdom’s testing strategy. Frail individuals with long-term conditions were more likely both to be tested and experience adverse outcomes. These confounders may not be well recorded in the routine data. With a new condition, such as COVID-19, other unknown confounders may also exist. The SCCS method eliminates intrapersonal time-invariant confounders because each person acts as their own control.12 The method has been widely used in epidemiological studies, including a study on influenza and MI.13
The study population comprised everyone in Scotland who had confirmed (positive real-time polymerase chain reaction test) COVID-19 infection and had experienced one or more thromboembolic event over the study period. The incidence rate ratio (IRR) of thromboembolic outcomes was derived from the ratio of incidence rates in risk and control intervals. The risk interval was defined as between 5 days before and 54 days after the sample was obtained for their first positive COVID-19 test. The risk interval was categorized into groups according to 5 to 1 day before, 0 to 7 days after, 8 to 28 days after, and 29 to 56 days after. The 5 days before confirmed infection patients were included in the risk period to take account of lags in symptom development and testing. The control interval was defined as the remaining study period. Because the UK COVID-19 pandemic started in March 2020, the majority of the control interval occurred before infection.
Conditional Poisson regression was used adjusting for participant age in quintile groups, the main time-varying confounder. Deriving rates for both the risk and control intervals from the same individual obviated the need to control statistically for time-invariant confounders. Because individuals who had fatal events before the pandemic had not had a chance to take a COVID-19 test, standard SCCS cannot be applied to fatal events, and the models were run initially for nonfatal hospitalizations. We then repeated the analyses for the composite outcome of hospitalization or death using the extended SCCS for event-dependent observation periods, which was described elsewhere.14
Subgroup analyses were conducted by COVID-19 admission (those with COVID-19 as primary diagnosis versus those without), age (≤75 versus >75 years), sex, and socioeconomic deprivation (SIMD quintile 1-3 vs SIMD quintile 4-5). P values for subgroup differences were calculated. Additional subgroup analysis was conducted for age (≤65, 66-80, and >80 years) to explore any age trends, even though the number of events were not sufficient to conduct formal tests. Three sensitivity analyses were conducted. Firstly, seasonality in 3-month categories was adjusted because cardiovascular diseases exhibit seasonal patterning. Secondly, we included an extended risk interval, 14 to 6 days before a positive test. If the elevated risk in this extended interval is lower than that in the immediate pretest interval, reverse causation is less likely. Thirdly, as COVID-19 infection was not tested before the 2020 pandemic, we restricted the analysis to cases with events after February 1, 2020. Lastly, we calculated the E values to investigate how robust our findings are regarding time-varying confounders.15 A high E-value suggests that only a strong time-varying confounder could nullify the findings.
