Abstract
Cholestatic liver disease is a disease that causes liver damage and fibrosis owing to bile stasis. It is represented by primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), but the pathophysiological pathways that cause bile stasis in both diseases are different. The pathogenesis of the disease is still unclear, although autoimmune mechanisms have been postulated and partially elucidated. Although the disease may progress slowly with only mild liver dysfunction, it may progress to liver cirrhosis or liver failure, which require liver transplantation. As a medical treatment, ursodeoxycholic acid is widely used for PBC and has proved to be very effective against disease progression in cases of PBC. On the other hand, its efficacy is limited in cases of PSC, and the research and development of various drugs are underway. Furthermore, the clinical course of both diseases is quite variable, making the design of clinical trials fairly difficult. In this review, we present the general natural history of PBC and PSC, and provide information on the latest drug therapies currently available and those that are under investigation.
Key Points
| Both primary biliary cholangitis and primary sclerosing cholangitis are slow progressive chronic liver diseases that are caused by bile duct destruction and fibrosis leading to cirrhosis. |
| However, the pathogenesis of the disease is often unclear, the cause is unknown, and effective treatments are limited. |
| In this review, promising treatments for cholestatic liver disease are described based on the latest findings and their perspectives. |
Introduction
Primary biliary cholangitis (PBC), previously called primary biliary cirrhosis, and primary sclerosing cholangitis (PSC) are both slow progressive chronic cholestatic liver diseases. PBC is mainly characterized by the granulomatous destruction of small intrahepatic bile ducts [1, 2], whereas PSC is characterized by inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, which lead to multiple bile duct stenoses [3, 4] (Fig. 1). In addition, many patients with PSC have concomitant inflammatory bowel disease (IBD), which is the strongest clinical risk factor [5, 6]. PSC has been suggested to be a cytotoxic disorder, as T cells are involved in the destruction of bile ducts [7]. Both PBC and PSC have autoimmune triggers for bile duct damage, and may lead to cirrhosis and liver failure after a certain period of time [8, 9]. Although some aspects of the pathogenesis have been clarified, PSC remains an intractable disease with limited effective treatment options. The prognosis of patients with liver failure is particularly poor, and there is no effective treatment other than liver transplantation; therefore, early diagnosis and therapeutic interventions are necessary. The development of new therapeutic agents by pharmaceutical companies is gradually progressing as the need for treatment becomes evident. This paper outlines the treatment options for PBC and PSC, citing the most recent literature. The aim of this review is to present the latest treatment options based on the pathogenesis of PBC and PSC.
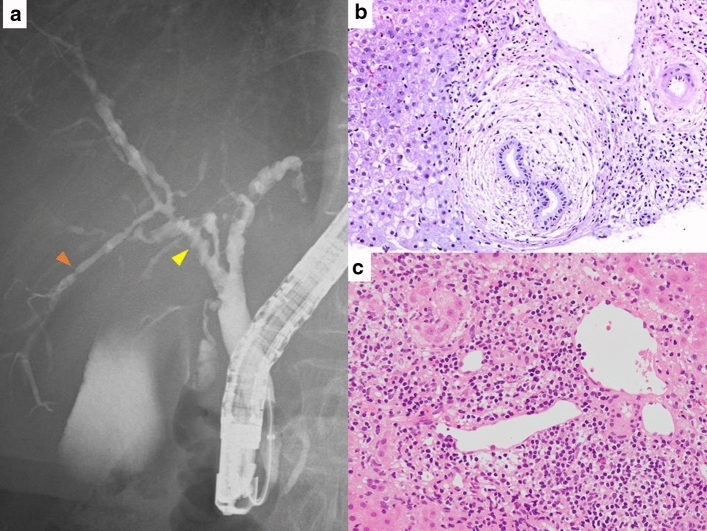
Fig. 1.
Clinical picture of PBC and PSC. a Cholangiography of the PSC by ERCP. There are multiple stenoses in the bile duct (yellow arrowhead, diverticulum-like outpunching; orange arrowhead, band-like stricture). b Histopathology of PSC obtained by liver biopsy. There is “onion-skin fibrosis” around intrahepatic small bile ducts. c Histopathology of PBC obtained by liver biopsy. The findings of chronic non-suppurative destructive cholangitis. ERCP endoscopic retrograde cholangiopancreatography, PBC primary biliary cholangitis, PSC primary sclerosing cholangitis
Natural History and Prognosis
Primary Biliary Cholangitis (PBC)
This condition has been recognized since at least 1851 and was named “primary biliary cirrhosis” in 1949 [10]. As cirrhosis is a feature only of advanced disease, a change in its name to “primary biliary cholangitis” was proposed by a patient advocacy group in 2014. PBC is a chronic liver disease that progresses over decades and has a high potential to cause cirrhosis. At initial diagnosis, most patients have both asymptomatic liver dysfunction and antimitochondrial antibodies [11, 12]. In terms of natural history, at onset, there is a silent phase with no liver dysfunction and only minute changes in liver histology, followed by a long period of asymptomatic liver dysfunction and, eventually, cirrhosis and end-stage liver failure. For patients diagnosed with cirrhosis, the median time to liver transplantation or death is 6–10 years without any intervention [13–16]. The use of ursodeoxycholic acid (UDCA) significantly improves survival in the majority of patients with PBC, with some reports suggesting a median survival time of 16–20 years [17, 18]. However, although the use of UDCA improves liver dysfunction, approximately one-third of patients are still at risk of progression to cirrhosis [11].
Primary Sclerosing Cholangitis (PSC)
The natural course of PSC varies. At initial diagnosis, 10–60% of patients are asymptomatic and are often diagnosed incidentally with hepatic biochemical abnormalities on blood tests [19–22]. In advanced cases, liver fibrosis may occur and lead to cirrhosis and liver failure [23, 24]. In the absence of drug treatment, liver transplantation is the only treatment for liver failure [25, 26]. Survival after the diagnosis of PSC without liver transplantation has been reported to be between 10 and 20 years [4, 19, 27–31], with variation by region and study period [32]. Patients with PSC are also at risk of biliary cancer during the course of the disease [4, 33–35]. The incidence of cholangiocarcinoma from PSC is 0.5–1%/year, with a median time to development of carcinoma before the onset of cirrhosis of 6 years [4]. Moreover, there are cases of hepatic dysfunction alone, which does not cause cirrhosis or carcinogenesis, with the degree of progression of PSC being variable [32].
Current Pharmacological Treatment
PBC
PBC treatment is mainly based on bile acid approaches and therapy [36]. Other approaches, such as immunosuppressants, have been studied, but there is a lack of evidence and immunosuppressants are not used in clinical practice. The progression of PBC is slow; thus, the endpoint of trials and studies are mainly not death or liver transplantation, but beneficial effects indicated by accepted surrogate biochemical parameters, such as alkaline phosphatase (ALP) and bilirubin levels.
