Abstract
Background
Mitochondrial dysfunction is one of the significant reasons for Alzheimer's disease (AD). Ginsenosides, natural molecules extracted from Panax ginseng, have been demonstrated to exert essential neuroprotective functions, which can ascribe to its anti-oxidative effect, enhancing central metabolism and improving mitochondrial function. However, a comprehensive analysis of cellular mitochondrial bioenergetics after ginsenoside treatment under Aβ-oxidative stress is missing.
Methods
The antioxidant activities of ginsenoside Rb1, Rd, Re, Rg1 were compared by measuring the cell survival and reactive oxygen species (ROS) formation. Next, the protective effects of ginsenosides of mitochondrial bioenergetics were examined by measuring oxygen consumption rate (OCR) in PC12 cells under Aβ-oxidative stress with an extracellular flux analyzer. Meanwhile, mitochondrial membrane potential (MMP) and mitochondrial dynamics were evaluated by confocal laser scanning microscopy.
Results
Ginsenoside Rg1 possessed the strongest anti-oxidative property, and which therefore provided the best protective function to PC12 cells under the Aβ oxidative stress by increasing ATP production to 3 folds, spare capacity to 2 folds, maximal respiration to 2 folds and non-mitochondrial respiration to 1.5 folds, as compared to Aβ cell model. Furthermore, ginsenoside Rg1 enhanced MMP and mitochondrial interconnectivity, and simultaneously reduced mitochondrial circularity.
Conclusion
In the present study, these results demonstrated that ginsenoside Rg1 could be the best natural compound, as compared with other ginsenosides, by modulating the OCR of cultured PC12 cells during oxidative phosphorylation, in regulating MMP and in improving mitochondria dynamics under Aβ-induced oxidative stress.
Keywords: Alzheimer disease, Extracellular flux analyzer, Panax ginseng, Mitochondrial dynamics, ROS
1. Introduction
Alzheimer's disease (AD) is classified as deterioration of learning, language and memory functions, especially in the elderly. In addition, AD is an irreversible and progressive neuronal failure. Amyloid plaque and neurofibrillary tangle are the significant biomarkers being identified in AD brain tissues [1]. Beta-amyloid peptide (Aβ), the basic unit of amyloid plaque, has been considered one of the essential elements in progression of AD [2]. Therefore, Aβ has been considered as one of the essential causative events during AD pathogenesis [3]. Although the detailed mechanism of Aβ-induced neurotoxicity has not entirely understood, several lines of evidence suggest that mitochondrial dysfunction could be caused by Aβ neurotoxicity driving to excessive ROS formation, as well as reduction of cytochrome c oxidase activity, mitochondrial respiratory chain and ATP formation [4]. As a result, therapeutic intervention related to mitochondrial bioenergetics may assist in preventing the Aβ-induced neurotoxicity in AD patients. Recently, the pharmacological treatment of AD patients primarily consists of two types of medicine, i.e., acetylcholinesterase inhibitor and glutamate modulator [5]. Unfortunately, practical approaches in slowing down the progression of AD have yet to be found. Searching for safer, better tolerated, less side-effect and effective medicine for AD treatment, therefore, remains an essential area of drug discovery.
The root of Panax ginseng is referring to Korean and Chinese ginseng, and which is a highly valued herbal medicine extensively utilized in Asian countries for different beneficial effects, including anti-inflammatory, anti-cancer, cardioprotective and reduction of peripheral vascular disease [6]. In the clinical practices, ginseng extracts attenuated ROS in patients suffering from cardiovascular diseases: these beneficial effects were suggested to be interceded by anti-oxidative and chelating functions of different ginsenosides [7]. The antioxidant property of ginsenoside depends on the function group on aglycone. For example, ginsenoside Rb1, Rb3, Rd, Re, Rg1 and Rh1 have been proposed as anti-oxidative compounds [8]. Currently, several studies have demonstrated significant efficacies of ginseng extract and its ginsenosides in AD treatment both in cell and animal models [9]. In clinical practices, AD patients receiving ginseng showed considerable improvement [10]. Besides, the treatment of ginsenoside showed remarkable protection of loss of memory in aged mouse model by reducing oxidative stress and modulating plasticity-related proteins and neurotrophic factors [11]. Ginsenoside Rb1, Rg1, Rg2, Rg3, as well as gintonin, showing strong effects against neuronal failure could be mediated by ROS signaling [12]. Apart from antioxidant properties, the protection of ATP-generation capacity was believed to be one of the action mechanisms of ginsenoside under Aβ-induced neurotoxicity [13]. In mitochondrial bioenergetics, ATP production is referring to spare capacity, which has been closely related to cell proliferation, health and flexibility [14]. Moreover, the maximal OCR of mitochondria is affected by basal respiration. Although the abilities of ginseng to reduce ROS formation and to enhance ATP synthesis in neurons have been shown [15], the functions of ginsenoside in mitochondrial bioenergetics of a live cell remains mysterious due to the technical constraint.
To obtain comprehensive understanding of ginsenoside in mitochondrial bioenergetics of neuron under Aβ treatment, the key elements of oxidative phosphorylation were measured with an extracellular flux analyzer by monitoring energy metabolism of living cell. Besides, MMP and mitochondrial dynamics were examined by using laser confocal scanning microscopy. Thus, the preventive measures of ginsenoside to Aβ-treated cells could be investigated and compared.
2. Methods
2.1. Chemicals
The standard compounds of ginsenoside Rb1, Rd, Re as well as Rg1, were purchased from Shanghai Research and Development Center for Standardization of Traditional Chinese Medicine (Shanghai, China). All these compounds were over 99% purity. Cell culture reagents and Aβ25-35 were purchased from Thermo Fisher Scientific (Waltham, MA). 3-(4,5-dimethylthioazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT) and nerve growth factor (NGF) were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals were analytical or standard grade.