A supplementary cohort analysis was conducted. Time-to-event (from testing positive in the exposed individual) to the thromboembolic events was regressed by positive COVID-19 test, controlling for age, sex, and deprivation using Cox proportional hazards model. Proportional hazards assumptions were checked using the Schoenfeld residuals. All analyses were conducted in R version 3.5.1 with the packages SCCS and survival.
Results
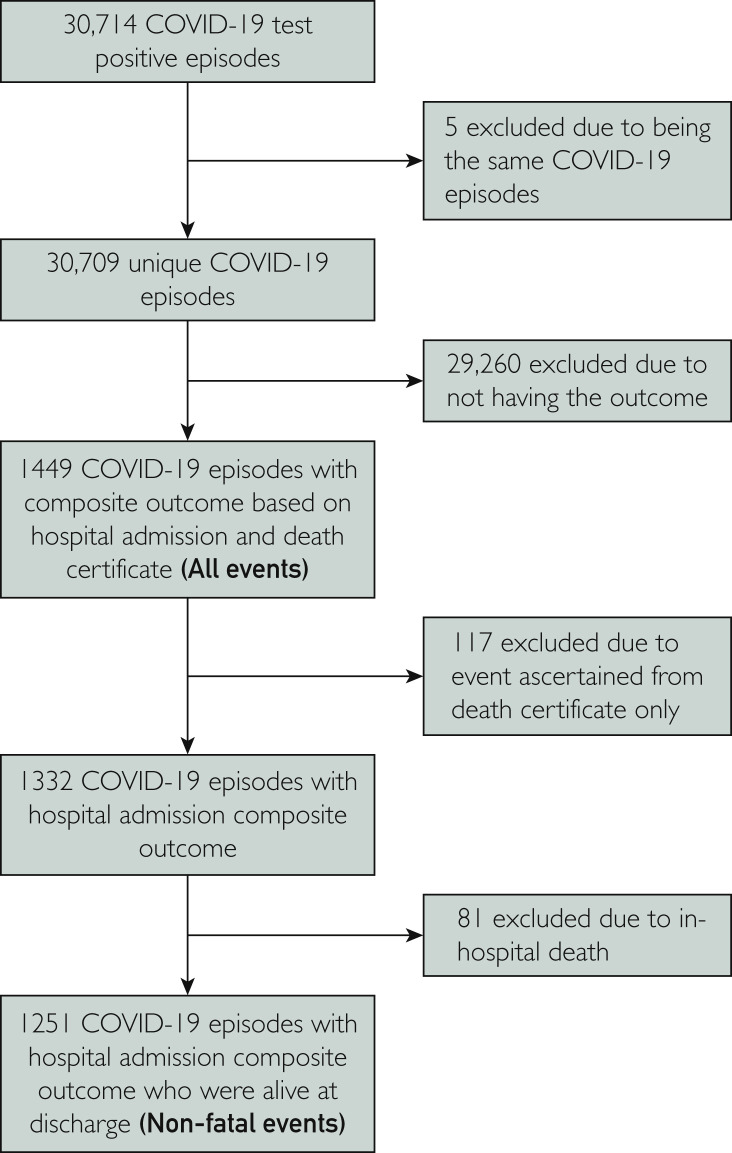
Of the 30,709 individuals who had at least one positive COVID-19 test (Figure 1 ) between March 1, 2020, and October 5, 2020, the incidence rates were 44.0, 67.0, 48.6, and 18.8 per 1000 person-years for MI, ischemic stroke, PE, and DVT, respectively. The SCCS analysis further excluded 29,260 individuals because they did not have thromboembolic events in the study period. Of the 1449 individuals who had thromboembolic events, 117 died out-of-hospital, 81 died in-hospital, and 1251 had nonfatal events. Less than one-third (31.5%) of the individuals had a COVID-19 primary diagnosis in hospital. Among people with nonfatal events, the median age was 77 years (interquartile range, 65-85 years), half were male, and 26.46% lived in the most deprived quintile (Table 1 ). The median age was older for ischemic stroke (82 years) and younger for PE (71 years) and DVT (73 years). Women accounted for a higher percentage (58.6%) of those with DVT.
Figure 1.
Participant flowchart. COVID-19, coronavirus disease 2019.
Table 1.
| Composite | Myocardial infarction | Ischemic stroke | Pulmonary embolism | Deep vein thrombosis | Elective surgeryc | |
|---|---|---|---|---|---|---|
| n for all events | 1449 | 376 | 560 | 417 | 179 | 123 |
| n for admissions only | 1332 | 337 | 505 | 391 | 174 | 123 |
| n for nonfatal admissions only | 1251 | 319 | 473 | 359 | 169 | 116 |
| COVID-19 as primary diagnosis in admission episode | 389 (31.5) | 104 (32.6) | 123 (26.0) | 145 (40.4) | 41 (26.6) | 14 (12.1) |
| Median (IQR) age, y | 77 (65-85) | 78 (67-85) | 82 (73-87) | 71 (59-81) | 73 (59-82) | 78 (70-85) |
| Sex | ||||||
| Female | 626 (50.04) | 128 (40.13) | 246 (52.01) | 180 (50.14) | 99 (58.58) | 45 (38.79) |
| Male | 625 (49.96) | 191 (59.87) | 227 (47.99) | 179 (49.86) | 70 (41.42) | 71 (61.21) |
| SIMD quintile | ||||||
| 1st (Most deprived) | 331 (26.46) | 91 (28.53) | 124 (26.22) | 84 (23.40) | 47 (27.81) | 34 (29.31) |
| 2nd | 282 (22.54) | 79 (24.76) | 100 (21.14) | 88 (24.51) | 32 (18.93) | 21 (18.10) |
| 3rd | 230 (18.39) | 55 (17.24) | 94 (19.87) | 65 (18.11) | 33 (19.53) | 21 (18.10) |
| 4th | 230 (18.39) | 53 (16.61) | 95 (20.08) | 68 (18.94) | 27 (15.98) | 25 (21.55) |
| 5th (Least deprived) | 178 (14.23) | 41 (12.85) | 60 (12.68) | 54 (15.04) | 30 (17.75) | 15 (12.93) |
COVID-19, coronavirus disease 2019; IQR, interquartile range; SIMD, Scottish Index of Multiple Deprivation.
n (%) are presented unless otherwise specified.
