Abstract
Background
A wide range of environmental factors, such as diseases, nutritional deficiencies, ageing, hormonal imbalances, stress, and ultraviolet (UV) radiation, may affect the structure and function of the skin that covers the entire surface of the human body. In this study, we investigated roles of red ginseng oil (RGO) in enhancing skin functions, including hair growth and skin protection, using mouse models.
Methods
For hair growth experiment, shaved dorsal skins of C57BL/6 mice were topically applied with vehicle, RGO, RGO's major compounds, or minoxidil for consecutive 21 days and skin tissues were examined the hair growth promoting capacity. For skin protection experiment, SKH-1 hairless mice were topically applied with vehicle or RGO twice a day for three days prior to exposure to UVC radiation at 20 kJ/cm2. Skin tissues were collected to evaluate skin protective effects of RGO.
Results
Topical application of RGO to C57BL/6 mice effectively promoted hair regeneration by inducing early telogen-to-anagen transition and significantly increasing the density and bulb diameter of hair follicles. Major compounds, including linoleic acids and β-sitosterol, contributed to RGO-promoted hair growth. Treatment with RGO as well as its major components upregulated expression of hair growth–related proteins. Furthermore, in SKH-1 hairless mice, RGO had a protective effect against UVC-induced skin damage by inhibiting inflammation and apoptosis, as well as inducing cytoprotective systems.
Conclusion
These data suggest that RGO may be a potent agent for improving skin health and thereby preventing and/or treating hair loss and protecting skin against UV radiation.
Keywords: Hair growth, Skin protection, Red ginseng oil, UV radiation
Graphical abstract
1. Introduction
The skin is the largest organ of the body that functions as a protective barrier from diverse environmental hazards such as ultraviolet (UV) radiation, microorganisms, and toxicants. It also contributes to the formation of several appendages, including hair, nails, and sebaceous and sweat glands [1]. Many internal and external factors, such as diseases, nutritional deficiencies, ageing, hormonal imbalances, stress, and UV radiation, may disturb the condition of healthy skin. In addition, impaired skin functions may affect the structure as well as the development of skin appendages, including hair.
Because of their many positive physiological benefits, plant oils have recently been attracting great interest for cosmetic and medical purposes related to the skin. Plant oils may improve skin health, enhance hair growth, fight against pathogens, and protect skin from sun damage. Additionally, topically applied plant oils penetrate well into the skin, and have a localised rather than systemic effect [2]. Red ginseng oil (RGO), extracted from red ginseng, one of the most popular folk medicines in East Asia, has been demonstrated to prevent the pathogenesis of various diseases and other chronic conditions. Our previous studies have shown that the antioxidant and hepatoprotective activities of RGO are associated with the upregulation of cellular antioxidant defence systems [3,4]. RGO reduces inflammation by inhibiting the production of pro-inflammatory mediators and cytokines [5]. Through the suppression of cell proliferation and induction of cytoprotective mechanisms, RGO exhibits an inhibitory effect on 12-O-tetradecanoylphorbol-13-acetate (TPA) induced neoplastic transformation of mouse epidermal cells [6]. Besides protecting against TPA-induced skin cancer, topical application of RGO was able to restore hair regeneration in an androgenic alopecia mouse model [7]. Our previous study indicated that supercritical CO2 fluid–extracted RGO is safe and nontoxic acutely [8]. These properties are especially present in RGO obtained by supercritical CO2 fluid extraction. Compared to conventional methods that use organic solvents, supercritical CO2 fluid extraction, which uses CO2 as a solvent, has the following advantages: minimisation of thermal degradation of valuable constituents; decreased contamination by solvent residues; and reduced formation of undesirable byproducts [9]. This method is also cheap, safe, and environmental friendly because CO2 is recyclable, nontoxic, and nonflammable [10]. We previously identified the presence of linoleic acid and β-sitosterol as major bioactive components [4]. Consistently, another study reported fatty acid composition of RGO extracted by supercritical CO2 fluid extraction with high content of linoleic acid [11]. Lipophilic fractions of red ginseng isolated by solvent extractions have also been indicated the presence of linoleic acid as well as β-sitosterol [12,13].
Although several studies on the bioactivities of RGO have been performed, potentials of RGO in maintaining skin functions have not been fully investigated. In addition, previous findings have the important implication that RGO might be a potential candidate for enhancing skin health. Therefore, the present study aimed to provide advanced results regarding the hair-promoting and photoprotective effects of RGO as well as the underlying mechanism.
2. Methods
2.1. Materials
Formalin, hematoxylin, eosin, linoleic acid, and β-sitosterol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies against β-catenin, Lef-1, Shh, Smo, patched, cyclin E, VEGF, IGF-1, SOD-2, GPx, 8-OHdG, β-actin, and peroxidase-conjugated secondary antigoat were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Catalase, cyclin D1, p-GSK3β, p-ERK, p-Akt, Bcl-2, Bax, cleaved caspase-3, cleaved caspase-9, cleaved-PARP, and peroxidase-conjugated secondary anti-rabbit antibodies were procured from Cell Signaling Technology (Boston, MA, USA). Anti-Gli1, 4HNE, and HO-1 antibodies were purchased from Abcam (Cambridge, UK). All other reagents were of the highest grade commercially available.
2.2. Preparation of RGO
Dried ginseng powder was loaded into the extractor of a pilot-scale supercritical CO2 fluid extraction system (Ilshin Autoclave Co., Ltd., Daejeon, Korea). The extraction system was set at 6,500 psi (relative to 450 bar) at a temperature of 65° C. Red ginseng oil was collected in an amber vial and stored at −20 ºC. The chemical compositions of RGO were analysed using GC/MS as previously described [4].
2.3. Animal experiments
For the hair growth experiment, six-week-old male C57BL/6 mice (18–20 g), purchased from Hyochang Science (Deagu, South Korea), were maintained under controlled humidity (50 ± 5%), 12 h light–dark cycle with free access to food and water. The experiment was performed according to protocols of the Institutional Animal Care and Use Committee (2014-08 and 2015-05) of Inje University (Gimhae, South Korea). At seven weeks of age, when all of their hair follicles were in the telogen phase, all animals were shaved with hair clippers and hair removal cream (Veet, Oxy Reckitt Benckiser, Chartres, France). The mice received topical applications of vehicle (polyethylene glycol (PEG):Ethanol:H2O = 50:20:30), 50% RGO, or 5% minoxidil (MNX, Hyundai Pharm. Co. Ltd, Chungnam, South Korea) once a day for 21 days. The back skin of the mice was photographed at days 0, 7, 10, 14, and 21, and the lengths of randomly plucked hairs were measured at days 14 and 21. At days 0, 7, 14, and 21, mice in each group were sacrificed to obtain skin tissues that were snap-frozen in liquid nitrogen and stored at −80 ºC for further experiments. To investigate the hair growth–promoting activity of major components of RGO, shaved C57BL/6 mice received topical applications of vehicle, 50% RGO, 5% LA, 5% SITOS, or 5% MNX once a day for 21 days. The dorsal skins were taken at days 0, 7, 10, 14, 17, and 21, and mice in each group were sacrificed to collect skin tissues at day 14.
For the skin protection experiment, six-week-old SKH-1 hairless mice (male, 18–20 g), purchased from Daehan Biolink (Eumseong, South Korea), were maintained according to protocols of the Institutional Animal Care and Use Committee (IACUC-2015-066) of Dongguk University (Seoul, South Korea). After a week of acclimation, dorsal skins of mice were treated with topical applications of vehicle or 1% RGO twice a day for three days. After that, mice were exposed to UVC radiation at 20 kJ/cm2. After 1 h, all mice were sacrificed to obtain dorsal skins that were stored at −80 ºC for further experiments.
