Abstract
Hereditary angioedema (HAE) is a rare disease that causes episodic attacks of subcutaneous and submucosal edema, which can be painful, incapacitating, and potentially fatal. These attacks are mediated by excessive bradykinin production, as a result of uncontrolled activation of the plasma kallikrein/kinin system, which is caused by a C1 esterase inhibitor deficiency or dysfunction in HAE types 1 and 2, respectively. For many years, treatment options were limited to therapies with substantial adverse effects, insufficient efficacy, or difficult routes of administration. Increased insights in the pathophysiology of HAE have paved the way for the development of new therapies with fewer side effects. In the last two decades, several targeted novel therapeutic strategies for HAE have been developed, for both long-term prophylaxis and on demand treatment of acute attacks. This article reviews the advances in the development of more effective and convenient treatment options for HAE and their anticipated effects on morbidity, mortality, and quality of life. The emergence of these improved treatment options will presumably change current HAE guidelines, but adherence to these recommendations may become restricted by high treatment costs. It will therefore be essential to determine the indications and identify the patients that will benefit most from these newest treatment generations. Ultimately, current preclinical research into gene therapies may eventually lead the way towards curative treatment options for HAE. In conclusion, an increasing shift towards the use of highly effective long-term prophylaxis is anticipated, which should drastically abate the burden on patients with hereditary angioedema.
Keywords: Hereditary angioedema, C1-inhibitor , Bradykinin, Contact activation system, Kallikrein/kinin system, Serine protease
Introduction
Hereditary angioedema (HAE) is a rare, disabling disorder, with symptoms ranging from disfiguring and incapacitating peripheral swellings, painful abdominal episodes, to potentially fatal laryngeal or oropharyngeal edema [1]. Before the availability of medication and adequate airway support, HAE-related mortality was estimated to be as high as 33 to 40% as a result of asphyxiation [2, 3]. For many years, treatment options were limited to fresh frozen plasma (FFP) infusions, antifibrinolytics, progestins, and attenuated androgens (AA). In the past decade various new targeted HAE therapeutics have been developed, with an emphasis on safety, efficacy, and easier routes of administration (including enhanced options for self-administration). Following a brief overview of the pathophysiology of HAE, this paper will review all currently available therapies and new treatment approaches in development for HAE with C1 esterase inhibitor (C1-INH) deficiency.
Pathophysiology
HAE is an autosomal dominant inherited disorder. Mutations in SERPING1, the gene that encodes the serine protease inhibitor (SERPIN) C1-INH, account for HAE with C1-INH deficiency (C1-INH-HAE). The two types of C1-INH-HAE, type 1 and type 2, are clinically indistinguishable but are caused by different mutations in the SERPING1 gene. In C1-INH-HAE type 1, the mutations result in truncated or misfolded proteins that are incompletely secreted, leading to decreased plasma levels of C1-INH which are less than 50%, generally 5 to 30%, of normal plasma levels. Mutations causing C1-INH-HAE type 2 are located at exon 8 or near the active site, leading to a fully secreted, but dysfunctional C1-INH protein. Hence, C1-INH functional levels in plasma are decreased in both types, whereas C1-INH antigenic levels are only decreased in C1-INH-HAE type 1 [4]. Other, even more rare, types of HAE are not associated with C1-INH deficiency (HAE with normal C1-INH; nC1-INH-HAE, formerly referred to as HAE type 3) and include those associated with mutations in the genes expressing factor XII [5], plasminogen [6], angiopoietin-1 [7], kininogen-1 [8], and myoferlin [9]. In one or more remaining types of nC1-INH-HAE, the genetic cause is still unknown. This review will focus on C1-INH-HAE types 1 and 2 as the clinical trials for new HAE therapies are predominantly focusing on these specific types.
C1-INH is a heavily glycosylated single chain SERPIN of 478 amino acid residues. It is the main inhibitor of various complement proteases (C1r, C1s, and mannose-binding lectin-associated serine protease; MASP 1 and 2), and contact system proteases (activated factor XII; FXIIa and plasma kallikrein; PKa). In addition, C1-INH is an efficient inhibitor of the intrinsic coagulation pathway (inhibition of activated factors XII and XI) and the fibrinolytic pathway (inhibition of plasmin). If C1-INH levels are decreased or the protein is defective, the kallikrein/kinin system (KKS) is inadequately controlled, leading to excessive release of the nonapeptide bradykinin (BK) which is the predominant mediator of enhanced vascular permeability in angioedema attacks [10, 11].
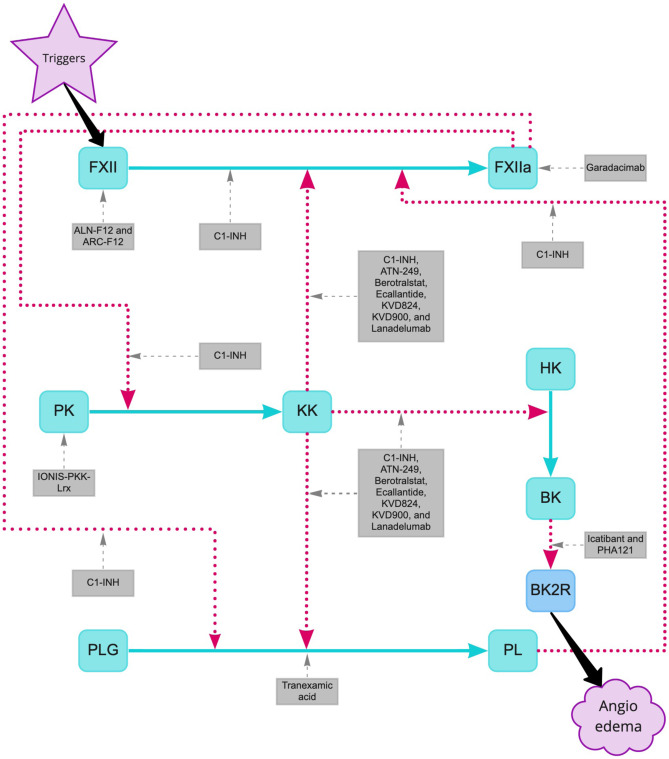
During attacks of C1-INH-HAE, activation of the contact system is initiated by autoactivation of factor XII (FXII), resulting in FXIIa. If uncontrolled by C1-INH FXIIa can activate prekallikrein (PK) to the proteolytic enzyme PKa, which in turn cleaves FXII to produce more FXIIa (see Fig. 1) In addition, the activated PKa liberates BK from high-molecular-weight kininogen (HK) [12, 13]. Once BK ligates the BK B2 receptor (BKRB2) which is constitutively expressed on endothelial cells, nitric oxide is produced leading to simultaneous smooth muscle cell relaxation and transmembrane vascular endothelial cadherin molecule degradation, causing vascular leakage between the endothelial cells [14, 15]. Moreover, FXIIa, PKa, and BK can activate plasmin from its zymogen plasminogen, thereby activating the fibrinolytic pathway. Plasmin is pivotal for the degradation of cross-linked fibrin. Therefore, plasmin activation results in increased fibrin degradation products including D-dimers that may be increased even in asymptomatic HAE patients. Indeed, D-dimer levels further increase during acute attacks [16]. Plasmin also stimulates the activation of FXII via positive feedback [17]. FXIIa in turn can activate factor XI, thereby initiating the intrinsic coagulation cascade. Nevertheless, HAE is not associated with an increased thrombotic risk.