Elective surgery included hernia repair, colonoscopy, cataract surgery, and hip and knee replacement, and is a negative control outcome.
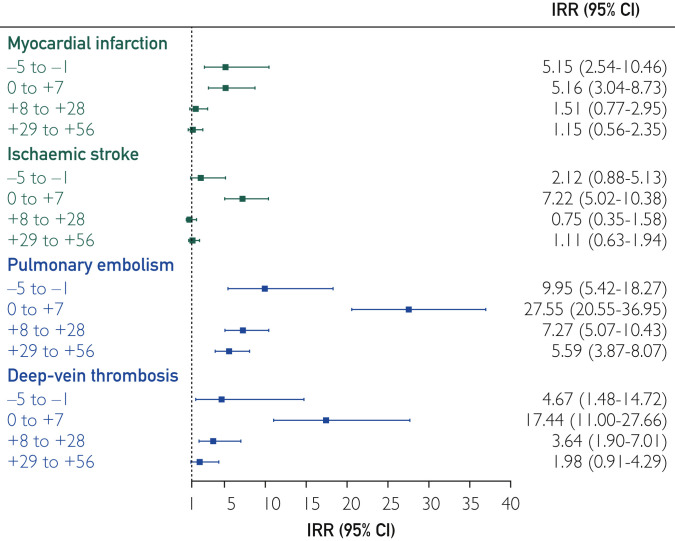
The risk of nonfatal thromboembolism was significantly higher over the whole risk interval and highest within the 7 days following the positive test (IRR, 12.01; 95% CI, 9.91 to 14.56) (Table 2 ). The associations were strongest for PE followed by DVT (Figure 2 ), which had similar risk patterns to overall thromboembolism. The associations with MI and ischemic stroke were smaller in magnitude but nonetheless significant in the 7 days following a positive test, as well as the previous 5 days for MI only. Except for MI, all IRRs in the 7-day post-test interval were significantly stronger than those in the pretest intervals (P<.04). As expected, there was no significant change in the risk of elective surgery before or after a positive COVID-19 test. The findings for the composite outcome of fatal and nonfatal thromboembolism were similar to those for nonfatal thromboembolism, after accounting for censoring.
Table 2.
| Outcome by risk intervals | Nonfatal events |
All eventsd |
||
|---|---|---|---|---|
| IRR (95% CI) | P | IRR (95% CI) | P | |
| Composite | ||||
| 5-1 days before | 4.77 (3.20-7.10) | <.0001 | 3.71 (2.50-5.49) | <.0001 |
| 0-7 days after | 12.01 (9.91-14.56) | <.0001 | 5.70 (4.72-6.89) | <.0001 |
| 8-28 days after | 2.82 (2.16-3.67) | <.0001 | 1.54 (1.22-1.94) | .0003 |
| 28-56 days after | 2.30 (1.77-3.00) | <.0001 | 1.51 (1.21-1.88) | .0002 |
| Myocardial infarction | ||||
| 5-1 days before | 5.15 (2.54-10.46) | <.0001 | 3.79 (1.86-7.71) | .0002 |
| 0-7 days after | 5.16 (3.04-8.73) | <.0001 | 1.98 (1.23-3.18) | .005 |
| 8-28 days after | 1.51 (0.77-2.95) | .23 | 0.85 (0.50-1.44) | .55 |
| 28-56 days after | 1.15 (0.56-2.35) | .70 | 0.90 (0.53-1.50) | .67 |
| Ischemic stroke | ||||
| 5-1 days before | 2.12 (0.88-5.13) | .10 | 1.58 (0.65-3.84) | .31 |
| 0-7 days after | 7.22 (5.02-10.38) | <.0001 | 3.25 (2.34-4.50) | <.0001 |
| 8-28 days after | 0.75 (0.35-1.58) | .45 | 0.69 (0.42-1.12) | .14 |
| 28-56 days after | 1.11 (0.63-1.94) | .72 | 0.94 (0.63-1.41) | .77 |
| Pulmonary embolism | ||||
| 5-1 days before | 9.95 (5.42-18.27) | <.0001 | 7.47 (4.13-13.51) | <.0001 |
| 0-7 days after | 27.55 (20.55-36.95) | <.0001 | 16.81 (12.46-22.69) | <.0001 |
| 8-28 days after | 7.27 (5.07-10.43) | <.0001 | 4.52 (3.21-6.35) | <.0001 |
| 28-56 days after | 5.59 (3.87-8.07) | <.0001 | 3.54 (2.54-4.93) | <.0001 |
| Deep vein thrombosis | ||||
| 5-1 days before | 4.67 (1.48-14.72) | .008 | 4.23 (1.34-13.32) | .01 |
| 0-7 days after | 17.44 (11.00-27.66) | <.0001 | 11.51 (7.30-18.16) | <.0001 |
| 8-28 days after | 3.64 (1.90-7.01) | .0001 | 2.43 (1.27-4.67) | .008 |
| 28-56 days after | 1.98 (0.91-4.29) | .08 | 1.77 (0.92-3.42) | .09 |
| Elective surgeriese | ||||
| 5-1 days before | — | — | — | — |
| 0-7 days after | 1.69 (0.41-6.88) | .47 | 1.28 (0.40-4.06) | .67 |
| 8-28 days after | 1.78 (0.65-4.90) | .26 | 0.94 (0.34-2.59) | .91 |
| 28-56 days after | 2.28 (0.98-5.32) | .06 | 1.19 (0.51-2.76) | .68 |
COVID-19, coronavirus disease 2019; IRR, incidence rate ratio.
