Abstract
Neurology practice has faced many challenges since Jean-Martin Charcot established its sacred tenets. Artificial Intelligence (AI) promises to revolutionize the time-tested neurology practice in unimaginable ways. AI can now diagnose stroke from CT/MRI scans, detect papilledema and diabetic retinopathy from retinal scans, interpret electroencephalogram (EEG) to prognosticate coma, detect seizure well before ictus, predict conversion of mild cognitive impairment to Alzheimer's dementia, classify neurodegenerative diseases based on gait and handwriting. Clinical practice would likely change in near future to accommodate AI as a complementary tool. The clinician should be prepared to change the perception of AI from nemesis to opportunity.
Keywords: Artificial intelligence, Machine learning, Stroke, Neurology
Introduction
The practice of neurology is firmly rooted in the time-tested clinical methods of history taking, meticulous examination and neurological localization. Neurology practice has faced many challenges since Charcot established its sacred tenets. The advent of Computed Tomography (CT) scans and Magnetic Resonance Imaging (MRI) in the twentieth century tempted the clinician to bypass the holy grail of time-tested clinical methods. The old guardians of neurology, while imparting training to budding neurologists, have stood resolute against such temptations. The risk of getting misled in a sea of incidental findings is often cited as the reason to stick with the traditional clinical methods. Artificial Intelligence (AI) aims to revolutionize neurology practice in ways unimaginable in the times of Jean-Martin Charcot. AI can now diagnose stroke from CT/MRI scans, detect papilledema and diabetic retinopathy from retinal scans, interpret electroencephalogram (EEG) to prognosticate coma, detect seizure well before ictus, predict conversion of mild cognitive impairment to Alzheimer's dementia, classify neurodegenerative diseases based on gait and handwriting.1 Importantly, AI does not bank on the clinical methods of yore to make deductions.
What is artificial intelligence?
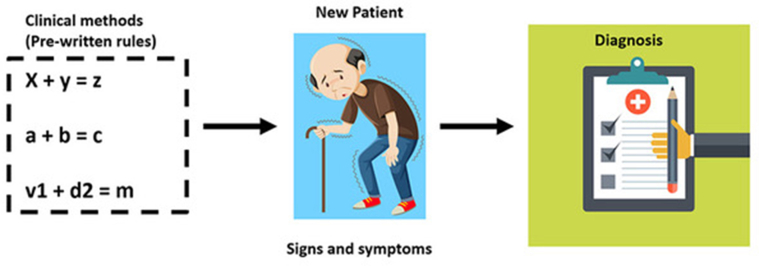
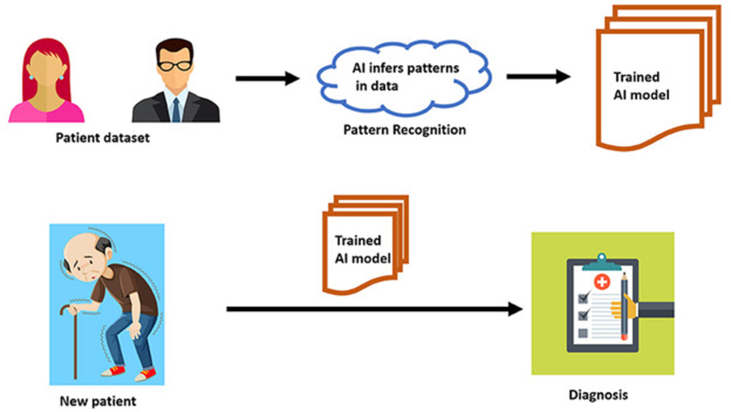
The undergraduate medical school relies heavily on rule-based training. The combination of hemiparesis and irregular pulse that invokes a differential diagnosis list is based on rules that have been formulated and refined by clinicians over centuries [Fig. 1]. In contrast, post-graduate residency encourages residents to infer patterns from continued clinical exposure rather than prewritten rules. Suspecting stroke in a disoriented patient is an example of the latter, which requires recall of clinical gestalt acquired during residency. AI algorithms emulate residency training by progressively learning from patterns that are inherent in large clinical datasets.2 These datasets currently exist in the form of CT/MRI images, retinal scans, electrophysiological tracings, electronic health records, histopathology slide images etc. These algorithms need not be programmed with prewritten explicit rules. They automatically learn on the go, generalizing from patterns hidden in large sets of examples, bypassing traditional clinical methods of heuristics [Fig. 2]. During the learning process, predictions of AI algorithms when compared against the gold standard determined by humans, it is referred to as supervised learning. In unsupervised learning, the algorithm learns without feedback from human input. AI applications in neurology practice are proving to be both disruptive and complementary. Common terminologies used in AI is summarized in Table 1.