Fig. 1.
Pathways inhibited by current HAE drugs and developmental treatment options. Activation of the contact system is initiated by activation of Factor XII (FXII), resulting in activated Factor XII (FXIIa). FXIIa can activate prekallikrein (PK) to plasma kallikrein (PKa), which in turn can activate FXII to produce more FXIIa. In addition, PKa cleaves bradykinin (BK) from high-molecular-weight kininogen (HK). Finally, BK ligates the bradykinin B2 receptor (BKRB2). Additionally, FXIIa and PKa can activate plasmin (PL) from plasminogen (PLG). PL further stimulates the activation of FXII. Activation is indicated by red big-dotted arrows and inhibition by HAE drugs by gray small-dotted arrows. C1-INH denotes C1 esterase inhibitor
Historical and Current Prophylactic Treatment
Treatment of HAE can be divided into two strategies: (1) treatment of acute attacks (on demand therapy; ODT) and (2) prevention of attacks, which includes short-term prophylaxis (STP) and long-term prophylaxis (LTP). It is notable that in the past the decision to initiate LTP used to be solely based on attack frequency, whereas it is nowadays very well recognized that not only burden of disease but also patient preferences should be taken into account [18]. STP is indicated before all procedures that can induce an attack, such as (dental) surgery, endotracheal intubation and endoscopy.
In contrast to angioedema with urticaria, which is predominantly histamine-mediated, antihistamines, corticosteroids, and epinephrine are ineffective in HAE [19]. For LTP, progestins were occasionally used to inhibit the effects of estrogens which may increase FXII, PKa, and HK levels, resulting in (over) consumption of C1-INH [20]. Progestins were reported to have a positive effect on the frequency of angioedema attacks in women with HAE. In most women they are well tolerated, but progestins carry the potential to cause breakthrough vaginal bleeding, pelvic discomfort, and mastalgia [21].
Until the first administration of intravenous plasma-derived C1-INH concentrate in 1979, and its Food and Drug Administration (FDA) approval in 2008, the only recommended options for LTP were attenuated androgens (AA) and antifibrinolytics [22, 23].
AA, such as danazol, oxandrolone, and stanozolol, are administered orally. Their mode of action is through induction of C1-INH synthesis in hepatocytes [24] and increased expression of C1-INH mRNA in peripheral blood mononuclear cells [25]. Furthermore, AA induces aminopeptidase P activity, which catabolizes BK [26]. Although AA are effective in a considerable proportion of HAE patients, their long-term use is limited due to a number of potential adverse effects [27]. AA are contraindicated in children and pregnant women (because of their virilising effects), as well as in patients with liver diseases, breast cancer, and prostate cancer [28]. In addition, AA use has been linked to increased cardiovascular risk and hepatocellular carcinoma. To minimize adverse effects, the optimal dose for danazol is ≤ 200 mg per day. AA has been recommended for STP in the past, but C1-INH concentrates are currently considered the pre-procedural prophylaxis of choice. Very frequent use of AA for STP may lead to similar side effects as seen with long-term use. Nevertheless, STP with AA is considered to be safe, even in children. AA are used for five days before until two to three days after the procedure [18] (see Table 1).
Table 1.
Current treatment options for hereditary angioedema with C1-inhibitor deficiency
| Drug (trade name) | Manufacturer | Mechanism of action | Indication | Administration | Age indications |
|---|---|---|---|---|---|
| Attenuated androgens: danazol, oxandrolone, and stanozolol | Generic manufacturers | AA induce aminopeptidase P activity and increase C1-INH synthesis and C1-INH mRNA expression | LTP, STP | Oral | ≥18 years |
| Berotralstat (Orladeyo®) | BioCryst Pharmaceuticals | Kallikrein inhibitor | LTP | Oral | FDA:≥12 years, under review for EMA approval |
| Plasma-derived C1-INH (Berinert®) | CSL Behring | Plasma-derived C1-INH concentrate | ODT, STP | Intravenous | All |
| Plasma-derived C1-INH (Cinryze®) | Takeda | Plasma-derived C1-INH concentrate | LTP, ODT, STP | Intravenous | LTP ≥6 years, ODT and STP ≥2 years |
| Conestat alfa (Ruconest®) | Pharming Group NV | Recombinant C1-INH concentrate | ODT | Intravenous | FDA: adolescents and adults EMA: ≥2 years |
| Ecallantide (Kalbitor®) | Takeda | Kallikrein inhibitor | ODT | Subcutaneous, no self-administration | ≥12 years |
| Plasma-derived C1-INH (Haegarda®) | CSL Behring | Plasma-derived C1-INH concentrate | LTP | Subcutaneous | ≥12 years |
| Icatibant (Firazyr®) | Takeda | BKRB2 antagonist | ODT | Subcutaneous |
FDA: ≥18 years EMA: ≥2 years |
| Lanadelumab (Takhzyro®) | Takeda | Kallikrein inhibitor | LTP | Subcutaneous | ≥12 years |
| Tranexamic acid (Cyklokapron®) | Generic manufacturers | Competitive inhibitor of plasminogen-mediated FXII activation | LTP | Oral | Adolescents and adults |
AA attenuated androgens, BKRB2 bradykinin receptor B2, C1-INH C1 esterase inhibitor, LTP long-term prophylaxis, ODT on demand treatment, STP short-term prophylaxis
Tranexamic acid is an orally available antifibrinolytic agent. The recommended dosage is 30–50 mg/kg daily, divided in two or three doses to a maximum of six grams per day. This lysine analogue holds a wide range of SERPIN activity, amongst which is the competitive inhibition of plasminogen. Thus, the activation of plasminogen into plasmin is reduced, thereby preventing plasminogen-mediated amplification of FXII activation. In most C1-INH-HAE patients, treatment with tranexamic acid is not sufficiently effective. Current international guidelines on HAE do recommend neither AA nor antifibrinolytics as a first-line therapy [29].