Patients' age quintile was adjusted.
Bold type indicates P < .05
Including both fatal and nonfatal events, with event-dependent observation handled using specialized method.
Elective surgery included hernia repair, colonoscopy, cataract surgery, and hip/knee replacement, and is a negative control outcome.
Figure 2.
Associations between COVID-19 and nonfatal outcomes. Incidence rate ratio (IRR) shown is the within IRR for outcomes. Incidence rates in the risk period (5 days before 56 after a positive coronavirus disease 2019 [COVID-19] test) were compared against the control period (all remaining time in study period) for each person.
Adjusting for seasonality did not alter the findings (Supplementary Table 1, available online at http://www.mayoclinicproceedings.org). The extended pretest risk interval generally had lower IRRs than the immediate pretest interval, and were nonsignificant for MI, ischemic stroke, and PE. Including only participants with thromboembolic events after February 2020 resulted in similar IRR estimates. The E values ranged from 5.53 (MI) to 40.59 (PE) for the lower bound of 95% CIs within 7 days of a positive test.
On subgroup analysis, the associations between a positive test and thromboembolism were significant regardless of COVID-19 admission, even though the elevation of risk was stronger among those admitted for COVID-19 (Table 3 ). A positive COVID-19 test was also associated with higher risk of thromboembolism regardless of age, but the magnitude of risk was significantly higher (P interaction<.0001) in people younger than 75 years. Compared with people aged older than 75 years, those younger had 23 and 47 times higher elevated thromboembolism and PE risk, respectively, within 7 days of a positive COVID-19 test (Table 3). There appears to be a dose-response trend by age even though insufficient sample size inhibited formal testing (Supplementary Table 2, available online at http://www.mayoclinicproceedings.org). A positive COVID-19 test was associated with higher risk of overall thromboembolism, PE, and DVT in both women and men, but the magnitude of risk was higher in men (P interaction<.006). The association between a positive COVID-19 test and ischemic stroke was significant in men only. There was no consistent evidence of socioeconomic deprivation being an effect modifier (Supplementary Table 2).
Table 3.
