Abstract
Background
Thrombosis of hepatic artery anastomosis (HAT) after liver transplantation is a catastrophic and dreaded complication. Early identification of HAT can salvage the situation. To monitor the anastomosis, conventional daily transcutaneous Doppler is performed. However, it has disadvantages of being noncontinuous, operator-dependent and technically difficult. Implantable Doppler probes wrapped around the anastomosed vessel giving continuous signal may be an important tool; however, very few studies are performed to study its efficacy after intra-abdominal vascular anastomosis, and its role is not clearly established.
Methods
Patients who underwent deceased donor liver transplant surgery were part of the study. On hepatic arterial anastomosis, implantable Doppler probe was fixed for monitoring. Conventional daily transcutaneous Doppler was also performed and the results were compared.
Results
A total of 40 hepatic arterial anastomoses were studied. The incidence of HAT was 10.53%. For the implantable Doppler probe monitoring, sensitivity and negative predictive value was 100%, whereas specificity was 94.44% and positive predictive value was 66.66% with an overall accuracy of 95%. A mean of 10 h of lead time was gained by implantable Doppler probe monitoring.
Conclusion
Our study showed that there was high sensitivity and negative predictive value of implantable Doppler probe monitoring system, which makes it ideal for post-operative vascular anastomoses surveillance monitoring; however, abnormal positive finding on implantable Doppler probe monitoring needs to be confirmed by conventional transcutaneous Doppler. The implantable Doppler probe monitoring, because of its round the clock and continuous nature gives us a good lead time in identifying vascular complication, which translates into graft salvage and reduction in morbidity and mortality.
Keywords: Hepatic arterial anastomosis, Implantable Doppler probe, Hepatic artery thrombosis, Liver transplantation
Introduction
Vascular anastomoses are possibly the most crucial part of liver transplant procedure. The intra-abdominal vascular anastomoses in liver transplantation include hepatic arterial, portal venous and hepatic venous anastomoses. The most common vascular complication in liver transplantation is hepatic artery thrombosis (HAT), with an incidence of 2–10%, followed by portal venous thrombosis and hepatic venous outflow obstruction.1
HAT is divided into early HAT (eHAT) and late HAT. By accepted definition, eHAT is thromboembolic occlusion of the hepatic artery that occurs within 2 months after liver transplantation. It is one of the most dreaded complications after liver transplantation and an important cause of graft loss and mortality. The natural history of eHAT starts immediately with bile duct ischemia and necrosis. This is followed by uncontrollable sepsis in which an already immune-compromised recipient may lead to mortality. Regular monitoring of patency of the anastomoses especially arterial anastomosis in early post-operative period is thus of immense importance.
Doppler ultrasound (DUS) that measures blood flow parameters in vessels has emerged as a very important screening tool to monitor the patency of anastomoses in post-operative period. The standard surveillance for vascular complications after liver transplantation is once daily transcutaneous DUS in first five post-operative days.2 There are, however, certain disadvantages of this current surveillance method. Firstly, being a non-continuous surveillance, there is obvious time lag before which abnormality can be detected. Then, it is operator-dependent and post-operative dressings and ileus may hamper correct interpretation.
To overcome these disadvantages, use of implantable miniature DUS probe for continuous monitoring was conceptualized. In this study, we wanted to ascertain the feasibility and utility of implantable DUS in monitoring intra-abdominal vascular anastomoses after liver transplantation in the immediate post-operative period. With this aim, we have compared this method with the conventional once daily transcutaneous DUS.
Materials and methods
Study population
This is a diagnostic cross-sectional study in which we included all patients who have undergone deceased donor liver transplant (DDLT) surgery at our center.