Fig. 1.
A diagram depicting traditional rule based learning.
Fig. 2.
A diagram showing the concept of machine learning in artificial intelligence.
Table 1.
Glossary of terminologies used in Artificial intelligence.
| Terminology | Description |
|---|---|
| Machine Learning (ML) | The process by which an algorithm encodes statistical regularities inherent in a database of examples, into parameter weights for future predictions |
| Supervised Learning | Training a machine learning algorithm by means of previously expert labelled training examples. |
| Unsupervised Learning | When machine learning algorithm discovers hidden patterns or data groupings without the need for human intervention. |
| Model | A trained machine learning algorithm, ready to make predictions from unseen data. |
| Training | Feeding a machine learning algorithm with examples from a training dataset so that it can derive useful parameters for future predictions. |
| Artificial Neural Network | A machine learning technique that processes information in an architecture comprising of a large number of layers, each layer extracting desired parameters incrementally from training data. |
| Deep Neural Network (DNN) | A deep learning architecture with multiple layers between input and output layers. |
| Convolutional Neural Network (CNN) | A class of DNN that display connectivity patterns that are analogous to that of the connectivity patterns and image processing in visual cortex. |
| Black Box | Human inability to explain the precise steps leading to the model's predictions, due to complex maze of parameters that is inscrutable to humans. |
AI applications in neurology practice
CT scan/MRI imaging
Stroke
CT imaging forms the bedrock of acute stroke management. Interpretations of emergency CT scans before stroke thrombolysis, and endovascular thrombectomy are plagued by a lack of available expertise and time delays. The emergency radiologist is often overwhelmed by the burden of normal CT scans before the abnormal catch his/her attention. The time delays to reporting intracranial hemorrhage or early signs of infarct often lead to loss of vital neurons before therapeutic interventions can be undertaken. Such delays usually manifest in permanent disability for the patient. For every 15 min saved after acute stroke to achieve a Large Vessel Occlusion (LVO) recanalization, 34 per 1000 treated patients have an improved disability outcome.3
Artificial intelligence can play a role in different aspects of the stroke treatment pathway, including infarct or haemorrhage detection, LVO detection, Alberta Stroke Program Early CT Score (ASPECTS) grading, and prognostication. ASPECTS is an extensively used severity grading score on non-contrast CT scan for assessing the extent of early ischemic stroke. A trained AI Machine Learning (ML) model for quantifying ASPECT scores on CT scans of acute stroke patients achieved a specificity of 91.8% and sensitivity of 66.2%.4 Another AI model for detecting hyperdense vessel sign in Middle Cerebral Artery (MCA), an early sign of stroke, showed 96% specificity in a 223 patient cohort with a mean algorithm run time of 1 min and 30 s.5 An AI system currently in use to detect stroke patterns of LVOs in acute stroke from non-contrast CT scans of the head, automatically alerts a stroke treatment team without human intervention. The alert is sent via a mobile phone application to the emergency room healthcare worker, neurologist, and neuro-interventional radiologist. The AI system has shown to save on average 52 min before emergency stroke intervention could be instituted.6
CT perfusion imaging to identify salvageable brain in acute stroke requires manual interpretation of data that is time-consuming and mandates specialized skill. An AI application RAPID (IschemaView, Menlo Park, CA) automatically calculates ischemic core and hypo-perfused tissue volumes from CT scans and gives information on brain tissue that can be salvaged with prompt intervention. The results are made available to the clinician within minutes. The software has been extensively tested and validated with high sensitivity and specificity.7, 8, 9
Perfusion-weighted MRI (PWI) is used for stroke diagnosis and segmenting the infarct tissue into ischemic core and salvageable penumbra. Huang et al used PWI-derived cerebral blood flow (CBF) and apparent diffusion coefficient (ADC) datasets for identifying salvageable brain tissue and achieved a ROC-AUC of 88%, 94%, and 97% after 30 min, after 60 min, and permanent MCA occlusion, respectively.10
A research version of AI application RAPID (Stanford University and iSchemaView) was used for detecting hypo-perfused but salvageable regions of the brain beyond the traditional window period for thrombolysis (4.5 h). Imaging techniques included CT perfusion imaging or perfusion-diffusion MRI, and images were processed using the research version of RAPID. A total of 225 patients were enrolled, of which 113 patients were randomly assigned to the alteplase group and 112 to the placebo group. The primary outcome (mRS 0–1) occurred in 40 patients (35.4%) in the alteplase group and in 33 patients (29.5%) in the placebo group (adjusted risk ratio, 1.44; 95% confidence interval [CI], 1.01 to 2.06; P = 0.04). This landmark trial made possible with a novel AI software is promising to push the boundaries of intravenous thrombolysis in whom the endovascular intervention is not an option.11
Multiple sclerosis
Multiple sclerosis (MS) has been primarily classified according to clinical symptoms instead of underlying pathological mechanisms. MS is classified into four phenotypes: relapsing-remitting MS (RRMS), clinically isolated syndrome (CIS), primary progressive MS (PPMS) and secondary progressive MS (SPMS).12,13 Disease activity and disability progression that guides the well-established classification cannot, however, predict relapse rate and confirmed disability progression with consistency. These lacunae in the classification have posed clinical dilemmas while commencing treatment and taking decision on its discontinuation.
