Abstract
Several professional organizations have recommended tramadol as one of the first-line or second-line therapies for patients with chronic noncancer pain and its prescription has been increasing rapidly worldwide; however, the safety profile of tramadol, such as risk of fracture, remains unclear. This study aimed to examine the association of tramadol with risk of hip fracture. Among individuals age 50 years or older without a history of hip fracture, cancer, or opioid use disorder in The Health Improvement Network (THIN) database in the United Kingdom general practice (2000–2017), five sequential propensity-score matched cohort studies were assembled, i.e., participants who initiated tramadol or those who initiated one of the following medications: codeine (n=146,956) (another commonly used weak opioid), naproxen (n=115,109) or ibuprofen (n=107,438) (commonly used nonselective non-steroidal anti-inflammatory drugs [NSAIDs]), celecoxib (n=43,130) or etoricoxib (n=27,689) (cyclooxygenase-2 inhibitors). The outcome was incident hip fracture over one year. After propensity-score matching, the included participants had a mean age of 65.7 years, and 56.9% were women. During the one-year follow-up, 518 hip fracture (3.7/1000 person-years) occurred in the tramadol cohort and 401 (2.9/1000 person-years) occurred in the codeine cohort. Compared with codeine, hazard ratio (HR) of hip fracture for tramadol was 1.28 (95% confidence interval[CI]:1.13–1.46). Risk of hip fracture was also higher in the tramadol cohort than in the naproxen (2.9/1000 person-years for tramadol, 1.7/1000 person-years for naproxen; HR=1.69, 95%CI:1.41–2.03), ibuprofen (3.4/1000 person-years for tramadol, 2.0/1000 person-years for ibuprofen; HR=1.65, 95%CI:1.39–1.96), celecoxib (3.4/1000 person-years for tramadol, 1.8/1000 person-years for celecoxib; HR=1.85, 95%CI:1.40–2.44), or etoricoxib (2.9/1000 person-years for tramadol, 1.5/1000 person-years for etoricoxib; HR=1.96, 95%CI:1.34–2.87) cohort. In this population-based cohort study, the initiation of tramadol was associated with a higher risk of hip fracture than initiation of codeine and commonly used NSAIDs, suggesting a need to re-visit several guidelines on tramadol use in clinical practice.
Keywords: Tramadol, Fracture, Cohort
INTRODUCTION
In the general population aged 50 years and older, about 20% of men and 50% of women are likely to sustain at least one fracture during the remainder of their lives, which often results in increased morbidity and mortality.(1,2) The healthcare burden related to fracture is expected to double by 2025.(3–5) Polypharmacy is common among elderly patients due to multiple comorbidities, and some medications may intensify the risk of fracture, either through their effect on increasing fall risk and/or through an effect on bone metabolism.(6–9)
Tramadol, a commonly used weak opioid for the treatment of pain,(10–14) has been considered an analgesic alternative, since its perceived risk of serious cardiovascular and gastrointestinal adverse effects was lower than that of non-steroidal anti-inflammatory drugs (NSAIDs),(15,16) and its risk of addiction and respiratory depression was lower than that of traditional opioids.(17,18) As a result, tramadol use has been increasing rapidly worldwide over the past decades.(10–14) For example, data from Truven Health Analytics MarketScan in the United States showed that prescriptions of tramadol increased by 22.8% between 2012 and 2015,(10) and tramadol dispensing rates increased in each of the provinces in Canada, with the highest in Nova Scotia increasing from 0.50/defined daily doses (DDD) in 2007 to 2.64/DDD in 2016.(11)
Nevertheless, a recently-published population-based cohort study reported a significantly higher all-cause mortality rate with tramadol use than with commonly used NSAIDs among patients with osteoarthritis;(14) however, the specific mechanisms linking tramadol use to an increased risk of mortality remains unclear. To date, several studies have reported that tramadol use might increase the risk of falls (a strong risk factor for fracture),(19–22) but only a few studies have addressed the potential relationship between tramadol use and the risk of fracture, and the results are inconclusive.(23–25) Furthermore, few, if any, studies have compared the risk of hip fracture, one that ranks among the top 10 leading causes of disability globally,(26,27) among tramadol initiators with that among initiators of other commonly used analgesics.