C1-INH concentrates supplement the deficient or replace the dysfunctional C1-INH in HAE patients, thus enabling inhibition of FXIIa and PKa in order to prevent excessive BK production. Plasma-derived C1-INH concentrates (Cinryze® and Berinert®) are an effective option for both LTP and STP [30–32]. The recommended dosages for STP with plasma-derived C1-INH concentrates are 1000 IU or 20 IU/kg, whereas the recommended LTP dose for Cinryze® is 1000 IU per 3–4 days. Berinert® has not been licensed for LTP. Although intravenous access is required for administration, many patients can be trained for self-administration which was found to be safe and highly effective [33]. It is of note however that long-term venous access may become complicated. Indwelling central catheters have been inserted in a subgroup of these patients, thereby risking serious complications such as thrombotic events and infections [34].
Haegarda® is a new generation of plasma-derived C1-INH concentrate that is administered subcutaneously. It was licensed in 2017 by the FDA for LTP in adolescents and adults. Clinical efficacy was demonstrated in the phase 3 Clinical Studies for Optimal Management in Preventing Angioedema with low-volume subcutaneous C1-inhibitor replacement Therapy (COMPACT) trial, in which a total of 83% of patients receiving the recommended dose of self-administered 60 IU/kg twice weekly were free of attacks in the long-term extension arm [35]. Moreover, this treatment improved HAE-related quality of life (QoL) [36].
Lanadelumab (Takhzyro®) is another subcutaneously administered drug that has been licensed for LTP in patients aged 12 years and older. This fully human IgG1 monoclonal antibody directed against PKa was approved by the FDA in 2018. In the phase 3 Hereditary Angioedema Long-term Prophylaxis (HELP) study, the average breakthrough attack rate was significantly lower in the groups that received 300 mg every 2 weeks and every 4 weeks as compared with placebo (respectively 0.26 and 0.53 versus 1.97 attacks per month) [37]. In addition, 66.7% of patients receiving 300 mg fortnightly reported a reduction of ≥ 90% of angioedema attacks during the treatment course of 26 weeks. Achievement of the minimal clinically important difference in total QoL-score was reported across all treatment arms (including 37% receiving placebo), with the greatest improvement (81%) shown in the 300 mg every 2 weeks arm which is the currently recommended dose. A tentative prolongation of the dosing interval to once every 4 weeks can be considered once clinical remission is achieved.
The most recent addition to the arsenal of LTP is the oral PKa inhibitor berotralstat (Orladeyo®, formerly BCX7353), approved for HAE patients aged 12 years and older by the FDA. The Efficacy and Safety Study of BCX7353 as an Oral Treatment for the Prevention of Attacks in HAE (APeX-2) study, a phase 3, randomized, double-blind, placebo-controlled study showed a 44% reduction in attack rate compared with placebo [38]. Notably, gastrointestinal side effects occurred frequently across all three study arms (42% in the group that received 110 mg, 50% in the 150 mg arm, and up to 36% in the placebo group) [39]. An open-label long-term safety study assessing the dose of 125 mg once daily is currently being conducted and an application for EMA approval has been submitted [40].
Historical and Current on Demand (acute) Treatment
Historically, HAE patients were treated with high volumes of fresh-frozen plasma (FFP) for acute attacks, because FFP are easily available and assumed to contain some amount of functional C1-INH from the donor’s plasma [41, 42]. Although its benefit has never been objectively established in randomized controlled trials, FFP might be effective provided that large volumes are used. However, FFP bears the risks of transmission of blood borne pathogens and could theoretically exacerbate an HAE attack or escalate its severity, due to plasma kininogens that may provide substrate for additional BK release [43]. Therefore, FFP is only recommended for life-threatening attacks when other ODT options are lacking [29].
Plasma-derived C1-INH concentrates (Cinryze® and Berinert®) are effective and recommended options for ODT, using 1000 IU of Cinryze® or 20 IU/kg of Berinert® in children and adults. Although the use of other plasma-derived products (e.g., clotting factor concentrates) has led to the transmission of blood borne viral infections such as human immunodeficiency virus and hepatitis C, the transmission risk has almost completely abated for C1-INH concentrates since the application of nanofiltration. This technique prevents enveloped and non-enveloped viruses and likely even prions from contaminating these plasma products.
In 2017, Shire Pharmaceuticals reported a supply shortage of Cinryze® in many European Union countries and the United States of America (USA) as the demand had exceeded the production capacity. Such shortages can be circumvented with the use of recombinant C1-INH concentrate conestat alfa (Ruconest®) which does not depend on plasma donors and can be produced in large quantities. Conestat alfa is isolated from the milk of transgenic rabbits harbouring genomic human C1-INH sequences. Its glycosylation pattern differs from plasma-derived C1-INH concentrate, resulting in a lower half-life, due to glycosylation-dependent clearance of C1-INH concentrate in the liver [44]. Currently, conestat alfa is only licensed for ODT, dosed at 50 IU/kg in patients < 84 kg or 4200 IU in patients with a body weight of ≥ 84 kg. Despite its lower half-life, conestat alfa has also shown efficacy for STP [45], as well as for LTP [46]. These findings suggest that the treatment effect does not solely depend on plasma concentrations of C1-INH. Theoretically, recombinant C1-INH may be bound to endothelial cells and still be biologically active though undetectable in plasma.