| Outcome by risk intervals | COVID-19 hospitalization |
Age, y |
Sex |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes |
No |
Pinteraction | ≤75 |
>75 |
Pinteraction | Female |
Male |
Pinteraction | |
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | ||||
| Composite | |||||||||
| 5-1 days before | 12.45 (7.37-21.03) | 2.48 (1.33-4.63) | .0001 | 3.80 (2.44-5.93) | 2.60 (1.81-3.72) | .19 | 2.22 (1.41-3.47) | 4.31 (3.02-6.15) | .03 |
| 0-7 days after | 36.97 (28.69-47.64) | 4.07 (2.83-5.85) | <.0001 | 22.78 (17.58-29.53) | 5.94 (4.35-8.12) | <.0001 | 6.36 (4.47-9.04) | 19.44 (15.38-24.58) | <.0001 |
| 8-28 days after | 6.16 (4.18-9.09) | 1.82 (1.26-2.63) | <.0001 | 5.79 (4.16-8.07) | 1.24 (0.78-1.97) | <.0001 | 2.64 (1.83-3.82) | 3.19 (2.18-4.66) | .50 |
| 28-56 days after | 4.85 (3.27-7.20) | 1.50 (1.04-2.17) | <.0001 | 4.27 (3.03-6.03) | 1.12 (0.72-1.74) | <.0001 | 2.28 (1.59-3.27) | 2.46 (1.66-3.65) | .79 |
| Myocardial infarction | |||||||||
| 5-1 days before | 3.58 (0.86-14.88) | 6.17 (2.73-13.95) | .52 | 4.14 (2.00-8.54) | 3.36 (1.80-6.26) | .67 | 4.29 (2.15-8.58) | 3.47 (1.82-6.60) | .66 |
| 0-7 days after | 8.09 (3.87-16.90) | 3.38 (1.50-7.65) | .12 | 6.19 (2.85-13.42) | 3.65 (1.69-7.88) | .34 | 3.35 (1.22-9.18) | 6.45 (3.46-12.00) | .28 |
| 8-28 days after | 1.00 (0.24-4.17) | 1.85 (0.86-3.96) | .46 | 2.49 (1.08-5.77) | 0.77 (0.24-2.45) | .11 | 1.58 (0.57-4.39) | 1.43 (0.58-3.50) | .89 |
| 28-56 days after | 1.28 (0.39-4.22) | 1.15 (0.47-2.81) | .89 | 1.01 (0.32-3.23) | 0.97 (0.35-2.64) | .96 | 1.41 (0.51-3.92) | 0.94 (0.34-2.57) | .60 |
| Ischemic stroke | |||||||||
| 5-1 days before | 3.23 (0.79-13.30) | 1.62 (0.52-5.06) | .45 | 1.70 (0.54-5.39) | 1.84 (1.00-3.37) | 1.00 | 1.21 (0.50-2.96) | 2.58 (1.32-5.08) | .18 |
| 0-7 days after | 14.03 (8.11-24.27) | 4.22 (2.50-7.12) | .0019 | 17.81 (10.67-29.72) | 3.63 (2.07-6.38) | <.0001 | 2.05 (0.84-5.00) | 13.27 (8.79-20.04) | .0002 |
| 8-28 days after | 1.26 (0.39-4.07) | 0.51 (0.19-1.37) | .25 | 0.45 (0.06-3.27) | 0.81 (0.36-1.84) | 1.00 | 0.75 (0.28-2.03) | 0.72 (0.23-2.26) | .96 |
| 28-56 days after | 2.37 (1.06-5.29) | 0.60 (0.27-1.36) | .02 | 1.46 (0.53-4.02) | 0.93 (0.47-1.83) | .47 | 1.20 (0.59-2.45) | 0.96 (0.39-2.37) | .75 |
| Pulmonary embolism | |||||||||
| 5-1 days before | 50.25 (24.26-104.07) | 2.09 (0.52-8.44) | .0001 | 5.36 (2.60-11.08) | 3.40 (1.64-7.03) | .39 | 1.98 (0.73-5.37) | 8.10 (4.42-14.86) | .02 |
| 0-7 days after | 135.97 (88.89-207.98) | 3.92 (1.83-8.40) | <.0001 | 46.84 (32.21-68.12) | 10.36 (5.99-17.91) | <.0001 | 15.22 (9.30-24.90) | 43.82 (29.78-64.48) | .001 |
| 8-28 days after | 23.97 (14.03-40.97) | 3.54 (2.03-6.16) | <.0001 | 12.64 (8.20-19.49) | 2.04 (0.93-4.48) | .0001 | 5.77 (3.42-9.71) | 9.35 (5.63-15.54) | .20 |
| 28-56 days after | 16.26 (9.35-28.27) | 2.99 (1.74-5.15) | <.0001 | 8.13 (5.16-12.81) | 1.88 (0.90-3.94) | .0009 | 4.98 (3.02-8.21) | 6.21 (3.58-10.77) | .57 |
| Deep vein thrombosis | |||||||||
| 5-1 days before | 24.07 (5.54-104.49) | 1.82 (0.25-13.20) | .04 | 2.20 (0.54-9.04) | 3.83 (1.51-9.69) | 1.00 | 3.18 (1.16-8.73) | 3.90 (1.20-12.65) | .80 |
| 0-7 days after | 92.44 (46.02-185.68) | 3.77 (1.36-10.46) | <.0001 | 24.21 (13.13-44.64) | 10.59 (5.11-21.97) | .09 | 8.04 (3.49-18.57) | 33.91 (18.80-61.18) | .006 |
| 8-28 days after | 9.54 (3.15-28.93) | 2.75 (1.18-6.40) | .08 | 5.30 (2.38-11.80) | 1.74 (0.53-5.68) | .13 | 4.89 (2.33-10.26) | 2.03 (0.49-8.45) | .33 |
| 28-56 days after | 1.46 (0.19-11.55) | 2.35 (1.01-5.46) | .67 | 2.85 (1.11-7.28) | 0.91 (0.22-3.82) | .19 | 1.41 (0.44-4.50) | 3.49 (1.23-9.90) | .26 |
COVID-19, coronavirus disease 2019; IRR: incidence rate ratio.
