Abstract
Background
This study aimed to investigate the effect of cold plasma treatment on the improvement of seed germination and surface sterilization of ginseng seeds.
Methods
Dehisced ginseng (Panax ginseng) seeds were exposed to dielectric barrier discharge (DBD) plasma operated in argon (Ar) or an argon/oxygen mixture (Ar/O2), and the resulting germination and surface sterilization were compared with those of an untreated control group. Bacterial and fungal detection assays were performed for plasma-treated ginseng seeds after serial dilution of surface-washed suspensions. The microbial colonies (fungi and bacteria) were classified according to their phenotypical morphologies and identified by molecular analysis. Furthermore, the effect of cold plasma treatment on the in vitro antifungal activity and suppression of Cylindrocarpon destructans in 4-year-old ginseng root discs was investigated.
Results
Seeds treated with plasma in Ar or Ar/O2 exhibited a higher germination rate (%) compared with the untreated controls. Furthermore, the plasma treatment exhibited bactericidal and fungicidal effects on the seed surface, and the latter effect was stronger than the former. In addition, plasma treatment exhibited in vitro antifungal activity against C. destructans and reduced the disease severity (%) of root rot in 4-year-old ginseng root discs. The results demonstrate the stimulatory effect of plasma treatment on seed germination, surface sterilization, and root rot disease suppression in ginseng.
Conclusion
The results of this study indicate that the cold plasma treatment can suppress the microbial community on the seed surface root rot in ginseng.
Keywords: cold plasma, dielectric barrier discharge, fungicidal, Korean ginseng, seed germination
Graphical abstract
1. Introduction
Korean ginseng (Panax ginseng Meyer) is an important medicinal and perennial herb, and has been used in Korean medicine for centuries [1]. The global demand for ginseng has been increasing because its root is considered to have health benefits [2]. Ginseng seeds possess a hard and impermeable seed coat, resulting in poor germination, subsequently leading to a reduced yield. In addition, the presence of seed-borne pathogens (biotic stress) can affect successful seedling growth. Several studies have attempted to improve ginseng seed germination using exogenous plant hormones [3,4], chemical methods [5], physical methods [6], chilling treatment [7], and the scarification method [8]. However, all these methods are time-consuming and labor-intensive and result in chemical residues and environmental pollution.
The cold plasma treatment was developed to overcome these issues. Plasma treatment of seeds is a new approach that has been proposed to facilitate seed germination and survival [9]. Plasma is defined as a neutral ionized gas and represents the fourth and the highest energy state of matter following solids, liquids, and gas [10]. Cold atmospheric plasma used in agriculture and food processing industries can be created by the dielectric barrier discharge (DBD) method [9]. DBD is the electrical discharge between two electrodes separated by an insulating dielectric barrier [11]. Several studies have reported enhanced seed germination and growth on the application of DBD plasma [12]. Recently, cold plasma has been successfully used for treating plant seeds, which can alter the wetting properties of seeds to achieve faster germination and greater yield [9]. Cold plasma treatment can effectively improve the germination of numerous seeds, seedling growth, and crop yield under a pollution-free controlled environment [9,13]. Furthermore, cold plasma treatment plays essential roles in several plant physiological processes, including seed surface sterilization to reduce or eliminate seed-borne pathogens [14,15]. This phenomenon has been demonstrated in several plants, including Asparagus acutifolius [16], Vigna mungo [17], Oryza sativa [18], Glycine max [19], and Mimosa caesalpiniaefolia [20]. A previous study reported that cold plasma treatment of rice seeds inactivated seed-borne pathogens and resulted in healthy emerging seedlings, whereas the seedlings from untreated seeds were heavily infected [21]. Cold plasma can thus inactivate seed-borne pathogenic fungi.
Another important advantage of plasma is the surface sterilization of seeds to eliminate several pathogenic fungi and bacteria [15,22]. Plant pathogens are mainly present in the seed coat, causing diseases in seedlings [23], and seeds are generally treated with insecticides and fungicides to remove these pathogens [24,25]. A limitation of traditional decontamination methods is that most chemicals, including fumigants such as ethylene oxide, have carcinogenic effects. This can be overcome by sterilization using cold plasma treatment [10,26]. Cold plasma treatment can eliminate surface pathogens due to the action of numerous ions and reactive species generated in the plasma [27]. In addition, plasma treatment can also improve plant metabolism [28,29]. Selcuk et al [15] reported a significant increase in grain and legume yields by cold plasma treatment. However, the detailed mechanisms underlying the stimulatory effects of cold plasma in improving seed germination and surface sterilization are still not fully understood. No study has been reported on the effects of cold plasma treatment on ginseng seeds till date. Therefore, in this study, we investigated the effects of cold plasma treatment on germination and surface sterilization of Korean ginseng seeds to overcome the limitations of traditional methods.
2. Materials and methods
2.1. Plasma apparatus and treatment
The atmospheric plasma treatment was performed in a self-designed planar-type DBD plasma reactor. A schematic diagram of the apparatus used to treat the seeds with DBD plasma is presented in Fig. 1, and it consisted of two 100 mm wide stainless-steel disks and a 5 mm thick glass dielectric barrier. The upper stainless-steel disk, with 16 evenly distributed needles, acted as the high-voltage electrode, whereas the lower stainless-steel disk covered by the glass dielectric barrier served as the ground electrode. The entire DBD reactor was enclosed in an acrylic chamber equipped with a gas inlet and outlet. The distance from the needle tip to the gas dielectric surface was 30 mm. On applying a high voltage alternating current (AC; operating frequency: 60 Hz) across the electrodes, the plasma generated at the needle tip propagated towards the ground electrode. The AC voltage of the primary power supply was set at 120 V, which was stepped up at a ratio of 1:70. Pure argon (Ar) or an argon/oxygen mixture (Ar/O2: 80%/20%) was used as the feed gas for the DBD plasma reactor. The flow rate of the feed gas was adjusted to 1 L/min.