Ursodeoxycholic Acid (UDCA)
Lifetime UDCA is the first-line therapy for PBC. Its use is recommended by the American Association for the Study of Liver Diseases (AASLD) [37] as well as the European Association for the Study of the Liver (EASL) [38]. UDCA accounts for approximately 1–3% of bile acids and is the predominant bile acid in drug therapy. The concentration of UDCA in bile correlates with improved serum liver function tests [39–42].
The recommended dosage in the AASLD guidance and EASL guidelines is 13–15 mg/kg/day. A study comparing three different dosages of UDCA (5–7 mg/kg/day, 13–15 mg/kg/day, and 23–25 mg/kg/day) showed that dosages of 13–15 mg/kg/day induce better biochemical responses and are more cost-effective [43]. Additionally, there is no need for dosage adjustment in patients with concomitant other liver or renal diseases.
UDCA acts via multiple mechanisms owing to its choleretic, cytoprotective, anti-inflammatory, and immune-modulatory properties [39]. In clinical practice, it also improves biochemical indices, delays histological progression, and improves survival without transplantation [43–45]. It is a safe drug at the recommended dose. Side effects are infrequently reported, such as weight gain of approximately 3 kg in the first 12 months, hair thinning, and diarrhea and bloating.
Obeticholic Acid (OCA)
Obeticholic acid (OCA) is the second-line therapy for PBC, approved by the Food and Drug Administration in May 2016, for use in combination with UDCA for those who have inadequate response to at least 1 year of treatment with UDCA, or as monotherapy for patients who are intolerant to UDCA. OCA is a farnesoid X receptor (FXR), which is a nuclear “ligand-activated” receptor abundantly expressed in tissues involved in the enterohepatic circulation of bile acids. Through FXR activation, OCA modulates bile acid synthesis, absorption, transport, secretion, and metabolism, with a net effect of choleresis [39, 45].
Studies examining the efficacy of OCA in patients with PBC are still ongoing, and the benefit of OCA in patients with decompensated liver disease has not yet been established. Based on trial results [46, 47], the recommended starting dosage for patients with preserved synthetic function and well-compensated PBC is 5 mg daily. After 3 months, the dosage can be increased to 10 mg daily if liver function tests remain abnormal and the patient tolerates the medication well. The most common side effect is pruritis, which may be severe and require treatment with an antihistamine or bile-acid binding resin, temporary treatment interruption, or dose reduction. Other side effects include fatigue, abdominal pain, and rash, but these are relatively rare.
PSC
Although there have been numerous clinical trials for PSC, no drugs have been shown to improve long-term outcomes. In addition, the natural course of PSC has not been elucidated, and the design of clinical trials is still under discussion. As with PBC, it is impossible to use death or liver transplantation as the primary endpoint in clinical trials. Therefore, some kind of primary endpoint must be established, and most clinical trials of new compounds use a decrease in serum ALP level as the endpoint. The reason for this is that prognosis is better in patients with decreased ALP at 1 year after the start of UDCA treatment [48]. However, ALP alone is not sufficient to verify efficacy, and the importance of pathological findings of liver fibrosis [49], a noninvasive assessment of liver fibrosis [50], and new prognostic models are being discussed. The combination of pathological findings, ALP levels, and transient elastography is the most promising surrogate endpoint. However, the results of a consensus process by the International PSC Study Group concluded that there are currently no biomarkers that can be used as a true measure of clinical efficacy and that there are insufficient data at this time [51]. One of the reasons why it is difficult to establish surrogate endpoints is that the natural history of PSC is diverse, and the diseases diagnosed as PSC are not homogeneous but rather a mixture of multiple disease groups. It is expected that the natural history of PSC will be clarified to establish an appropriate primary endpoint.
UDCA
The most frequently used drug for PSC is UDCA. The administration of UDCA often improves serum biliary enzyme levels [52], and numerous clinical trials have been conducted in patients with PSC. Although these studies have shown a biochemical improvement effect, the long-term improvement in the prognosis of patients with PSC has not yet been clarified. Moreover, a previous article reported that the long-term prognosis is worsened by the administration of a high dosage of UDCA (> 28 mg/kg/day), and adverse events were also more frequent [53]. Therefore, guidelines, such as the American Society of Hepatology Clinical Practice Guidelines (2010), American College of Gastroenterology Guidelines (2015), and European Hepatology Society Clinical Practice Guidelines (2009), have not unified policies regarding UDCA recommendations for patients with PSC. The long-term prognostic improvement effect of UDCA will remain uncertain until clinical trials with well-defined endpoints are conducted. There is no evidence yet that UDCA worsens prognosis at standard dosages of 13–15 mg/kg/day, and there is no other alternative currently. It has recently been confirmed that the long-term prognosis is good in cases in which ALP is reduced by the administration of UDCA [48]. As of now, it seems appropriate to use UDCA as the first-line drug in clinical practice.
Overlap Syndrome with Autoimmune Hepatitis
PBC and PSC are rarely associated with autoimmune hepatitis (AIH), which is also known as “overlap syndrome.”
PBC-AIH occurs in 1–3% of patients with PBC [54], and PSC-AIH occurs in 1.4–8% of patients with PSC [55–57]. In both cases, corticosteroids and immunosuppressive agents are available as treatment options for AIH, but there have been few reports and a low level of evidence regarding the recommended treatment and no optimal treatment guideline. In PBC-AIH overlap syndrome, the combination of corticosteroid and UDCA is often used [58–60] because of reported cases of poor prognosis from patients treated with UDCA alone. A meta-analysis comparing the combination of corticosteroid (with or without immunosuppressive agent) and UDCA, UDCA alone, and corticosteroid with or without immunosuppressive agent found biochemical improvement and higher transplant-free survival with the combination [61]. However, caution is needed with the use of corticosteroids for PBC because of the risk of worsening osteoporosis. Similar to PBC-AIH, there have been reports that the combination therapy of corticosteroids and UDCA is effective in patients with the overlap syndrome of PSC-AIH [62, 63]. However, due to the lack of evidence from randomized controlled trials, the PSC guidelines of AASLD recommend corticosteroids and other immunosuppressive agents, but the efficacy of these agents remains unclear [64].
Symptoms and Management of Complications
Complications of cholestatic liver disease can affect the extrahepatic organs. In particular, the most common pathologies associated with chronic cholestatic liver disease include deficiency of fat-soluble vitamins, metabolic bone disease, dyslipidemia, pruritus, and various diseases associated with portal hypertension. Although many of the complications can be evaluated by clinical and imaging studies, pruritus without jaundice can only be evaluated by visual examination, such as scratch marks or specific questioning.