Hardware
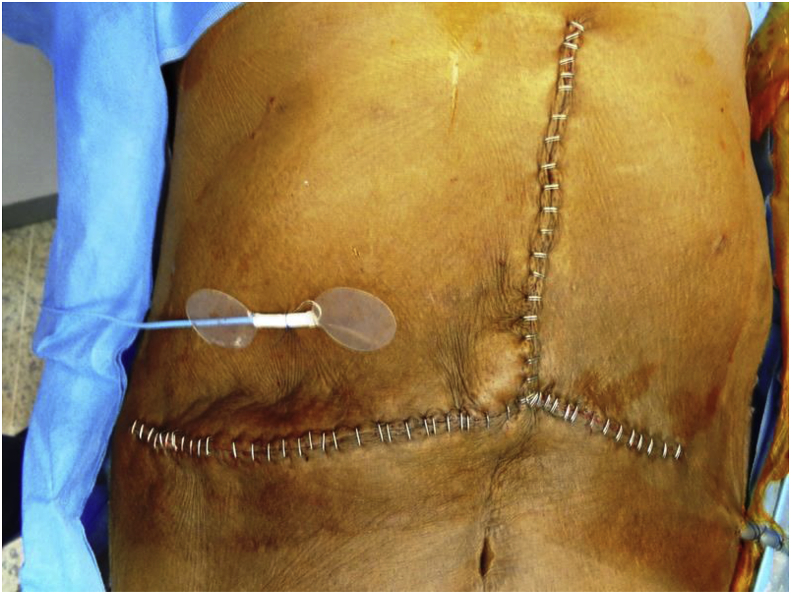
The Cook–Swartz implantable Doppler monitor system is the only proprietary device currently in the market that allows such monitoring. It consists of a 20 MHz ultrasonic Doppler crystal, a silicone cuff and a monitor unit. This cuff that is secured to the Doppler crystal (probe) is fixed to the target vessel at the time of the operation (Fig. 1, Fig. 2, Fig. 3). This Doppler probe is connected via a very thin cable to a bedside monitor, which gives continuous audio and visual signals of blood flow for monitoring (Fig. 4). The probe can be kept for as long as the monitoring is required. The probe can be very easily removed by simple traction on the connecting cable with a minimal force of less than 0.1 lb without disturbing the anastomosis.3,4
Fig. 1.
Doppler probe being implanted distal to the anastomosis.
Fig. 2.
Cable of implanted Doppler being anchored to skin using metal staples.
Fig. 3.
Position of implantable Doppler leaving skin in relation to main wound.
Fig. 4.
Implantable Doppler monitor with continuous audio-visual signal.
Data
Demographic data collected was of age, sex, body mass index (BMI), diagnosis, description of comorbidities if any and history of tobacco smoking. The pre-operative investigations studied included serum hemoglobin, albumin, bilirubin, creatinine and international normalized ratio. Per-operative findings of the donor's vascular anatomy and approximate estimate of the diameter of vascular anastomosis were noted. We also noted time taken for completion of the vascular anastomosis and total duration of surgery.
The implantable DUS probe was anchored on the vessel downstream of the anastomosis and connected via extension cable to the bed side Doppler monitor, which gave a continuous audio-visual recording of Doppler signal for first five post-operative days. Any abnormal or absent audio-visual signal was recorded as a positive finding.
All these cases were also monitored, with classical once a day transcutaneous DUS performed by a team of formally trained sonologists of our institution. Findings were recorded as normal, viz. negative or abnormal, viz. positive finding. The sonologists were blinded of the results of implantable DUS monitoring findings. Additional transcutaneous DUS examinations were performed on clinical suspicion of a vascular complication as deemed necessary.
Sensitivity, specificity, positive predictive value, negative predictive value and accuracy were calculated for the study modality as well as for the standard reference modality.
Results
A total of 40 patients who had undergone hepatic arterial anastomosis as part of liver transplant procedure were studied. The mean age of all patients was 46.4 years, with a range of 28–62 years. Of a total of 40 patients, 32 (80%) were of male gender and 8 (20%) were of female gender. Mean BMI of all patients was 30.42 kg/m2 (24.78–36.46 kg/m2). The pre-operative investigations studied are as per Table 1.
Table 1.