Fig. 1.
A schematic representation of the experimental set-up of the dielectric barrier discharges (DBD) plasma reactor for plasma treatment of ginseng seeds.
Dehisced P. ginseng seeds were placed on a Petri dish and exposed to plasma for 10 min. This treatment was performed once a day for 3 d. Ginseng seeds in a Petri dish without exposure to plasma were regarded as the untreated control. After each treatment, the seeds were maintained at 10 °C, and after the last treatment, they were collected for microbial analysis. The effect of plasma treatment on seed surface sterilization was determined as the percentage of colony-forming units (CFU) in plasma-treated seeds relative to that in the untreated controls.
2.2. Plant material and germination tests
Dehisced ginseng seeds were purchased from a local market in Kumsan, South Korea, and stored at 4 °C until further analyses. The seeds were subjected to plasma treatment with Ar or Ar/O2. Seeds without plasma treatment were regarded as the untreated controls. For each treatment, 150 seeds were placed in 3 Petri dishes (50 seeds each) on two layers of filter paper. Ten milliliters of sterile distilled water (SDW) was added to each Petri dish to maintain the moisture level during the experiments. Seed germination (%) and root emergence were observed on days 1, 3, 5, 7, and 10 of incubation. The seed germination percentage (%) was calculated using the following equation: Germination (%) = N/Nt × 100, where N is the number of germinated seeds in each Petri dish and Nt is the total number of seeds in each Petri dish.
2.3. Microbial detection assay
Plasma-treated ginseng seeds (20 seeds) were added to 10 mL of SDW, and vortexed to obtain a microbial suspension. The seeds were removed, and the remaining water was spread on appropriate solid media after 10-fold serial dilution. The microbial suspensions were used for both bacterial and fungal detection assays. For bacterial detection, the suspensions were spread on nutrient agar (NA) medium and incubated at 28 °C for 30 h. The number of bacterial CFU was determined to estimate the population density. The colonies formed on the NA were classified according to their phenotypical morphologies, and all isolates were subjected to molecular identification using sequence homology of their 16S rRNA gene [30]. Genomic DNA was isolated using a bacterial Genomic DNA Extraction Kit (iNtRON Biotechnology, Seongnam, South Korea), following the manufacturer’s instructions. The 16S rRNA gene was amplified by polymerase chain reaction (PCR) using Taq DNA polymerase and the general primer pair 27F/1492R [31]. The obtained PCR products were sequenced using an automated sequencer (Genetic Analyzer 3130; Applied Biosystems, Foster City, CA, USA) with the same primers. The sequences were compared for similarity with the bacterial reference species sequences in the genomic database using the National Center for Biotechnology Information (NCBI)-BLAST tool.
For fungal detection, the serially diluted microbial suspensions were plated on potato dextrose agar (PDA; Difco, USA) acidified with lactic acid (PDAA; 1 mL 85% lactic acid/L PDA) to avoid bacterial growth and incubated at 20 °C for 10 d. The colonies formed on PDAA were classified according to their morphological characteristics. All isolates were subjected to molecular identification using sequence homology of their internal transcribed spacer (ITS) regions. The total genomic DNA of each isolate was extracted following the method developed by Chi et al [32]. The ITS region was amplified using the fungal-specific primers ITS1 and ITS4 [33]. The ITS sequences were used to retrieve the most closely-associated fungal isolates from the GenBank database (http://www.ncbi.nlm.nih.gov), using the NCBI BLAST tool.
2.4. Suppression of Cylindrocarpon destructans by plasma treatment of ginseng root discs
The suppression of C. destructans, which causes root rot disease in ginseng, was evaluated by cold plasma treatment of 4-year-old ginseng root discs inoculated with conidium suspensions. To prepare C. destructans conidium suspensions, the fungus was cultured on PDA plates at 21 °C for 2 wk in darkness to induce sporulation. Conidia were harvested from the plates by gently rubbing the surface mycelium with a rubber swab, and the spores were collected in SDW. Hyphal debris was removed from the conidia by centrifuging the crude conidium preparation, during which the conidia settled at the bottom of the tube. The conidium suspensions were adjusted to a concentration of 1 × 105 conidia/mL using a hemocytometer.
For the in vitro test, the PDA plates were spread with 100 μL of conidial suspensions (105 conidia/mL), and irradiated with cold plasma after 3 h, for 10 min at 120 V. Conidia without plasma treatment was regarded as the untreated control. Conidial germination was recorded after incubation at 21 °C 10 d. For the in-planta test, 4-year-old ginseng roots were surface-sterilized with 70% ethanol for 5 min, rinsed 2-3 times with SDW, and then treated with 1% NaOCl for 5 min and rinsed twice with SDW. The roots were sectioned into discs (0.5 cm thick and 2.5 cm wide) with a sterilized razor blade and placed in Petri dishes containing water-soaked filter paper for moisture. C. destructans conidial suspensions (105 conidia/mL) were deposited on the center of the prepared root discs. Plasma treatment was performed as previously described, except only Ar/O2 was used as the feed gas. Root discs were examined for root rot symptoms after incubation at 21 °C for 10 d. Disease severity (%) was calculated based on the disease index scale, as described by Song et al [34].
2.5. Statistical analysis
Data were subjected to analysis of variance (ANOVA) using JMP ® version 3.1 (SAS Institute Inc., 1995). Significant differences between treatment means were determined using the least significant difference (LSD) test, at p < 0.05. All experiments were performed at least twice. Data from each experiment were analyzed separately. The results of one representative experiment are shown.