Metabolic Bone Disease
Metabolic bone disease is a common complication in patients with cholestatic liver disease. In 80% of cases, especially in patients with advanced PBC or PSC, there is a decrease in bone mineral density, and the condition is diagnosed as osteopenia or osteoporosis [49]. Therefore, it is advisable to administer calcium (1200 mg/day) and vitamin D3 (1000–2000 IU/day cholecalciferol) to patients with PBC and PSC unless there is a condition that contraindicates this, such as renal stones. In patients who meet the criteria for osteoporosis, bisphosphonates should be considered to restore bone mass. Bisphosphonates are safe, but gastrointestinal disturbances occur rarely [50].
Pruritus
It was reported that pruritus is present in more than 80% of patients with cholestatic liver disease [43]. Although pruritus is not directly related to prognosis, it can have a variety of effects, such as decreased concentration, sleep disturbance, decreased work capacity, and decreased appetite, significantly reducing the patient’s quality of life. The potent neuronal activator lysophosphatidic acid (LPA) and its forming enzyme autotaxin (ATX) may be potential mediators of cholestatic pruritus. ATX activity correlated with itch severity and effectiveness of several anti-pruritic therapeutic interventions in cholestatic patients [65]. The ATX-LPA signaling axis may represent a key element in the pathogenesis of pruritus in chronic cholestatic liver diseases [51]. Bile acid-binding resins, such as cholestyramine or colestipol (4–16 g/day), are generally used for pruritus caused by cholestatic liver disease. Other treatments include the antibiotic rifampin (150–600 mg/day), the opioid antagonist naloxone (25–50 mg), and the selective serotonin reuptake inhibitor sertraline (75–100 mg/day). In addition, bezafibrate, a peroxisome proliferator-activated receptor (PPAR) agonist, is known to reduce pruritus by reducing bile congestion and damage. A randomized controlled trial reported that bezafibrate (400 mg/day) was effective in improving symptoms in patients with cholestatic diseases with severe pruritus [66].
Dyslipidemia
Patients with cholestatic liver disease frequently have abnormal lipid profiles. Physical findings may include yellow spots around the eyelids, called xanthelasma, and around the palms of the hands, soles of the feet, and tendons, called xanthomas. Statins and fibrates can be used safely in patients with cholestatic liver disease [67]. Bile acid-binding resins also have the potential to improve dyslipidemia [68, 69].
Fat-Soluble Vitamin Deficiency
Patients with cholestatic liver disease are prone to fat-soluble vitamin deficiency because bile plays an important role in the absorption of fat-soluble vitamins in the intestinal tract [51]. In particular, patients with PBC or PSC with bile stasis, such as bilirubin above 2 mg/dL, should be evaluated for fat-soluble vitamins annually. If there is fat-soluble vitamin deficiency at that time, vitamin replacement therapy is necessary.
New Therapeutic Targets
PBC
Although there are only limited treatment options for PBC available today, novel agents are under investigation in clinical trials. Generally speaking, therapeutic options target what could be considered as the “upstream” immune response, “midstream” biliary injury, and “downstream” fibrotic processes [70].
The main target of therapeutic approaches is “midstream” biliary injury and cholestasis, which have central roles in disease progression. As anti-cholestatic agents, FXR, PPAR, and fibroblast growth factor 19 (FGF19) agonists are being investigated (Table 1).
Table 1.
Therapeutic targets for PBC
| Drug | Target | Trial design | Patients | Duration | Outcome |
|---|---|---|---|---|---|
| EDP-305 [71] | FXR | Randomized, placebo-controlled | 68 | 12 weeks | 20% median decrease in ALP at 12 weeks |
| Fenofibrate [76] | PPAR | Open-label | 10 | 8 weeks | 32% median decrease in ALP at 8 weeks |
| Fenofibrate [77] | PPAR | Open-label | 22 | 3 months | 68% of the patients reached a normal ALP level at 3 months |
| Bezafibrate [79] | PPAR | Randomized, placebo-controlled | 100 | 24 months | 31% of the patients reached a normal level of T-bil, ALP, AST, ALT, and albumin |
| Seladelpar [80] | PPAR | Randomized, placebo-controlled | 70 | 12 weeks | 53% median decrease in ALP at 12 weeks |
| Elafibranor [81] | PPAR | Randomized, placebo-controlled | 45 | 12 weeks | 40% median decrease in ALP at 12 weeks |
| NGM282 [82] | FGF19 | Randomized, placebo-controlled | 45 | 4 weeks | 15% median decrease in ALP at 4 weeks |
| Rituximab [83] | CD20 | Open-label | 6 | 52 weeks | 14% median decrease in ALP at 36 weeks |
| Ustekinumab [85] | IL-12/23 | Open-label | 20 | 28 weeks | 12% median decrease in ALP at 28 weeks |
| Abatacept [86] | CD80/86 | Open-label | 16 | 24 weeks | No significant change in ALP |
| Setanaxib | NOX4 | Randomized, placebo-controlled | 92 | 6 weeks | 17% median decrease in ALP and 23% median decrease in γGTP |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, FGF19 fibroblast growth factor 19, FXR farnesoid X receptor, IL interleukin, NOX4 anti-NOX4 antibody, PBC primary biliary cholangitis, PPAR peroxisome proliferator-activated receptor, T-bil total bilirubin, γGTP gamma-glutamyl transpeptidase
Farnesoid X Receptor Agonists
In addition to the aforementioned OCA, tropifexor, cilofexor, and EDP-305 have been studied as semi-synthetic bile acid and non-bile acid FXR agonists. In a phase II study in patients with PBC, liver biochemical tests (ALP, alanine aminotransferase [ALT], aspartate aminotransferase [AST], and gamma-glutamyl transpeptidase [γGTP]) were significantly improved from baseline after 12 weeks in the 1 mg or 2.5 mg of EDP-305 treatment groups compared with the placebo group [71]. Tropifexor and cilofexor are also in phase II trials, respectively [72, 73].