To fill this knowledge gap, we compared the risk of incident hip fracture among tramadol initiators with the initiators of one of the following medications: codeine (another commonly used weak opioid), naproxen or ibuprofen (commonly used nonselective NSAIDs), celecoxib or etoricoxib (cyclooxygenase-2 [COX-2] inhibitors) by conducting five propensity-score matched cohort studies.
MATERIALS AND METHODS
Data Source
This study was based on the data retrieved from The Health Improvement Network (THIN), which contains medical records of about 17 million individuals from 770 general practices in the United Kingdom (UK). In THIN, the following data was recorded for each patient: anthropometrics, socio-demographics, lifestyle habits, GP visit details, diagnoses from specialists’ evaluations and hospital admissions, as well as laboratory testing results. All the diagnoses in THIN were coded by the Read classification system (28) while the medications were coded by Multilex classification system.(29) Previous studies have demonstrated that THIN data was valid for both epidemiological and clinical studies.(30)
Study Design and Cohort Definition
Included in this analysis were participants who were 50 years or older between January 2000 and December 2016 and had not been prescribed tramadol or its active comparator (i.e., codeine, naproxen, ibuprofen, celecoxib, or etoricoxib) over one year or more before entering this study. Individuals who had a history of hip fracture, cancer, or opioid use disorder prior to entry into this study cohort were excluded.
We compared the risk of incident hip fracture between participants who initiated tramadol and those who initiated one of the following pain-relief medications: codeine (another commonly used weak opioid), naproxen or ibuprofen (commonly used nonselective NSAIDs), celecoxib or etoricoxib (COX-2 inhibitors). The index date is defined as the date of initiating either tramadol or the comparator for the corresponding participants. The total time interval from January 2000 to December 2016 was divided into 17 one-year blocks. Within each time block, we identified tramadol or the comparator initiators and calculated the propensity-score for tramadol initiation using logistic regression. Propensity score is defined as the probability of treatment assignment conditional on observed baseline characteristics,(31) which can be calculated based on the following variables: age at index date, sex, Townsend Deprivation Index(32), body mass index (BMI), alcohol drinking habits, smoking status, comorbidities and medication use before the index date, and healthcare utilization (i.e., number of hospitalization, general practice visit, and specialist referral during the past year prior to the index date (variables listed in Table 1). Within each time block tramadol initiators were matched 1:1 to the comparator initiators using the greedy-matching algorithm, i.e., for each tramadol initiator, a comparator initiator with the closest propensity score was selected(31). Propensity score matching is used to balance many covariates in epidemiological studies and to reduce the effect of confounding by indication.(31) We adopted this method to assemble five propensity-score matched cohort studies: tramadol vs. codeine, tramadol vs. naproxen, tramadol vs. ibuprofen, tramadol vs. celecoxib, and tramadol vs. etoricoxib, respectively.
Table 1.
Basic Characteristics of Tramadol Cohort Compared with Codeine Cohort