3. Results
3.1. Effect of cold plasma treatment on seed germination
The effect of cold plasma treatment under two conditions, i. e., Ar and Ar/O2, on the germination ability of ginseng seeds was examined. The seed germination rate (%) was higher for seeds exposed to plasma with either Ar or Ar/O2 than for the untreated controls. However, no significant difference could be detected in the germination rate (%) between the Ar and Ar/O2 plasma treatments from 7 d onwards. Fig. 2A shows the germination stages of dehisced ginseng seeds after the plasma treatment. The germination rate (%) was 90.2 ± 1.6% and 93.5% ± 2.1% on treatment with Ar and Ar/O2, respectively, after 10 d of incubation (Fig. 2B). The germination rate (%) exhibited a sharp increase on the third day, in both Ar and Ar/O2 treatments, which was followed by minor increases up to day 10 when the highest germination rate (%) was achieved. In addition, plasma treatment also increased seedling root length. The Ar/O2 plasma treatment yielded greater root length (23.5 mm) when compared to the plasma treatment with Ar (16.7 cm) on day 10 (Fig. 2C). However, no significant difference could be detected between the Ar treatment and untreated controls. These results indicate that plasma treatment can enhance seed germinability and seedling growth in ginseng.
Fig. 2.
Effect of plasma treatment with Ar or Ar/O2 on germination of ginseng seeds. (A) A series of stages in ginseng seed germination and effects of plasma. 1, dehiscent ginseng seed; 2 and 3, seeds just before germination; 4, germinating seed with emerging radicle. (B) Seed germination percentage and (C) root length of ginseng seeds subjected to plasma-treatment with 100% Ar or Ar/O2 (80%/20%). The two treatments were compared with the untreated controls. Values are expressed as mean ± SD of 3 replicates with 20 seeds each. Ra, radicle; Sc, seed coat. Scale bar, 1.0 mm.
3.2. Bactericidal effect of plasma treatment on the ginseng seed surface
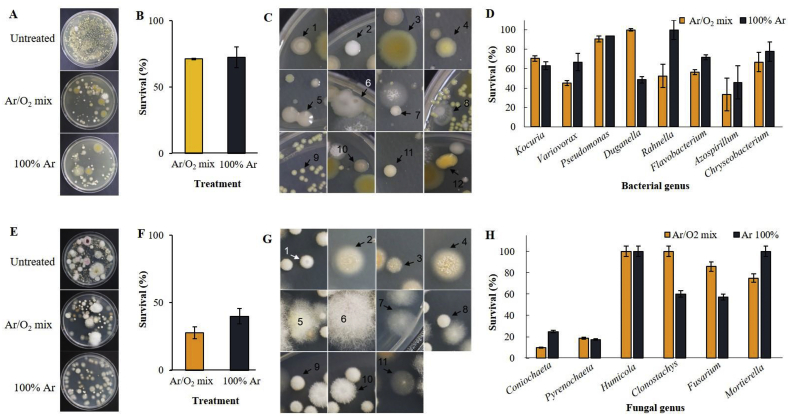
Bacteria present on the ginseng seed were grown on NA plates and then identified. On an average, 1.85 × 109 CFU/seed were recovered from the untreated controls. The total number of bacterial colonies was reduced by both plasma treatments (Fig. 3A). The two plasma treatments for decontamination of ginseng seeds exhibited similar effects. Bacterial populations of 1.31 × 109 CFU/seed and 1.34 × 109 CFU/seed were detected for seeds subjected to plasma treatment with Ar/O2 and Ar, respectively, exhibiting survival rates of 71.2% and 72.5%, respectively (Fig. 3B). In the untreated controls, a total of 8 bacterial genera were isolated from the seed surface and identified using 16S rRNA sequences (Fig. 3C; Table 1). The identity percentage ranged between 96–100% for all strains. The dominant genera were represented by Kocuria spp. (23.2%), Variovorax spp. (20.5%), and Pseudomonas spp. (18.5%) (Table 1). Bacterial survival (%) rates were observed at different levels for seeds treated with Ar or Ar/O2 (Fig. 3D). The genus Kocuria includes gram-positive bacteria, which are known as human pathogens. Bacterial survival rates of 62.9% and 70.5% were observed for seeds treated with plasma in Ar or Ar/O2, respectively. Interestingly, the genus Pseudomonas was not significantly affected by plasma treatment, exhibiting survival rates of 93.8% and 90.4% on plasma treatment with Ar and Ar/O2, respectively. Duganella is non-pathogenic to plants, and in the present study, its survival rates depended on the gas source and were recorded as 48.5% and 100% in Ar and Ar/O2, respectively, but Azospirillum, a non-pathogenic genus was inhibited drastically using plasma treatment both with Ar or Ar/O2.
Fig. 3.
Bactericidal and fungicidal effects of cold plasma treatment on ginseng seeds. (A) Bacterial colonies were generated by spreading the surface washed suspensions of untreated controls, plasma-treated seeds with Ar/O2, and plasma treated seeds with Ar on NA plates. (B) Survival (%) of bacteria on the surface of ginseng seeds after plasma treatment. (C) Various bacterial colonies were detected from the seed surface that were generated by spreading untreated seed suspensions on NA plates and incubating at 28 °C for 30 h. (D) Survival (%) of various bacterial genera on the surface of ginseng seeds after plasma treatment with Ar/O2 or Ar gases. (E) Fungal colonies were generated by spreading the surface washed suspensions of untreated controls, plasma-treated seeds with Ar/O2, and plasma-treated seeds with Ar on PDAA plates. (F) Survival (%) of fungi on the surface of ginseng seeds after plasma treatment. (G) The fungal colonies were generated by spreading the untreated seed suspensions on PDAA plates and incubating at 20 °C for 10 d. (H) Survival (%) of various fungal genera on the surface of ginseng seeds after plasma treatment with Ar/O2 or Ar gases. The experiment was performed at least twice, with three replicates (Petri dishes) per treatment.