Unsupervised ML algorithm, a form of AI, has been used to study MRI images from known patients of MS to define new subtypes. The algorithm named Subtype and Staging Inference (SuStaIn) has uncovered data-driven disease subtypes which show distinct temporal progression patterns.14 The newly defined MS subtypes: cortex-led, normal-appearing white matter-led, and lesion-led are characteristically different from the well-established clinical subtypes. Patients with the lesion-led subtype have been postulated to have the highest risk of confirmed disability progression and the highest relapse rate. Lesion-led MS subtype also shows positive treatment response in selected clinical trials. An independent cohort of 3068 patients was used for validation of these new findings.15
Epilepsy
ML techniques have been widely applied in the field of epilepsy for analysing imaging data. An ML algorithm was used in 41 patients with focal cortical dysplasias (FCDs) and matched controls to achieve 98% accuracy in distinguishing histologic subtypes. The ML algorithm showed 92% and 86% accuracy (for type I and II FCDs, respectively) in lateralization of the lesion, and 92% and 82% accuracy (for type I and II FCDs, respectively) in predicting Engel I seizure freedom at an average of 4 years of follow-up.16 Another AI algorithm (artificial neural network), when applied to an MRI dataset of 61 patients with type II FCDs as well as 120 controls from three different epilepsy centres, showed a sensitivity of 73.7% and specificity of 90.0% in detecting solitary FCDs.17
A Support Vector Machine (SVM) algorithm, a form of AI, could accurately distinguish patients with active epilepsy from those in remission (seizure-free for 12 months while not on medications) as well as from controls by examining MRI imaging characteristics (fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity in diffusion tensor imaging data) from 20 paediatric patients and 29 controls.18 ML algorithms have also been used to lateralize refractory temporal lobe epilepsies before epilepsy surgery.19,20
Dementia and neurodegenerative diseases
AI has been shown to improve the diagnostic accuracy of several neurodegenerative diseases, including dementia. ML algorithms have been successfully employed to automatically discriminate Alzheimer disease (AD) from Vascular Dementia (VD), reaching a classification accuracy greater than 84%.21 MRI images have been used to generate nuanced neuroimaging signatures for AD diagnosis. An AI algorithm was trained using the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset (n = 417) and validated on three independent cohorts: the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) (n = 382), the Framingham Heart Study (n = 102), and the National Alzheimer's Coordinating Center (NACC) (n = 582), with a mean area under curve values of 0.996, 0.974, 0.876 and 0.954 respectively. The high-risk cerebral regions predicted by the AI algorithm closely adhered to post-mortem histopathological findings. The AI algorithms' prediction exceeded the diagnostic performance of a multi-institutional team of practising neurologists (n = 11).22
At present, the diagnosis of Parkinson Disease (PD) is highly dependent on clinical presentation. Dopamine transporter positron emitted topography employed in its diagnosis is not cost-effective and cannot be routinely employed. Neuromelanin sensitive magnetic resonance imaging (NMS-MRI) has shown abnormalities in the substantia nigra pars compacta (SNc) in Parkinson's disease (PD). An AI algorithm (Convolutional Neural Network) has been used for diagnosing PD with high testing accuracy (80%), using the neuromelanin signal in MRI imaging data. The algorithm also discriminates PD from atypical parkinsonian syndromes (85.7% test accuracy) while locating the most discriminative regions on the neuromelanin contrast images.23
Electrophysiology
Seizures