2.4. Histological analysis
The treated dorsal skins were collected and fixed in 10% formalin and embedded in OTC compound. Sections were stained with hematoxylin and eosin (H&E), and digital photomicrographs were taken from representative areas using a digital camera (Paxcam, Iowa, USA).
2.5. Immunohistochemical analysis
Mouse dorsal skins were embedded in OTC compound, sectioned and attached onto slide glass. Sections were blocked and hybridised with primary antibodies overnight at 4 ºC. After washing, the sections were incubated with fluorescein isothiocyanate (FITC) conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA). The fluorescent images were captured using a C2 confocal microscope (Nikon, Seoul, South Korea).
2.6. Alkaline phosphatase (ALP) activity
ALP activity assay was performed using an Alkaline Phosphatase Assay kit according to the instructions of BioAssay Systems (Vienna, Austria).
2.7. Western blotting
Dorsal skin tissues were homogenised in radioimmunoprecipitation assay (RIPA) buffer. After centrifugation, supernatants were collected and total protein concentration was measured using BCA Protein Assay Kits (Thermo Scientific, Waltham, MA, USA). An equal amount of proteins was resolved in 10–12% SDS-PAGE and then transferred onto polyvinylidene fluoride membranes using a semidry transfer system (Bio-Rad, Hercules, CA, USA). Membranes were blocked with 5% non-fat milk in 0.1% Tween 20 in phosphate buffered saline and hybridised with appropriate primary antibodies overnight. The membranes were then incubated with horseradish peroxidase–conjugated secondary antibodies for 3 h. Finally, protein bands were detected using enhanced chemiluminescence western blotting reagents (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
2.8. Statistical analysis
Results are presented as means ± standard deviation (SD). Statistical analysis was done using one-way analysis of variance and Tukey's post hoc test. P values less than 0.05 were considered statistically significant.
3. Results
3.1. RGO promotes hair growth in C57BL/6 mouse model
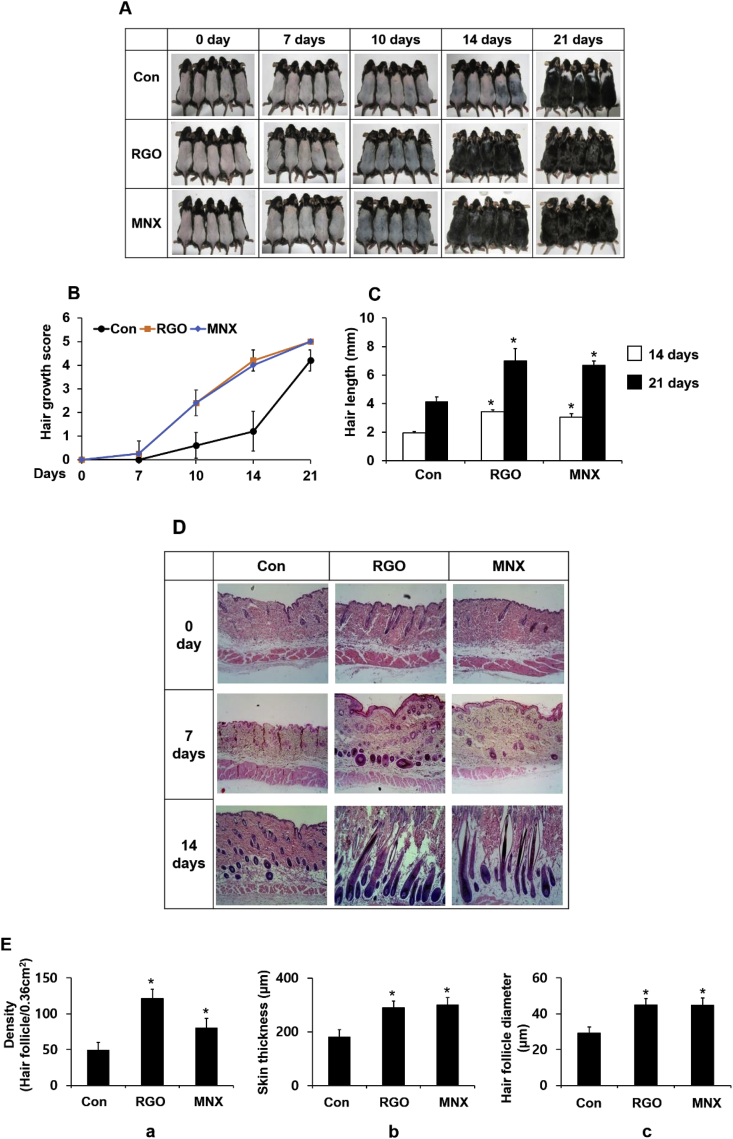
To evaluate the role of RGO in promoting hair growth, vehicle, RGO, or MNX (minoxidil, positive control) were topically applied, for 21 consecutive days, to shaved skins of C57BL/6 mice. Results showed that the RGO and MNX groups exhibited grey skin at day 10 and visible hair shafts after 14 days of depilation (Fig. 1A and B). Mice treated with vehicle retained pink skin at day 10 and had large areas without pigmentation by day 14. RGO or MNX treatment also increased hair shaft length at days 14 and 21, when compared to vehicle-treated control (Fig. 1C). In addition, histological analysis revealed that RGO treatment promoted telogen-to-anagen transition of hair follicles in mouse skin (Fig. 1D). Hair follicles in the RGO group entered anagen prematurely compared with those in the vehicle-treated group, even in the MNX-treated mice. Hair follicles that were treated with RGO saw progression from telogen to early/middle anagen after 7 days of treatment. Specifically, some of the hair follicles entered anagen III/IV by day 7 and part of the hair follicles exhibited anagen V/VI by day 14, with hair shafts erupting out of the epidermis. However, hair follicles in the vehicle-treated group appeared to be in anagen I/II by day 7 and anagen III/IV by day 14. Furthermore, RGO treatment resulted in a significant gain in the density of hair follicles, diameter of hair bulbs, and skin thickness when compared with vehicle treatment (Fig. Ea-c). Similar results were observed in the MNX-treated group, a positive control in this experiment. Overall, these results suggest that RGO can stimulate early progression of hair follicles into the anagen phase of the hair cycle and promote development of hair follicles, thereby enhancing hair regeneration.
Fig. 1.
Hair growth–promoting effect of RGO in C57BL/6 mice. The dorsal skins of male C57BL/6 mice were shaved and treated with a topical application of RGO or MNX once a day for 21 days. (A) The back skin was photographed at 0, 7, 10, 14, and 21 days. (B) Hair growth efficacy was scored as 0, 2, 3, 4, and 5 in correspondence to hair growth of 0%, 0–20%, 20–40%, 40–60%, 60–80%, and 80–100%. (C) The lengths of randomly plucked hairs were measured after 14 and 21 days of depilation. (D) Representative photomicrographs of H&E staining at days 0, 7, and 14. (E) Histological analysis: (E-a) density of hair follicle, (E-b) skin thickness, and (E-c) diameter of hair bulb. Data are presented as the mean ± SD of at least three independent experiments. ∗P < 0.05 significantly different when compared with the control.