To date, ecallantide (Kalbitor®, formerly DX-88) is the only direct PKa inhibitor available for ODT in patients aged 12 years and older (USA only), with a recommended subcutaneous dosage of 30 mg [47]. In the Evaluation of DX-88′s Effects in Mitigating Angioedema (EDEMA) 4 trial, a phase 3, double-blind, placebo-controlled randomized trial, ecallantide showed a significant reduction in mean symptom complex severity score and was associated with a significantly larger mean treatment outcome score compared with placebo [48]. Since anaphylaxis was reported in 3.5% of patients, ecallantide contains a black box warning in the USA and may only be administered by a healthcare professional in a setting with adequate medical support [49, 50].
More downstream inhibition of the KKS and the effects of BK on the vessel wall can be achieved with icatibant (Firazyr®), a synthetic decapeptide which acts as a competitive selective antagonist of the BK2 receptor. The For Angioedema Subcutaneous Treatment (FAST)-3 trial, a randomized, double-blind, placebo-controlled phase 3 study demonstrated a significantly shorter time to onset of symptom relief with icatibant (2.0 h) than with placebo (19.8; P < 0.0001) [51]. An important observation in further observational studies was the faster resolution of an angioedema attack when icatibant was administered early compared with late treatment [52]. Icatibant can be self-administered subcutaneously at a 30-mg dose and is licensed for ODT in patients aged 2 years and older by the European Medicines Agency (EMA), although the FDA approved icatibant only in adults.
Future HAE Treatment Strategies
As long as absolute curation of HAE remains impossible, the development of newer drugs is focusing on increased efficacy, tolerability, and enhanced ease of administration. The newest LTP HAE drugs currently under development are highly specific (targeting a single protein of the KKS or contact system) and are either administered less frequent (once monthly or less) or orally. Likewise, some of the newest ODT drugs in clinical development are administered orally.
Several LTP therapies currently in development are specifically targeting FXII(a), the initiator of the BK-producing pathway. Garadacimab® (formerly CSL312) is the first fully human monoclonal antibody directed against FXIIa. The first clinical trial was initiated in 2017 and analysis of the first randomization period of the phase 2 trial showed an almost 99% reduction in breakthrough attacks compared with placebo, with a favourable safety profile [53] (see Table 2). Another mode of FXII inhibition is through small interfering RNA (siRNA), a sophisticated technique in which intracellular translation of FXII mRNA into the FXII protein is prevented by ~ 20–30 nucleotide RNA molecules. Two siRNAs are in preclinical development: ALN-F12® and ARC-F12® [54, 55]. Treatment with siRNAs seems promising, as the very long half-lives allow dosing as infrequent as twice annually [56].
Table 2.
Developmental treatments for hereditary angioedema with C1-inhibitor deficiency
| Drug (trade name) | Manufacturer | Mechanism of action | Planned indication | Administration | Regulatory status |
|---|---|---|---|---|---|
| ALN-F12® | Alnylam Pharmaceuticals | RNA interference targeted at FXII | LTP | Subcutaneous | Preclinical development |
| ARC-F12® | Arrowhead Pharmaceuticals | RNA interference targeted at FXII | LTP | Subcutaneous | Preclinical development |
| ATN-249® | Attune Pharmaceuticals | Kallikrein inhibitor | LTP | Oral | Phase 1 trial is complete |
| Berotralstat (BCX7353) | BioCryst Pharmaceuticals | Kallikrein inhibitor | ODT | Oral | Phase 2 trial is complete |
| BMN 331® | BioMarin | Adeno-associated virus-mediated antibody delivery gene therapy | LTP | Intravenous | Preclinical development |
| Conestat alfa (Ruconest®) | Pharming Group NV | Recombinant C1-INH concentrate | LTP | Intravenous | Phase 2 trial is complete |
| Garadacimab® | CSL Behring | Humanised anti-FXIIa monoclonal antibody | LTP | Subcutaneous | Phase 2 trial is recruiting |
| IONIS-PKK-LRx® | IONIS Pharmaceuticals | Antisense oligonucleotide targeted at prekallikrein | LTP | Subcutaneous | Phase 2 results are expected Q2 2021 |
| KVD824® | KalVista Pharmaceuticals | Kallikrein inhibitor | LTP | Oral | Phase 2 trial is expected to start in 2021 |
| KVD900® | KalVista Pharmaceuticals | Kallikrein inhibitor | ODT | Oral | Phase 2 trial data are expected in Q1 2021 |
| NTLA-2002® | Intellia Therapeutics | CRISPR/Cas9 editing of KLKB1 | LTP | Intravenous | Preclinical development |
| PHA022121® | Pharvaris | BKRB2 antagonist | LTP, ODT | Oral | Phase 2 trial is in progress |
BKRB2 bradykinin receptor B2, C1-INH C1 esterase inhibitor, LTP long-term prophylaxis, ODT on demand treatment
More downstream inhibition of the KKS is accomplished by targeting plasma PK. IONIS-PKK-LRx® is a second-generation antisense oligonucleotide that can selectively reduce hepatic plasma prekallikrein mRNA synthesis. The phase 1 study with IONIS-PKK-LRx® showed a well-tolerated safety profile with reductions in plasma prekallikrein up to 94%. In addition, two patients with severe BK-mediated angioedema, including one patient with C1-INH-HAE, have been treated with IONIS-PKK-LRx® under a compassionate use protocol and their attack rate was effectively reduced, with a good tolerability of the drug [57]. A phase 2 trial comparing subcutaneous IONIS-PKK-LRx® 80 mg once monthly to placebo is ongoing [58]. Another investigational preparation targeting prekallikrein is NTLA-2002®, which is in preclinical development. Intellia Therapeutics has designed this treatment to knockout the KLKB1 gene—encoding plasma PK—using CRISPR/Cas9 technology. Hereby NTLA-2002® aims to prevent the production of prekallikrein long-term through a single course of treatment [59].
Berotralstat, the first approved oral PKa inhibitor for LTP, was investigated in liquid formulation for ODT in the ZENITH-1 study. This phase 2 trial showed that a single dose of 750 mg reduced symptom severity and use of additional rescue medication, with a favourable safety profile [60]. Three other oral PKa inhibitors under development are ATN-249®, KVD824®, and KVD900® [61, 62]. ATN-249®, developed for LTP, has recently successfully completed a phase 1 trial [63]. The results of the phase 2 trial of KVD900®, investigating its effectiveness as ODT, are expected in 2021 [64]. KVD824® has a markedly longer half-life than KVD900®, and therefore, its intended use is LTP. KVD824® has successfully completed its first-in-human study and a phase 2 trial is expected to start soon [65].