Peroxisome Proliferator-Activated Receptor Agonists
PPARs are members of the nuclear receptor superfamily of ligand-activated transcription factors and contain three isotypes encoded by PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3) genes [74]. Each gene exhibits isoform-specific distribution patterns and functions in tissues [75]. Fenofibrate is classified as a PPARα agonist, and bezafibrate is considered a pan-PPAR agonist with a similar affinity for the three isoforms. Although the evidence for fenofibrate on PBC treatment is low [76–78], bezafibrate as PBC treatment is supported by a randomized controlled trial (BEZURSO trial) [79], and in patients with an inadequate response to UDCA, the addition of bezafibrate (400 mg/day) is associated with a significantly higher percentage of normalization of ALP, AST, ALT, total bilirubin (T-bil), albumin, and prothrombin time at 24 months compared with placebo. Seladelpar is a selective PPARδ agonist. In a phase II randomized controlled trial of patients with an inadequate response to UDCA, compared with those in the placebo group, ALP levels were significantly lower in the group treated with seladelpar (50 mg, 200 mg/day), but ALT levels increased and the trial was interrupted [80]. Subsequent additional validation showed that elevated ALT levels were not related to seladelpar, and a phase III randomized controlled trial (ENHANCE study) is currently underway. Elafibranor is a dual PPARα/δ agonist and was evaluated in a phase II study in PBC. The addition of elafibranor (80 mg and 120 mg) to the treatment of patients who had an inadequate response to UDCA significantly decreased serum ALP and T-bil levels at 12 weeks compared with placebo, and there was no worsening of pruritus or pruritus sensation [81].
Fibroblast Growth Factor 19 Agonists
FGF19 is an endocrine hormone induced in the intestine by FXR activation, and the administration of FGF19 inhibits an enzyme that catalyzes the rate-limiting step in the bile acid synthesis pathway and protects against liver damage. As an FGF19 agonist, a synthetic analog NGM282 was developed and tested in randomized, double-blind, placebo-controlled trials in PBC [82]. The absolute values of ALP and transaminases were decreased from baseline after 28 days in the NGM282 treatment group compared with the placebo group.
Immunosuppressive Agents
Therapeutic agents that target “upstream” immune response, rituximab [83, 84], ustekinumab [85], abatacept [86], and baricitinib, were studied in PBC. Rituximab, an antibody against CD20 antigen on the surface of B cells, is thought to be effective in improving the condition of PBC. The results of a clinical trial showed that rituximab significantly improved ALP levels, but the effect was limited. Ustekinumab, an anti-interleukin-12/23 monoclonal antibody, improved ALP levels at 28 weeks, but the effect was limited. Abatacept, which targets CD80/86, did not significantly improve ALP levels in an open-label trial.
Anti-fibrotic Drugs
Setanaxib is an anti-fibrotic agent that targets “downstream” fibrotic processes and has been evaluated in a multicenter, randomized, double-blind, placebo-controlled, phase II study in PBC. After 6 weeks, serum ALP and γGTP levels decreased significantly in a setanaxib dose-dependent manner, and no significant side effects were observed.
PSC
The treatment of PSCs is challenging and complex. There have been studies using various immunosuppressive and anti-inflammatory drugs and antibiotics in patients with PSC, including UDCA, glucocorticoids, cyclosporine, methotrexate, etanercept, and vancomycin (Table 2). However, treatments that have been tested have not proven to be beneficial.
Table 2.
New therapeutic targets for PSC
| Drug | Target | Trial design | Patients | Duration | Outcome |
|---|---|---|---|---|---|
| NorUDCA [87] | Bile acid | Randomized, placebo-controlled | 161 | 16 weeks | Improvement in serum ALP |
| OCA [89] | FXR | Randomized, placebo-controlled | 76 | 24 weeks | Improvement in serum ALP |
| Budesonide [91] | Glucocorticoid | Open-label | 21 | 1 year | Slight improvement in serum ALP and AST at 1 year |
| Methotrexate [93] | Folic acid | Randomized, placebo-controlled | 24 | 2 years | No significant change in ALP |
| Etanercept [95] | TNFα | Open-label | 10 | 6 months | No significant change in T-bil |
| Infliximab [96] | TNFα | Randomized, placebo-controlled | 24 | 1 year | No significant change in ALP |
| Vancomycin [99] | Antibiotics | Open-label | 14 | - | Improvement in serum ALT and γGTP |
| Probiotics [101] | Probiotics | Randomized, placebo-controlled | 14 | 3 months | No significant change in ALP, γGTP, AST, and ALT |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, FXR farnesoid X receptor, norUDCA norursodeoxycholic acid, OCA obeticholic acid, PSC primary sclerosing cholangitis, T-bil total bilirubin, TNFα tumor necrosis factor-α, γGTP gamma-glutamyl transpeptidase
Norursodoxycholic Acid
Norursodoxycholic Acid (norUDCA), a homolog of UCDA, is an artificially synthesized bile acid with a shorter side chain structure than UDCA. Compared to UDCA, norUDCA is less susceptible to conjugation by taurine and glycine and is therefore easily reabsorbed by bile duct cells after being discharged into bile as a bile acid. Due to this function, it is expected to have protective effects on the bile ducts and liver. In a randomized controlled trial for patients with PSC, norUDCA significantly reduced serum ALP levels compared with those in the placebo group and was regarded safe with no changes in adverse events [87]. The use of norUDCA in patients with PSC is promising, and a phase III study is underway [88].
OCA
As mentioned above, OCA is an FXR-selective agonist and has proven to be effective in patients with PBC with inadequate response to UDCA. In patients with PSC who were evaluated in a randomized, double-blind, placebo-controlled, phase II study, ALP levels were significantly lower in those treated with 5–10 mg OCA compared with the placebo group at 24 weeks [89]. The drug was well tolerated despite the moderate side effect of pruritus, and further studies are needed.
Bezafibrate
Bezafibrate is often used for patients with PSC for whom UDCA is inadequately effective. Bezafibrate, a PPAR agonist, is indicated for dyslipidemia. Prospective studies have shown that it has a short-term biochemical improvement effect on PSC, such as a decrease in hepatic enzymes [90]. However, the effect of improving long-term prognosis is still unclear, and research investigating long-term efficacy is expected. Rhabdomyolysis may occur as a side effect, and caution should be exercised in patients with renal dysfunction.
Glucocorticoids
As budesonide is a corticosteroid with high affinity for receptors involved in hepatic metabolism and is effective against diseases with immunological involvement, it was presumed to be effective in PSC. In a pilot study of 21 patients treated with oral budesonide, there was a slight improvement in serum ALP and AST levels, but significant femoral neck bone loss was observed [91]. Patients with PSC tend to have bone loss that can cause osteoporosis, which may be exacerbated by glucocorticoid therapy [92].
Immunosuppressive Agents
Immunosuppressive agents are presumed to contribute to improvement because immunological responses contribute to the pathogenesis of PSC as well as PBC, and clinical trials have been conducted with these agents. In a prospective, placebo-controlled, double-blind study of methotrexate in PSC, only a decrease in serum ALP was observed in the methotrexate-treated group, but there were no significant changes in liver histological results, endoscopic retrograde cholangiopancreatography (ERCP) findings, or liver function tests [93]. In recent years, there have been no reports demonstrating the efficacy of methotrexate for the treatment of PBC, and there are no prospects for it as a treatment option. In a case report, the combination of cyclosporine and methylprednisolone resulted in significant improvement in cholangiography and pancreatography, although the patient also received UDCA [94]. Immunosuppressants alone have not been shown to be effective in patients with PSC.