3.2. RGO upregulates hair growth-related genes in C57BL/6 mouse skins
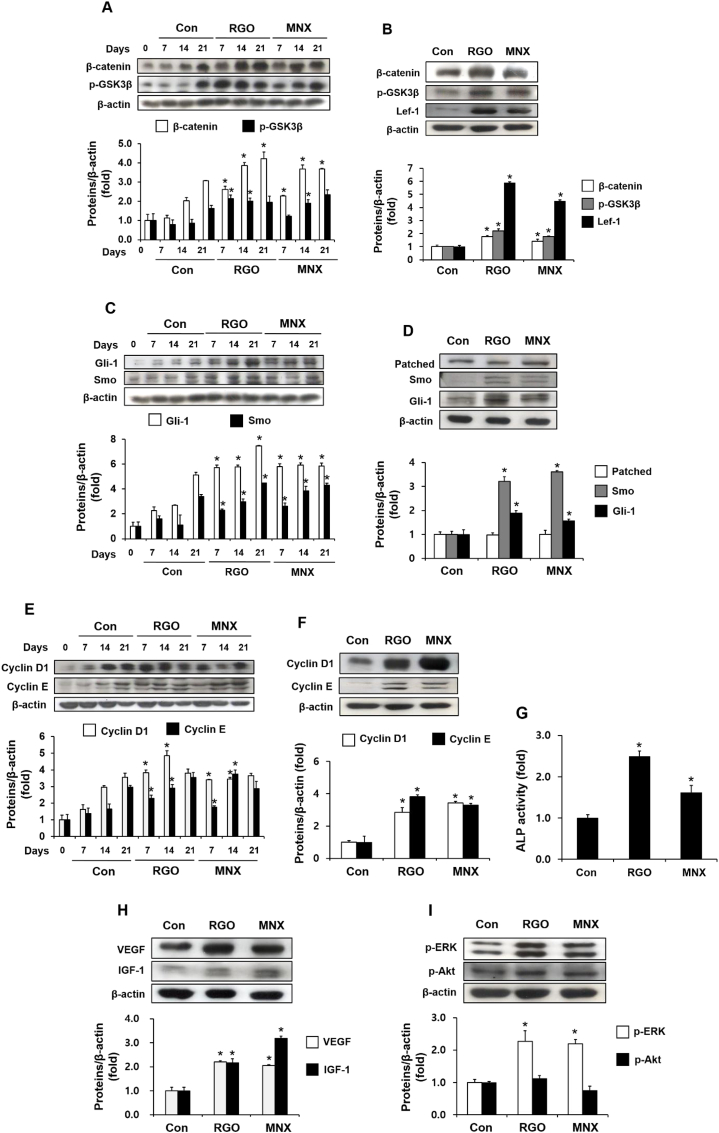
To better understand the possible hair regeneration mechanisms of RGO, we analysed the expression of hair promotion–associated genes in the skin tissues. Treatment with RGO significantly upregulated protein levels of β-catenin, and phospho-glycogen synthase kinase 3 beta (p-GSK3β) by day 7, and the expression of these proteins continuously increased at days 14 and 21, when compared to vehicle-treated mice (Fig. 2A). At day 14, the RGO-treated group exhibited greater protein expression of β-catenin, p-GSK3β, and lymphoid enhancer-binding factor 1 (Lef-1) than the control group, and was comparable to the MNX-treated group, suggesting the necessity of the Wnt/β-catenin pathway for RGO-mediated activity (Fig. 2B). Similarly, RGO induced early expression of Gli-1 and smoothened (Smo) at day 7, and the increased tendency for expression was sustained after 14 and 21 days (Fig. 2C). At day 7, topical application of RGO significantly increased the protein levels of Gli-1 and Smo but failed to induce patched expression, implying an enhanced sonic hedgehog (Shh)/Gli-1 pathway in mice treated with RGO (Fig. 2D). Consequently, the cyclin E and cyclin D1, Wnt/β-catenin and Shh/Gli-1 pathway–targeted genes were also upregulated by treatment with RGO or MNX (Fig. 2E and F). In addition, levels of dermal alkaline phosphatase (ALP), a biomarker of hair growth, significantly increased in the mice treated with RGO when compared with the mice treated with vehicle only (Fig. 2G). ALP activity in the RGO-treated group was even greater than that of the MNX-treated group. Also, RGO treatment resulted in a significant rise in the protein levels of growth factors, such as vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1), in skin tissues compared with vehicle treatment only (Fig. 2H). Topical application of RGO activated a downstream kinase by enhancing the phosphorylation level of extracellular-signal-regulated kinase (ERK) but failed to induce Akt kinase (Fig. 2I). These results suggest that topical application of RGO could promote hair growth by upregulating the expression of hair growth–related genes in mouse skin tissues.
Fig. 2.
RGO induces the expression of hair growth–associated genes in mouse skin. (A) Time-dependent protein expression of β-catenin and p-GSK3β. (B) Protein expression of β-catenin, p-GSK3β, and Lef-1 at day 14. (C) Time-dependent protein expression of Smo and Gli-1. (D) Protein expression of patched, Smo, and Gli-1 at day 14. (E) Time-dependent protein expression of cyclin D1 and cyclin E. (F) Protein expression of cyclin D1 and cyclin E at day 14. (G) ALP activity at day 14. (H) Protein expression of growth factors IGF-1 and VEGF at day 14. (I) The expression levels of p-ERK and p-Akt proteins at day 14. Data are presented as the mean ± SD of at least three independent experiments. ∗P < 0.05 significantly different when compared with the control at the same indicated time.
3.3. Hair growth–promoting activity of major components of RGO in C57BL/6 mouse models
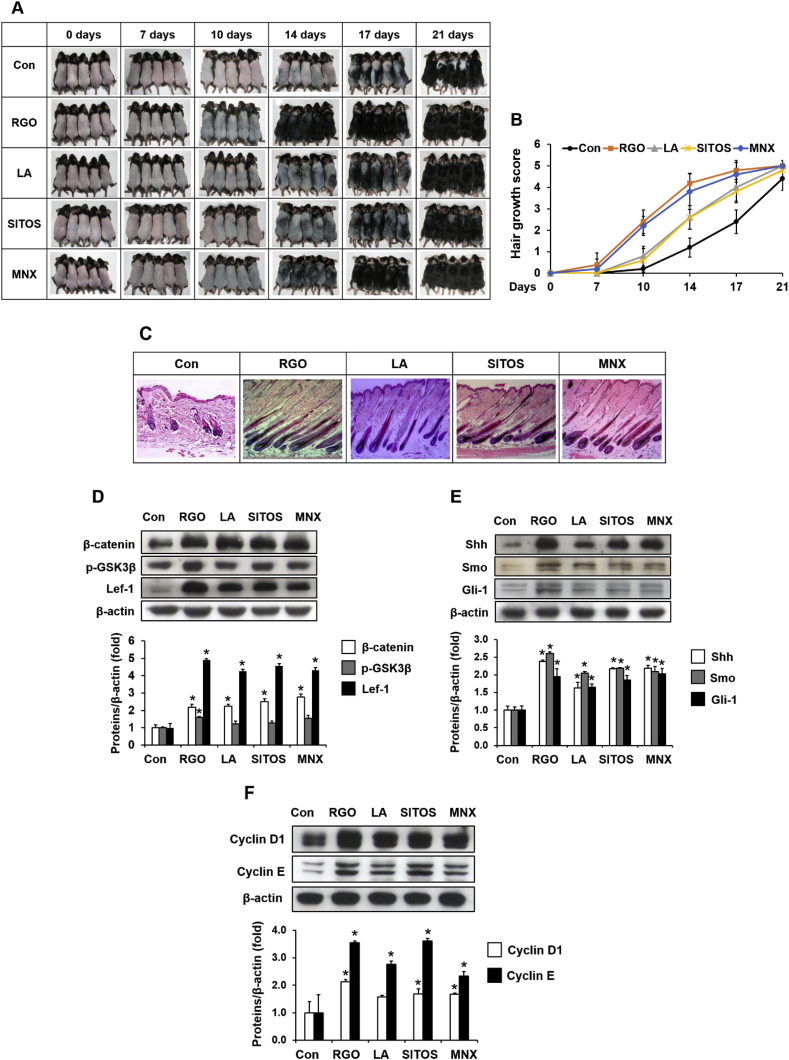
To examine the hair growth–promoting role of major components in RGO, C57BL/6 mice were topically treated with RGO, linoleic acid (LA) or β-sitosterol (SITOS) for 21 days. As shown in Fig. 3A and B, LA or SITOS treatment produced effective hair growth–promoting activity when compared with vehicle treatment. Both the LA- and SITOS-treated groups exhibited light grey skin by day 10 and dark grey skin with small hair shafts by day 14 of treatment, whereas the control group had large areas of back skin without pigmentation at day 14. Dorsal skins of mice in the LA and SITOS groups had visible hair shafts at day 17 and were fully coated with long hair shafts after 21 days of treatment. Histological analysis indicated a premature telogen-to-anagen transition of hair follicles in the mice treated with RGO, LA, SITOS or MNX compared to the mice treated with vehicle (Fig. 3C). The LA- and SITOS-treated groups had hair follicles in deep subcutis at anagen V/VI of the hair cycle with hair shafts erupting out of the epidermis. In addition, LA and SITOS treatment activated the Wnt/β-catenin and Shh/Gli pathways, as evident from enhanced expression of hair growth induction–related genes, such as β-catenin, Lef-1, p-GSK3β, Shh, Smo, Gli-1, cyclin D1, and cyclin E, in mouse skin tissues after 14 days of depilation (Fig. 3D, E and 3F). More interestingly, topical application of RGO seemed to possess greater hair growth–promoting potential than treatment with its single major components. These data suggest that RGO has great hair growth efficacy, possibly through the synergistic induction of its major components, including LA and SITOS.