2.2. Cell culture
Rat pheochromocytoma cells (PC-12 cells) were purchased from the American Type Culture Collection (Manassas, VA). PC-12 cells were cultured in high-glucose Dulbecco's modified Eagle's medium, supplemented with 6% horse serum, 6% fetal bovine serum and 100 units/ml concentration of penicillin and streptomycin in a water-saturated CO2 (7.5%) incubator at 37°C. The cells were grown up to 70-90% confluency for experimental purposes [16]. PC12 cell is an excellent neuronal model for AD in previous studies [17,18]
2.3. Cell viability assay
The cell survival ability was examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT, Invitrogen) assay. In brief, PC12 cells were seeded at a concentration of 1 × 104 cells per well. After 24 hours of drug exposure, cells in each well were incubated with 10μL MTT solution (5mg/mL in 1XPBS) at a final concentration of 0.5mg/mL for 3 hours at 37 °C. After the medium was removed, dimethyl sulfoxide (DMSO) was used to dissolve the organic crystal inside the cells, and the absorbance was measured using a microplate reader at a wavelength at 570nm. The cell viability was determined as the percentage of absorbance value of vehicle control; while the value of control was 100%. Thus, the effect of different ginsenosides on the reduction of Aβ-induced neurotoxicity was measured. After cells were exposed to fresh medium for 3 hours, cells were treated with 0.1–10μM of ginsenosides for 24 hours before Aβ exposure. Fifty μM of Aβ25-35 (diluted in 1X PBS) was added to the culture medium and incubated for 24 hours. To assess cell survival ability, Aβ25-35-containing media was discarded, and each well was washed twice with 1 X PBS. The cell viability was examined by MTT assay [19].
2.4. ROS formation assay
The detection of intracellular ROS content was conducted by using 2′,7′-dichlorofluorescin diacetate (DCFH-DA, Sigma-Aldrich), a ROS-sensitive compound. Cultured PC12 cells (1 × 104 cells/well) in a 96-well plate were exposed with standard compounds for 24 hours, and the cells were stained with 100μM DCFH-DA in 1X PBS for 1 hour at 37°C in a CO2 incubator. After washing with 1X PBS, the cells were then treated with 50μM Aβ for 24 hours at 37°C. Next, the amount of intracellular ROS under Aβ-induced oxidative stress was examined by photoluminescence spectroscopy with excitation wavelength at 485 nm and emission wavelength at 530 nm at 37°C [20].
2.5. Mitochondrial bioenergetic analysis
Mitochondrial bioenergetics of PC12 cell was detected by a Seahorse Bioscience XFp extracellular flux analyzer (Agilent, Santa Clara, CA), which determined the amount of oxygen change by oxidative phosphorylation in live cells. In the present studies, the seeding concentration of PC12 cells was set at 1 × 104 cells per well. Mitochondrial complex inhibitors (Sigma-Aldrich) were pre-optimized at 1μM oligomycin (ATP synthase inhibitor), 1μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (mitochondrial uncoupler), and 1μM rotenone/antimycin A (inhibitors of complex I and complex III) to elicit maximal effects on mitochondrial respiration. Background correction wells were used to calibrate the background noise. Cultured PC12 cells were cultured on the XFp cell culture mini-plates and exposed with ginsenoside for 24 hours. After the drug treatment, the fluorescent probe cartridges of the XFp analyzer were hydrated in an incubator at 37°C without CO2. Before fluorescent probe calibration, cells were exposed with 1μM Aβ25-35 for 24 hours and then equilibrated in 37°C incubator without CO2 in XF Base Medium (10 mM glucose, 1 mM pyruvate and 2 mM L-glutamine, pH 7.4 at 37°C) for another 1 hour. After calibrating the fluorescent probe cartridges, the plate was put into XFp extracellular flux analyzer for Mito Stress Test. OCRs were detected and normalized to the protein concentration/well and corrected for extra-mitochondrial oxygen change from the environment. Eventually, six key indicators of mitochondrial bioenergetic function were calculated from the bioenergetics profile, i.e., basal respiration, ATP production, proton leak, maximal respiration, spare capacity and non-mitochondrial respiration [21].
2.6. MMP analysis
PC12 cultured on an autoclaved coverslip in 6-well plates were incubated with tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (10μg/mL) in fresh culture medium for 10min at 37°C and 5% CO2 before the analysis. After treated with JC-1, the cells were washed three times with 1X PBS and mounted the coverslips onto microscope slides. Images were taken using a Zeiss laser scanning confocal microscope. JC-1 monomer (green) was observed with a 505-550nm emission filter under 488nm laser illumination. JC-1 aggregates (red) were observed with a 585nm filter under 568nm laser illumination. The MMP was detected by laser scanning confocal microscopy using a 63 X lens (NA = 1.4) and analyzed by Zen software [22].
2.7. Mitochondrial dynamic analysis
Cultured PC12 cells were loaded with mitochondrial indicator by incubation with MitoTracker™ Red FM in the culture medium at 37°C for 30 min in a CO2 incubator after different drug treatments. Mitochondrial dynamics was observed using laser scanning confocal microscopy. The signal of MitoTracker™ was then analyzed by ImageJ program (National Institutes of Mental Health, Bethesda, MD) with Mito-Morphology software for mitochondrial circularity, interconnectivity (area/perimeter), mitochondrion content and minor axis as previously described [23]. The mitochondrial interconnectivity and circularity were reflected as key indicators for mitochondrial morphological changes [24].
2.8. Statistical analysis
The mitochondrial bioenergetics was showed on Wave Desktop software 2.3.0. The data acquisition of the confocal image was conducted by Zen black edition software. All results were expressed as Mean ± Standard Error of the Mean (SEM). Statistically, significant tests were conducted with Dunnett's test (one-way analysis of variance with multiple comparisons, SPSS, version 13). Statistically, the difference was defined as (∗), where p < 0.05, (∗∗) where p < 0.01 and (∗∗∗) where p < 0.001.