Demographic and pre-operative data.
| Variables | Mean | Standard deviation |
|---|---|---|
| Age (year) | 46.40 | 9.16 |
| BMI (kg/m2) | 30.42 | 2.95 |
| Hb (gm %) | 9.43 | 0.95 |
| Serum bilirubin (mg %) | 2.47 | 0.65 |
| Serum albumin (gm %) | 2.24 | 0.51 |
| Serum creatinine (mg %) | 1.09 | 0.46 |
| INR | 2.11 | 0.44 |
INR, international normalized ratio.
The details of a total of 40 arterial anastomoses between donor and recipient arteries made during DDLT surgeries in patients diagnosed with end-stage liver disease (ESLD) were studied. The different etiologies of ESLD in these patients are as shown in Table 2.
Table 2.
Etiology of cirrhosis.
| Etiology | Number |
|---|---|
| Ethanol | 15 |
| Cryptogenic | 9 |
| HCV | 4 |
| HCV + HCC | 3 |
| HBV | 3 |
| HBV + HCC | 2 |
| Primary biliary cirrhosis | 1 |
| Primary hyperoxaluria | 1 |
| Primary sclerosing cholangitis | 1 |
| Wilson disease | 1 |
| Total | 40 |
HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Thorough benching of the donor liver demonstrated normal arterial anatomy in 36 livers (90%). In these patients, celiac artery of donor liver was anastomosed to recipient's right hepatic artery (24, 60%), left hepatic artery (7, 17.5%) or hepatic artery proper (5, 12.5%). Four of donor livers (10%) demonstrated arterial variations. Two donor livers had accessory right hepatic artery (donor celiac artery to recipient right hepatic artery and donor accessory right hepatic artery to donor gastro duodenal artery anastomosis), whereas one had accessory left hepatic artery (donor celiac artery to recipient right hepatic artery anastomosis) and the remaining had replaced right hepatic artery (donor celiac artery to recipient right hepatic artery and donor replaced right hepatic artery to donor gastro duodenal artery anastomosis).
Mean diameter of arterial anastomosis was 4.58 mm. The anastomosis was fashioned with satisfactory flow in the first time in a total of 26 anastomoses, whereas 14 anastomoses required a redo to achieve satisfactory flow.
Different suture materials were used depending on surgeon's personal preference. CV GORE-TEX 8-0 suture was used in 15 anastomoses (37.5%) in a continuous fashion, whereas polypropylene 8-0 suture was used in 25 anastomoses (62.5%) in interrupted fashion.
Mean total blood loss during entire surgery was 1.55 L. Mean number of units of packed RBC transfused was 3.33, whereas mean number of units of fresh frozen plasma transfused was 9.48.
Time taken for completion of vascular anastomosis ranged from 19 to 57 min, with a mean of 36.83 min. Cold ischemia time was calculated from the time the donor portal vein was infused with cold preservative solution up to removal of the ‘benched’ liver graft from the cold preservative solution. Mean cold ischemia time was 8.5 h, whereas mean warm ischemia time was 48.42 min and was calculated from the time of removal of the ‘benched’ liver graft from the cold preservative solution up to the completion of portal vein anastomosis. All DDLT surgeries were of piggyback kind and sequence of vascular anastomoses included donor suprahepatic inferior vena cava with recipient hepatic venous cuff (outflow anastomosis), followed by portal vein anastomosis, followed by hepatic arterial anastomosis (inflow anastomoses). Biliary ductal anastomoses were the last to perform as a protocol. The total duration of a DDLT surgery was a mean of 8.87 h.
Of a total of 40 anastomoses, six cases showed an abnormal, viz. positive finding with either diminished or absent signal on implantable Doppler probe monitoring. These six cases were subjected to a classical transcutaneous DUS examination. Four of these six cases confirmed findings suggestive of hepatic artery thrombosis on transcutaneous DUS examination and they required re-exploration. Two cases were managed with Fogarty thrombectomy through splenic artery stump, whereas the remaining two cases required a re-do anastomosis. None of the patients required explantation of the liver graft with re-transplants. Thus, the incidence of HAT in our series was 10.53%. The two cases in which implantable Doppler probe monitoring finding was abnormal but normal transcutaneous Doppler were treated conservatively with a close vigil and ultimately had expectant good outcome.