Table 1.
NCBI-BLAST Homology Sequence Identity of the Detected Microbial Species Isolated From the Surface of Wild Ginseng Seeds
| NCBI BLAST Homology sequence identity |
Colony Serial no. | GenBank accession No. from our isolates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Genus | Relative abundance (%) | Species | GenBank accession No. | Identity (%) | Query length | E value | |||
| Bacteria | Kocuria | 23.2 | marina | KM099465.1 | 99.3 | 873 | 0.0 | 9 | MW205759 |
| Variovorax | 20.5 | paradoxus | MK371082.1 | 98.2 | 1405 | 0.0 | 4 | MW205754 | |
| boronicumulans | MT758347.1 | 99.7 | 961 | 0.0 | 10 | MW205760 | |||
| Pseudomonas | 18.5 | rhodesiae | CP054205.1 | 99.9 | 1482 | 0.0 | 1 | MW205751 | |
| brenneri | MF957286.1 | 96.8 | 1385 | 0.0 | 6 | MW205756 | |||
| tolaasii | NR114795.1 | 98.9 | 1213 | 0.0 | 7 | MW205757 | |||
| mandelii | MH587023.1 | 99.4 | 821 | 0.0 | 8 | MW205758 | |||
| Duganella | 14.6 | zoogloeoides | MH259953.1 | 96.6 | 922 | 0.0 | 5 | MW205755 | |
| Rahnella | 9.3 | aquatilis | MK072684.1 | 98.9 | 1265 | 0.0 | 2 | MW205752 | |
| Flavobacterium | 8.6 | pectinovorum | KY077148.1 | 99.1 | 1178 | 0.0 | 3 | MW205753 | |
| Azospirillum | 4.0 | melinis | KY465732.1 | 97.9 | 825 | 0.0 | 11 | MW205768 | |
| Chryseobacterium | 1.3 | yeoncheonense | MT225904.1 | 98.7 | 684 | 0.0 | 12 | MW205761 | |
| Fungi | Coniochaeta | 55.1 | fasciculata | KT876721.1 | 99.6 | 537 | 0.0 | 2 | MW199080 |
| fasciculata | KT876721.1 | 97.6 | 537 | 0.0 | 3 | MW199081 | |||
| fasciculata | KT876721.1 | 99.8 | 538 | 0.0 | 4 | MW199082 | |||
| fasciculata | KT876721.1 | 100.0 | 538 | 0.0 | 11 | MW199089 | |||
| Pyrenochaeta | 36.8 | - | HQ914829.1 | 99.6 | 522 | 0.0 | 1 | MW199079 | |
| - | HQ914829.1 | 100.0 | 522 | 0.0 | 8 | MW199086 | |||
| Humicola | 3.6 | fuscoatra | MK967561.1 | 99.6 | 530 | 0.0 | 7 | MW199085 | |
| fuscoatra | MK791665.1 | 99.4 | 515 | 0.0 | 9 | MW199087 | |||
| Clonostachys | 2.1 | rossmaniae | KC806298.1 | 99.8 | 532 | 0.0 | 10 | MW199088 | |
| Fusarium | 1.5 | proliferatum | MT447507.1 | 99.8 | 522 | 0 | 6 | MW199084 | |
| Mortierella | 0.9 | hyalina | MT003094.1 | 98.5 | 615 | 0 | 5 | MW199083 | |
3.3. Fungicidal effect of plasma treatment on the ginseng seed surface
Numerous fungi present on the seed surface were detected by culturing on PDAA media (Fig. 3E). On an average, 7.88 × 103 CFU/seed were recovered from the seed surface of the untreated controls. The plasma treatment could effectively sterilize the seed surface by inactivating the fungi to a greater extent than bacteria. The total number of fungal colonies was remarkably lower for the plasma-treated seeds both with Ar or Ar/O2 than for the untreated controls. Fungal populations of 2.18 × 103 CFU/seed and 3.15 × 103 CFU/seed were recovered from the seed surface following plasma treatments with Ar and Ar/O2, respectively, exhibiting survival rates of 27.7% and 40.0%, respectively (Fig. 3F). We isolated 6 fungal genera from the untreated seed surfaces, which were then identified based on ITS sequence analysis (Fig. 3G and Table 1). The identity percentage ranged between 97–100% for all fungal species. The most dominant fungal genera were Coniochaeta (55.1%) and Pyrenochaeta (36.8%) (Table 1). In overall, The genera Coniochaeta and Pyrenochaeta were remarkably affected by plasma treatment to the highest level with both Ar/O2 and Ar, while Fusarium and Clonostachys genera were affected to the next highest level by plasma treatment better with Ar than Ar/O2. On the other hand, Humicola spp., were not affected by plasma treatment either with Ar or Ar/O2. The survival rate of Mortierella hyalina was observed as 100% and 75%, following plasma treatment both with Ar and Ar/O2, respectively (Fig. 3H). Except the fungal genera of Humicola and Pyrenochaeta, the survival (%) rate for the remaining four fungal genera varied upon plasma treatment with Ar or Ar/O2. These results show that survival rate of pathogenic fungi by plasma treatment varies from species to species.