3. Results
3.1. Ginsenoside protects Aβ-induced cell damage
PC12 cell is a commonly used neuronal cell line in testing the protective function of drugs. Besides, this cell line showed a robust and fast reaction to various stimuli during mitochondrial respiration. Aβ25-35, a neurotoxicity inducer of synthetic peptide fragment from Aβ protein, induced cell damage of cultured PC12 cells in a dose-dependent manner: the result showed a saturated cell death starting at 50μM Aβ25-35 (Fig. S1A). Here, 50μM Aβ25-35 was used for subsequent experiments. This Aβ-induced cell death model was employed to reveal possible protective function of major ginsenosides from ginseng, i.e., Rb1, Rd, Re and Rg1 (Fig. 1A). In cultured PC12 cells, Aβ25-35 was incubated with or without NGF, and then which was used to treat the cells for 24 hours. The treatment with Aβ25-35 significantly decreased cell viability by ~40% at 50 μM Aβ25-35 (Fig. 1B). In comparison to the Aβ25-35 application, the co-treatment of NGF increased cell survival to over 80% of control. The treatment of Aβ25-35 with ginsenoside Rd, Re and Rg1 showed less toxic to the cultured cells, like NGF (Fig. 1B). The protective ability of ginsenoside against Aβ toxicity was in a dose-dependent manner: the highest modulation was conducted at 10μM of ginsenoside in most cases. Rg1 showed a better effect in relieving Aβ25-35-induced cell toxicity. In contrast, Rb1 was the weakest one in protecting cell toxicity. Oxidative stress is one of the reasons for neuro-damage, triggered by Aβ. In cultured PC 12 cells, the application of Aβ25-35 induced ROS production, showing a maximal modulation significantly to 250% at 50μM, as compared to vehicle control (Fig. 1B). The application of NGF in culture showed a protective function against Aβ-induced cell death. By applying ginsenoside before Aβ25-35 exposure, the intracellular ROS was decreased, and which was in a dose-dependent manner (Fig. 1B). The administration of Rg1 showed the best protective effects having maximal protection of over 150%, as compared with that of control (Fig. 1B). Again, Rb1 was the weakest ginsenoside to alter the ROS formation.
Fig. 1.
Protective effects of ginsenosides to PC12 cells under Aβ-induced stress. (A) The compound structures of ginsenosides, Rb1, Rd, Re as well as Rg1. (B) Cultured PC12 cells (1 × 104 cells/well) were treated to Aβ25-35 (50μM), and NGF (50ng/mL) served as a vehicle positive control. A dose-dependent reaction was examined by pre-treating the cultures with Rb1, Rd, Re, and Rg1 for 24 hours before the addition of Aβ25-35. The cell survival ability was examined by MTT assay after 48 hours (left panel). The concentration of intracellular ROS was detected by DCFH-DA after 1-hour staining. The response was like the left panel. Data are expressed as the percentage of control (untreated culture), in Mean ± SEM, n = 5, each with triplicate samples. Statistical comparison was made with the control group, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.2. Ginsenoside enhances mitochondrial bioenergetics
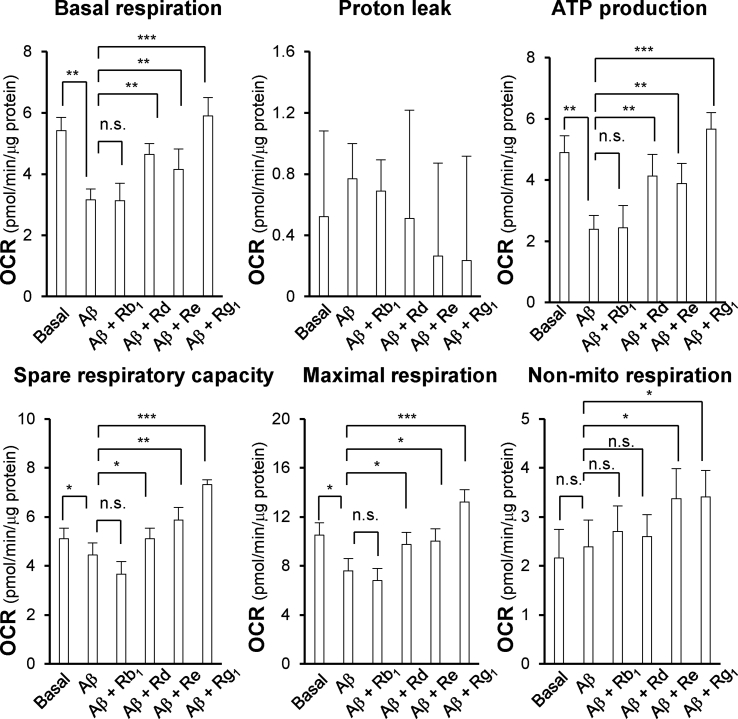
Various indicators of mitochondrial bioenergetics were measured by an extracellular flux analyzer and calculated accordingly (Fig. S1B). In cultured PC12 cells, the cell density for seeding and the concentration of FCCP were firstly identified to detect the cellular metabolic functions. The optimal concentration of PC12 cells was set at 10 × 103 cells/well, as to adjust basal OCR to an appropriate range (100 - 160 pmol/min) (Fig. 2A). The concentrations of oligomycin (1μM) and rotenone/antimycin A (1μM) were set according to the manufacturer's guideline. Application of Aβ in cultured PC12 cells reduced the basal OCR in a dose-dependently manner, and the dosage of Aβ25-35 was optimized to 1 μM to detect a measurable OCR value (Fig. 2A). The applied FCCP was also revealed and optimized (Fig. 2C). The Aβ-treated PC12 cells showed different mitochondrial dysfunctions, resulting in significant reduction in basal respiration (~50%), ATP formation (~60%) and maximal respiration (~30%) (Fig. 2D), as compared with the control group. The pre-treatment of ginsenoside in Aβ-treated PC12 cells maintained mitochondrial OCR. Ginsenosides could increase basal respiration, ATP formation, spare capacity, maximal respiration and non-mitochondria respiration to different degree; however, Rb1 did not show such effects. Amongst these ginsenosides, Rg1 was the best one in modulating mitochondrial bioenergetics, and therefore the applied dose of Rg1 was determined. Ten mM ginsenoside was used thereafter. In the extracellular flux analyzer, Rd and Re could recover the stressed cells to normal status by enhancing spare capacity and maximal respiration to a reasonable level, while Rg1 could further increase the value to over 2 folds of the normal state (Fig. 2D). The parameters of proton leak and non-mitochondrial respiration changed irregularly, properly due to the detection limit of present method was not sensitive enough to measure these parameters, precisely.