Patients' age quintile was adjusted.
Bold type indicates Pinteraction < .05
The findings from cohort analysis were consistent with those from SCCS. Individuals who had a COVID-19 infection were at a higher risk of all of the outcomes, with strongest association with PE (hazard ratio [HR], 24.04; 95% CI, 18.49 to 31.33), followed by DVT (HR, 10.45; 95% CI, 7.02 to 15.56), ischemic stroke (HR, 4.40; 95% CI, 3.44 to 5.63), and MI (HR, 3.31; 95% CI, 2.59 to 4.22).
Discussion
In this national, general population study including hospitalized and community-dwelling individuals, we showed an elevated risk of thromboembolism in temporal proximity to confirmed COVID-19 infection. In the week following a positive test, participants were at significantly increased risk of MI, ischemic stroke, PE, and DVT, with the increased risk of the latter two being marked (day 0 to +7 IRRs of >27- and >17-fold, respectively) — with risk ratios substantially exceeding those previously associated with upper respiratory infections16 — and elevated risk continuing for some time thereafter. The risk ratios were even higher in younger people and in men. The clear implication of this work is that PE/DVT risks are substantially elevated in hospitalized patients as compared with more modest and shorter atherothrombotic risks. However, there appears a broader thrombotic impact not confined to hospitalized populations, albeit at a lower risk level.
The associations were also significant in individuals not hospitalized for COVID-19. Although the IRRs were modest compared with the hospitalized group, the excess risk for PE was sustained at near three-fold for more than 1 to 2 months after the initial COVID-19 infection. This modest excess risk may also be applicable to a large number of people who were infected with COVID-19 but not hospitalized, which could mean a sizeable population burden. The annual incidence of PE in the UK general population was 0.98 per 1000.17 If the IRR on this study (3.92 in the first 7 days of the nonhospitalized group) is applicable to the general population, this would translate to a rate difference of 3.84 in 1000. There were 4.27 million people who tested positive for COVID-19 in the UK as of March 16, 2021, indicating that at least 16,400 new PE cases could have been caused by COVID-19.
At the present time, unpublished results from intensive care unit COVID-19 populations have led to early stopping of anticoagulant therapeutic arms because of signals suggestive of harm.6 Conversely, the same collated international studies have intimated a significant decreased need for life support and improved results from less severe hospitalized patients.7 Such heterogenous results could be related to the severity of COVID-19, as well as the timing of administering pharmacologic prophylaxis. Given the potentially treatable nature of thrombotic events, urgent work must be undertaken in prevention and treatment trial design to a consider risk stratification strategy that includes COVID-19 severity, age, and sex.
Our new findings are in line with but meaningfully extend previous COVID-19 studies, including another national cohort from Denmark.9 A meta-analysis of more than 100,000 COVID-19 patients reported that 1.2% developed ischemic stroke18; a large proportion even considering their age and vascular risk profile. A hospital-based case-control study of 123 patients found an association (odds ratio, 3.9) between COVID-19 infection and acute ischemic stroke, after controlling for age, sex, and vascular risk factors.19 Similarly, two meta-analyses reported high rates of PE and DVT in patients with COVID-19.3 , 4 Traditional thromboembolic risk factors were not significantly associated with PE in COVID-19 patients, suggesting that the pathways may be different.20 Previous studies21 have shown that the PE found in severe COVID-19 patients might actually be primarily caused by pulmonary thrombi rather than pulmonary emboli, which warrants further investigation.
This study’s association pattern for MI is similar to that for influenza, with five to six times higher risk in the first 7 days after a positive test.13 However, the association of COVID-19 with venous thromboembolism appeared to be much stronger than that of other infections. For example, a study using the same SCCS method found the elevated risk of DVT was much lower (IRR, 1.91 in the first 2 weeks) for upper respiratory infections.16 The same study also found that the risk of PE elevated (IRR, 2.11 in the first 4 weeks) following urinary tract infection. These suggest that COVID-19 may have either different mechanisms, or a stronger systemic inflammation (in keeping with the cytokine storm), leading to an exponential difference in the risk of PE/DVT compared with other infections, while having similar elevation in MI risk.