Fig. 3.
Effect of major compounds in RGO on hair growth in a C57BL/6 mouse model. The dorsal skins of male C57/BL mice were shaved and treated with topical applications of RGO, LA, SITOS or MNX once a day for 21 days. (A) The back skin was photographed at 0, 7, 10, 14, and 21 days. (B) Hair growth efficacy was scored as 0, 2, 3, 4, and 5 in correspondence to hair growth of 0%, 0–20%, 20–40%, 40–60%, 60–80%, and 80–100%. (C) Representative photomicrographs of H&E staining at day 14. Protein expression of (D) β-catenin, p-GSK3β and Lef-1, (E) Shh, Smo, and Gli-1, and (F) cyclin D1 and cyclin E at day 14. Data are presented as the mean ± SD of at least three independent experiments. ∗P < 0.05 significantly different when compared with the control.
3.4. RGO suppresses UVC-induced apoptosis in mice
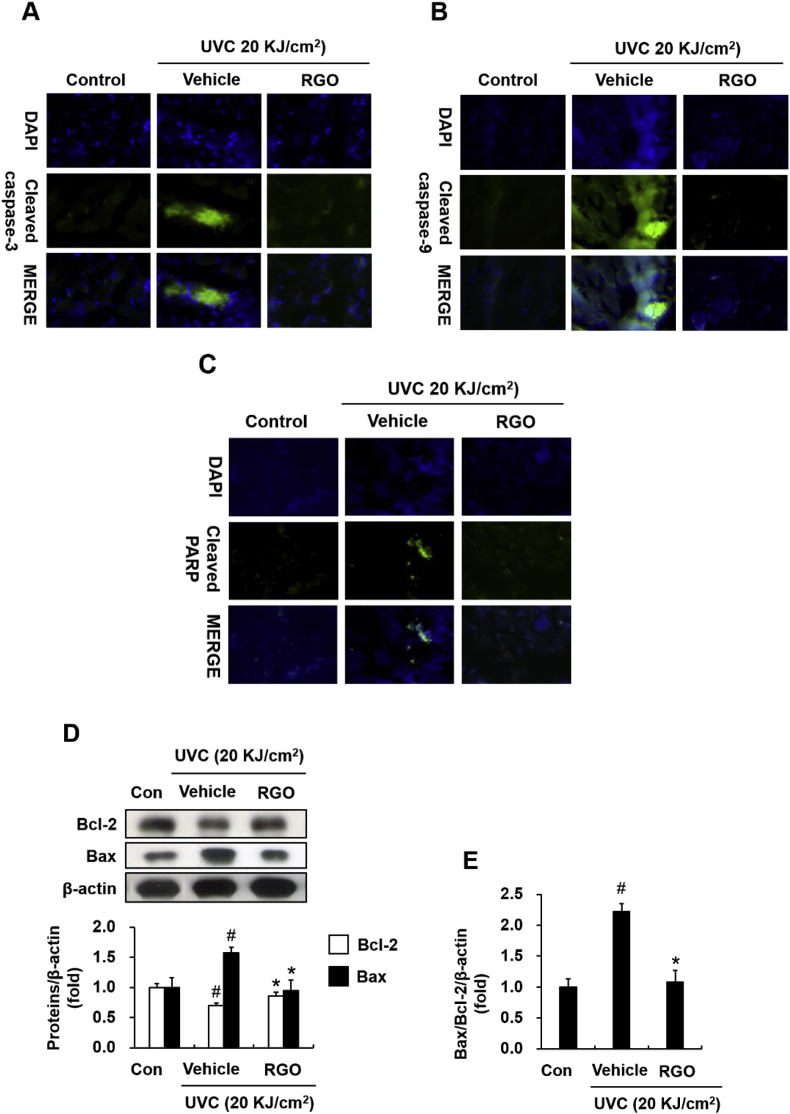
Both acute and chronic exposure to UV radiation are believed to have serious effects on the structure and function of skin, such as sunburn, inflammation, sustained immunosuppression, and carcinogenesis of the skin [14,15]. Therefore, we investigated whether RGO has a protective effect against UVC-induced apoptosis. Immunohistochemical analysis revealed that UVC exposure activated the intrinsic apoptotic pathway by enhancing levels of cleaved-caspase-3 and cleaved-caspase-9 in the mouse skin tissues. However, the RGO-treated group exhibited a significant reduction in the level of UVC-induced, active forms of caspase-3 and caspase-9 (Fig. 4A and B). UVC also stimulated the formation of poly-ADP ribose polymerase (PARP) proteolytic cleavage fragments, which was inhibited in the mice that received topical applications of RGO (Fig. 4C). In addition, anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins appear to act as regulators of the mitochondria-mediated apoptosis process. UVC irradiation decreased Bcl-2 expression and increased Bax expression, leading to a substantial elevation of the Bax/Bcl-2 ratio in the UVC-treated group when compared with the control group (Fig. 4D and E). These results suggest that alterations in the Bax/Bcl-2 ratio caused by UVC are involved in mitochondrial dysfunction. However, RGO topical application diminished the Bax/Bcl-2 ratio by increasing Bcl-2 expression and decreasing Bax expression. Overall, these data suggest that, in addition to hair growth promotion, RGO has a protective effect against UVC-induced apoptosis.
Fig. 4.
Inhibitory effect of RGO on UVC-induced apoptosis in SKH-1 hairless mice. The mice were treated with topical applications of vehicle or 1% RGO for three days prior to irradiating with 20 kJ/cm2 UVC. Dorsal skin tissues were collected and subjected to western blotting and immunofluorescence. Representative immunohistochemical staining of (A) cleaved-caspase-3, (B) cleaved-caspase 9, and (C) cleaved-PARP in mouse skins. (D) Protein expression of Bcl-2 and Bax. (E) Ratio of Bax/Bcl-2. Data are presented as the mean ± SD of at least three independent experiments. #P < 0.05 significantly different when compared to the UVC-treated group. ∗P < 0.05 significantly different when compared with the control group.