Fig. 2.
Mitochondrial bioenergetics function of ginsenosides to Aβ-treated PC12 cells. (A) Cultured PC12 cells with different cell concentrations were cultured in XFp culture miniplate and seeded for 48 hours before detection. (B) Cultured PC12 cells (10 × 103 cells/well) were treated to Aβ25-35 at different concentrations for 24 hours, and OCR was detected. The OCR values were normalized with protein concentration. (C) PC12 cells (10 × 103 cells/well) were seeded for 48 hours, then treated with 1μM oligomycin and three serial addition of FCCP at various concentrations (a high concentration range of 1, 1.25, 1.5μM and a low concentration range of 0.5, 0.75, 1μM). (D) Cultured PC12 cells (10 × 103 cells/well) were pre-treated with ginsenoside at 10μM for 24 hours before exposure to Aβ25-35 (1μM) for another 24 hours. (E) Cultured PC12 cells (10 × 103 cells/well) were treated with ginsenoside Rg1 (0.1 to 10μM) for 24 hours before Aβ addition. Oligomycin (1μM, ATP synthase inhibitor), FCCP (1μM, uncoupling agent), and rotenone/antimycin A (R&A at 1μM, complex I and complex III inhibitors) applied onto the wells during seahorse XFp operation. The OCR value was normalized with protein concentration/well. Values are expressed as Mean ± SEM, n = 3 - 4, each with triplicate samples. Statistical comparison was made with Aβ experimental group, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
By quantifying the mitochondrial bioenergetics from the extracellular flux analyzer, different indicators could be compared amongst four ginsenosides, i.e., Rb1, Rd, Re and Rg1. These bioenergetic parameters could be altered by applied ginsenosides, except the proton leak (Fig. 3). Rg1 showed the best induction in basal respiration, ATP formation, spare capacity, maximal respiration and non-mitochondrial respiration, as compared to other ginsenosides. The maximal induction, triggered by Rg1, could increase to ~2 folds in basal respiration, ~3 folds in ATP formation, ~2 folds in spare capacity, ~2 folds in maximal respiration and ~1.5 folds in non-mitochondrial respiration, as compared to the Aβ-treated group (Fig. 3). In addition, the Rg1-induced different parameters of mitochondrial bioenergetics were enhanced in dose-dependently manners (Fig. 2E). The robust efficacy of Rg1 in mitochondrial bioenergetics was similar to the scenario in cell survival assay.
Fig. 3.
Ginsenosides alter oxygen consumption of Aβ-treated PC12 cells. Cultured PC12 cells (10 × 103 cells/well) were pre-treated with ginsenosides at 10μM for 24 hours before exposure to Aβ25-35 (1μM) for another 24 hours. The OCR value was normalized with protein concentration/well. The basal respiration, proton leak, ATP formation, spare capacity, maximal respiration, and non-mitochondrial respiration were measured. Values are expressed as Mean ± SEM, n = 3, each with triplicate samples. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
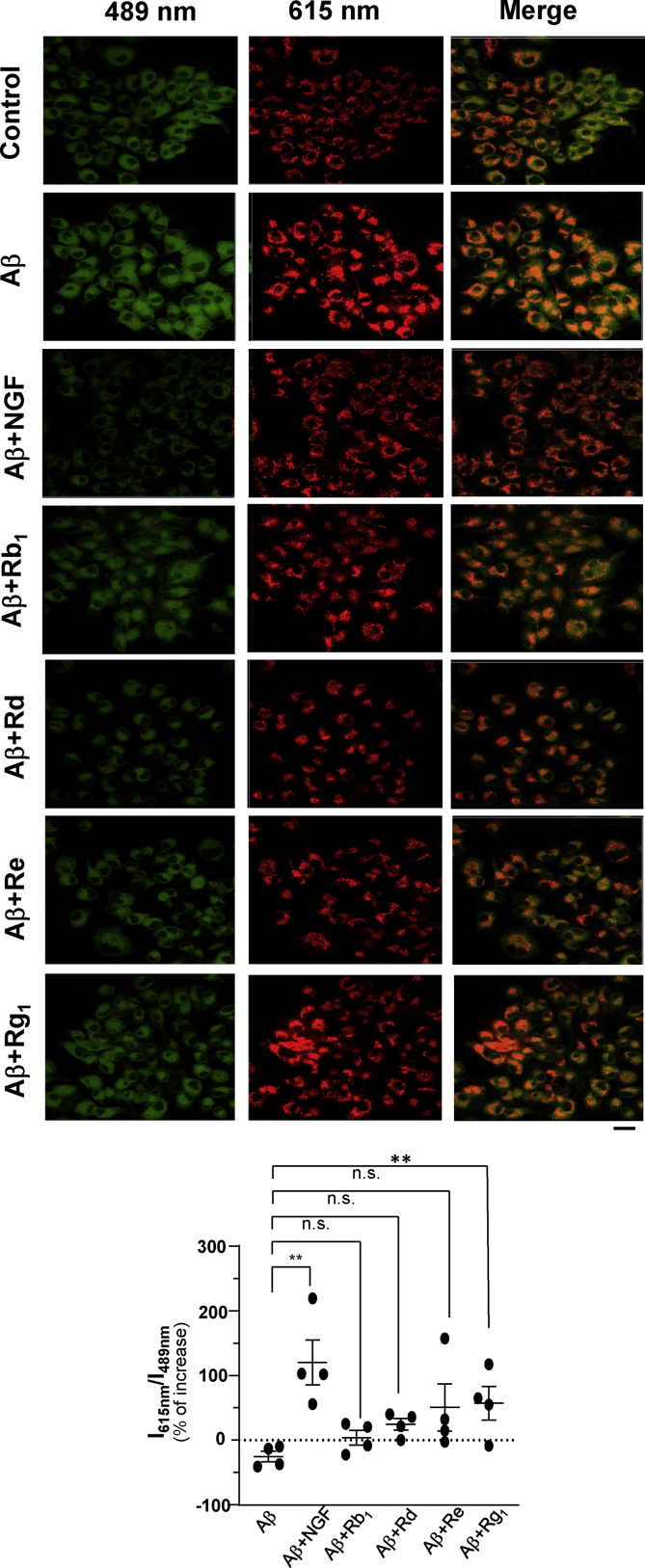
3.3. Ginsenoside modulates MMP and mitochondrial dynamics
Mitochondrial damage can directly trigger intrinsic mechanism of apoptosis, reduction of MMP, disruption of electron transport chain (ETC), formation of oxidative stress, as well as changes of apoptotic proteins. The protective effect of ginsenoside under Aβ-induced mitochondrial dysfunction was firstly examined by examining MMP using JC-1 staining (Fig. 4). JC-1 probe can indicate the change of MMP. The monomer of JC-1 can penetrate into cytoplasm, and which thereafter is being aggregated in mitochondria forming red J-aggregate. The fluorescence transition of JC-1 from red to green indicates reduction of MMP and mitochondrial injury. The ratio of signal intensity of red J-aggregate and green monomer of JC-1 could be used to determine MMP. We found that the MMP was decreased significantly after Aβ treatment. (Fig. 4). The treatment with Aβ25-35 at 50μM significantly decreased MMP to ~25%, as compared to control. NGF served as a positive control and showed a protective function under Aβ-induced cell damage in maintaining or even in potentiating MMP (Fig. 4). As expected, Rg1 showed significant induction in MMP of the Aβ-treated culture. The MMP was increased significantly to ~25% after Rg1 treatment, as compared with the Aβ treatment (Fig. 4). Ginsenoside Rb1, Rd and Re did not significantly alter the JC-1 staining, even though showing minimal effect.