PHA022121® is the first oral drug (in the form of soft gel capsules) targeted at the BKRB2 receptor for use in acute attacks of HAE (ODT). This is a selective, competitive receptor antagonist that was shown to be highly potent in preclinical studies [66]. The phase 1 trial started in July 2019 and a phase 2 trial is expected to start soon.
Ambitiously, the first gene therapy strategies are already in preclinical development. An adeno-associated virus-mediated antibody delivery gene therapy demonstrated the induction of sustained and adequate plasma levels of C1-INH for at least 24 weeks in a murine model of HAE, by introducing an extrachromosomal copy of SERPING1 into the cells [67]. BioMarin (BMN 331®) [68] and Regenxbio [69] are currently conducting preclinical experiments with these novel adeno-associated virus gene therapies for HAE. The potential long-term protection after a single treatment with such therapies is promising. Naturally, clinical safety and efficacy studies will be necessary before any further conclusions about these new therapeutic strategies can be drawn. Another challenge is the high levels of functional C1-INH levels required to effectively reduce breakthrough attacks [70]. The residual functional C1-INH in most C1-INH-HAE patients is 10–15%, while the minimal amount of C1-INH required to effectively prevent attacks is above 38% [71]. This contrasts to congenital factor IX deficiency (haemophilia B) for instance, in which only small antigenic increases of factor IX by gene therapy can induce marked reductions in disease severity [72].
Discussion
This article focused on the current and future therapeutic options for C1-INH-HAE. With the development of highly effective therapies that are more easily administered and cause fewer side-effects than the conventional drugs, treatment decisions for C1-INH-HAE are expected to shift towards more personalized preferences with better disease control, provided that these drugs are affordable and accessible.
Within the last few years, two new LTP options became available and tremendously changed the field of C1-INH-HAE treatment. These options concern the subcutaneous C1-INH concentrate and the PKa inhibitor lanadelumab. Both are highly effective in the prevention of breakthrough attacks and showed impressive results, with high proportions of patients experiencing a complete lack of HAE attacks in their respective trials.
The present shift in treatment options will raise new clinical questions. Although the International/Canadian HAE guideline recommends both subcutaneous C1-INH or lanadelumab as first-line therapy [29], adherence to this recommendation may become restricted by the high costs of treatment. It will therefore be essential to determine the indications and identify patients that may benefit the most from this newest generation of treatments. Furthermore, it is currently unknown whether STP prior to invasive procedures remains necessary in patients with well controlled HAE under highly effective LTP. Moreover, once patients require parenteral STP or ODT less often, they might lose their skills to self-administer these drugs. Thus, better disease control could seemingly adversely lead to more emergency department visits by C1-INH-HAE patients, when they require intravenous agents for ODT. In addition, orally administered ODT may pose other drawbacks, such as impaired swallowing during laryngeal or oropharyngeal angioedema attacks, or abdominal attacks (due to vomiting), and a potentially reduced absorption during abdominal attacks. In the latter case, oral drugs may work less rapid than parenteral administered therapies. Nonetheless, oral ODT would certainly shorten the time to administration in patients who are currently dependent on healthcare professionals for the administration of parenteral ODT and in patients with reluctance to injections.
Another potential unexplored issue concerns the concomitant use of both ODT and LTP with the same mechanism of action. Whether PKa inhibitors developed for ODT will be effective in patients on LTP targeting the same protein remains to be elucidated. Moreover, it is still uncertain whether current limitations for C1-INH-HAE patients, such as the avoidance of estrogen-containing contraceptives, will remain necessary.
Even though the therapeutic options have widely expanded, the number of treatment options remains limited in certain subgroups of patients. For pregnant or breastfeeding patients, as well as children under the age of 12 years, the licensed therapies were virtually unchanged in recent years. This review focused on patients with C1-INH-HAE, since the aforementioned drugs have been primarily investigated in this population. Whether their clinical efficacy is similar in patients with other BK-mediated angioedema (such as nC1-INH-HAE or angiotensin-converting enzyme inhibitor-induced angioedema) needs to be assessed in future trials. Thinking further ahead, the first steps have been made in the domain of gene therapy for C1-INH-HAE. This opens the field for completely novel treatment options.
Conclusion
Increased insights in the pathophysiology of HAE have paved the way for the development of new and more targeted therapies. The availability of more easily administered ODT options has improved both patients’ safety and quality of life. The anticipated shift towards highly effective, less frequently administered, and easily used LTP will hopefully further reduce the frequency and severity of HAE attacks and ameliorate the burden on HAE patients.
Abbreviations
- AA
Attenuated androgens
- BK
Bradykinin
- BKRB2
Bradykinin receptor B2
- C1-INH
C1 esterase inhibitor
- C1-INH-HAE
HAE due to C1-INH deficiency
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- FFP
Fresh frozen plasma
- FXII
Factor XII
- FXIIa
Activated factor XII
- HAE
Hereditary angioedema
- HK
High-molecular-weight kininogen
- KKS
Kallikrein/kinin system
- LTP
Long-term prophylaxis
- MASP
Mannose-binding lectin-associated serine protease
- nC1-INH-HAE
HAE with normal C1-INH
- ODT
On demand therapy
- PKa
Plasma kallikrein
- QoL
Quality of life
- SERPIN
Serine protease inhibitor
- SiRNA
Small interfering RNA
- STP
Short-term prophylaxis
Authors’ Contributions
All authors conceived the presented idea. L.F. performed the literature search and drafted the manuscript. D.C. and K.B critically revised the work. All authors contributed to the final manuscript.
Compliance with Ethical Standards
Conflicts of Interest
K.B. has received grant research support and/or speaker fees from CSL Behring and Shire (a Takeda company), outside the submitted work.
D.C. reports consultancy fees from BioCryst, CSL Behring, Pharming, Pharvaris and Shire (a Takeda company), outside the submitted work.