3.5. RGO inhibits COX-2, AP-1 and MAPK signalling pathways in UVC-irradiated mice
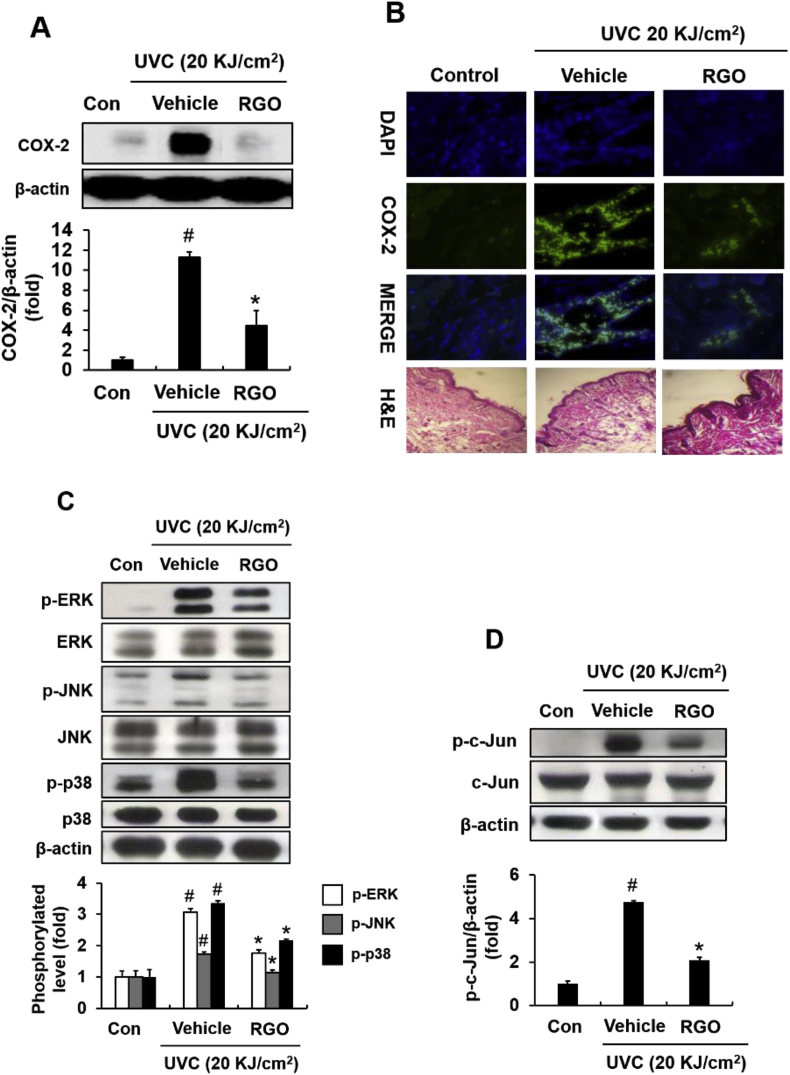
To assess the anti-inflammatory property of RGO, COX-2 expression was examined in UVC-irradiated mouse skin. As illustrated in Fig. 5A, exposure of mice to UVC radiation (20 kJ/cm2) markedly induced COX-2 expression in skin tissues. However, UVC-induced COX-2 expression was significantly reduced in the presence of RGO. Immunohistological analysis also confirmed UVC-enhanced COX-2 expression in skin tissues, which was obviously suppressed by topical application of RGO (Fig. 5B). Also, we evaluated effects of RGO on mitogen-activated protein kinase (MAPK) and activator protein 1 (AP-1) signalling pathways in UVC-irradiated mice. Results showed that RGO pretreatment significantly attenuated UVC-induced phosphorylation of ERK, c-Jun N-terminal kinase (JNK), and p38 MAPK in mouse skin tissues (Fig. 5C). Similar to MAPK signalling pathways, transcription factor AP-1 was provoked by UVC radiation by inducing the phosphorylation of c-Jun/AP-1, which was suppressed in the RGO-pretreated mice (Fig. 5D).
Fig. 5.
Effect of RGO on COX-2, MAPK and AP-1 pathways in UVC-exposed SKH-1 hairless mice. COX-2 expression in UVC-exposed SKH-1 hairless mice. The mice were treated with topical applications of vehicle or 1% RGO for three days prior to irradiating with 20 kJ/cm2 UVC. Dorsal skin tissues were collected and subjected to western blotting and immunofluorescence. (A) Protein expression of COX-2. (B) Representative immunohistochemical staining of COX-2 in mouse skins. (C) Phosphorylation levels of ERK, JNK, and p-p38. (D) Protein expression of p-c-Jun/AP-1. Data are presented as the mean ± SD of at least three independent experiments. #P < 0.05 significantly different when compared to the UVC-treated group. ∗P < 0.05 significantly different when compared with the control group.
3.6. RGO protects skin from UVC-induced oxidative damage through the upregulation of cytoprotective enzymes
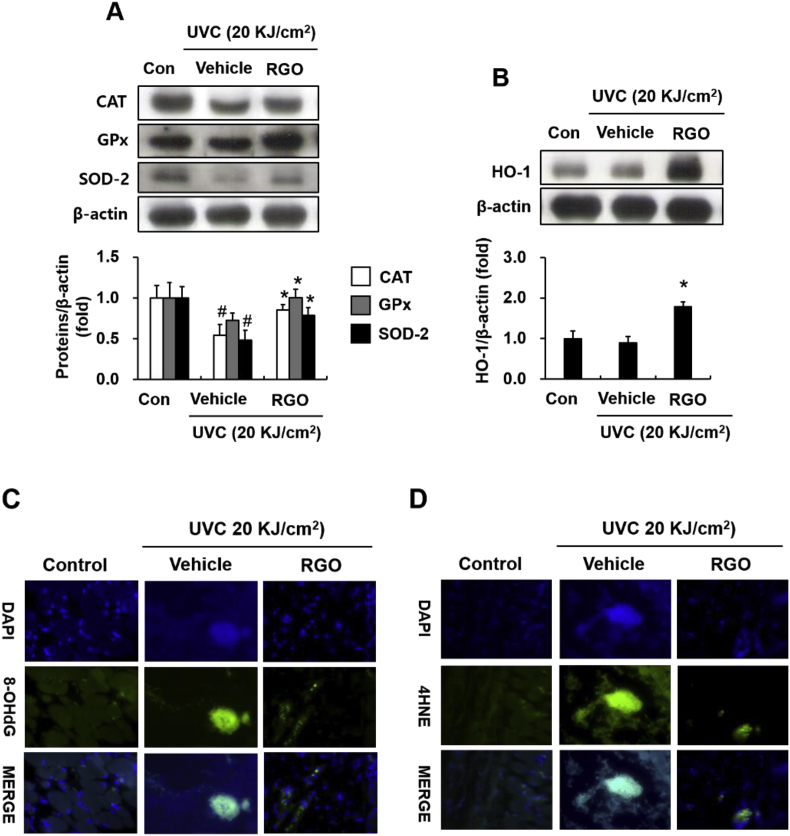
To further investigate whether the protective effect of RGO was associated with the upregulation of cytoprotective systems, we inspected the expression of antioxidant enzymes in the mouse skin tissues. UVC exposure resulted in decreased expression levels of primary antioxidant enzymes, including catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase 2 (SOD-2), implying enhanced oxidative stress in UVC-irradiated mouse skin (Fig. 6A). However, RGO restored the levels of these antioxidant enzymes in the UVC-exposed mice. In addition, RGO considerably catalysed the expression of phase II antioxidant heme oxygenase 1 (HO-1) enzyme in skin tissues when compared to the control and UVC-treated groups (Fig. 6B). Consequently, enhanced antioxidant defence systems are able to contribute to the protection of skin cells/skin tissues against DNA damage and lipid oxidation caused by UVC exposure. In this study, RGO exhibited protective effects against UVC-induced DNA and lipid oxidation in mouse skin tissues, as demonstrated by reduced 8-OHdG and 4HNE levels (Fig. 6C and D).