The cases that were suspected to have HAT on implantable Doppler probe monitoring had detection of abnormal signal at odd times out of hospital working hours and at a mean of 10 h (8–12 h) earlier to next scheduled transcutaneous DUS examination. This was the mean lead time given to us by implantable Doppler probe monitoring system, well before any clinical or biochemical suspicion of HAT.
Sensitivity, specificity, positive predictive value, negative predictive value and accuracy, viz. efficacy were calculated for the study modality (implantable Doppler probe monitoring) as well as for the standard reference modality (classical transcutaneous DUS examination) and compared. For the implantable Doppler probe monitoring, sensitivity and negative predictive value was 100% each, whereas specificity was 94.44% and positive predictive value was 66.66% with an overall accuracy of 95% (Table 3). Same values for the classical transcutaneous DUS examination were 100% (Table 4).
Table 3.
Implantable Doppler monitoring data.
| Normal on Doppler (negative) |
Abnormal on Doppler (positive) |
||
| 34 |
6 |
||
| No thrombosis |
Thrombosis |
No thrombosis |
Thrombosis |
| 34 |
0 |
2 |
4 |
| TN |
FN |
FP |
TP |
| Sensitivity = TP/TP+FN = 4/4 = 100% | |||
| Specificity = TN/TN+FP = 34/36 = 94.44% | |||
| PPV = TP/TP+FP = 4/6 = 66.66% | |||
| NPV = TN/TN+FN = 34/34 = 100% | |||
| Accuracy (efficacy) = TP+TN/TP+TN+FP+FN = 38/40 = 95% | |||
FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive.
Table 4.
Transcutaneous Doppler monitoring data.
| Normal on Doppler (negative) |
Abnormal on Doppler (positive) |
||
| 36 |
4 |
||
| No thrombosis |
Thrombosis |
No thrombosis |
Thrombosis |
| 36 |
0 |
0 |
4 |
| TN |
FN |
FP |
TP |
| Sensitivity = TP/TP+FN = 4/4 = 100% | |||
| Specificity = TN/TN+FP = 36/36 = 100% | |||
| PPV = TP/TP+FP = 4/4 = 100% | |||
| NPV = TN/TN+FN = 36/36 = 100% | |||
| Accuracy (efficacy) = TP+TN/TP+TN+FP+FN = 40/40 = 100% | |||
FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive.
Discussion
Intra-abdominal vascular anastomoses form an integral part of work in a gastrointestinal surgical department. The commonest and most dangerous vascular complication in liver transplantation is eHAT. If eHAT occurs, it evolves rapidly causing a cascade of potentially lethal events. Furthermore, the clinical picture of eHAT and primary graft dysfunction is quite similar. Hence, early and correct identification of eHAT assumes great importance.5 Monitoring of vascular anastomoses with different methods ranges in complexity, invasiveness and efficacy. But as yet there is no consensus on the standard accepted modality. The reconstructive microvascular surgical studies show possible utility of 20 MHz implantable Doppler probe monitoring of vascular anastomoses.6, 7, 8
Post-operative protocol-based transcutaneous ultrasonic Doppler monitoring of these intra-abdominal vascular anastomoses are the current reference standard method of surveillance monitoring. However, there are some obvious inherent limitations of this trans-cutaneous once daily ultrasound monitoring like being non-continuous there is inherent time lag between occurrence of a complication and its detection, availability of poor sonologic window because of post-operative ileus and heavy abdominal dressings and inter-observer bias.