EEG electrodes facilitate the recording of non-overlapping band signals such as delta, theta, alpha, and beta frequencies allowing interrogation of various disease and health states. An AI application for seizure detection using scalp EEG showed a sensitivity of 93% and specificity of 94%.24 Another AI algorithm (ML) uses intracranial electrode recordings for real-time detection of seizures.25 Electric activity of seizure spreads abruptly, simultaneously propagating over large cortical areas making the determination of epileptogenic zone difficult. Dian et al employed intracranial EEG from 6 patients undergoing resection surgery to train an AI algorithm to detect epileptogenic zone. The system used high frequency and low-frequency oscillations in the recordings for seizure detection, correctly identifying zones for resection from a patient.26 Another area where AI is playing a role is in predicting seizures in patients well before ictus. A novel AI (deep learning algorithm) using long-term scalp EEG data for patient-specific epileptic seizure prediction has shown an accuracy of 99.6%, a sensitivity of 99.72%, a specificity of 99.60%, a false alarm rate of 0.004 per hour and a prediction time of 1 h prior to the seizure onset.27
Coma
AI algorithms have been used in the diagnosis and prognosis of coma. Supervised learning algorithms (AI) for discovering evidence of covert consciousness in unresponsive individuals following an acute brain injury detected that up to 15% had detectable brain activity in response to verbal commands.28 Another AI algorithm (deep-learning artificial neural network) predicted six-month functional outcome from EEG data in comatose patients 12 h following cardiac arrest. It predicted a 6-month functional outcome as good (48% accuracy and 0% false-positive rate) versus bad (58% accuracy and 5% false-positive rate).29
Retinal and fundus scans
An ML algorithm to detect papilledema was trained on 14,341 fundus photographs using a retrospective dataset and externally tested the model with 1505 fundus photographs. The algorithm showed an AUC for the detection of papilledema of 0.96 (95% CI, 0.95 to 0.97), a sensitivity of 96.4% (95% CI, 93.9 to 98.3), and a specificity of 84.7% (95% CI, 82.3 to 87.1) in the external testing dataset.30,31 Another AI algorithm to detect diabetic retinopathy (DR), trained on 30,000 expert labelled images from 3 retrospective datasets (DiaRetDB1, Kaggle (EyePACS) and Australian Tele-eye care DR), was externally tested using a prospective dataset obtained over six months. The system tested on the 193 patients in primary care practice judged 17 as having diabetic retinopathy of sufficient severity to require referral. The resulting specificity was 92% (95% CI, 87%–96%), which compared favourably with the gold standard of ophthalmologist evaluation.32
Limitations
AI algorithms are good at learning specific tasks that vastly differ from the sponge-like learning displayed by humans.2 For e.g., an algorithm trained to detect papilledema from fundus photographs will fail miserably when tasked to detect optic atrophy instead of papilledema. Given an abundance of time and information, the expert clinician should be able to deliver comparable predictions to AI, a useful benchmark while evaluating the performance of AI tools. Performance metrics of AI that seem too good to be true may be just that. The outcomes of standalone in silico experiments with outstanding performance metrics often contrast with the messy world of usual clinical practice. Does the AI tool make a dent in patient outcomes? Or will it become a fancy tool when the novelty wears off? Answers may be found through prospective randomized controlled trials in clinical settings, by comparing the output of AI and clinicians. AI applications bank heavily on the integrity of enormous training data and is prone to human bias during the training process.
The more complex the AI tool, the more inscrutable is its inner workings. The Black Box problem in AI refers to human inability in explaining the precise steps leading to the AI tools’ predictions.33 Performance metrics should be viewed as an entity delinked from patient safety. Healthy individuals misclassified with erroneous diagnosis may become a burden on healthcare resources with unnecessary investigations, while a diseased individual misclassified as healthy may result in dire consequences. Fixing accountability for erroneous decisions flowing from AI and the potential ethical-legal ramifications are subjects of active debate. Due caution should be exercised while deploying the AI tools at scale.1 Food and Drug Administration (FDA) has given stringent benchmarks for approval of AI applications for clinical use.