Anti-tumor Necrosis Factor Agents
Tumor necrosis factor is released by Kupffer cells in the liver and it is speculated to be involved in liver damage in PSC. In a pilot study of 10 patients, etanercept was ineffective [95], and in a double-blind, placebo-controlled study of 24 patients, infliximab also showed no effect [96].
Antibiotics
Since immune mechanisms in the intestine and toxins released by enteric bacteria are involved in the pathogenesis of PSC, the use of antibiotics in the treatment of PSC has received much attention. Especially in patients with complicated IBD, the use of antibiotics may reduce the exposure of pathogens to the biliary epithelium by decreasing the number of Escherischia coli and endotoxins. The rationale that antibiotics may be applied in the treatment of PSC relies on studies in rats with a similar condition to PSC owing to an increase in enteric bacteria [97]. Oral vancomycin is barely absorbed systemically, concentrates in the gut, and decreases cytokine release from T cells [98]. Because of this effect, there have been studies reporting improvements in PSC symptoms with oral vancomycin. An observational study found that oral vancomycin improves liver biochemical tests (ALT and γGTP) and symptoms in children with PSC, particularly in those without cirrhosis [99]. In addition, a randomized controlled trial of adult patients with PSC showed that oral vancomycin significantly reduced ALP levels compared with that in the placebo group, indicating acceptable efficacy [100]. Further studies of vancomycin treatment for PSC are expected.
Probiotics
As probiotics are beneficial bacteria that regulate the intestinal microflora and have been shown to be useful for patients with IBD, it was presumed that they would also be useful for PSC with frequent complications of IBD. A randomized, placebo-controlled study of probiotics in patients with PSC showed no benefit to PSC symptoms (fatigue, pruritus, or frequency of stools), liver biochemistry, or liver function [101]. Recently, the mechanism by which the gut microbiota activates T helper 17 (TH17) cells in the liver and causes an excessive immune response in the liver in patients with PSC was studied [102]. In mice, elimination of intestinal bacteria by antimicrobial administration has been shown to attenuate the TH17 response, and it is expected that new therapeutic and diagnostic agents targeting intestinal bacteria will be developed in the future.
Discussion
Both PSC and PBC are slow progressive chronic liver diseases caused by bile duct destruction and fibrosis leading to cirrhosis. However, the pathogenesis of the disease is unclear, the cause is unknown, and effective treatments are limited. In this review, we described promising treatments for cholestatic liver disease based on the latest findings and their perspectives (Fig. 2). In both PBC and PSC, UDCA is widely used as the standard first-line treatment. In PBC, drug treatments such as PPAR agonists, bile acid inhibitors, anti-fibrotic drugs, and anti-inflammatory drugs are used. The results suggest that these drugs are useful in improving the condition of the disease. Various drugs, such as immunosuppressants, antibacterials, and glucocorticoids, have been tested in clinical trials for PSC, but the efficacy of these drugs has not been confirmed. PSC is a chronic disease with a long-term course that varies widely from case to case. This makes it difficult to use true endpoints such as death or liver transplantation in clinical trials, which hinders the development of drug therapies. Recently, the mechanism of liver inflammation caused by intestinal bacteria has been elucidated in basic research, and there are high expectations for the development of new drugs for PSC targeting intestinal bacteria. As there are still few effective treatments for cholestatic liver disease, we hope to prove the efficacy of the drugs under development and expect to see long-term clinical effects.
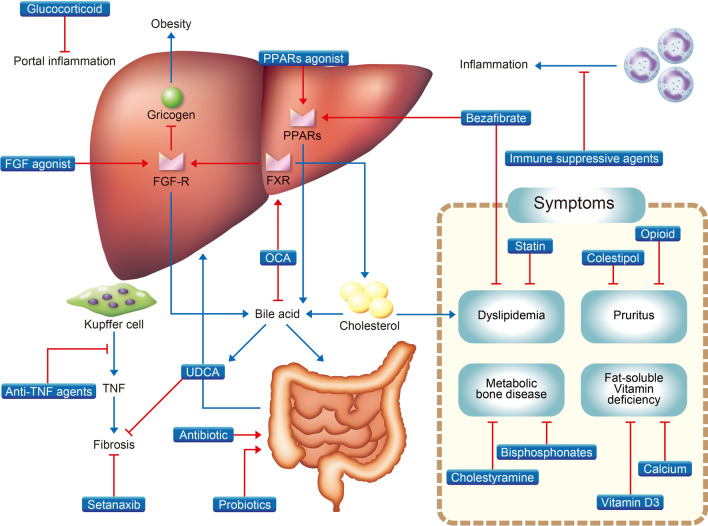
Fig. 2.
Pharmacological treatment for cholestatic liver disease. FGF fibroblast growth factor, FGF-R fibroblast growth factor receptor, FXR farnesoid X receptor, OCA obeticholic acid, PPAR peroxisome proliferator-activated receptor, TNF tumor necrosis factor, UDCA ursodeoxycholic acid
Acknowledgements
The authors are grateful to N. Kobayashi, A. Ujiie, Y. Yamazaki, and K. Kato from Yokohama City University for their assistance with this article.
Declarations
Funding
No sources of funding were used in the preparation of this article.
Conflict of interest/Competing interest
The authors have no conflict of interest related to this article.
Author contributions
SH, YK, A. Nogami, and YH participated in the writing of the article, and MY, KH, and A. Nakajima participated in the critical revision of the manuscript.