Fig. 6.
Induction of cytoprotective proteins by RGO prevents UVC-induced oxidative damage in SKH-1 hairless mice. The mice were treated with topical applications of vehicle or 1% RGO for three days prior to irradiating with 20 KJ/cm2 UVC. Dorsal skin tissues were collected and subjected to western blotting and immunofluorescence. (A) Protein expression of primary antioxidant enzymes CAT, GPx, and SOD-2 in mouse skin tissues. (B) Protein expression of phase antioxidant enzyme HO-1 in mouse skin tissues. Representative images of (C) 8-OHdG and (D) 4HNE staining in mouse skin tissues. Data are presented as the mean ± SD of at least three independent experiments. #P < 0.05 significantly different when compared to the UVC-treated group. ∗P < 0.05 significantly different when compared with the control group.
4. Discussion
Impaired skin health may cause decreased skin barrier function, enhanced susceptibility to irritation, and disturbances to the development of skin appendages; the need to seek novel skin care agents is thus emphasised. Currently, the use of botanical agents as skin care products has been attracting great attention for maintaining skin functions. Improving skin conditions contribute not only to preventing injury caused by harmful factors but also to supporting normal development of skin appendages. In this study, we provided evidence that supercritical CO2-extracted RGO may be a strong candidate for recovery of skin functions, such as hair growth and skin protection. RGO induced the expression of proteins related to the promotion of hair growth, resulting in a premature telogen-to-anagen transition in hair follicles, and effectively enhanced hair growth. Furthermore, we found that topical application of RGO, through the inhibition of apoptosis and inflammation, and induction of cytoprotective systems, had protective effects against UVC-caused skin damage.
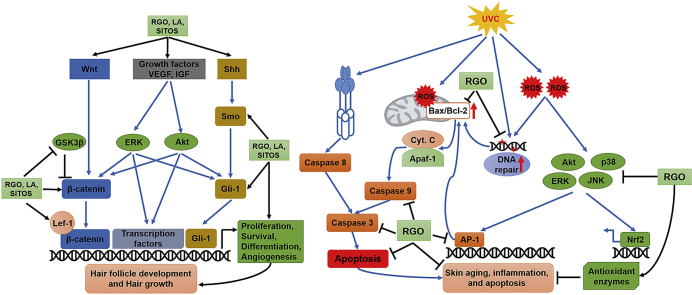
Hair is an important part of the body that originates from the ectoderm of the skin. Hair shafts are synthesised by hair follicles, which undergo repetitive phases of growth (anagen), regression (catagen), and resting (telogen) [16,17]. Regulation of hair growth is a complicated process that involves a variety of signalling pathways in the skin. Of them, the Wnt/β-catenin pathway is one of the most important signalling pathways triggering hair follicle formation and hair growth. Previous studies have demonstrated that ablation of β-catenin results in a significant decrease in its target genes, thereby leading to the lessening of cell proliferation, rapid regression of anagen hair follicles and, finally, hair loss [18,19]. In addition, Wnt10b overexpression has been reported to activate the Wnt/β-catenin signalling pathway to promote hair regeneration, whereas suppression of β-catenin by siRNA abolishes hair follicle regeneration, even when Wnt10b is overexpressed [20]. In contrast, epidermal overexpression of β-catenin in transgenic mice induces de novo hair follicle formation in adults [21,22]. Mice lacking Lef-1, a co-transcription factor of β-catenin in the Wnt signalling pathway, also exhibit arrested follicle development as well as a lack of vibrissae and body hair [23]. Reduced levels of Wnt10a and Lef-1 have been observed in balding hair follicles from the frontal scalp, and β-catenin expression in balding dermal papilla cells is lower than that in non-balding dermal papilla cells [24]. In addition to inducing telogen-to-anagen transition, the Wnt/β-catenin signalling pathway also plays a critical role in maintaining the anagen phase and regulating the behaviour of keratinocytes in hair follicles during hair cycling [25]. In the present study, a significant upregulation of Wnt/β-catenin pathway–related proteins was observed in skin tissues of mice treated with RGO or its major compounds when compared to the control group.
Along with the Wnt/β-catenin pathway, several additional signalling pathways, such as growth factor, bone morphogenetic protein, Notch and Shh pathways are involved in hair follicle formation, morphogenesis and development [26]. The Shh pathway makes an important contribution to follicle morphogenesis and development [27]. In a model of chemotherapy-induced alopecia, mice with overexpression of Shh, which was mediated by an adenovirus (AdShh) vector, showed accelerated initiation of the anagen phase of hair follicle development and hair regrowth when compared with naïve mice or AdNull-treated mice [28]. Blockade of the Shh signal leads to the reduced expression of its target genes, thereby inhibiting ingrowth of hair follicles and consequent hair morphogenesis at many stages, indicating that the Shh pathway is essential for the growth and morphogenesis of hair follicles throughout the growth cycle [27,29,30]. Moreover, several growth factors such as IGF-1 and VEGF have been documented to contribute to the growth and development of hair follicles [31,32]. VEGF has been demonstrated to elevate hair follicle progression into the anagen phase and promote hair growth through the induction of angiogenesis, which is essential to the increased nutrition and oxygen required for rapid cell division during the anagen phase [33]. IGF-1 is involved in hair development via regulating cellular survival and proliferation, tissue remodelling, the hair cycle and follicular differentiation [34,35]. The production of IGF-1 in dermal papilla cells acquired from balding scalp is lower than that from non-balding scalp [36,37]. In this study, RGO along with its major components activated the Shh/Gli-1 pathway as well as growth factor in a mouse model, consequently triggering the anagen phase and hair growth.
In addition to its hair growth–promoting effect, we evaluated the role of RGO in protecting skin from environmental hazards. In this study, we used UVC-exposed SKH-1 hairless mice as a model to investigate the protective effects of RGO since UVC causes more serious biological injuries due to its higher energy compared to UVB and UVA. Our studies revealed that topical application of RGO significantly decreased inflammation, inhibited apoptosis, and enhanced the cytoprotective system, leading to a lessening of UVC-caused damage. The mice pre-treated with RGO prior to UVC exposure had diminished skin inflammation, as evident from their reduced COX-2 expression. UV-induced COX-2 expression results in erythema, cutaneous inflammation, enhanced production of pro-inflammatory cytokines, and infiltration of inflammatory cells into damaged skin tissues [38,39]. COX-2-deficient mice exhibited a significant attenuation in UV-induced skin tumorigenesis, whereas COX-2 overexpression in transgenic mice enhanced tumorigenesis [40]. In animal models, suppression of COX-2 expression has been demonstrated to minimise cutaneous inflammation as well as UV-induced skin tumour [41,42]. Therefore, inhibition of COX-2 in skin treated with RGO could contribute to the prevention of skin disorders caused by UV radiation.