L.F. declares no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267–274. doi: 10.1016/j.amjmed.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 2.Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol. 2012;130(3):692–697. doi: 10.1016/j.jaci.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 3.Bork K, Siedlecki K, Bosch S, Schopf RE, Kreuz W. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc. 2000;75(4):349–354. doi: 10.4065/75.4.349. [DOI] [PubMed] [Google Scholar]
- 4.Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K, et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69(5):602–616. doi: 10.1111/all.12380. [DOI] [PubMed] [Google Scholar]
- 5.Dewald G, Bork K. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun. 2006;343(4):1286–1289. doi: 10.1016/j.bbrc.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 6.Bork K, Wulff K, Steinmuller-Magin L, Braenne I, Staubach-Renz P, Witzke G, et al. Hereditary angioedema with a mutation in the plasminogen gene. Allergy. 2018;73(2):442–450. doi: 10.1111/all.13270. [DOI] [PubMed] [Google Scholar]
- 7.Bafunno V, Firinu D, D'Apolito M, Cordisco G, Loffredo S, Leccese A, et al. Mutation of the angiopoietin-1 gene (ANGPT1) associates with a new type of hereditary angioedema. J Allergy Clin Immunol. 2018;141(3):1009–1017. doi: 10.1016/j.jaci.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Bork K, Wulff K, Rossmann H, Steinmuller-Magin L, Braenne I, Witzke G, et al. Hereditary angioedema cosegregating with a novel kininogen 1 gene mutation changing the N-terminal cleavage site of bradykinin. Allergy. 2019;74(12):2479–2481. doi: 10.1111/all.13869. [DOI] [PubMed] [Google Scholar]
- 9.Ariano A, D'Apolito M, Bova M, Bellanti F, Loffredo S, D'Andrea G, et al. A myoferlin gain-of-function variant associates with a new type of hereditary angioedema. Allergy. 2020 doi: 10.1111/all.14454. [DOI] [PubMed] [Google Scholar]
- 10.Zeerleder S. C1-inhibitor: more than a serine protease inhibitor. Semin Thromb Hemost. 2011;37(4):362–374. doi: 10.1055/s-0031-1276585. [DOI] [PubMed] [Google Scholar]
- 11.Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14(1):28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 12.Colman RW (1984) Surface-mediated defense reactions. The plasma contact activation system. J Clin Invest, 73(5), 1249–1253. 10.1172/jci111326 [DOI] [PMC free article] [PubMed]
- 13.Kaplan AP, Joseph K. Pathogenic mechanisms of bradykinin mediated diseases: dysregulation of an innate inflammatory pathway. Adv Immunol. 2014;121:41–89. doi: 10.1016/b978-0-12-800100-4.00002-7. [DOI] [PubMed] [Google Scholar]
- 14.Zuraw BL, Christiansen SC. HAE pathophysiology and underlying mechanisms. Clin Rev Allergy Immunol. 2016;51(2):216–229. doi: 10.1007/s12016-016-8561-8. [DOI] [PubMed] [Google Scholar]
- 15.Campbell DJ. The kallikrein-kinin system in humans. Clin Exp Pharmacol Physiol. 2001;28(12):1060–1065. doi: 10.1046/j.1440-1681.2001.03564.x. [DOI] [PubMed] [Google Scholar]
- 16.Cugno M, Zanichelli A, Bellatorre AG, Griffini S, Cicardi M. Plasma biomarkers of acute attacks in patients with angioedema due to C1-inhibitor deficiency. Allergy. 2009;64(2):254–257. doi: 10.1111/j.1398-9995.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 17.Parsopoulou F, Charignon D, Tengo M, Psarros F, Maas C, Gonzalez-Quevedo T, et al (2020) Plasminogen glycoforms alteration and activation susceptibility associated with the missense variant p.Lys330Glu in HAE-PLG patients. Allergy. 10.1111/all.14280 [DOI] [PubMed]
- 18.Maurer M, Magerl M, Ansotegui I, Aygoren-Pursun E, Betschel S, Bork K, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2017 revision and update. Allergy. 2018 doi: 10.1111/all.13384. [DOI] [PubMed] [Google Scholar]
- 19.Agostoni A, Aygoren-Pursun E, Binkley KE, Blanch A, Bork K, Bouillet L, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114(3 Suppl):S51–131. doi: 10.1016/j.jaci.2004.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoem NO, Johannesen S, Hauge G, Rud AC, Sandem S, Briseid K. Contact activation factors in plasma from women using oral contraceptives–increased levels of factor XII, kinin-free high molecular weight kininogen and acetone-activated kallikrein. Thromb Res. 1991;64(4):427–434. doi: 10.1016/0049-3848(91)90343-u. [DOI] [PubMed] [Google Scholar]
- 21.Caballero T, Farkas H, Bouillet L, Bowen T, Gompel A, Fagerberg C, et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol. 2012;129(2):308–320. doi: 10.1016/j.jaci.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Bork K, Witzke G. Long-term prophylaxis with C1-inhibitor (C1 INH) concentrate in patients with recurrent angioedema caused by hereditary and acquired C1-inhibitor deficiency. J Allergy Clin Immunol. 1989;83(3):677–682. doi: 10.1016/0091-6749(89)90082-1. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan AP, Pawaskar D, Chiao J. C1 inhibitor activity and angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2020;8(3):892–900. doi: 10.1016/j.jaip.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Birjmohun RS, Kees Hovingh G, Stroes ES, Hofstra JJ, Dallinga-Thie GM, Meijers JC, et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. Clin Ther. 2008;30(12):2314–2323. doi: 10.1016/j.clinthera.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Pappalardo E, Zingale LC, Cicardi M. Increased expression of C1-inhibitor mRNA in patients with hereditary angioedema treated with Danazol. Immunol Lett. 2003;86(3):271–276. doi: 10.1016/s0165-2478(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 26.Drouet C, Desormeaux A, Robillard J, Ponard D, Bouillet L, Martin L, et al. Metallopeptidase activities in hereditary angioedema: effect of androgen prophylaxis on plasma aminopeptidase P. J Allergy Clin Immunol. 2008;121(2):429–433. doi: 10.1016/j.jaci.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100(2):153–161. doi: 10.1016/s1081-1206(10)60424-3. [DOI] [PubMed] [Google Scholar]