3.4. Suppression of C. destructans by cold plasma treatment
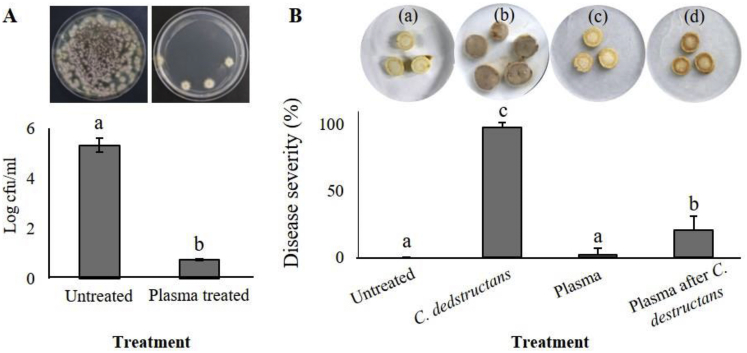
The plasma treatment exhibited antifungal activity against C. destructans, which causes root rot in ginseng. The plasma treatment effectively suppressed the growth of C. destructans on PDA, yielding only 5 conidia/mL for 10 d, whereas the number of fungal colonies in the untreated controls was 3 × 105 conidia/mL (Fig. 4A). Under in vitro conditions, the water-treated ginseng roots (untreated controls) did not exhibit any disease symptoms, whereas severe root rot symptoms were observed on ginseng root discs inoculated with C. destructans conidium suspensions without plasma treatment. Similar to the water-treated control, no root rot symptoms were observed on ginseng root discs treated only with cold plasma (Fig. 4B). However, root discs treated with cold plasma 3 h after inoculation with C. destructans conidium suspensions exhibited brownish discoloration or mild rot symptoms, with an average disease severity of 20.4%, whereas the disease severity was 97.9% in root discs without plasma treatment. These results suggest that the plasma treatment could effectively control the root rot of ginseng caused by C. destructans.
Fig. 4.
Effect of cold plasma (Ar/O2) treatment on in vitro antifungal activity and in planta suppression of C. destructans in 4-year-old ginseng root discs. (A) In vitro fungicidal effect of cold plasma treatment on suppression of C. destructans in comparison with untreated controls on PDAA plates. (B) Disease severity (%) of C. destructans on 4-year-old ginseng root discs following cold plasma treatment. The treatments were as follows: (a) water only (untreated control), (b) negative control (inoculation with C. destructans conidium suspensions). (c) cold plasma treated only followed by incubation at 25 °C. (d) cold plasma treatment 3 h after inoculation with C. destructans conidia suspensions. Rot severity was examined 10 d after inoculation at 25 °C. The disease severity was calculated based on the disease index rating scale from 0–4. The experiment was performed at least twice, with five replicates (root discs) per treatment. Bars with the same letters are not significantly different based on the least significant difference (p < 0.05).
4. Discussion
This study describes the effect of cold plasma treatment in improving the germination success rate and surface sterilization of Korean ginseng seeds under laboratory conditions. Ginseng seeds were subjected to cold plasma treatments with Ar or Ar/O2 gases. Plasma treatment is inexpensive and is thus suitable for commercial-scale applications. Our data demonstrate that cold plasma treatment can improve ginseng and seed germination. Plasma treatments have previously been reported to increase the seed germination rate in spring wheat, maize, oat, and barley [35]. Furthermore, cold plasma treatment significantly improved germination in Chenopodium album [36]. The increase in germinability may be attributed to the rupturing of the outer seed lipid layer by the plasma treatment, resulting in higher water uptake through the seed coat and enhanced germination. In our study, the improvement of seed germination of ginseng seeds by cold plasma treatment might be due to any of the following reasons, such as changes in physiology or mechanical damage to breaking seed dormancy or seed imbibition. In addition, the cold plasma treatment can increase crop yield and shorten the harvesting time. Several previous studies have suggested that plasma treatment can enhance seed germination and seedling growth rate by improving water uptake [[37], [38], [39], [40]]. These results are consistent with those of Jiang et al [41], who found that plasma treatment significantly improved seedling height, root length, and fresh weight in wheat crops. However, the mode of action of plasma-induced changes in seeds remains unclear. There may be an indirect association between seed germination and water uptake.
In addition to improving seed germination by breaking the dormancy, cold plasma treatment has previously been reported to inactivate seed-borne pathogenic microorganisms [15,42]. Several studies have shown that low-temperature plasma can inhibit the growth of food pathogens [15,43,44] in addition to phytopathogenic fungi [45]. On the contrary, this study analyzes the survival of microbiome on the surface of ginseng seeds upon treatment with cold plasma using Ar or Ar/O2, that effect on bacteria and fungi at various levels at the same time. When plasma was generated with 100% Ar, the effects were considered due to excited state and when oxygen is added to the Ar gas, the reactive species of oxygen work together, then the excited O2 and Ar induced by Ar/O2 can exhibit high energy [46]. The results of these studies are in accordance with our results, where the fungicidal effect of plasma was observed to be greater than its bactericidal effect, even though, the fungal cell wall is constituted of chitin and pigments such as melanins [47], which may provide protection against damaging agents, including gamma irradiation, extreme temperatures, and free oxygen radicals [48]. A previous study by Moman et al [49] reported a similar effect of plasma treatment on Pseudomonas spp., which are widespread bacteria in agricultural soils and have both beneficial and harmful effects on plants [50]. The findings in our study underlie the hypothesis that the exposure of cold plasma may associate with the decontamination of surface pathogens on ginseng seeds, which depends on characteristics of target microorganism. This might be achieved via rupturing the bacterial cell wall or disruption of intracellular components or generating various reactive species or virulence factors such as prevention of biofilm formation leading to cell death.