AI applications approved by FDA
FDA approvals for AI/ML-based medical technologies fall into three categories viz de novo pathway, premarket approval and 510(k) clearance. De novo classification is used to classify those novel medical devices for which there are no legally marketed counterparts, but which offer adequate safety and effectiveness with general controls. The FDA performs a risk-based assessment of the device in question before approval and allowing the device to be marketed. Premarket approval is issued to algorithms that can have a large impact on human health and as such, their evaluation undergoes more thorough scientific and regulatory processes to determine their safety and effectiveness. In order to approve an application, the FDA determines that the device's safety and effectiveness is supported by satisfactory scientific evidence. Upon approval, the applicant can proceed with marketing the product. A 510(k) clearance for an algorithm is granted by FDA when it has been shown to be at least as safe and effective as another similar, legally marketed algorithm. The submitter seeking this clearance must provide substantial proof of equivalence in their application. Without an approval of being substantially equivalent to the other algorithm, the one pending approval cannot be legally marketed.34
EnsoSleep (EnsoData, Inc.), an AI application, was approved by FDA via 510(k) premarket notification (approval number K162627) in 2017 for diagnosis of sleep disorders. The tool analyses recorded physiological signals and automatically scores sleep study results, including the staging of sleep, detection of arousals, leg movements, and sleep disordered breathing events including obstructive apneas.34
IDx (IDx LLC.) was approved via de novo pathway (FDA approval number, DEN180001) for detection of diabetic retinopathy. IDx, is a an AI algorithm to analyze images of the eye taken with a retinal camera called the Topcon NW400. A doctor uploads the digital images of the patient's retinas to a cloud server on which IDx is installed. If the images are of sufficient quality, the software provides the doctor with one of two results: (1) “more than mild diabetic retinopathy detected: refer to an eye care professional” or (2) “negative for more than mild diabetic retinopathy; rescreen in 12 months.”34
ContaCT (Viz.AI.) was approved in 2018 via de novo pathway (FDA approval number, DEN170073) for stroke detection on CT scan. The device analyses CT angiogram images of the brain acquired in the acute setting, and sends notifications to a neurovascular specialist that a suspected large vessel occlusion has been identified and recommends review of those images. Images can be previewed through a mobile application.34 Rapid LVO (iSchemaView, Inc.) was approved in 2020 via 510(k) premarket notification (FDA approval number, K200941). Rapid LVO gives results in under 3 min by using a vessel tracker in conjunction with assessment of brain regions with reduced blood vessel density on CT scans, to identify suspected LVOs, with a sensitivity of 97% and a specificity of 96%. Since stroke arising out of LVOs are amenable to emergency endovascular interventions, stroke team members are immediately notified when a suspected LVO is detected.34
Icobrain (icometrix NV) approved via 510(k) premarket notification for MRI brain interpretation (FDA approval number, K181939) is intended for use of automatic labelling, visualisation, and volumetric quantification of segmentable brain structures from MRI images.34 VBrain (Vysioneer Inc.) approved in 2021 uses an AI algorithm (deep neural networks) to contour (segment) brain tumour on MRI images during radiation therapy treatment planning.
Future applications
The AI pioneers of the 1950s had envisaged a future with machines that could sense, reason and think like people (general AI), a concept that is likely to remain in the realms of science fiction in the foreseeable future. Clinicians and clinical methods are likely to remain relevant in the future even as AI applications bring new set of tools to complement traditional clinical methods for triage, diagnosis, treatment decisions and prognosis. AI tools may find use in seizure classification, for e.g., differentiating epileptic seizures from non-epileptic seizures or classifying the phenotype of complex genetic epilepsies. Novel algorithms may detect early signatures of disease in large population-based datasets, aid in quick and meaningful interpretations of whole genome or exome sequencing.1 AI algorithms are likely to be employed in syndromic diagnosis of rare genetic disorders, neurocutaneous syndromic diagnosis and arriving at a pathological diagnosis from biopsy specimens. AI tools of future may also assist with predicting response to therapeutics.