The need of continuous monitoring of the vascular anastomoses in early post-operative period after liver transplantation and its probable utility in reconstructive procedures was the reason for taking up the present study. The role of implantable Doppler probe monitoring vascular anastomosis in liver transplantation is still not clearly established. Few authors like Kaneko et al., Yokoyama and Yukito Tabuchi9 affirmed positively the utility of such monitoring. However, there are few studies which question the routine use of implantable probes like those by Rosenberg et al., Smit et al. and Whitaker et al.10,11
In liver transplant surgeries, we studied only arterial anastomoses and not portal venous anastomoses, because portal venous thrombosis is rare to occur in early post-operative period. Also, we did not study outflow vascular anastomoses, as all liver transplants at our institute were of piggyback type and it is technically difficult to apply the Doppler probe in the restricted and relatively inaccessible area.
The incidence of HAT in our series was four cases out of 38 hepatic arterial anastomoses (10.53%). The literature review by Mourad et al. in 2014 found the incidence to be 9%, whereas in their own study it was 7%.12 The incidence of HAT in our series is in concordance with existing literature. However, the small sample size precluded us from the calculation of confidence intervals in our study.
Sensitivity, specificity, positive predictive value, negative predictive value and accuracy were used as parameters of efficacy. For the implantable Doppler probe monitoring system, sensitivity and negative predictive value was 100%, whereas specificity was 94.44% and positive predictive value was 66.66% with an overall accuracy of 95% (Table 4). The high sensitivity and negative predictive value of implantable Doppler probe monitoring system makes it ideal for post-operative vascular anastomoses surveillance monitoring. The two abnormal, that is, positive findings on implantable Doppler probe monitoring which turned out to be normal, that is, negative findings on classical transcutaneous DUS examination with stable or improving clinical and biochemical picture (false positives) were possibly due to inadvertent loss of contact, viz. dislodgement of silastic cuff of the implantable Doppler probe with the target vessel. These patients were followed up expectantly with a constant vigil and the clinical outcome was good.
Jennifer P. Guillemaud et al. in a consecutive series of 351 patients who underwent free flap reconstruction of head and neck defects and were monitored post-operatively with implantable Doppler probes showed sensitivity of 65.8% and specificity of 98.2% for the detection of flap compromise, whereas Edward Chang et al. in a very recent study published in March 2016 showed that overall sensitivity and specificity of implantable Doppler probes were 77.8% and 88.4%, respectively, during free flap monitoring.13
As regards to its utility in liver transplantation, in a prospective observational study by Bekker et al., hepatic arterial signals were checked using implantable miniature probe at least six times per day for the first 10 days after transplantation and then were compared with percutaneous DUS screening.14 Their conclusion was that the accuracy for detection of eHAT with continuous monitoring was 99%.9 Our study also corroborates this finding.
The four patients with suspicion of HAT on implantable Doppler probe and immediate confirmation on classical transcutaneous DUS examination underwent exploratory laparotomy and remedial action. Two cases were managed with Fogarty thrombectomy through splenic artery stump and other two cases with re-do anastomosis. The cases that were suspected to have HAT on implantable Doppler probe monitoring had detection of abnormal signal at odd times out of hospital working hours and at a mean of 10 h earlier to next scheduled transcutaneous DUS examination. This was the mean lead time given to us by implantable Doppler probe monitoring system, well before any clinical or biochemical suspicion of HAT. Also, none of the patients required explantation of the liver graft with re-transplants as the action taken subsequent to the warning by implantable Doppler probe monitoring was immediate, resulting into a salvage rate of 100%. We found this as the real advantage of a continuous 24 h surveillance monitoring.
Conclusion
The high sensitivity and negative predictive value of implantable Doppler probe monitoring system make it ideal for post-operative vascular anastomoses surveillance monitoring. However, an abnormal positive finding on implantable Doppler probe monitoring system needs to be confirmed on conventional transcutaneous DUS system, before the patient is considered for re-exploration.
The implantable Doppler probe monitoring system, because of its inherent robust round the clock, reliable and cost-effective continuous monitoring nature, lends us with a good lead time by identification of a vascular complication well before the irreversible cascade of clinical catastrophe has set in.
Disclosure of competing interest
The authors have none to declare.