Varying effects of the cold plasma treatment, either with Ar or Ar/O2, were observed on the survival rate (%) of various fungal pathogens present on the surface of ginseng seeds. However, plasma treatment with Ar/O2 was more effective in inactivating Coniochaeta sp. and Pyrenochaeta sp. than with Ar alone, resulting in a lower survival rate (%) of these fungi when compared with other fungal species. A similar reduction was observed in the survival of Fusarium sp., Humicola sp. and Clonostachys sp. on plasma treatment with Ar. These results are supported by the observed inactivation of different microorganisms in cold atmospheric pressure plasma [51]. Similarly, a previous study by Panngom et al [52] reported that non-thermal plasma treatment with Ar could diminish the fungal pathogens by inactivating the fungal spores of Fusarium oxysporum f. sp. lycopersici. Although bacteria and fungi on the surface of ginseng seeds were successfully inactivated by cold plasma treatment with Ar/O2 or Ar, the inactivation mechanisms are still poorly understood, because of the complexity of non-thermal plasma treatments. The genus Pyrenochaeta was also abundant on the seeds (36.4%) and has previously been isolated from ginseng [53,54]. However, the precise mechanism of microorganismal inactivation by cold atmospheric plasma treatment remains controversial [55,56].
Based on the in vitro antifungal activity of the two cold plasma treatments, only the plasma treatment with Ar/O2 was subjected used for testing the fungicidal effect against the C. destructans, which causes primary root-rot disease in ginseng, using an in-planta study on 4-year-old ginseng root discs [57]. The root rot disease is one of the most serious diseases in ginseng, as it can greatly reduce the yield and damage the quality of roots [58]. Previous studies have shown that this pathogen is genetically diverse and consists of several species [59]. Many studies have reported that cold plasma treatment can effectively control bacterial and fungal contamination of seeds, thus improving the germination quality [15,60]. Plasma treatment may further activate the host defenses, providing additional protection against pathogenic infections [52]. Plasma treatment is one of the eco-friendly and cost-effective techniques to control root rot in ginseng for sustainable agriculture.
5. Conclusion
In summary, non-thermal plasma is an emerging and promising agricultural technology, as it represents an efficient, eco-friendly, and inexpensive alternative to traditional methods for improvement of seed germination and surface sterilization. To the best of our knowledge, this study is the first attempted application of cold plasma for treating ginseng seeds. Cold plasma treatment with Ar/O2 and Ar enhanced seed germination. We demonstrated that cold plasma treatment by DBD with Ar/O2 can effectively reduce the survival rate (%) of bacteria and fungi on the surface of ginseng seeds, including phytopathogens. This study confirms that the low-temperature plasma is an effective tool for improving germination and root growth, exerting fungicidal and bactericidal effects on ginseng seeds, particularly in the control of ginseng root rot disease. Several applications of plasma in agriculture have recently been reported, including the proposed application of plasma-activated water and soil sterilization. Therefore, plasma can be used not only for the treatment of ginseng seeds but also during the entire field cultivation period. However, we only assessed two kinds of plasma sources and one irradiation condition for enhancing the germination and surface sterilization of ginseng seeds in this study. Further studies are thus required to optimize the effects of applied plasma.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Science, ICT and Future Planning, Korea (Grant No. 2016R1A2A2A05920703).
References
- 1.Yun T.K. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci 2019. 2001;16 doi: 10.3346/jkms.2001.16.S.S3. S3–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee T.K., Johnke R.M., Allison R.R., O’Brie K.F., Dobbs L.J. Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.H., Ahn I.O., Khan A.L., Kamran M., Waqas M., Lee J.S., Kim D.H., Jang S.W., Lee I.J. Regulation of endogenous gibberellins and abscisic acid levels during different seed collection periods in Panax ginseng. Hortic Environ Biotechnol. 2014;55:166–174. [Google Scholar]
- 4.Lee J.W., Jo I.H., Kim J.U., Hong C.E., Kim Y.C., Kim D.H., Park Y.D. Improvement of seed dehiscence and germination in ginseng by stratification, gibberellin, and/or kinetin treatments. Hortic Environ Biotech. 2018;59:293–301. [Google Scholar]
- 5.Blaszczak W., Doblado R., Frias J., Vidal-Valverde C., Jadwiga Sadowska J., Fornal J. Microstructural and biochemical changes in raw and germinated cowpea seeds upon high-pressure treatment. Food Res Int. 2007;40:415–423. [Google Scholar]
- 6.Al-Bachir M. Effect of gamma irradiation on microbial load and sensory characteristics of aniseed (Pimpinella anisum) Bioresource Technol. 2007;98:1871–1876. doi: 10.1016/j.biortech.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Kwon W.S., Lee J.H., Lee M.G. Optimum chilling terms for germination of the dehisced ginseng (Panax ginseng C. A. Meyer) seed. J Ginseng Res. 2001;25:167–170. [Google Scholar]
- 8.Li T.S.C., Bedford K.E., Sholberg P.L. Improved germination of American ginseng seeds under controlled environments. HortTechnol. 2000;10:131–135. [Google Scholar]