| Before propensity-score matched | Propensity-score matched | |||||
|---|---|---|---|---|---|---|
| Tramadol | Codeine | Standard difference | Tramadol | Codeine | Standard difference | |
| Participants, n | 202,003 | 170,369 | 146,956 | 146,956 | ||
| Demographics | ||||||
| Age, mean (SD), y | 65.9 (10.0) | 67.1 (10.3) | 0.112 | 66.5 (10.1) | 66.5 (10.1) | 0.001 |
| Socioeconomic deprivation index, mean (SD) † | 2.8 (1.4) | 2.6 (1.3) | 0.122 | 2.7 (1.3) | 2.7 (1.3) | 0.001 |
| Female (%) | 57.5 | 57.9 | 0.009 | 57.4 | 57.5 | 0.001 |
| BMI, mean (SD), kg/m2 | 28.5 (5.8) | 27.8 (5.4) | 0.126 | 28.1 (5.5) | 28.1 (5.5) | 0.001 |
| Lifestyle factors | ||||||
| Drinking (%) | 0.030 | 0.002 | ||||
| None | 21.1 | 19.9 | 20.1 | 20.2 | ||
| Past | 3.0 | 2.9 | 2.8 | 2.8 | ||
| Current | 75.9 | 77.2 | 77.1 | 77.0 | ||
| Smoking (%) | 0.132 | 0.003 | ||||
| None | 47.2 | 52.0 | 50.3 | 50.4 | ||
| Past | 31.8 | 31.9 | 32.1 | 32.1 | ||
| Current | 20.9 | 16.1 | 17.6 | 17.5 | ||
| Comorbidity (%) | ||||||
| Other fracture# | 8.3 | 7.7 | 0.022 | 7.9 | 7.8 | 0.001 |
| Fall | 11.2 | 12.6 | 0.044 | 11.9 | 11.8 | <0.001 |
| Osteoporosis | 9.0 | 7.9 | 0.038 | 8.2 | 8.2 | 0.001 |
| Seizure | 0.6 | 0.7 | 0.010 | 0.6 | 0.6 | 0.001 |
| Diabetes | 15.2 | 14.9 | 0.009 | 14.8 | 14.8 | 0.001 |
| Hypertension | 45.8 | 46.4 | 0.010 | 46.0 | 46.1 | 0.001 |
| Liver disease | 2.5 | 2.5 | 0.001 | 2.5 | 2.5 | 0.001 |
| Chronic kidney disease | 8.3 | 9.2 | 0.033 | 8.7 | 8.7 | 0.001 |
| Transient ischaemic attack | 3.2 | 3.5 | 0.013 | 3.3 | 3.3 | 0.001 |
| Ischaemic heart disease | 16.6 | 16.0 | 0.018 | 16.1 | 16.0 | 0.002 |
| Congestive heart failure | 3.6 | 3.9 | 0.017 | 3.6 | 3.6 | 0.001 |
| Myocardial infarction | 6.5 | 6.3 | 0.007 | 6.4 | 6.3 | 0.001 |
| Stroke | 4.0 | 4.4 | 0.025 | 4.1 | 4.1 | 0.001 |
| Angina | 10.5 | 10.2 | 0.010 | 10.2 | 10.2 | <0.001 |
| Peripheral vascular disease | 2.4 | 1.8 | 0.044 | 2.0 | 2.0 | 0.003 |
| Venous thromboembolism | 3.6 | 3.3 | 0.014 | 3.4 | 3.4 | 0.001 |
| Pneumonia or infection | 7.4 | 7.4 | 0.002 | 7.4 | 7.3 | 0.001 |
| Hyperlipidaemia | 15.3 | 14.6 | 0.020 | 14.7 | 14.8 | 0.001 |
| Dementia | 0.7 | 1.4 | 0.074 | 0.8 | 0.9 | 0.002 |
| Varicose veins | 10.2 | 10.3 | 0.001 | 10.3 | 10.3 | 0.001 |
| Other circulatory disease | 28.3 | 28.8 | 0.010 | 28.6 | 28.5 | 0.001 |
| Osteoarthritis | 33.9 | 28.5 | 0.116 | 30.4 | 30.5 | 0.001 |
| Rheumatoid arthritis | 2.8 | 1.9 | 0.057 | 2.2 | 2.1 | 0.004 |
| Depression | 15.1 | 13.0 | 0.062 | 13.5 | 13.5 | 0.001 |
| Chronic obstructive pulmonary disease | 7.2 | 6.0 | 0.046 | 6.4 | 6.3 | 0.003 |
| Atrial fibrillation | 5.3 | 6.4 | 0.046 | 5.8 | 5.8 | <0.001 |
| Anxiety | 15.8 | 15.0 | 0.023 | 15.1 | 15.1 | <0.001 |
| Sleep disorder or sleep apnea | 1.9 | 1.6 | 0.020 | 1.7 | 1.7 | <0.001 |
| Peptic ulcer | 7.8 | 6.6 | 0.047 | 7.0 | 6.9 | 0.003 |
| Alcohol abuse | 3.6 | 2.6 | 0.058 | 2.8 | 2.8 | 0.002 |
| Medication (%) | ||||||
| Other opioids* | 19.0 | 11.0 | 0.227 | 12.8 | 12.4 | 0.010 |
| Other NSAIDs* | 79.1 | 69.9 | 0.212 | 74.5 | 74.7 | 0.005 |
| Aspirin | 34.9 | 34.2 | 0.016 | 34.2 | 34.0 | 0.003 |
| Bisphosphonates | 8.1 | 6.7 | 0.052 | 7.1 | 7.0 | 0.004 |
| Statin | 40.6 | 38.2 | 0.050 | 38.8 | 38.8 | <0.001 |
| Glucocorticoids | 23.6 | 21.7 | 0.047 | 22.4 | 22.2 | 0.005 |
| Nitrates | 15.4 | 14.4 | 0.028 | 14.6 | 14.5 | 0.003 |
| Antihypertensive medicine | 64.7 | 63.1 | 0.035 | 63.4 | 63.4 | <0.001 |
| Antidiabetic medicine | 11.5 | 10.9 | 0.017 | 11.1 | 11.0 | 0.001 |