Apoptosis is the process of programmed cell death through the activation of intrinsic and extrinsic apoptotic pathways [43]. UV irradiation can trigger the apoptotic process through various pathways, such as DNA damage, death receptor activation, and reactive oxygen species (ROS) accumulation in an independent and/or synergistic manner [42,43]. The Bcl-2 family that regulates the intrinsic apoptotic pathway consists of several homologous proteins, such as anti-apoptotic Bcl-2 and pro-apoptotic Bax protein [44,45]. Typically, the Bax/Bcl-2 ratio, which is related to the regulation of mitochondrial function, has been considered an index for determining sensitivity to apoptosis [46]. In extrinsic pathways, caspase cascades, including initiator (caspase-8 and caspase-9) and effector caspases (caspase-3, and caspase-7), play an important role in the transduction of apoptotic signals [47]. Caspase-3 is the most common executioner of apoptosis in both intrinsic and extrinsic pathways and plays a central role in the process of UV-induced apoptosis [48,49]. This study indicated that UVC-induced apoptosis via caspase cascades was suppressed by RGO pretreatment as evident from reduced levels of cleaved-caspase-3, cleaved-caspase-9, and PARP. In addition, in UVC-exposed mice, RGO decreased the Bax/Bcl-2 ratio, whereby the Bcl-2 level was restored close to baseline and Bax expression was mitigated.
The MAPK signalling pathway plays a crucial role in transmitting extracellular stimuli into intracellular responses, which trigger a variety of cellular events, such as cell survival, proliferation, differentiation, and apoptosis [46]. Accumulating evidence has shown that UVC may stimulate all three MAPK signalling pathways [50,51]. Both JNK and p38 MAPK have been shown to take part in UVC-induced, mitochondria-mediated apoptosis [51,52]. Moreover, the activation of signal transduction pathways by UVC irradiation also results in the induction of the transcription factor AP-1, which itself regulates the processes of cell proliferation, inflammation, transformation and tumorigenesis [53,54]. In this study, we found that RGO suppressed the phosphorylation of all three MAPKs in skin tissues of UVC-exposed mice. Inhibition of UVC-induced AP-1 by RGO was observed in the form of decreased phosphorylation of c-Jun. These results suggest that RGO could attenuate UVC-induced acute inflammation and apoptosis by mitigating the activation of the MAPK and AP-1 signalling pathways.
UV radiation brought about the intracellular generation of large quantities of reactive oxygen species (ROS), such as singlet oxygen, superoxide radicals, hydroxyl radicals, and hydrogen peroxide, which subsequently cause damage to cellular components, such as proteins, nucleic acids, lipid membranes and mitochondria [46,55]. UV-induced oxidative damage contributes to inflammation, gene mutations and immunosuppression, resulting in a range of skin disorders, such as photoaging, skin wrinkling, and skin cancer [55]. The skin is equipped with a complex defence system including enzymatic and non-enzymatic components involved in UV absorbance, DNA repair, ROS neutralisation, and carcinogen detoxification and elimination [49]. Nevertheless, endogenous protective systems may be depleted by prolonged UV exposure and/or high energy UV radiation; thus, it is essential to develop additional types of photoprotection. One approach to protecting human skin from the harmful influence of UV radiation is the enhancement of cellular antioxidant defence systems, which maintain cellular redox homeostasis [56]. Additionally, the phase II antioxidant enzyme HO-1 plays an important role in the protection of cells from oxidative damage through the neutralisation of ROS [57]. As expected, our results showed that, in UVC-irradiated mice, topical application of RGO increased the level of both primary antioxidant enzymes, such as CAT, GPx, and SOD-2, and the phase II antioxidant HO-1. Consequently, RGO reduced UVC-induced oxidative damage to lipids and DNA, as evident in the decreased levels of 4HNE and 8-OHdG, respectively.
In summary, the data in our study demonstrate the role of supercritical CO2-extracted RGO in improving skin function and plausible underlying mechanisms for RGO-mediated activities. Our results reveal that RGO stimulates the biological progression of hair follicles from telogen to anagen and promotes hair growth through activation of the Wnt/β-catenin and Shh/Gli-1 signalling pathways as well as induction of growth factors. RGO also exhibits pharmacological action through its anti-apoptotic, anti-inflammatory and antioxidant properties that protect the skin from the adverse effects of UV irradiation. Therefore, RGO may be a promising candidate for dermatological formulations that promote hair growth and provide skin photoprotection against UVC irradiation.
Author contributions
W.S.J. contributed to conception, design of the project, discussed the data with V.L.T and revised the manuscript. V.L.T designed the experiment, conducted the experiments, analysed the results, and wrote the manuscript. All authors reviewed the manuscript.
Additional information
Correspondence should be addressed to W.S.J.
Conflicts of interest
The authors declare no competing interests.
Acknowledgements
We are grateful to the Korea Ginseng Corporation for the preparation of RGO. This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (NRF- 2014R1A2A1A11050006, 2020R1F1A1073595, and 2021R1A2C2006745).
References
- 1.Montagna W., Parakkal P.F. The structure & function of skin. ed. Academic Press; 1974. An introduction to skin; pp. 1–17. [Google Scholar]
- 2.Lin T.-K., Zhong L., Santiago J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2018;19:70. doi: 10.3390/ijms19010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bak M.-J., Jun M., Jeong W.-S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H(2)O(2)-treated HepG2 cells and CCl(4)-treated mice. Int J Mol Sci. 2012;13:2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bak M.J., Truong V.-L., Ko S.-Y., Nguyen X.N.G., Jun M., Hong S.-G., Lee J.-W., Jeong W.-S. Induction of Nrf2/ARE-mediated cytoprotective genes by red ginseng oil through ASK1–MKK4/7–JNK and p38 MAPK signaling pathways in HepG2 cells. J Ginseng Res. 2016;40:423–430. doi: 10.1016/j.jgr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bak M.-J., Hong S.-G., Lee J.-W., Jeong W.-S. Red ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van-Long T., Ng K.A., Woo-Sik J. Red ginseng oil inhibits TPA-induced transformation of skin epidermal JB6 cells. J Med Food. 2018;21:380–389. doi: 10.1089/jmf.2017.4082. [DOI] [PubMed] [Google Scholar]
- 7.Truong V.-L., Bak M.J., Lee C., Jun M., Jeong W.-S. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22:1505. doi: 10.3390/molecules22091505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bak M.-J., Kim K.-B., Jun M., Jeong W.-S. Safety of red ginseng oil for single oral administration in Sprague–Dawley rats. J Ginseng Res. 2014;38:78–81. doi: 10.1016/j.jgr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J.-S., Jeon M.-H., Moon W.-S., Moon J.-N., Cheon E.J., Kim J.-W., Jung S.K., Ji Y.-H., Son S.W., Kim M.-R. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol Pharm Bull. 2014;37:44–53. doi: 10.1248/bpb.b13-00528. [DOI] [PubMed] [Google Scholar]
- 10.Sahena F., Zaidul I.S.M., Jinap S., Karim A.A., Abbas K.A., Norulaini N.A.N., Omar A.K.M. Application of supercritical CO2 in lipid extraction – a review. J Food Eng. 2009;95:240–253. [Google Scholar]
- 11.Seo H.W., Suh J.H., So S.-H., Kyung J.-S., Kim Y.-S., Han C.-K. Subacute oral toxicity and bacterial mutagenicity study of Korean Red Ginseng oil. J Ginseng Res. 2017;41:595–601. doi: 10.1016/j.jgr.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park H.J., Lee J.H., Song Y.B., Park K.H. Effects of dietary supplementation of lipophilic fraction from Panax ginseng on cGMP and cAMP in rat platelets and on blood coagulation. Biol Pharm Bull. 1996;19:1434–1439. doi: 10.1248/bpb.19.1434. [DOI] [PubMed] [Google Scholar]