Fig. 4.
Ginsenoside Rg1 enhances the MMP in Aβ-treated cells. Confocal laser scanning microscope of JC-1 stained cell after different ginsenosides treatment (10μM) and post-treatment with Aβ25-35 (50μM). Red fluorescence signal (emission: 610 nm) and green fluorescence signal (emission: 570 nm) were determined by using a confocal laser scanning microscope. One representative result is shown.; Scale bar = 20 μm. The ratio of red fluorescence signal 610 nm and green fluorescence signal at 570 nm was calculated by the Zen software blue edition. Data are expressed as percentage of increase, as compared to untreated culture, in Mean ± SEM, where n = 4. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
To determine the relationship between mitochondrial dynamics and neurotoxicity induced by Aβ, the mitochondria in cultured PC12 cells were stained by MitoTracker red. The MitoTracker red signal monitors the change of mitochondrial morphology in cultured PC12 cells. The MitoTracker red-labeled mitochondria could be analyzed by a well-established ImageJ software for circularity, area/perimeter, mitochondrion content and minor axis. The area/perimeter, minor axis, and content of mitochondria were decreased; while the circularity of mitochondria was increased significantly under Aβ-treatment (Fig. 5). In contrast, an apparent decrease of area/perimeter of mitochondria was observed under applied Aβ25-35. NGF served as positive control and showed significant protective function under Aβ-induced cell damage in mitochondrial dynamics. By applying ginsenoside before Aβ25-35 application, the mitochondrial dynamics was mostly resorted back to background level (Fig. 5). Rg1 showed the best induction in mitochondrial content, interconnectivity and minor axis, as well as the best reduction in circularity, as compared to other ginsenosides. The maximal induction, triggered by ginsenoside Rg1, could increase to ~50% in interconnectivity, ~25% in mitochondrial content, ~75% in spare respiratory capacity and decrease to ~10% in circularity compared to Aβ-treatment (Fig. 5). Rb1 did not show such an effect at all.
Fig. 5.
Ginsenoside Rg1 improves the mitochondrial dynamics in Aβ-treated cells. Cultured PC12 cells were pretreated with 10 μM of different ginsenosides for 24 hours and treated with 50μM of Aβ25-35 for 24 hours. Then, the treated cells were incubated with 1μM MitoTracker Red in 1X PBS at 37°C for 1 hour. Micrographs were taken by the laser scanning confocal microscopy. One representative picture result was shown. Scale bar = 20μm. The quantification of mitochondria content, circularity, interconnectivity, and minor axis were calculated by the imageJ. Values were expressed as % of increase, as compared to untreated culture, in Mean ± SEM, where n = 4. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
4. Discussion
The ROS and Aβ mis-location have been well characterized as biomarkers of both AD and Aβ neurotoxicity. Here in cultured PC12 cells, the exposure of Aβ resulted in a dose-dependent change of ROS formation in parallel to published observation [25]. Amongst those ginsenosides in protecting Aβ-induced oxidative stress, Rg1 displayed the best protective effect in cultured PC12 cells, which was mainly mediated by inhibiting ROS formation. By pre-treating cells with Rg1, the Aβ-induced damage could be reduced. Moreover, Rg1 inhibited NF-kB signaling, and which diminished the apoptosis of PC12 cells under hydrogen peroxide (H2O2)-induced cell death, as reported previously [26]. Besides, this protective function could be induced, at least partly, by Keap1-Nrf2-ARE signaling pathway [27]. Besides, Rg1 could protect the ROS-induced cell death via myosin-IIA actin-related reorganization of cytoskeletons in cultured PC12 cells and cortical neurons [28]. These lines of evidence are consistent with our current results.