- 28.Lumry WR. Current and emerging therapies to prevent hereditary angioedema attacks. Am J Manag Care. 2018;24(14 Suppl):S299–s307. [PubMed] [Google Scholar]
- 29.Betschel S, Badiou J, Binkley K, Borici-Mazi R, Hebert J, Kanani A, et al. The International/Canadian Hereditary Angioedema Guideline. Allergy Asthma Clin Immunol. 2019;15:72. doi: 10.1186/s13223-019-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig T, Shapiro R, Vegh A, Baker JW, Bernstein JA, Busse P, et al. Efficacy and safety of an intravenous C1-inhibitor concentrate for long-term prophylaxis in hereditary angioedema. Allergy Rhinol (Providence) 2017;8(1):13–19. doi: 10.2500/ar.2017.8.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavigan G, Yang WH, Santucci S, Harrison R, Karsh J. The prophylactic use of C1 inhibitor in hereditary angioedema patients undergoing invasive surgical procedures: a retrospective study. Allergy Asthma Clin Immunol. 2014;10(1):17. doi: 10.1186/1710-1492-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bork K, Hardt J, Staubach-Renz P, Witzke G. Risk of laryngeal edema and facial swellings after tooth extraction in patients with hereditary angioedema with and without prophylaxis with C1 inhibitor concentrate: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(1):58–64. doi: 10.1016/j.tripleo.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 33.Levi M, Choi G, Picavet C, Hack CE. Self-administration of C1-inhibitor concentrate in patients with hereditary or acquired angioedema caused by C1-inhibitor deficiency. J Allergy Clin Immunol. 2006;117(4):904–908. doi: 10.1016/j.jaci.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Riedl MA, Bygum A, Lumry W, Magerl M, Bernstein JA, Busse P, et al. Safety and usage of C1-inhibitor in hereditary angioedema: Berinert Registry Data. J Allergy Clin Immunol Pract. 2016;4(5):963–971. doi: 10.1016/j.jaip.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Craig T, Zuraw B, Longhurst H, Cicardi M, Bork K, Grattan C, et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2019;7(6):1793–1802.e1792. doi: 10.1016/j.jaip.2019.01.054. [DOI] [PubMed] [Google Scholar]
- 36.Lumry WR, Craig T, Zuraw B, Longhurst H, Baker J, Li HH, et al. Health-related quality of life with subcutaneous C1-inhibitor for prevention of attacks of hereditary angioedema. J Allergy Clin Immunol Pract. 2018;6(5):1733–1741.e1733. doi: 10.1016/j.jaip.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 37.Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108–2121. doi: 10.1001/jama.2018.16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuraw B, Lumry WR, Johnston DT, Aygören-Pürsün E, Banerji A, Bernstein JA, et al. Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: a randomized, double-blind, placebo-controlled phase 3 trial. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Riedl M, Lumry W, Banerji A, Aygoren-Pursun E, Bernstein J, Maurer M, et al. P154 safety and tolerability of once-daily oral kallikrein inhibitor BCX7353 in Phase 3 APEX-2 HAE study. Ann Allergy Asthma Immunol. 2019;123(5):S28–S29. doi: 10.1016/j.anai.2019.08.258. [DOI] [Google Scholar]
- 40.U. S. National Library of Medicine. (2018, 2019 Dec 23). A long term safety study of BCX7353 in hereditary angioedema (APeX-S). Retrieved from https://clinicaltrials.gov/ct2/show/NCT03472040?term=bcx7353&draw=2&rank=1
- 41.Jaffe CJ, Atkinson JP, Gelfand JA, Frank MM. Hereditary angioedema: the use of fresh frozen plasma for prophylaxis in patients undergoing oral surgery. J Allergy Clin Immunol. 1975;55(6):386–393. doi: 10.1016/0091-6749(75)90077-9. [DOI] [PubMed] [Google Scholar]
- 42.Pickering RJ, Good RA, Kelly JR, & Gewurz H (1969) Replacement therapy in hereditary angioedema. Successful treatment of two patients with fresh frozen plasma. Lancet, 1(7590), 326–330. 10.1016/s0140-6736(69)91295-1 [DOI] [PubMed]
- 43.Zuraw BL. Clinical practice. Hereditary angioedema N Engl J Med. 2008;359(10):1027–1036. doi: 10.1056/NEJMcp0803977. [DOI] [PubMed] [Google Scholar]
- 44.Koles K, van Berkel PH, Pieper FR, Nuijens JH, Mannesse ML, Vliegenthart JF, et al. N- and O-glycans of recombinant human C1 inhibitor expressed in the milk of transgenic rabbits. Glycobiology. 2004;14(1):51–64. doi: 10.1093/glycob/cwh010. [DOI] [PubMed] [Google Scholar]
- 45.Valerieva A, Staevska M, Jesenak M, Hrubiskova K, Sobotkova M, Zachova R, et al. Recombinant human C1 esterase inhibitor as short-term prophylaxis in patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2020;8(2):799–802. doi: 10.1016/j.jaip.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Riedl MA, Grivcheva-Panovska V, Moldovan D, Baker J, Yang WH, Giannetti BM, et al. Recombinant human C1 esterase inhibitor for prophylaxis of hereditary angio-oedema: a phase 2, multicentre, randomised, double-blind, placebo-controlled crossover trial. Lancet. 2017;390(10102):1595–1602. doi: 10.1016/s0140-6736(17)31963-3. [DOI] [PubMed] [Google Scholar]
- 47.Cicardi M, Levy RJ, McNeil DL, Li HH, Sheffer AL, Campion M, et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363(6):523–531. doi: 10.1056/NEJMoa0905079. [DOI] [PubMed] [Google Scholar]