References
- 1.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ, et al. Primary biliary cirrhosis. Hepatology. 2009;50(1):291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Bernstein D, Shiffman ML, Kwo P, Kim WR, Kowdley KV, et al. Diagnosis and management of primary biliary cholangitis. Am J Gastroenterol. 2019;114(1):48–63. doi: 10.1038/s41395-018-0390-3. [DOI] [PubMed] [Google Scholar]
- 3.Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med. 2016;375(12):1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58(6):2045–2055. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
- 5.Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65(10):1681–1689. doi: 10.1136/gutjnl-2015-311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10(4):430–436. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 7.Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E, et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology. 2013;58(3):1084–1093. doi: 10.1002/hep.26447. [DOI] [PubMed] [Google Scholar]
- 8.Purohit T, Cappell MS. Primary biliary cirrhosis: pathophysiology, clinical presentation and therapy. World J Hepatol. 2015;7(7):926–941. doi: 10.4254/wjh.v7.i7.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Sugarman PA. Schizophrenia among Afro-Caribbeans. Br J Psychiatry. 1992;160:712–713. doi: 10.1192/bjp.160.5.712b. [DOI] [PubMed] [Google Scholar]
- 11.Carbone M, Mells GF, Pells G, Dawwas MF, Newton JL, Heneghan MA, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144(3):560–9.e7. doi: 10.1053/j.gastro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramaniam K, Grambsch PM, Wiesner RH, Lindor KD, Dickson ER. Diminished survival in asymptomatic primary biliary cirrhosis. A prospective study. Gastroenterology. 1990;98(6):1567–1571. doi: 10.1016/0016-5085(90)91091-J. [DOI] [PubMed] [Google Scholar]
- 13.Prince M, Chetwynd A, Newman W, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology. 2002;123(4):1044–1051. doi: 10.1053/gast.2002.36027. [DOI] [PubMed] [Google Scholar]
- 14.Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10(1):1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 15.Christensen E, Crowe J, Doniach D, Popper H, Ranek L, Rodés J, et al. Clinical pattern and course of disease in primary biliary cirrhosis based on an analysis of 236 patients. Gastroenterology. 1980;78(2):236–246. doi: 10.1016/0016-5085(80)90571-5. [DOI] [PubMed] [Google Scholar]
- 16.Locke GR, Therneau TM, Ludwig J, Dickson ER, Lindor KD. Time course of histological progression in primary biliary cirrhosis. Hepatology. 1996;23(1):52–56. doi: 10.1002/hep.510230108. [DOI] [PubMed] [Google Scholar]
- 17.Mahl TC, Shockcor W, Boyer JL. Primary biliary cirrhosis: survival of a large cohort of symptomatic and asymptomatic patients followed for 24 years. J Hepatol. 1994;20(6):707–713. doi: 10.1016/S0168-8278(05)80139-4. [DOI] [PubMed] [Google Scholar]
- 18.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386(10003):1565–1575. doi: 10.1016/S0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 19.Porayko MK, Wiesner RH, LaRusso NF, Ludwig J, MacCarty RL, Steiner BL, et al. Patients with asymptomatic primary sclerosing cholangitis frequently have progressive disease. Gastroenterology. 1990;98(6):1594–1602. doi: 10.1016/0016-5085(90)91096-O. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007;102(5):1042–1049. doi: 10.1111/j.1572-0241.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 21.Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107–114. doi: 10.1111/j.1572-0241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 22.Yanai H, Matalon S, Rosenblatt A, Awadie H, Berdichevski T, Snir Y, et al. Prognosis of primary sclerosing cholangitis in Israel is independent of coexisting inflammatory bowel Disease. J Crohns Colitis. 2015;9(2):177–184. doi: 10.1093/ecco-jcc/jju013. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg DS, Camp A, Martinez-Camacho A, Forman L, Fortune B, Reddy KR. Risk of waitlist mortality in patients with primary sclerosing cholangitis and bacterial cholangitis. Liver Transpl. 2013;19(3):250–258. doi: 10.1002/lt.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125(5):1364–1369. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145(3):521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J Hepatol. 2008;48(6):939–944. doi: 10.1016/j.jhep.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Hadizadeh M, Abedi SH, Malekpour H, Radinnia E, Jabbehdari S, Padashi M, et al. Prevalence of inflammatory bowel disease among patients with primary sclerosing cholangitis in Iran. Arab J Gastroenterol. 2016;17(1):17–19. doi: 10.1016/j.ajg.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology. 2017;152(8):1975–84.e8. doi: 10.1053/j.gastro.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindkvist B, Benito de Valle M, Gullberg B, Björnsson E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology. 2010;52(2):571–577. doi: 10.1002/hep.23678. [DOI] [PubMed] [Google Scholar]
- 30.Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33(1):99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka A, Takikawa H. Geoepidemiology of primary sclerosing cholangitis: a critical review. J Autoimmun. 2013;46:35–40. doi: 10.1016/j.jaut.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Takakura WR, Tabibian JH, Bowlus CL. The evolution of natural history of primary sclerosing cholangitis. Curr Opin Gastroenterol. 2017;33(2):71–77. doi: 10.1097/MOG.0000000000000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toy E, Balasubramanian S, Selmi C, Li CS, Bowlus CL. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011;11:83. doi: 10.1186/1471-230X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingham JG, Kochar N, Gravenor MB. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004;126(7):1929–1930. doi: 10.1053/j.gastro.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 35.Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, Rajaram R, Rauws EA, Mulder CJ, et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51(4):562–566. doi: 10.1136/gut.51.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindor K. Ursodeoxycholic acid for the treatment of primary biliary cirrhosis. N Engl J Med. 2007;357(15):1524–1529. doi: 10.1056/NEJMct074694. [DOI] [PubMed] [Google Scholar]
- 37.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69(1):394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 38.easloffice@easloffice.eu EAftSotLEa, Liver EAftSot EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Beuers U, Trauner M, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015;62(1 Suppl):S25–37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Beuers U. Drug insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(6):318–328. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 41.Gohlke H, Schmitz B, Sommerfeld A, Reinehr R, Häussinger D. α5 β1-integrins are sensors for tauroursodeoxycholic acid in hepatocytes. Hepatology. 2013;57(3):1117–1129. doi: 10.1002/hep.25992. [DOI] [PubMed] [Google Scholar]