Mitochondrion is a power factory in cell, which generates most of cellular ATP. The organelle produces ATP via the coupling of oxidative phosphorylation with respiration. There are several studies about the protective effect of ginsenosides in neurons by reducing the mitochondria-mediated cell death [29]. However, most of these researchers are focusing on ATP formation and intracellular ROS production [30]; while the influence of other indicators in mitochondrial bioenergetics is often negated. Among these parameters, spare capacity has been considered as a crucial parameter of bioenergetic profile in cells that are corresponding to the supply of substrate during increased demand for energy consumption. Here, ginsenoside Rd, Re and Rg1 showed enhancement of spare capacity and ATP formation under the insult of applied Aβ: this observation was consistent with an increase of pathway related to energy production after treatment of ginseng extract [31]. Amongst these ginsenosides, Rg1 displayed the best protective effects on the culture under Aβ-induced mitochondrial toxicity. The mechanism of Rg1 in preventing Aβ-induced mitochondrial dysfunction could be accounted for down-regulating caspase-3 and up-regulating cytochrome c oxidase. Cytochrome c oxidase is a crucial enzyme in the ETC. In the mechanism of oxidation phosphorylation, cytochrome c binds four hydrogen ion from the inner aqueous phase to make 2 water molecules with oxygen, and then which can translocate another 4 hydrogen ion across the membrane, as to increase MMP and to increase ATP formation [32]. This phenomenon is in line with our reported result here. In addition, Rg1 could enhance mitochondrial bioenergetics by up-regulating the expressions of PGC-1α, NRF-1, TFAM-1, mitochondrial complex lll, and complex IV [9]: these modulations accounted the enhancement of basal respiration, spare capacity, maximal respiration and non-mitochondrial respiration in Aβ-induced stress.
Mitochondrial dynamics are kept balanced by fission and fusion that plays a vital role in mitochondrial function [33]. Indeed, mitochondrial dysfunction is an early and causal event in neurodegeneration. To meet high energy requirement, the mitochondrial dynamics related pathway is being triggered, and the balance of dynamics is therefore shifted. Increasing of circularity, reduction of mitochondrial length and connectivity are indicative markers of fragmentation (mitochondrial fission), as a means to protect mitochondrial health [34]. Here, a novel approach using cultured PC12 cells, stained by MitoTracker red, was used as to examine the change in mitochondrial morphology. With this approach, we found that in PC12 culture under Aβ exposure, the mitochondrial morphology was markedly affected. As expected, the interconnectivity, minor axis and content of mitochondria were increased; while the circularity was decreased after ginsenoside treatment in Aβ-treated cell model, i.e., possible increase of mitochondrial fragmentation. In previous findings, Rg1 could attenuate the injury of myocardial hypoxia/reoxygenation in cardiomyocytes (H9C2 cells) by modulating the balance of mitochondrial dynamics via mitofusin-2 protein (MFN2), a member of large GTPases family involving in mitochondrial fission and fusion [35]. The function of MFN2 is to control mitochondrial metabolism, and the loss of this function leads to reduction in protein synthesis of complexe I, II, III, V, and coenzyme Q [36]: the final outcome is inhibition of respiratory chain function. Besides, MFN1 and MFN2 could regulate the morphology of mitochondrial [37]. We hypothesize that Rg1 could reduce the mitochondrial dynamics via regulation of MFN. Thus, the mRNA and protein expressions of MFN1 and MFN2 should be analyzed in future.
Rg1 is a key saponin in ginseng extract and has been proposed to have excellent efficacy in neuroprotection. The first criterion for being a neuroprotective drug is the permeability of blood-brain barrier (BBB). After oral administration of G. biloba extract, the ginsenosides could be identified and measured by liquid chromatography-mass spectrometry in the brain tissues of rats [38,39]. In addition, the absorption of ginsenosides by the brain cells was reported to be improved by activating adenosine signaling in a rat model [39]. In aged mouse model, oral administration of Rg1 up-regulated the protein expression of brain-derived neurotrophic factor via activation of protein kinase A and cyclic adenosine monophosphate-response element-binding protein (CREB) phosphorylation in the brain, and thereafter the memory loss in aged mouse could be recovered [40]. In the β-sitosterol β-D-glucoside-induced Parkinson animal model, the oral administration of ginseng extract could reduce the protein expression of α-synuclein in the striatum, and which prevented the loss of dopaminergic neuron in substantia nigra [41]. Therefore, these lines of evidence strongly support the possible application of ginsenoside in treating degenerative brain disease. In addition, clinical trial on the use of medicine containing Rg1 has passed the safety evaluation [42].
In summary, this study demonstrated the pharmacological effects of ginsenosides in Aβ-induced PC12 cell line. Meanwhile, we found that the protecting mechanism of ginsenosides could be involved the reduction of ROS formation, enhancement of different parameters in mitochondrial bioenergetics, MMP and mitochondrial morphology under Aβ-induced cell model. As a result, Rg1 is the best pharmacological drug being identified here, and it could be further developed for clinical treatment of disease correlating with AD.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgment
This work is supported by GBA Institute of Collaborate Innovation (GICI-022); Special project of Foshan University of science and technology in 2019 (FSUST19-SRI10); Shenzhen Science and Technology Innovation Committee (ZDSYS201707281432317; JCYJ20170413173747440; JCYJ20180306174903174), Zhongshan Municipal Bureau of Science and Technology (ZSST20SC03); Guangzhou Science and Technology Committee Research Grant (GZSTI16SC02; GZSTI17SC02); Hong Kong RGC Theme-based Research Scheme (T13-605/18-W); Hong Kong Innovation Technology Fund (UIM/340, UIM/385, ITS/500/18FP; TUYF19SC02; PD18SC01 and HMRF18SC06).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2020.09.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
The effects of Aβ25-35 dosage to cell viability of PC12 cells. Cultured PC12 cells (1 × 104 cells/well) were treated with Aβ25-35 (1-100μM) for one day. Cell survival ability was determined by MTT assay. Values are expressed as Mean ± SEM, n = 4, each with triplicate samples, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (B) Schematic diagram of key indicators of mitochondrial bioenergetics detected by Seahorse Bioscience XFp extracellular flux analyzer. Basal respiration shows OCR of the mitochondria under normal levels. Proton leak represents the difference of OCR after oligomycin and rotenone/antimycin A addition. ATP formation shows the fraction of basal OCR that is being used to activate ATP formation. Maximal respiration represents the highest OCR that the cell can achieve, which is detected as the OCR after FCCP addition. Spare capacity is the difference between the highest and normal OCR and can be considered as biomarker of cell flexibility and health state. The non-mitochondrial rate was subtracted from all other rates, which is a result of a subset of cellular enzymes that continue to consume oxygen after rotenone/antimycin A addition.