Acknowledgements
This article is based on Armed Forces Medical Research Committee Project No 4561/2014 granted by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
References
- 1.Oh C.K., Pelletier S.J., Sawyer R.G. Uni- and multi-variate analysis of risk factors for early and late hepatic artery thrombosis after liver transplantation. Transplantation. 2001;71:767–772. doi: 10.1097/00007890-200103270-00014. [DOI] [PubMed] [Google Scholar]
- 2.Crossin J.D., Muradali D., Wilson S.R. US of liver transplants: normal and abnormal. Radiographics. 2003;23:1093–1114. doi: 10.1148/rg.235035031. [DOI] [PubMed] [Google Scholar]
- 3.Swartz WM, Jones NF, Cherup L, Klein A. Direct monitoring of micro vascular anastomoses with the 20-MHz ultrasonic Doppler probe: an experimental and clinical study. Plast Reconstr Surg. Feb 1988:149–153. doi: 10.1097/00006534-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Smit J.M., Whitaker I.S., Liss A.G. Post-operative monitoring of micro vascular breast reconstructions using the implantable Cook-Swartz Doppler system: a study of 145 probes & technical discussion. J Plast Reconstr Aesthetic Surg. 2009;62:1286–1292. doi: 10.1016/j.bjps.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda H., Yagi T., Sadamori H. Complications of arterial reconstruction in living donor liver transplantation: a single-center experience. Surg Today. 2006;36:245–251. doi: 10.1007/s00595-005-3131-3. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko T., Nakao A., Tabuchi Y., Matsunaka T., Ohi S., Takagi H. An experimental study on implantable Doppler miniprobe. Jpn J Gastroenterol Surg. 1995;28:1055–1061. [Google Scholar]
- 7.Paydar K.Z., Hansen S.L., Chang D.S., Hoffman W.Y., Leon P. Implantable venous Doppler monitoring in head and neck free flap reconstruction increases the salvage rate. Plast Reconstr Surg. 2010;125:1129. doi: 10.1097/PRS.0b013e3181d0ab23. [DOI] [PubMed] [Google Scholar]
- 8.Schmulder A., Gur E., Zaretski A. Eight-year experience of the Cook-Swartz Doppler in free-flap operations: microsurgical and re-exploration results with regard to a wide spectrum of surgeries. Microsurgery. 2011 Jan;31(1):1–6. doi: 10.1002/micr.20816. [DOI] [PubMed] [Google Scholar]
- 9.de Jong Koert P., Bekker Jasper, van Laarhoven Stijn. Implantable continuous Doppler monitoring device for detection of hepatic artery thrombosis after liver transplantation. Transplantation. 2012;94 doi: 10.1097/TP.0b013e318269e6ad. [DOI] [PubMed] [Google Scholar]
- 10.Jason J., Rosenberg M.D., Bruno D., Fornage M.D., Chevray Pierre M. Monitoring buried free flaps: limitations of the implantable Doppler and use of color duplex sonography as a confirmatory test. Plast Reconstr Surg. 2006;118:109. doi: 10.1097/01.prs.0000221113.78244.8c. [DOI] [PubMed] [Google Scholar]
- 11.Smit Jeroen M., Werker Paul M.N., Liss Anders G. Introduction of the implantable Doppler system did not lead to an increased salvage rate of compromised flaps: a multivariate analysis. Plast Reconstr Surg. 2010;125:1710. doi: 10.1097/PRS.0b013e3181d0ace8. [DOI] [PubMed] [Google Scholar]
- 12.Piardi Tullio, Martin Lhuaire, Bruno Onorina. Vascular complications following liver transplantation: a literature review of advances in 2015. World J Hepatol. 2016 January 8;8(1):36–57. doi: 10.4254/wjh.v8.i1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang E.I., Ibrahim A., Zhang H. March 2016. Deciphering the Sensitivity and Specificity of the Implantable Doppler Probe in Free Flap Monitoring. Plastic and Reconstructive Surgery. [DOI] [PubMed] [Google Scholar]
- 14.Bekker J., Ploem S., de Jong K.P. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746–757. doi: 10.1111/j.1600-6143.2008.02541.x. [DOI] [PubMed] [Google Scholar]