- 48.Levy RJ, Lumry WR, McNeil DL, Li HH, Campion M, Horn PT, et al. EDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104(6):523–529. doi: 10.1016/j.anai.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Craig TJ, Li HH, Riedl M, Bernstein JA, Lumry WR, MacGinnitie AJ, et al. Characterization of anaphylaxis after ecallantide treatment of hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2015;3(2):206–212.e204. doi: 10.1016/j.jaip.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Duffey H, Firszt R. Management of acute attacks of hereditary angioedema: role of ecallantide. J Blood Med. 2015;6:115–123. doi: 10.2147/jbm.S66825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lumry WR, Li HH, Levy RJ, Potter PC, Farkas H, Moldovan D, et al. Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol. 2011;107(6):529–537. doi: 10.1016/j.anai.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Maurer M, Aberer W, Bouillet L, Caballero T, Fabien V, Kanny G, et al. Hereditary angioedema attacks resolve faster and are shorter after early icatibant treatment. PLoS ONE. 2013;8(2):e53773. doi: 10.1371/journal.pone.0053773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohn DM, Zuraw BL, Craig T, Bork K, Feuersenger H, Jacobs I, et al A multicenter, randomized, double-blind, dose-ranging phase 2 study investigating the efficacy, pharmacokinetics and safety of prophylaxis with the anti-factor XIIa monoclonal antibody garadacimab in patients with hereditary angioedema [Conference presentation]. Paper presented at the HAE Global Conference 2020. https://haei.org/gc2020/#scientific
- 54.Liu J, Qin J, Borodovsky A, Racie T, Castoreno A, Schlegel M, et al. An investigational RNAi therapeutic targeting Factor XII (ALN-F12) for the treatment of hereditary angioedema. RNA. 2019;25(2):255–263. doi: 10.1261/rna.068916.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrowhead Pharmaceuticals. (2016). Arrowhead pharmaceuticals presents new data on ARC-F12 and ARC-LPA using DPCsqTM subcutaneous RNAi delivery vehicle. Retrieved from http://ir.arrowheadpharma.com/news-releases/news-release-details/arrowhead-pharmaceuticals-presents-new-data-arc-f12-and-arc-lpa
- 56.Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 57.Cohn DM, Viney NJ, Fijen LM, Schneider E, Alexander VJ, Xia S, et al. Antisense inhibition of prekallikrein to control hereditary angioedema. N Engl J Med. 2020;383(13):1242–1247. doi: 10.1056/NEJMoa1915035. [DOI] [PubMed] [Google Scholar]
- 58.U. S. National Library of Medicine. (2020, February 20, 2020). A study to assess the clinical efficacy of IONIS-PKK-LRx in participants with hereditary angioedema. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04030598?term=ionis-pkk-lrx&draw=2
- 59.Intellia Therapeutics. (2020). In vivo therapies. Retrieved from https://www.intelliatx.com/in-vivo-therapies/
- 60.Longhurst H, Moldovan D, Bygum A, Cicardi M, Huissoon A, Aygoren-Pursun E, et al (2019) Oral plasma kallikrein inhibitor BCX7353 is safe and effective as an on-demand treatment of angioedema attacks in hereditary angioedema (HAE) patients: results of the ZENITH-1 Trial. Paper presented at the AAAAI, San Francisco CA. https://ir.biocryst.com/static-files/2b3e13b9-ad24-432c-9ac5-15711d954d88
- 61.Kalfus I, McDonald A, Qian S (2017) Potency, selectivity, and exposure evaluation of ATN-249, a new oral kallikrein inhibitor for hereditary angioedema. J Allergy Clin Immunol, 139(2), AB378. 10.1016/j.jaci.2016.12.905
- 62.Hampton SL, De Donatis GM, Murugesan NI, Rushbrooke LJ, Li L, Duckworth E, et al (2019) KVD900 as a single dose, rapid, oral plasma kallikrein inhibitor for the on-demand treatment of hereditary angioedema attacks: pharmacokinetic and pharmacodynamic results from a phase 1 single ascending dose study. Journal of Allergy and Clinical Immunology, 143(2), AB39. 10.1016/j.jaci.2018.12.118
- 63.Attune Pharmaceuticals. (2019, 2019 Jan 24). Pharmacokinetics and safety of ATN-249, a novel oral plasma kallikrein inhibitor for hereditary angioedema. Retrieved from http://attunepharma.com/assets/Attune-WSAAI-Poster-Jan-2019.pdf
- 64.U. S. National Library of Medicine. (2019, 2020 Feb 28). A phase II, cross-over clinical trial evaluating the efficacy and safety of KVD900 in the on-demand treatment of angioedema attacks in adult subjects with hereditary angioedema type I or II. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04208412?term=kvd900&draw=2&rank=1
- 65.KalVista. (2020). KalVista for HAE. Retrieved from https://www.kalvista.com/products-pipeline/kalvista-hae
- 66.Pharvaris. (2020). HAE. Retrieved from https://pharvaris.com/science/
- 67.Qiu T, Chiuchiolo MJ, Whaley AS, Russo AR, Sondhi D, Kaminsky SM, et al. Gene therapy for C1 esterase inhibitor deficiency in a murine model of hereditary angioedema. Allergy. 2019;74(6):1081–1089. doi: 10.1111/all.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.BioMarin. (2019, 2019 Nov 14). Poised for significant growth and profitability, biomarin shares company highlights during R&D day on November 14th in New York. Retrieved from https://investors.biomarin.com/2019-11-14-Poised-for-Significant-Growth-and-Profitability-BioMarin-Shares-Company-Highlights-During-R-D-Day-on-November-14th-in-New-York
- 69.Regenxbio. (2019, 2019 Jul 24). Regenxbio expands pipeline using NAV vectors to deliver therapeutic antibodies for the treatment of hereditary angioedema and neurodegenerative diseases. Retrieved from http://ir.regenxbio.com/news-releases/news-release-details/regenxbio-expands-pipeline-using-nav-vectors-deliver-therapeutic/
- 70.Zhang Y, Tortorici MA, Pawaskar D, Pragst I, Machnig T, Hutmacher M, et al. Exposure-response model of subcutaneous C1-inhibitor concentrate to estimate the risk of attacks in patients with hereditary angioedema. CPT Pharmacometrics Syst Pharmacol. 2018;7(3):158–165. doi: 10.1002/psp4.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Späth PJ, Wüthrich B, Bütler R. Quantification of C1-inhibitor functional activities by immunodiffusion assay in plasma of patients with hereditary angioedema–evidence of a functionally critical level of C1-inhibitor concentration. Complement. 1984;1(3):147–159. doi: 10.1159/000467830. [DOI] [PubMed] [Google Scholar]
- 72.Miesbach W, Meijer K, Coppens M, Kampmann P, Klamroth R, Schutgens R, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131(9):1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]