References
- 1.Eikelenboom P., Rozemuller A.J., Hoozemans J.J., Veerhuis R., Van Gool W.A. Neuroinflammation and Alzheimer disease: clinical and therapeutic implications. Alzheimer Dis Assoc Disord. 2000;14(Supplement):S54–S61. doi: 10.1097/00002093-200000001-00009. [DOI] [PubMed] [Google Scholar]
- 2.LeBlanc A. The role of β-amyloid peptide in Alzheimer's disease. Metab Brain Dis. 1994;9(1):3–31. doi: 10.1007/BF01996071. [DOI] [PubMed] [Google Scholar]
- 3.Brothers H.M., Gosztyla M.L., Robinson S.R. The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer's disease. Front Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagani L., Eckert A. Amyloid-beta interaction with mitochondria. Int J Alzheimers Dis. 2011:1–12. doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheikh-Bahaei N., Sajjadi S.A., Pierce A.L. Current role for biomarkers in clinical diagnosis of Alzheimer disease and frontotemporal dementia. Curr Treat Options Neurol. 2017;19(12) doi: 10.1007/s11940-017-0484-z. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42(3):264–269. doi: 10.1016/j.jgr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi L.W., Wang C.Z., Yuan C.S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72(8):689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saw C.L.L., Yang A.Y.Y., Cheng D.C., Boyanapalli S.S.S., Su Z.Y., Khor T.O., Gao S., Wang J., Jiang Z.H., Kong A.N.T. Pharmacodynamics of ginsenosides: antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem Res Toxicol. 2012;25(8):1574–1580. doi: 10.1021/tx2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang T., Fang F., Chen L., Zhu Y., Zhang J., Chen X., Shidu Yan S.S. Ginsenoside Rg1 attenuates oligomeric Aβ1-42-induced mitochondrial dysfunction. Curr Alzheimer Res. 2012;9(3):388–395. doi: 10.2174/156720512800107636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J., Kim S.H., Lee D.S., Lee D.J., Kim S.H., Chung S., Yang H.O. Effects of fermented ginseng on memory impairment and β-amyloid reduction in Alzheimer's disease experimental models. J Ginseng Res. 2013;37(1):100–107. doi: 10.5142/jgr.2013.37.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H., Li Q., Zhang Z., Pei X., Wang J., Li Y. Long-term ginsenoside consumption prevents memory loss in aged samp8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Mohanan P., Subramaniyam S., Mathiyalagan R., Yang D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42(2):123–132. doi: 10.1016/j.jgr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan B.L., Norhaizan M.E., Liew W.P.P., Sulaiman Rahman H.S. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desler C., Hansen T.L., Frederiksen J.B., Marcker M.L., Singh K.K., Rasmussen L.J. Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res. 2012;2012:1–9. doi: 10.1155/2012/192503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kausar S., Wang F., Cui H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells. 2018;7(12):274. doi: 10.3390/cells7120274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu W., Mak S., Zheng Z., Xia Y., Xu M., Duan R., Dong T., Li S., Zhan C., Shang X. Shexiang Baoxin Pill, a Traditional Chinese herbal formula, rescues the cognitive impairments in APP/PS1 transgenic mice. Front Pharmacol. 2020;11:1045. doi: 10.3389/fphar.2020.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X., Li Y., Mu X. Effect of quercetin on PC12 Alzheimer’s disease cell model induced by Aβ25-35 and its mechanism based on Sirtuin1/Nrf2/HO-1 pathway. Biomed Res Int. 2020;2020:1–10. doi: 10.1155/2020/8210578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou B., Li L., Qiu X., Wu J., Xu L., Shao W. Long non-coding RNA ANRIL knockdown suppresses apoptosis and pro-inflammatory cytokines while enhancing neurite outgrowth via binding microRNA-125a in a cellular model of Alzheimer's disease. Mol Med Rep. 2020;22:1489–1497. doi: 10.3892/mmr.2020.11203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Sun P., Bao Y., Liu J., An L. Cytotoxicity of single-walled carbon nanotubes on PC12 cells. Toxicol In Vitro. 2011;25:242–250. doi: 10.1016/j.tiv.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Gao J., Liu S., Xu F., Liu Y., Lv C., Deng Y., Shi J., Gong Q. Trilobatin protects against oxidative injury in neuronal PC12 cells through regulating mitochondrial ROS homeostasis mediated by AMPK/Nrf2/Sirt3 signaling pathway. Front Mol Neurosci. 2018;11:267. doi: 10.3389/fnmol.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrick D., Neilson A., Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Zhu X., Chen M., Ge Q., Shen Y., Pan S. Resveratrol protects PC12 cells against OGD/R-induced apoptosis via the mitochondrial-mediated signaling pathway. Acta Biochim Biophys Sin. 2016;48:342–353. doi: 10.1093/abbs/gmw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwan K.K.L., Huang Y., Leung K.W., Dong T.T.X., Tsim K.W.K. Danggui Buxue Tang, A Chinese herbal decoction containing Astragali Radix and Angelicae Sinensis Radix, modulates mitochondrial bioenergetics in cultured cardiomyoblasts. Front Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dagda R.K., Cherra S.J., Kulich S.M., Tandon A., Park D., Chu C.T. Loss of pink1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284(20):13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong Y., Bai L., Gong R., Chuan J.L., Duan X.M., Zhu Y.X. Shikonin protects PC12 cells against β-amyloid peptide-induced cell injury through antioxidant and antiapoptotic activities. Sci Rep. 2018;8(1) doi: 10.1038/s41598-017-18058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting Nf-kB activation. Neurochem Int. 2011;58(1):119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Liu D., Wu J.F., Zhang D., Cheng B.B., Zhang Y.N., Yin Z.F., Wang Y., Du J., Ling C. Ginsenoside Rg1 attenuates ultraviolet β-induced glucocortisides resistance in keratinocytes via Nrf2/hdac2 signaling. Sci Rep. 2016;6(1) doi: 10.1038/srep39336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Liu Q., Xu Y.Q., Zhang Y.Y., Lv Y.N., Tan Y., Jiang N., Cao G.S., Ma X.N., Wang J.R. Ginsenoside Rg1 protects against oxidative stress-induced neuronal apoptosis through myosin IIA-actin related cytoskeletal reorganization. Int J Biol Sci. 2016;12(11):1341–1356. doi: 10.7150/ijbs.