AI has the potential to turn the drug discovery paradigm upside down by using patient-driven data from sources such as research papers, patents, clinical trials and patient records, rather than the traditional trial-and-error approach. AI's potential is being harnessed in an attempt to treat amyotrophic lateral sclerosis (ALS), a hitherto untreatable and fatal disease. A London-based start-up firm BenevolentBio, has an AI platform that has flagged around 100 existing compounds with potential for treatment for ALS. From these, scientists selected five compounds to undergo tests using patient-derived cells at the Sheffield Institute of Translational Neuroscience, UK. The research, presented at the International Symposium on ALS/MND in Boston, Massachusetts, in December 2017, found that four of these compounds had promise, and one was shown to delay neurological symptoms in mice.35
Conclusion
AI tools would be worth their salt if they can make a dent in clinical outcomes. Otherwise, it will become another fancy instrument that will quickly fade into obsolescence. AI technologies will have to prove their mettle in prospective randomized control trials in clinical settings that pit AI against humans. Considering the current advances in AI, the clinical practice would likely change to accommodate AI into its workflow as a complementary tool. The challenge to adopting these new tools in the clinical practice are due to hindrances, posed by regulatory frameworks and trust issues with new technologies, from both the physicians and patients alike. To begin with clinician's perception of AI should change from nemesis to opportunity. In change lies the essence of life, and in adaptability to the change, survival.
Disclosure of competing interest
The authors have none to declare.
Acknowledgments
V.Y.V. is supported by a Medical Research Council (UK) strategic award to establish an International Centre for Genomic Medicine in Neuromuscular Diseases (MR/S005021/1).
References
- 1.Auger S.D., Jacobs B.M., Dobson R. Big data, machine learning and artificial intelligence: a neurologist's guide. Practical Neurol. 2021;21:4–11. doi: 10.1136/practneurol-2020-002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vishnu V.Y., Vinny P.W. The neurologist and artificial intelligence: Titans at crossroads. Ann Indian Acad Neurol. Jul-Sep 2019;22(3):264–266. doi: 10.4103/aian.AIAN_493_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheth S.A., Jahan R., Gralla J. Time to endovascular reperfusion and degree of disability in acute stroke. Ann Neurol. 2015;78(4):584–593. doi: 10.1002/ana.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuang H., Najm M., Chakraborty D. Automated aspects on noncontrast CT scans in patients with acute ischemic stroke using ma- chine learning. Am J Neuroradiol. 2019;40:33–38. doi: 10.3174/ajnr.A5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung C. Abstract WP76: automated detection of hyperdense MCA sign and auto- mated notification of large vessel occlusion using artificial intelligence. https://insights.ovid.com/stroke/stro/2019/02/001/abstract-wp76-automated-detection- hyperdense-mca/372/00007670.
- 6.Barreira C.M., Bouslama M., Haussen D.C. Abstract wP61: automated large artery occlusion detection iN stroke imaging - ALADiN study. Stroke. 2018;49:AwP61. [Google Scholar]
- 7.Campbell B.C., Mitchell P.J., Kleinig T.J. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira R.G., Jadhav A.P., Haussen D.C. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 9.Albers G.W., Lansberg M.G., Kemp S. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3) Int J Stroke. 2017;12:896–905. doi: 10.1177/1747493017701147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S., Shen Q., Duong T.Q. Quantitative prediction of acute ischemic tissue fate using support vector machine. Brain Res. 2011;1405:77–84. doi: 10.1016/j.brainres.2011.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H., Campbell B.C.V., Parsons M.W. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–1803. doi: 10.1056/NEJMoa1813046. [DOI] [PubMed] [Google Scholar]
- 12.Thompson A.J., Baranzini S.E., Geurts J., Hemmer B., Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622–1636. doi: 10.1016/S0140-6736(18)30481-1. [DOI] [PubMed] [Google Scholar]