| ACE inhibitors | 34.3 | 34.8 | 0.010 | 34.5 | 34.5 | 0.001 |
| Beta receptor inhibitors | 35.2 | 35.3 | 0.001 | 35.1 | 35.1 | <0.001 |
| Calcium channel blockers | 32.0 | 31.3 | 0.015 | 31.4 | 31.3 | 0.001 |
| Loop diuretics | 19.2 | 17.5 | 0.044 | 17.7 | 17.6 | 0.002 |
| Thiazide diuretics | 33.0 | 32.7 | 0.006 | 32.7 | 32.8 | 0.001 |
| Potassium-sparing diuretics | 8.5 | 7.6 | 0.034 | 7.8 | 7.7 | 0.003 |
| Angiotensin receptor blocker | 12.4 | 12.0 | 0.011 | 12.3 | 12.0 | 0.009 |
| Insulin | 3.4 | 3.0 | 0.019 | 3.1 | 3.1 | <0.001 |
| Anticoagulants | 7.1 | 7.8 | 0.025 | 7.4 | 7.3 | 0.001 |
| Benzodiazepines | 41.0 | 32.5 | 0.178 | 35.2 | 35.1 | 0.002 |
| SSRI | 26.8 | 22.0 | 0.113 | 23.2 | 23.2 | <0.001 |
| SNRI | 7.9 | 5.7 | 0.085 | 6.2 | 6.2 | 0.002 |
| Antiepileptic medicine | 10.7 | 7.5 | 0.112 | 8.3 | 8.2 | 0.004 |
| Estrogen | 19.2 | 18.0 | 0.029 | 18.5 | 18.6 | 0.002 |
| PPIs | 54.0 | 46.7 | 0.148 | 49.3 | 49.2 | 0.003 |
| H2 blockers | 24.7 | 21.5 | 0.076 | 22.7 | 22.5 | 0.005 |
| Healthcare utilization, mean (SD) | ||||||
| Hospitalizations‡ | 0.5 (1.2) | 0.4 (1.1) | 0.037 | 0.4 (1.1) | 0.4 (1.1) | 0.005 |
| General practice visits‡ | 7.2 (6.6) | 6.9 (6.5) | 0.045 | 7.0 (6.6) | 7.0 (6.4) | 0.003 |
| Specialist referrals‡ | 0.6 (1.1) | 0.5 (1.0) | 0.095 | 0.6 (1.0) | 0.6 (1.0) | 0.004 |
BMI, body mass index; n, number; y, years; SD, standard deviation; NSAID, non-steroidal anti-inflammatory drug; ACE, angiotensin converting enzyme; SSRI, Selective serotonin reuptake inhibitor; SNRI, Serotonin-norepinephrine reuptake inhibitor; PPIs, proton pump inhibitor; H2 blockers, histamine-2 blockers.
The Socio-Economic Deprivation Index (i.e., Townsend Deprivation Index) was grouped into quintiles from 1 (least deprived) to 5 (most deprived).
Other fracture refers to spine and wrist fracture.
Other NSAIDs or opioids means other NSAIDs or opioids use prior to the index date.
Frequency during the past one year.
Assessment of Outcome
The incident hip fracture during a one-year follow-up period was the primary outcome of the study. Hip fracture was identified by using Read Codes as previous studies have done in THIN.(33–35)
Statistical Analysis
The baseline characteristics of the tramadol cohort were compared with that of each of the active comparison cohorts, i.e., codeine, naproxen, ibuprofen, celecoxib, or etoricoxib cohort. We adopted an “intention-to-treat” analysis method to compute the follow-up time for each participant, while person-years of follow-up for each participant were calculated as the time frame from the index date to the earliest occurrence of the following: incident hip fracture, disenrollment from a GP practice, age of ninety, death, or the end of one year follow-up. We computed the rate of incident hip fracture for each cohort and plotted cumulative incidence curves of hip fracture. We calculated the absolute rate difference (RD) in incident hip fracture between the tramadol cohort and each of the active comparison cohorts according to the following formula: RD = rate (tramadol) - rate (comparison). The hazard ratio (HR) of incident hip fracture for the tramadol initiation was obtained using cause-specific Cox proportional hazard models accounting for the competing risk of death when compared with each comparator.(36) We performed the sex-specific analyses to test whether the relation of tramadol initiation to the risk of hip fracture in men differed from that in women.