- 9.Randeniya L.K., de Groot G.J.J.B. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: a review. Plasma Process Polymers. 2015;12:608–623. [Google Scholar]
- 10.Moreau M., Orange N., Feuilloley M.G.J. Non-thermal plasma technologies: new tools for bio-decontamination. Biotech Adv. 2008;26:610–617. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Ananth A., Gandhi M.S., Mok Y.S. A dielectric barrier discharge (DBD) plasma reactor: an efficient tool to prepare novel RuO2 nanorods. J Phys D Appl Phys. 2013;46:155202. [Google Scholar]
- 12.Henselova M., Slovakova L., Martinka M., Zahoranova A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia. 2012;67:490–497. [Google Scholar]
- 13.Tong J.Y., He R., Zhang X.L., Zhan R.T., Chen W.W., Yang S.Z. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci Technol. 2014;16:260. [Google Scholar]
- 14.Zhou Z.W., Huang Y.F., Yang S.Z., Chen W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agri Sci. 2011;2:23–27. [Google Scholar]
- 15.Selcuk M., Oksuz L., Basaran P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresource Technol. 2008;99:5104–5109. doi: 10.1016/j.biortech.2007.09.076. [DOI] [PubMed] [Google Scholar]
- 16.Porto C.L., Sergio L., Boari F., Logrieco A.F., Cantore V. Cold plasma pretreatment improves the germination of wild asparagus (Asparagus acutifolius L.) seeds. Scientia Horti. 2019;256:108554. [Google Scholar]
- 17.Billah M., Sajib S.A., Roy N.C., Rashid M.M., Talukder M.R. Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo L.) seed germination and growth. Arch Biochem Biophy. 2020;681:108253. doi: 10.1016/j.abb.2020.108253. [DOI] [PubMed] [Google Scholar]
- 18.Yodpitak S., Mahatheeranont S., Boonyawan D., Sookwong P., Roytrakul S., Norkaew O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019;289:328–339. doi: 10.1016/j.foodchem.2019.03.061. [DOI] [PubMed] [Google Scholar]
- 19.Pérez Pizá M.C., Prevosto L., Zilli C., Cejas E., Kelly H., Balestrasse K. Effects of non–thermal plasmas on seed-borne Diaporthe/Phomopsis complex and germination parameters of soybean seeds. Innov Food Sci Emerg Technol. 2018;49:82–91. [Google Scholar]
- 20.da Silva A.R.M., Farias M.L., da Silva D.L.S., Vitoriano J.O., de Sousa R.C., Alves-Juni C. Using atmospheric plasma to increase wettability, imbibition and germination of physically dormant seeds of Mimosa Caesalpiniafolia. Colloids and Surfaces B: Biointerfaces. 2017;157:280–285. doi: 10.1016/j.colsurfb.2017.05.063. [DOI] [PubMed] [Google Scholar]
- 21.Khamsen N., Onwimol D., Teerakawanich N., Dechanupaprittha S., Kanokbannakorn W., Hongesombut K., Srisonphan S. Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl Mater Interfaces. 2016;8:19268–19275. doi: 10.1021/acsami.6b04555. [DOI] [PubMed] [Google Scholar]
- 22.Dobrynin D., Fridman G., Mukhin Y.V., Wynosky-Dolfi M.A., Rieger J., Rest R.F., Gutsol A.F., Fridman A. Cold plasma inactivation of Bacillus cereus and Bacillus anthracis (Anthrax) spores. IEEE T Plasma Sci. 2010;38:1878–1884. [Google Scholar]
- 23.Tsedaley B. Review on Seed health tests and detection methods of seedborne diseases. J Biol Agri Healthcare. 2015;5:176–184. [Google Scholar]
- 24.Wang H., Zhou B., Feng H. Surface characteristics of fresh produce and their impact on attachment and removal of human pathogens. Produce contamination. In: Gomez-Lopez V.M., editor. Decontamination of fresh and minimally processed produce. Wiley-Blackwell Publishing; USA: 2012. pp. 43–55. [Google Scholar]
- 25.Sharma K.K., Singh U.S., Sharma P., Kumar A., Sharma L. Seed treatments for sustainable agriculture-A review. J Appl Nat Sci 2015. 2015;7:521–539. [Google Scholar]
- 26.Mosovska S., Medvecka V., Halaszova N., Durina P., Valik L., Mikulajova A., Zahoranova A. Cold atmospheric pressure ambient air plasma inhibition of pathogenic bacteria on the surface of black pepper. Food Res Int. 2018;106:862–869. doi: 10.1016/j.foodres.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 27.Choi J.H., Han I., Baik H.K., Lee M.H., Han D.W., Park J.C., Lee I.S., Song K.M., Lim Y.S. Analysis of sterilization effect by pulsed dielectric barrier discharge. J Electrostat. 2006;64:17–22. [Google Scholar]
- 28.Yin M.Q., Huang M.J., Ma B.Z., Ma T.C. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci Technol. 2005;7:3143–3147. [Google Scholar]
- 29.Jiang J.F., Lu Y.F., Li J.G., Li L., He X., Shao H.L., Dong Y.H. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (bacterial wilt) Plos One. 2014;9:1–6. doi: 10.1371/journal.pone.0097753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank J.A., Reich C.I., Sharma S., Weisbaum J.S., Wilson B.A., Olsen G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74:2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi M.H., Park S.Y., Lee Y.H. A quick and safe method for fungal DNA extraction. Plant Pathol J. 2009;25:108–111. [Google Scholar]
- 33.White T.J., Bruns T., Lee S., Taylor J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols; a quide to methods and applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- 34.Song M., Yun H.Y., Kim Y.H. Antagonistic Bacillus species as a biological control of ginseng root rot caused by Fusarium cf. incarnatum. J Ginseng Res. 2014;38:136–145. doi: 10.1016/j.jgr.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubinov A.E., Lazarenko E.M., Selemir V.D. Effect of glow discharge air plasma on grain crops seed. IEEE Trans Plasma Sci. 2000;28:180–183. [Google Scholar]