Our study showed that the association with ischemic stroke was significantly stronger in younger (≤75 years) individuals. This is consistent with previous reports of relatively young people (mean age, 53 to 60 years) with COVID-19 requiring thrombectomy.22, 23, 24 In addition, among stroke patients, those who tested positive for COVID-19 were on average 7 to 15 years younger than those who tested negative.25 , 26 The underlying mechanism warrants further investigation but could relate to cytokine storm, at least in some people.27 Historical reports showed that healthy young people were more likely to experience cytokine storm following viral infections,27 and cytokine storm in COVID-19 patients leading to hypercoagulability was a hypothesized mechanism for thromboembolism.28 The finding that COVID-19 is associated with a higher risk of thromboembolism in men than women may partially explain our previous finding that men have worse case fatality following COVID-19 infection.29 This hypothesis requires further study.
Our study has several strengths. Firstly, it was unselective, covering the whole of Scotland and all confirmed COVID-19 cases regardless of whether they were hospitalized. This avoided the selection bias intrinsic to hospital-based studies. Because both COVID-19 infection and thromboembolism increase the chance of hospitalization, selecting only hospital cases inevitably results in collider bias.8 Secondly, time-invariant confounders, including unknown and unmeasured confounders, were perfectly controlled by using participants as their own controls. The key time-varying confounders, age and seasonality, were adjusted for in the model.12 The use of E-values showed that the elevated risk within 7 days of a positive test would only be meaningfully nullified if there were very strong time-varying confounders that could increase/decrease the risk of positive test and thromboembolic events by 5 to 20 times. Thirdly, we were able to separately analyze nonfatal events using the standard SCCS method, and all events, using a specific method designed for censored data.14 The two approaches produced consistent findings. This, along with the sensitivity analysis including only events shortly before the COVID-19 pandemic, suggest that the results should be robust against immortal time biases.
However, the findings of this study are still subject to the following limitations. To ensure internal validity, this study opted for the SCCS method, which only included patients with at least one thromboembolism during the study period. This may limit the generalizability of the findings to people with lower risk of these events even though our supplementary cohort analysis showed similar results. If the elevated risk of PE is truly causal, the estimates that we provided could be an underestimate. The IRR for the latest category in the risk interval was still significantly greater than one, suggesting a long tail of risk elevation and thus some of the pre- and post-infection control interval could be misspecified. Patients with no or mild symptoms from COVID-19 infection are less likely to have been tested, especially at the beginning of the pandemic when testing capacity was lower. The increased risk of thromboembolism demonstrated in the days before confirmed infection is likely to reflect the time lag between actual date of infection and our proxy measure of it, which was the date of specimen collection. Reverse causation is possible in some patients, for example, nosocomial infection of patients hospitalized for thromboembolic events. However, the lack of an association with elective surgery suggests that any reverse causation is unlikely to fully explain our findings. The lowered risk in extended pretest interval for outcomes except MI also does not support strong reverse causation. It is highly likely that there was underreporting of events from the first wave. There were 1465 individuals who died of suspected COVID-19 (ICD-10, U07.2) without any tests, suggesting that individuals who had COVID-19 but were untested were only a small proportion (4.8%) compared with those tested and unlikely to change our conclusion. Although there was no role for routine computerized tomographic scanning in COVID-1930 and data on rates of advanced imaging are not yet clear, it is our expectation that more extensive imaging in subsequent waves is highly likely to increase pickup of thrombus.
In conclusion, COVID-19 infection was associated with substantially elevated risk of PE and DVT, with excess PE risk lasting at least 8 weeks postinfection. These complications should be addressed through prophylaxis and early detection; clinicians should be alerted to the possibility of PEs in community-treated patients with residual or prolonged symptoms. Clinical trials to prevent thrombotic events should consider the post-hospital convalescent stage where we have demonstrated ongoing increased risk in addition to younger individuals with COVID-19.