- 13.Lublin F.D., Reingold S.C., Cohen J.A. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young A.L., Marinescu R.V., Oxtoby N.P. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun. 2018 Oct 15;9(1):4273. doi: 10.1038/s41467-018-05892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eshaghi A., Young A.L., Wijeratne P.A. Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat Commun. 2021;12:2078. doi: 10.1038/s41467-021-22265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong S.-J., Bernhardt B.C., Schrader D.S., Bernasconi N., Bernasconi A. Whole-brain MRI phenotyping in dysplasia-related frontal lobe epilepsy. Neurology. 2016;86:643–650. doi: 10.1212/WNL.0000000000002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin B., Krishnan B., Adler S. Automated detection of focal cortical dysplasia type II with sur- face-based magnetic resonance imaging postprocessing and ma- chine learning. Epilepsia. 2018;59:982–992. doi: 10.1111/epi.14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amarreh I., Meyerand M.E., Stafstrom C., Hermann B.P., Birn R.M. Individual classification of children with epilepsy using support vector machine with multiple indices of diffusion tensor imaging. Neuroimage Clin. 2014;4:757–764. doi: 10.1016/j.nicl.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakken I.J., Axelson D., Kvistad K.A. Applications of neural network analyses to in vivo 1H magnetic resonance spectroscopy of epilepsy patients. Epilepsy Res. 1999;35:245–252. doi: 10.1016/s0920-1211(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.S., Lee D.S., Kim S.K. Localization of epileptogenic zones in F-18 FDG brain PET of patients with temporal lobe epilepsy using artificial neural net- work. IEEE Trans Med Imaging. 2000;19:347–355. doi: 10.1109/42.848185. [DOI] [PubMed] [Google Scholar]
- 21.Castellazzi G., Cuzzoni M.G., Cotta Ramusino M. A machine learning approach for the differential diagnosis of Alzheimer and vascular dementia fed by MRI selected features. Front Neuroinform. 2020;14:25. doi: 10.3389/fninf.2020.00025. Published 2020 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu Shangran, Joshi Prajakta S., Miller Matthew I. Development and validation of an interpretable deep learning framework for Alzheimer's disease classification. Brain. June 2020;143(6):1920–1933. doi: 10.1093/brain/awaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinde Sumeet, Prasad Shweta, Saboo Yash. Predictive markers for Parkinson's disease using deep neural nets on neuromelanin sensitive MRI. NeuroImage: Clinical. 2019;22 doi: 10.1016/j.nicl.2019.101748. 101748, ISSN 2213-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fergus P., Hignett D., Hussain A., Al-Jumeily D., Abdel-Aziz D. Automatic epileptic seizure detection using scalp EEG and advanced artificial intelligence techniques. BioMed Res Int. 2015;2015:986736. doi: 10.1155/2015/986736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharbouch A., Shoeb A., Guttag J. An algorithm for seizure onset detection using intracranial EEG. Epilepsy Behav. 2011;22:S29–S35. doi: 10.1016/j.yebeh.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dian J.A., Colic S., Chinvarun Y., Carlen P.L., Bardakjian B.L. Identification of brain regions of interest for epilepsy surgery planning using support vector machines. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:6590–6593. doi: 10.1109/EMBC.2015.7319903. [DOI] [PubMed] [Google Scholar]
- 27.Daoud H., Bayoumi M.A. Efficient epileptic seizure prediction based on deep learning. IEEE Trans Biomed Circuits Syst. 2019;13:804–813. doi: 10.1109/TBCAS.2019.2929053. [DOI] [PubMed] [Google Scholar]
- 28.Claassen J., Doyle K., Matory A. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380:2497–2505. doi: 10.1056/NEJMoa1812757. [DOI] [PubMed] [Google Scholar]
- 29.Tjepkema-Cloostermans M.C., da Silva Lourenço C., Ruijter B.J. Outcome prediction in postanoxic coma with deep learning∗. Crit Care Med. 2019;47:1424–1432. doi: 10.1097/CCM.0000000000003854. [DOI] [PubMed] [Google Scholar]
- 30.Kohane I. AI for the eye - automated assistance for clinicians screening for papilledema. N Engl J Med. 2020 Apr 30;382(18):1760–1761. doi: 10.1056/NEJMe2004551. [DOI] [PubMed] [Google Scholar]
- 31.Milea D., Najjar R.P., Zhubo J. Artificial intelligence to detect papilledema from ocular fundus photographs. N Engl J Med. 2020 doi: 10.1056/NEJMoa1917130. [DOI] [PubMed] [Google Scholar]
- 32.Kanagasingam Y., Xiao D., Vignarajan J., Preetham A., Tay-Kearney M., Mehrotra A. Evaluation of artificial intelligence-based grading of diabetic retinopathy in primary care. JAMA Netw Open. 2018 Sep 7;1(5) doi: 10.1001/jamanetworkopen.2018.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castelvecchi D. Can we open the black box of AI? Nature. 2016 Oct 6;538(7623):20–23. doi: 10.1038/538020a. [DOI] [PubMed] [Google Scholar]
- 34.Benjamens S., Dhunnoo P., Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. npj Digit Med. 2020;3:118. doi: 10.1038/s41746-020-00324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stopford M., Myszczynska M., Markus N. C29 Harnessing Machine Learning and artificial intelligence to identify novel ALS therapeutics. Sessions 1 - 11. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 2):1–73. doi: 10.1080/21678421.2017.1368577. [DOI] [Google Scholar]