A total of seven sensitivity analyses were performed to test the robustness of findings. Firstly, we excluded the participants with a propensity-score above the 97.5th percentile of the propensity-score of the comparator cohort and below the 2.5th percentile of the propensity-score of the tramadol cohort.(37) Secondly, we restricted our analyses to the participants who were not prescribed other opioids before index date to minimize the residual confounding effect by indication. Thirdly, we performed missing data imputation analyses and imputed five datasets in total. We calculated the effect estimates and their confidence intervals (CIs) from each imputed dataset. Then, we calculated the overall effect estimate and its confidence intervals from five imputed datasets using Rubin’s rules.(38) Fourthly, we performed an “as-treated” analysis to account for non-adherence of medications under investigation throughout study period. Specifically, individuals were followed from the index date until the earliest occurrence of the following: an incident hip fracture, disenrollment from a GP practice, age of ninety, death, the end of a one-year follow-up period, drug discontinuation or change of initiated medication (e.g., swapping from tramadol to codeine or vice versa, while comparing the two). If a participant had not refilled a prescription for a period of more than 60 days,(39) the follow-up time would be censored at that time. Fifthly, we conducted quantitative sensitivity analyses to evaluate the minimum unmeasured confounding effect that would explain away an association observed in previous analyses.(40) Sixthly, we conducted a sensitivity analysis for atraumatic hip fracture. Specifically, the atraumatic hip fracture was considered as the outcome, and cause-specific Cox proportional hazard models accounting for the competing risk of death were performed when compared with each comparison cohort. Lastly, we performed a sensitivity analysis restricted to individuals aged 60 years or older.
All statistical analyses were performed on SAS V.9.4 with P < 0.05 as statistical significance.
RESULTS