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M., Bai X.Y., Yu S.T., Zhao W.X., Qiao J.H., Liu Y., Zhao D.Q., Wang J.W., Wang S. Ginsenoside Re Inhibits ROS/ASK-1 dependent mitochondrial apoptosis pathway and activation of Nrf2-antioxidant response in beta-amyloid-challenged SH-SY5Y Cells. Molecules. 2019;24(15):2687. doi: 10.3390/molecules24152687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu M., Ma Q., Fan C.L., Chen X., Zhang H.M., Tang M. Ginsenosides Rb1 and Rg1 protect primary cultured astrocytes against oxygen-glucose deprivation/reoxygenation-induced injury via improving mitochondrial function. Int J Mol Sci. 2019;20(23):6086. doi: 10.3390/ijms20236086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin E.J., Jo S.G., Choi S.B., Cho C.W., Lim W.C., Hong H.D., Lim T.G., Jang Y.J., Jang M., Byun S. Red ginseng improves exercise endurance by promoting mitochondrial biogenesis and myoblast differentiation. Molecules. 2020;25(4):865. doi: 10.3390/molecules25040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hüttemann M., Helling S., Sanderson T.H., Sinkler C., Samavati L., Mahapatra G., Varughese A., Lu G.R., Liu J., Ramzan R. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome C oxidase and cytochrome C in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 2012;1817(4):598–609. doi: 10.1016/j.bbabio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archer S.L. Mitochondrial dynamics — mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369(23):2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 34.Manczak M., Kandimalla R., Yin X.L., Reddy P.H. Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity. Hum Mol Genet. 2018;28(2):177–199. doi: 10.1093/hmg/ddy335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong G.T., Chen T.B., Ren X.C., Zhang Z.F., Huang W.X., Liu L., Luo P., Zhou H. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion. 2016;26:7–18. doi: 10.1016/j.mito.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Filadi R., Pendin D., Pizzo P. Mitofusin 2: from functions to disease. Cell Death Dis. 2018;9(3) doi: 10.1038/s41419-017-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong S.B., Kalkhoran S.B., Cabrera-Fuentes H.A., Hausenloy D.J. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur J Pharmacol. 2015;763:104–114. doi: 10.1016/j.ejphar.2015.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W., Liao Q.P., Quan L.H., Liu C.Y., Chang Q., Liu X.M., Liao Y.H. The effect of Acorus gramineus on the bio-availabilities and brain concentrations of ginsenosides Rg1, Re and Rb1 after oral administration of Kai-Xin-San preparations in rats. J Ethnopharmacol. 2010;131(2):313–320. doi: 10.1016/j.jep.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 39.Liang W.Y., Xu W., Zhu J., Zhu Y.D., Gu Q.L., Li Y.P., Guo C.J., Huang Y.J., Yu J.F., Wang W.X. Ginkgo biloba extract improves brain uptake of ginsenosides by increasing blood-brain barrier permeability via activating A1 adenosine receptor signaling pathway. J Ethnopharmacol. 2020;246:112243. doi: 10.1016/j.jep.2019.112243. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z., Qi Y., Cheng Z., Zhu X., Fan C., Yu S.Y. The effects of ginsenoside Rg1 on chronic stress induced depression-like behaviors, BDNF expression and the phosphorylation of PKA and CREB in rats. Neuroscience. 2016;322:358–369. doi: 10.1016/j.neuroscience.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 41.Van Kampen J.M., Baranowski D.B., Shaw C.A., Kay D.G. Panax ginseng is neuroprotective in a novel progressive model of Parkinson's disease. Exp Gerontol. 2014;50:95–105. doi: 10.1016/j.exger.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Tian J., Shi J., Wei M., Qin R., Ni J., Zhang X., Li T., Wang Y. The efficacy and safety of Fufangdanshen tablets (Radix Salviae miltiorrhizae formula tablets) for mild to moderate vascular dementia: a study protocol for a randomized controlled trial. Trials. 2016;17:281. doi: 10.1186/s13063-016-1410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effects of Aβ25-35 dosage to cell viability of PC12 cells. Cultured PC12 cells (1 × 104 cells/well) were treated with Aβ25-35 (1-100μM) for one day. Cell survival ability was determined by MTT assay. Values are expressed as Mean ± SEM, n = 4, each with triplicate samples, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (B) Schematic diagram of key indicators of mitochondrial bioenergetics detected by Seahorse Bioscience XFp extracellular flux analyzer. Basal respiration shows OCR of the mitochondria under normal levels. Proton leak represents the difference of OCR after oligomycin and rotenone/antimycin A addition. ATP formation shows the fraction of basal OCR that is being used to activate ATP formation. Maximal respiration represents the highest OCR that the cell can achieve, which is detected as the OCR after FCCP addition. Spare capacity is the difference between the highest and normal OCR and can be considered as biomarker of cell flexibility and health state. The non-mitochondrial rate was subtracted from all other rates, which is a result of a subset of cellular enzymes that continue to consume oxygen after rotenone/antimycin A addition.