- 36.Sera B., Gajdova I., Cernak M., Gavril B., Hnatiuc E., Kovacik D., Kriha V., Slama J., Sery M., Spatenka P. How various plasma sources may affect seed germination and growth. IEEE Plasma Science. 2012;39:1365–1369. [Google Scholar]
- 37.Bormashenko E., Shapira Y., Grynyov R., Whyman G., Bormashenko Y., Drori E. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris) J Exp Bot. 2015;66:4013–4021. doi: 10.1093/jxb/erv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park Y., Oh K.S., Oh J., Seok D.C., Kim S.B., Yoo S.J., Lee M.J. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process Polymers. 2016;15 [Google Scholar]
- 39.Wang X.Q., Zhou R.W., Groot G.D., Bazaka K., Murphy A.B., Ostrikov K. Spectral characteristics of cotton seeds treated by a dielectric barrier discharge plasma. Sci Rep. 2017. 2017;7:5601. doi: 10.1038/s41598-017-04963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adhikari B., Adhikari M., Park G.S. The effects of plasma on plant growth, development, and sustainability. Appl Sci. 2020;10:6045. [Google Scholar]
- 41.Jiang J., He X., Li L., Li J., Shao H., Xu Q., Ye R., Dong Y. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Science Technol. 2014;16:54–58. [Google Scholar]
- 42.Schnabel U., Niquet R., Krohmann U., Winter J., Schluter O., Weltmann K.D., Ehlbeck J. Decontamination of microbiologically contaminated specimen by direct and indirect plasma treatment. Plasma Process Polymers. 2012;9:569–575. [Google Scholar]
- 43.Dasan B.G., Boyaci I.H., Mutlu M. Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control. 2016;70:1–8. doi: 10.1016/j.ijfoodmicro.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Butscher D., Loon H.V., Waskow A., von Rohr P.R., Schuppler M. Plasma inactivation of microorganisms on sprout seeds in a dielectric barrier discharge. Int J Food Microbiol. 2016;238:222–232. doi: 10.1016/j.ijfoodmicro.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X., Liu D., Zhou R., Song Y., Sun Y., Zhang Q., Niu J., Fan H., Yang S.Z. Atmospheric cold plasma jet for plant disease treatment. Appl Phys Lett. 2014;104 [Google Scholar]
- 46.Salarieh S., Dorranian D. Sterilization of turmeric by atmospheric pressure dielectric barrier discharge plasma. Plasma Science Technol. 2013;15 doi: 10.1088/1009-0630/15/11/09. [DOI] [Google Scholar]
- 47.Nimrichter L., Rodrigues M.L., Rodrigues E.G., Travassos L.R. The multitude of targets for the immune system and drug therapy in the fungal cell wall. Microbes Infect. 2005;7:789–798. doi: 10.1016/j.micinf.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Latgé J.P., Beauvais A. Functional duality of the cell wall. Curr Opin Microbiol. 2014;20:111–117. doi: 10.1016/j.mib.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Moman R.M., Najmaldeen H. The bactgericidal efficacy of cold atmospheric plasma technology on some bacterial strains. Egyp Acad J Biology Sci. 2010;2:43–47. [Google Scholar]
- 50.Noori M.S.S., Saud H.M. Potential plant growth-promoting activity of Pseudomonas sp isolated from paddy soil in Malaysia as biocontrol agent. J Plant Pathol Microbiol. 2012;3:1–4. [Google Scholar]
- 51.Lee K., Paek K.H., Ju W.T., Lee Y. Sterilization of bacteria, yeast, and bacterial endospores by atmospheric-pressure cold plasma using helium and oxygen. J Microbiol. 2006;44:269–275. [PubMed] [Google Scholar]
- 52.Panngom K., Lee S.H., Park D.H., Sim G.B., Kim Y.H., Uhm H.S., Park G.S., Choi E.H. Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. Plos One. 2014;9 doi: 10.1371/journal.pone.0099300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park S.U., Lim H.S., Par K.C., Par Y.H., Bae H. Fungal endophytes from three cultivars of panax ginseng Meyer cultivated in Korea. J Ginseng Res. 2012;36:107–113. doi: 10.5142/jgr.2012.36.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H., Yang H.Y., You X.L., Li Y.H. Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. SpringerPlus. 2013;2:107. doi: 10.1186/2193-1801-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boudam M.K., Moisan M., Saoudi B., Popovici C., Gherardi N., Massines F. Bacterial spore inactivation by atmospheric-pressure plasmas in the presence or absence of UV photons as obtained with the same gas mixture. J Physi D Appl Phys. 2006;39:3494–3507. [Google Scholar]
- 56.Hertwig C., Meneses N., Mathys A. Cold atmospheric pressure plasma and low energy electron beam as alternative nonthermal decontamination technologies for dry food surfaces: a review. Trends Food Sci Technol. 2018;77:131–142. [Google Scholar]
- 57.Kang Y.H., Lee S.H., Lee J.K. Development of a selective medium for the fungal pathogen Cylindrocarpon destructans using radicicol. Plant Pathol J. 2014;30:432–436. doi: 10.5423/PPJ.NT.08.2014.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rahman M., Punja Z.K. Factors influencing development of root rot on ginseng caused by Cylindrocarpon destructans. Phytopathol. 2005;95:1381–1390. doi: 10.1094/PHYTO-95-1381. [DOI] [PubMed] [Google Scholar]
- 59.Cabral A., Groenewald J.Z., Rego C., Oliveira H., Crous P.W. Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol Prog. 2012;11:655–688. [Google Scholar]
- 60.Zahoranova A., Henselova M., Hudecova D., Kalinakova B., Kovacik D., Medvecka V., Cernak M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seed surface. Plasma Chem Plasma Process. 2016;36:397–414. [Google Scholar]