Acknowledgments
This study was supported by the Wellcome Trust ISSF COVID Response Fund in the University of Glasgow. The authors would like to acknowledge the support of the Electronic Data Research and Innovation Service Team (Public Health Scotland), especially Ms Johanna Bruce, for their involvement in obtaining approvals, provisioning and linking data and the use of the secure analytical platform within the National Safe Haven. Drs Sattar and Pell are joint senior authors
Footnotes
Potential Competing Interests: The authors report no potential competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W., Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh Y.J., Hong H., Ohana M. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298(2):E70–E80. doi: 10.1148/radiol.2020203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NICE COVID-19 rapid guideline: reducing the risk of venous thromboembolism in over 16s with COVID-19. NICE guideline [NG186] Web site. https://www.nice.org.uk/guidance/ng186 Published 2020. [PubMed]
- 6.Statement from the REMAP-CAP trial on blood thinners in COVID-19 patients [press release] Imperial College London; 2020. https://www.imperial.ac.uk/news/211713/statement-from-remap-cap-trial-blood-thinners/ [Google Scholar]
- 7.NIH . Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients [press release] NIH; 2021. https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients [Google Scholar]
- 8.Griffith G., Morris T.T., Tudball M. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11(1):5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalager-Pedersen M., Lund L.C., Mariager T. Venous thromboembolism and major bleeding in patients with COVID-19: a nationwide population-based cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay D.F., Nelson S.M., Haw S.J., Pell J.P. Impact of Scotland's smoke-free legislation on pregnancy complications: retrospective cohort study. PLoS Med. 2012;9(3):e1001175. doi: 10.1371/journal.pmed.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner S., Mackay D., Dick S., Semple S., Pell J.P. Associations between a smoke-free homes intervention and childhood admissions to hospital in Scotland: an interrupted time-series analysis of whole-population data. Lancet Public Health. 2020;5(9):e493–e500. doi: 10.1016/S2468-2667(20)30178-X. [DOI] [PubMed] [Google Scholar]
- 12.Whitaker H.J., Paddy Farrington C., Spiessens B., Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 13.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 14.Farrington C.P., Whitaker H.J., Hocine M.N. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics. 2009;10(1):3–16. doi: 10.1093/biostatistics/kxn013. [DOI] [PubMed] [Google Scholar]
- 15.Haneuse S., VanderWeele T.J., Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 16.Smeeth L., Cook C., Thomas S., Hall A.J., Hubbard R., Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367(9516):1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 17.Kempny A., McCabe C., Dimopoulos K. Incidence, mortality and bleeding rates associated with pulmonary embolism in England between 1997 and 2015. Int J Cardiol. 2019;277:229–234. doi: 10.1016/j.ijcard.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Nannoni S., de Groot R., Bell S., Markus H.S. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16(2):137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belani P., Schefflein J., Kihira S. COVID-19 is an independent risk factor for acute ischemic stroke. AJNR Am J Neuroradiol. 2020;41(8):1361–1364. doi: 10.3174/ajnr.A6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fauvel C., Weizman O., Trimaille A. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41(32):3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattaneo M., Bertinato E.M., Birocchi S. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang A., Mandigo G.K., Yim P.D., Meyers P.M., Lavine S.D. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerven Surg. 2020;12(7):648–653. doi: 10.1136/neurintsurg-2020-016220. [DOI] [PubMed] [Google Scholar]
- 23.Sweid A., Hammoud B., Bekelis K. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke. 2020;15(7):733–742. doi: 10.1177/1747493020937189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escalard S., Maïer B., Redjem H. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: experience from Paris. Stroke. 2020;51(8):2540–2543. doi: 10.1161/STROKEAHA.120.030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaghi S., Ishida K., Torres J. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majidi S., Fifi J.T., Ladner T.R. Emergent large vessel occlusion stroke during New York City’s COVID-19 outbreak: clinical characteristics and paraclinical findings. Stroke. 2020;51(9):2656–2663. doi: 10.1161/STROKEAHA.120.030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J., Dushoff J., Earn D.J. Age-specific mortality risk from pandemic influenza. J Theor Biol. 2011;288:29–34. doi: 10.1016/j.jtbi.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Ellul M., Benjamin L., Singh B. Neurological Associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jani B.D., Ho F.K., Lowe D.J. Comparison of COVID-19 outcomes among shielded and non-shielded populations: a general population cohort study of 1.3 million. medRxiv. 2020 doi: 10.1101/2020.09.17.20196436. [DOI] [Google Scholar]
- 30.The Royal College of Radiologists . The role of CT in patients suspected with COVID-19 infection [press release] The Royal College of Radiologists; 2020. https://www.rcr.ac.uk/posts/role-ct-patients-suspected-covid-19-infection-12-march-2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.