- 42.Dilger K, Hohenester S, Winkler-Budenhofer U, Bastiaansen BA, Schaap FG, Rust C, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57(1):133–140. doi: 10.1016/j.jhep.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Angulo P, Dickson ER, Therneau TM, Jorgensen RA, Smith C, DeSotel CK, et al. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomized trial. J Hepatol. 1999;30(5):830–835. doi: 10.1016/S0168-8278(99)80136-6. [DOI] [PubMed] [Google Scholar]
- 44.Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology. 2000;32(6):1196–1199. doi: 10.1053/jhep.2000.20240. [DOI] [PubMed] [Google Scholar]
- 45.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7(8):678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 46.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375(7):631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 47.Samur S, Klebanoff M, Banken R, Pratt DS, Chapman R, Ollendorf DA, et al. Long-term clinical impact and cost-effectiveness of obeticholic acid for the treatment of primary biliary cholangitis. Hepatology. 2017;65(3):920–928. doi: 10.1002/hep.28932. [DOI] [PubMed] [Google Scholar]
- 48.de Vries EM, Wang J, Leeflang MM, Boonstra K, Weersma RK, Beuers UH, et al. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: evaluation of prognostic value. Liver Int. 2016;36(12):1867–1875. doi: 10.1111/liv.13110. [DOI] [PubMed] [Google Scholar]
- 49.de Vries EMG, Färkkilä M, Milkiewicz P, Hov JR, Eksteen B, Thorburn D, et al. Enhanced liver fibrosis test predicts transplant-free survival in primary sclerosing cholangitis, a multi-centre study. Liver Int. 2017;37(10):1554–1561. doi: 10.1111/liv.13402. [DOI] [PubMed] [Google Scholar]
- 50.de Vries EM, de Krijger M, Färkkilä M, Arola J, Schirmacher P, Gotthardt D, et al. Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: an international cohort study. Hepatology. 2017;65(3):907–919. doi: 10.1002/hep.28963. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Zhang W, Evans JF, Floreani A, Zou Z, Nishio Y, et al. Autotaxin, pruritus and primary biliary cholangitis (PBC) Autoimmun Rev. 2016;15(8):795–800. doi: 10.1016/j.autrev.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Triantos CK, Koukias NM, Nikolopoulou VN, Burroughs AK. Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther. 2011;34(8):901–910. doi: 10.1111/j.1365-2036.2011.04822.x. [DOI] [PubMed] [Google Scholar]
- 53.Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50(3):808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonder A, Retana A, Winston DM, Leung J, Kaplan MM. Prevalence of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol. 2011;9(7):609–612. doi: 10.1016/j.cgh.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Al-Chalabi T, Portmann BC, Bernal W, McFarlane IG, Heneghan MA. Autoimmune hepatitis overlap syndromes: an evaluation of treatment response, long-term outcome and survival. Aliment Pharmacol Ther. 2008;28(2):209–220. doi: 10.1111/j.1365-2036.2008.03722.x. [DOI] [PubMed] [Google Scholar]
- 56.Kaya M, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary sclerosing cholangitis: an evaluation of a modified scoring system. J Hepatol. 2000;33(4):537–542. doi: 10.1016/S0168-8278(00)80004-5. [DOI] [PubMed] [Google Scholar]
- 57.van Buuren HR, van Hoogstraten HJE, Terkivatan T, Schalm SW, Vleggaar FP. High prevalence of autoimmune hepatitis among patients with primary sclerosing cholangitis. J Hepatol. 2000;33(4):543–548. doi: 10.1016/S0168-8278(00)80005-7. [DOI] [PubMed] [Google Scholar]
- 58.Ozaslan E, Efe C, Heurgué-Berlot A, Kav T, Masi C, Purnak T, et al. Factors associated with response to therapy and outcome of patients with primary biliary cirrhosis with features of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2014;12(5):863–869. doi: 10.1016/j.cgh.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 59.Yoshioka Y, Taniai M, Hashimoto E, Haruta I, Shiratori K. Clinical profile of primary biliary cirrhosis with features of autoimmune hepatitis: importance of corticosteroid therapy. Hepatol Res. 2014;44(9):947–955. doi: 10.1111/hepr.12210. [DOI] [PubMed] [Google Scholar]
- 60.Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28(2):296–301. doi: 10.1002/hep.510280203. [DOI] [PubMed] [Google Scholar]
- 61.Freedman BL, Danford CJ, Patwardhan V, Bonder A. Treatment of overlap syndromes in autoimmune liver disease: a systematic review and meta-analysis. J Clin Med. 2020;9(5):1449. doi: 10.3390/jcm9051449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lüth S, Kanzler S, Frenzel C, Kasper HU, Dienes HP, Schramm C, et al. Characteristics and long-term prognosis of the autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. J Clin Gastroenterol. 2009;43(1):75–80. doi: 10.1097/MCG.0b013e318157c614. [DOI] [PubMed] [Google Scholar]
- 63.Floreani A, Rizzotto ER, Ferrara F, Carderi I, Caroli D, Blasone L, et al. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100(7):1516–1522. doi: 10.1111/j.1572-0241.2005.41841.x. [DOI] [PubMed] [Google Scholar]
- 64.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 65.Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56(4):1391–1400. doi: 10.1002/hep.25748. [DOI] [PubMed] [Google Scholar]
- 66.de Vries E, Bolier R, Goet J, Parés A, Verbeek J, de Vree M, et al. Fibrates for Itch (FITCH) in fibrosing cholangiopathies: a double-blind, randomized, placebo-controlled trial. Gastroenterology. 2021;160(3):734–43.e6. doi: 10.1053/j.gastro.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Dubrovsky AMK, Bowlus CL. Statins, fibrates, and other peroxisome proliferator-activated receptor agonists for the treatment of cholestatic liver diseases. Gastroenterol Hepatol (N Y). 2020;16(1):31–38. [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki T, Oba K, Igari Y, Watanabe K, Matsumura N, Futami-Suda S, et al. Effects of bile-acid-binding resin (colestimide) on blood glucose and visceral fat in Japanese patients with type 2 diabetes mellitus and hypercholesterolemia: an open-label, randomized, case-control, crossover study. J Diabetes Complications. 2012;26(1):34–39. doi: 10.1016/j.jdiacomp.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 69.Kishimoto N, Fujii S, Chiba H, Sakuma I, Tsutsui H. Colestimide, an anion exchange resin agent, can decrease the number of LDL particles without affecting their size in patients with hyperlipidemia. J Cardiol. 2010;55(1):65–68. doi: 10.1016/j.jjcc.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Dyson JK, Hirschfield GM, Adams DH, Beuers U, Mann DA, Lindor KD, et al. Novel therapeutic targets in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. 2015;12(3):147–158. doi: 10.1038/nrgastro.2015.12. [DOI] [PubMed] [Google Scholar]
- 71.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29—. Identifier NCT03394924, A Study to Assess the Safety, Tolerability, Pharmacokinetics and Efficacy of EDP-305 in Subjects With Primary Biliary Cholangitis. 2020. https://clinicaltrials.gov/ct2/show/NCT03394924. Accessed 17 May 2021