- 13.Lee D.G., Lee J., Kim K.-T., Lee S.-W., Kim Y.-O., Cho I.-H., Kim H.-J., Park C.-G., Lee S. High-performance liquid chromatography analysis of phytosterols in Panax ginseng root grown under different conditions. J Ginseng Res. 2018;42:16–20. doi: 10.1016/j.jgr.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura Y., Ananthaswamy H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 15.D'Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider M.R., Schmidt-Ullrich R., Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:132–142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Cotsarelis G., Millar S.E. Towards a molecular understanding of hair loss and its treatment. Trends Mol Med. 2001;7:293–301. doi: 10.1016/s1471-4914(01)02027-5. [DOI] [PubMed] [Google Scholar]
- 18.Choi Y.S., Zhang Y., Xu M., Yang Y., Ito M., Peng T., Cui Z., Nagy A., Hadjantonakis A.-K., Lang R.A. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huelsken J., Vogel R., Erdmann B., Cotsarelis G., Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 20.Li Y.-H., Zhang K., Yang K., Ye J.-X., Xing Y.-Z., Guo H.-Y., Deng F., Lian X.-H., Yang T. Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. J Invest Dermatol. 2013;133:42–48. doi: 10.1038/jid.2012.235. [DOI] [PubMed] [Google Scholar]
- 21.Gat U., DasGupta R., Degenstein L., Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 22.Celso C.L., Prowse D.M., Watt F.M. Transient activation of β-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 23.Van Genderen C., Okamura R.M., Fariñas I., Quo R.G., Parslow T.G., Bruhn L., Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 24.Lu G.-Q., Wu Z.-B., Chu X.-Y., Bi Z.-G., Fan W.-X. An investigation of crosstalk between Wnt/β-catenin and transforming growth factor-β signaling in androgenetic alopecia. Medicine. 2016;95:e4297. doi: 10.1097/MD.0000000000004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enshell-Seijffers D., Lindon C., Kashiwagi M., Morgan B.A. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rishikaysh P., Dev K., Diaz D., Qureshi W.M.S., Filip S., Mokry J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014;15:1647–1670. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang C., Swan R.Z., Grachtchouk M., Bolinger M., Litingtung Y., Robertson E.K., Cooper M.K., Gaffield W., Westphal H., Beachy P.A. Essential role for sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 28.Sato N., Leopold P.L., Crystal R.G. Effect of adenovirus-mediated expression of Sonic hedgehog gene on hair regrowth in mice with chemotherapy-induced alopecia. J Natl Cancer Inst. 2001;93:1858–1864. doi: 10.1093/jnci/93.24.1858. [DOI] [PubMed] [Google Scholar]
- 29.St-Jacques B., Dassule H.R., Karavanova I., Botchkarev V.A., Li J., Danielian P.S., McMahon J.A., Lewis P.M., Paus R., McMahon A.P. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1069. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 30.Oro A.E., Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 31.Peus D., Pittelkow M.R. Growth factors in hair organ development and the hair growth cycle. Dermatol Clin. 1996;14:559–572. doi: 10.1016/s0733-8635(05)70384-3. [DOI] [PubMed] [Google Scholar]
- 32.Stenn K.S., Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 33.Yano K., Brown L.F., Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weger N., Schlake T. IGF-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125:873–882. doi: 10.1111/j.0022-202X.2005.23946.x. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Yang Z., Li Z., Gu L., Wang Y., Sung C. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-β1 in C57BL/6 mice in vivo. Growth Horm IGF Res. 2014;24:89–94. doi: 10.1016/j.ghir.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Panchaprateep R., Asawanonda P. Insulin-like growth factor-1: roles in androgenetic alopecia. Exp Dermatol. 2014;23:216–218. doi: 10.1111/exd.12339. [DOI] [PubMed] [Google Scholar]
- 37.Trüeb R.M. Further clinical evidence for the effect of IGF-1 on hair growth and alopecia. Skin Appendage Disord. 2018;4:90–95. doi: 10.1159/000479333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buonanno M., Stanislauskas M., Ponnaiya B., Bigelow A.W., Randers-Pehrson G., Xu Y., Shuryak I., Smilenov L., Owens D.M., Brenner D.J. 207-nm UV light—a promising tool for safe low-cost reduction of surgical site infections. II: in-vivo safety studies. PLOS ONE. 2016;11 doi: 10.1371/journal.pone.0138418. e0138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripp C.S., Blomme E.A.G., Chinn K.S., Hardy M.M., LaCelle P., Pentland A.P. Epidermal COX-2 induction following ultraviolet irradiation: suggested mechanism for the role of COX-2 inhibition in photoprotection. J Invest Dermatol. 2003;121:853–861. doi: 10.1046/j.1523-1747.2003.12495.x. [DOI] [PubMed] [Google Scholar]
- 40.Rundhaug J.E., Fischer S.M. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis†. Photochem Photobiol. 2008;84:322–329. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee J.L., Mukhtar H., Bickers D.R., Kopelovich L., Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003;192:294–306. doi: 10.1016/s0041-008x(03)00301-6. [DOI] [PubMed] [Google Scholar]
- 42.Wu N.-L., Fang J.-Y., Chen M., Wu C.-J., Huang C.-C., Hung C.-F. Chrysin protects epidermal keratinocytes from UVA- and UVB-induced damage. J Agric Food Chem. 2011;59:8391–8400. doi: 10.1021/jf200931t. [DOI] [PubMed] [Google Scholar]
- 43.Lee C.-H., Wu S.-B., Hong C.-H., Yu H.-S., Wei Y.-H. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: the implication in UV-based phototherapy. Int J Mol Sci. 2013;14:6414–6435. doi: 10.3390/ijms14036414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cory S., Adams J.M. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 45.Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 46.Shin S.W., Jung E., Kim S., Kim J.-H., Kim E.-G., Lee J., Park D. Antagonizing effects and mechanisms of afzelin against UVB-induced cell damage. PLOS ONE. 2013;8 doi: 10.1371/journal.pone.0061971. e61971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ola M.S., Nawaz M., Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 48.Porter A.G., Jänicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 49.Yoshihisa Y., Mu Rehman, Shimizu T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp. Dermatol. 2014;23:178–183. doi: 10.1111/exd.12347. [DOI] [PubMed] [Google Scholar]
- 50.Huang C., Li J., Ding M., Leonard S.S., Wang L., Castranova V., Vallyathan V., Shi X. UV induces phosphorylation of protein kinase B (Akt) at ser-473 and thr-308 in mouse epidermal Cl 41 cells through hydrogen peroxide. J Biol Chem. 2001;276:40234–40240. doi: 10.1074/jbc.M103684200. [DOI] [PubMed] [Google Scholar]
- 51.Bode A.M., Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci STKE. 2003;2003:re2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 52.Oliveira M.M., Ratti B.A., Daré R.G., Silva S.O., Truiti MdCT., Ueda-Nakamura T., Auzély-Velty R., Nakamura C.V. Dihydrocaffeic acid prevents UVB-induced oxidative stress leading to the inhibition of apoptosis and MMP-1 expression via p38 signaling pathway. Oxid Med Cell Longev. 2019;2019:1–14. doi: 10.1155/2019/2419096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong Z., Huang C., Ma W.-Y., Malewicz B., Baumann W.J., Kiss Z. Increased synthesis of phosphocholine is required for UV-induced AP-1 activation. Oncogene. 1998;17:1845. doi: 10.1038/sj.onc.1202084. [DOI] [PubMed] [Google Scholar]
- 54.Ma W.Y., Huang C., Dong Z. Inhibition of ultraviolet C irradiation-induced AP-1 activity by aspirin is through inhibition of JNKs but not erks or P38 MAP kinase. Int J Oncol. 1998;12:565–573. doi: 10.3892/ijo.12.3.565. [DOI] [PubMed] [Google Scholar]
- 55.Halliday G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Truong V.-L., Jun M., Jeong W.-S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors. 2018;44:36–49. doi: 10.1002/biof.1399. [DOI] [PubMed] [Google Scholar]
- 57.Lin Q., Weis S., Yang G., Weng Y.-H., Helston R., Rish K., Smith A., Bordner J., Polte T., Gaunitz F. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]