- 72.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29—. Identifier NCT02516605, A Multi-part, Double Blind Study to Assess Safety, Tolerability and Efficacy of Tropifexor (LJN452) in PBC Patients; 2021. https://clinicaltrials.gov/ct2/show/NCT02516605. Accessed 17 May 2021
- 73.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29 - . Identifier NCT02943447, Study to Evaluate the Safety, Tolerability, and Efficacy of Cilofexor in Adults With Primary Biliary Cholangitis Without Cirrhosis (PBC-Phase 2). 2020. https://clinicaltrials.gov/ct2/show/NCT02943447. Accessed 17 May 2021
- 74.Aagaard MM, Siersbæk R, Mandrup S. Molecular basis for gene-specific transactivation by nuclear receptors. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2011;1812(8):824–835. doi: 10.1016/j.bbadis.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 75.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liberopoulos EN, Florentin M, Elisaf MS, Mikhailidis DP, Tsianos E. Fenofibrate in primary biliary cirrhosis: a pilot study. Open Cardiovasc Med J. 2010;28(4):120–126. doi: 10.2174/1874192401004010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han XF, Wang QX, Liu Y, You ZR, Bian ZL, Qiu DK, et al. Efficacy of fenofibrate in Chinese patients with primary biliary cirrhosis partially responding to ursodeoxycholic acid therapy. J Dig Dis. 2012;13(4):219–224. doi: 10.1111/j.1751-2980.2012.00574.x. [DOI] [PubMed] [Google Scholar]
- 78.Hegade VS, Khanna A, Walker LJ, Wong LL, Dyson JK, Jones DEJ. Long-term fenofibrate treatment in primary biliary cholangitis improves biochemistry but not the UK-PBC Risk Score. Dig Dis Sci. 2016;61(10):3037–3044. doi: 10.1007/s10620-016-4250-y. [DOI] [PubMed] [Google Scholar]
- 79.Corpechot C, Chazouillères O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378(23):2171–2181. doi: 10.1056/NEJMoa1714519. [DOI] [PubMed] [Google Scholar]
- 80.Jones D, Boudes PF, Swain MG, Bowlus CL, Galambos MR, Bacon BR, et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2(10):716–726. doi: 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 81.Schattenberg JM, Pares A, Kowdley KV, Heneghan MA, Caldwell S, Pratt D, et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol. 2021;74:1344–1354. doi: 10.1016/j.jhep.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 82.Mayo MJ, Wigg AJ, Leggett BA, Arnold H, Thompson AJ, Weltman M, et al. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun. 2018;2(9):1037–1050. doi: 10.1002/hep4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsuda M, Moritoki Y, Lian ZX, Zhang W, Yoshida K, Wakabayashi K, et al. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55(2):512–521. doi: 10.1002/hep.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myers RP, Swain MG, Lee SS, Shaheen AA, Burak KW. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013;108(6):933–941. doi: 10.1038/ajg.2013.51. [DOI] [PubMed] [Google Scholar]
- 85.Hirschfield GM, Gershwin ME, Strauss R, Mayo MJ, Levy C, Zou B, et al. Ustekinumab for patients with primary biliary cholangitis who have an inadequate response to ursodeoxycholic acid: a proof-of-concept study. Hepatology. 2016;64(1):189–199. doi: 10.1002/hep.28359. [DOI] [PubMed] [Google Scholar]
- 86.Bowlus CL, Yang GX, Liu CH, Johnson CR, Dhaliwal SS, Frank D, et al. Therapeutic trials of biologics in primary biliary cholangitis: an open label study of abatacept and review of the literature. J Autoimmun. 2019;101:26–34. doi: 10.1016/j.jaut.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Färkkilä M, et al. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67(3):549–558. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 88.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29—. Identifier NCT 03872921, norUrsodeoxycholic Acid vs Placebo in PSC; 2019. https://ClinicalTrials.gov/show/NCT03872921. Accessed 17 May 2021.
- 89.Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73(1):94–101. doi: 10.1016/j.jhep.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mizuno S, Hirano K, Isayama H, Watanabe T, Yamamoto N, Nakai Y, et al. Prospective study of bezafibrate for the treatment of primary sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2015;22(10):766–770. doi: 10.1002/jhbp.281. [DOI] [PubMed] [Google Scholar]
- 91.Angulo P, Batts KP, Jorgensen RA, LaRusso NA, Lindor KD. Oral budesonide in the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95(9):2333–2337. doi: 10.1111/j.1572-0241.2000.02323.x. [DOI] [PubMed] [Google Scholar]
- 92.Hay JE, Lindor KD, Wiesner RH, Dickson ER, Krom RA, LaRusso NF. The metabolic bone disease of primary sclerosing cholangitis. Hepatology. 1991;14(2):257–261. doi: 10.1002/hep.1840140209. [DOI] [PubMed] [Google Scholar]
- 93.Knox TA, Kaplan MM. A double-blind controlled trial of oral-pulse methotrexate therapy in the treatment of primary sclerosing cholangitis. Gastroenterology. 1994;106(2):494–499. doi: 10.1016/0016-5085(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 94.Kyokane K, Ichihara T, Horisawa M, Suzuki N, Ichihara S, Suga S, et al. Successful treatment of primary sclerosing cholangitis with cyclosporine and corticosteroid. Hepatogastroenterology. 1994;41(5):449–452. [PubMed] [Google Scholar]
- 95.Epstein MP, Kaplan MM. A pilot study of etanercept in the treatment of primary sclerosing cholangitis. Dig Dis Sci. 2004;49(1):1–4. doi: 10.1023/B:DDAS.0000011827.87103.2e. [DOI] [PubMed] [Google Scholar]
- 96.Hommes DW, Erkelens W, Ponsioen C, Stokkers P, Rauws E, van der Spek M, et al. A double-blind, placebo-controlled, randomized study of infliximab in primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42(5):522–526. doi: 10.1097/MCG.0b013e3181662426. [DOI] [PubMed] [Google Scholar]
- 97.Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98(2):414–423. doi: 10.1016/0016-5085(90)90833-M. [DOI] [PubMed] [Google Scholar]
- 98.Damman JL, Rodriguez EA, Ali AH, Buness CW, Cox KL, Carey EJ, et al. Review article: the evidence that vancomycin is a therapeutic option for primary sclerosing cholangitis. Aliment Pharmacol Ther. 2018;47(7):886–895. doi: 10.1111/apt.14540. [DOI] [PubMed] [Google Scholar]
- 99.Davies YK, Cox KM, Abdullah BA, Safta A, Terry AB, Cox KL. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. J Pediatr Gastroenterol Nutr. 2008;47(1):61–67. doi: 10.1097/MPG.0b013e31816fee95. [DOI] [PubMed] [Google Scholar]
- 100.Rahimpour S, Nasiri-Toosi M, Khalili H, Ebrahimi-Daryani N, Nouri-Taromlou MK, Azizi Z. A triple blinded, randomized, placebo-controlled clinical trial to evaluate the efficacy and safety of oral vancomycin in primary sclerosing cholangitis: a pilot study. J Gastrointestin Liver Dis. 2016;25(4):457–464. doi: 10.15403/jgld.2014.1121.254.rah. [DOI] [PubMed] [Google Scholar]
- 101.Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: a randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol. 2008;20(7):688–692. doi: 10.1097/MEG.0b013e3282f5197e. [DOI] [PubMed] [Google Scholar]
- 102.Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T, et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol. 2019;4(3):492–503. doi: 10.1038/s41564-018-0333-1. [DOI] [PubMed] [Google Scholar]