Of 3,755,932 patients who met the inclusion criteria, 612,981 patients initiated with either tramadol (n= 337,167) or codeine (n= 275,814) treatment without prescription history of both drugs before entering this study. We excluded 102,483 patients who had a history of cancer, opioid use disorder, or hip fracture, and 138,126 patients who had missing information on BMI, smoking status, alcohol drinking, or Townsend Deprivation Index Score. Of the remaining (n=372,372), 146,956 initiators of tramadol (72.7%) were matched to the same number of initiators of codeine by propensity-score (Figure 1). The selection process for the other four propensity-score matched cohorts is illustrated in the Appendix.
Figure 1.
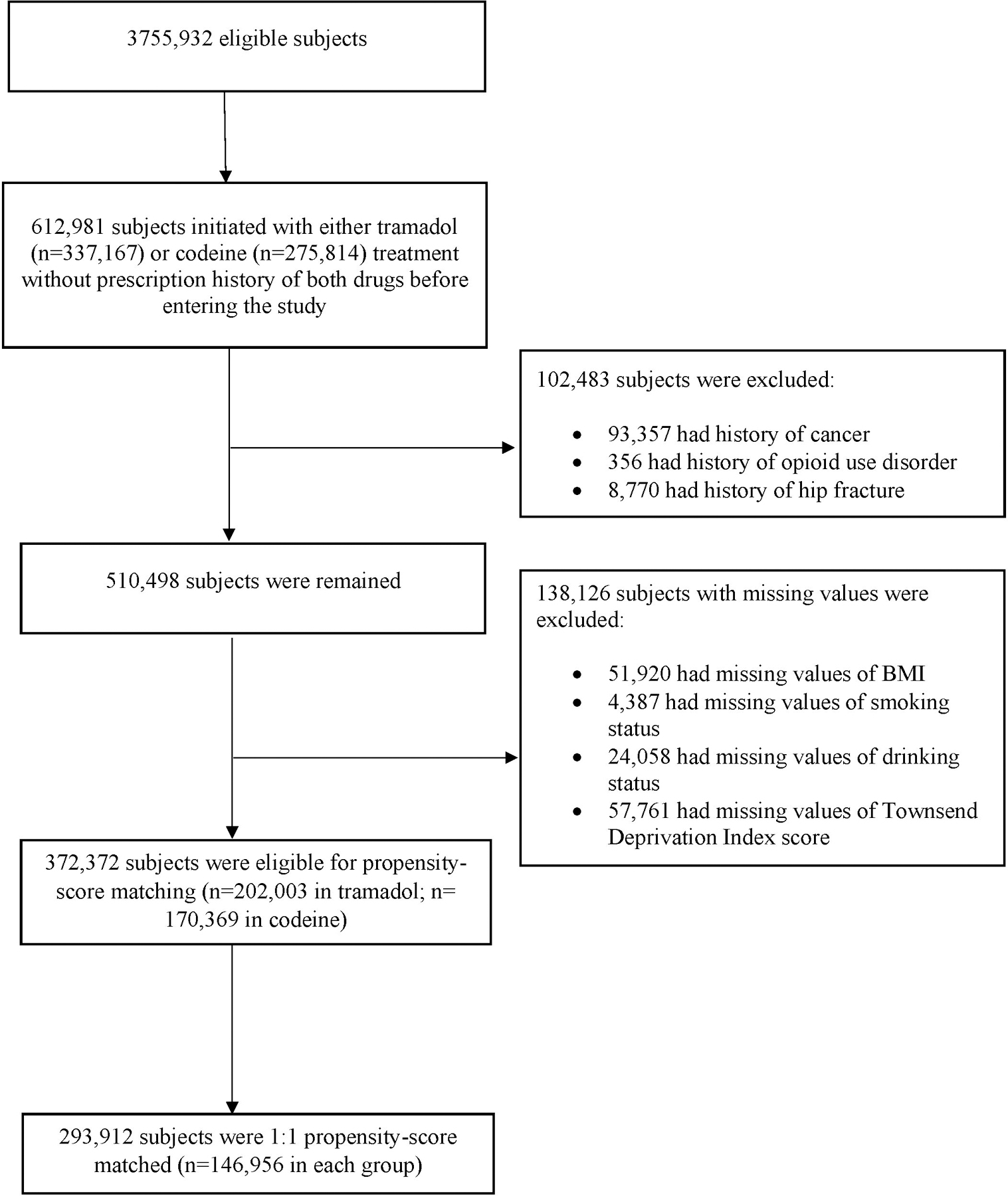
Selection Process of Propensity-score Matched Cohorts of Patients with Noncancer Pain and Tramadol Initiation Comparing with Initiation of Codeine.
The baseline characteristics of each before and after propensity-score matched cohort are presented in Table 1 and Appendix. The mean age was between 65.0 and 66.5 years in different propensity-score matched cohorts, and approximately 60% were women. Overall, the characteristics across the propensity-score matched cohorts were balanced, with all of the standardized differences < 0.1(41).
The tramadol cohort had a higher risk of incident hip fracture than did the codeine cohort (Figure 2). As shown in Table 2, a total of 518 cases of hip fracture (3.7/1000 person-years) were reported in the tramadol cohort and 401 cases (2.9/1000 person-years) were reported in the codeine cohort during the one year follow-up. The RD of incident hip fracture in the tramadol cohort vs. that in the codeine cohort was 0.8 (95% CI: 0.4 to 1.2) /1000 person-years and the corresponding HR was 1.28 (95% CI: 1.13 to 1.46) (Figure 3). Meanwhile, the tramadol cohort also exhibited a higher risk of incident hip fracture than did the codeine cohort among both the female (HR = 1.18, 95% CI: 1.02 to 1.38) and male subgroup (HR = 1.60, 95% CI: 1.24 to 2.06) (Figure 3, RDs were showed in Appendix). The results of sensitivity analyses (i.e., propensity-score trimming, restricting analyses among the participants without history of other opioids use, missing data imputation, “as-treated” approach, or restricting outcome to atraumatic hip fracture) did not change materially (Appendix). Furthermore, according to the quantitative sensitivity analyses, the observed association (i.e., HR = 1.28) might be explained by the residual confounding effect if there is an unmeasured covariate with HR≥1.88 with both tramadol use and risk of hip fracture.
Figure 2.
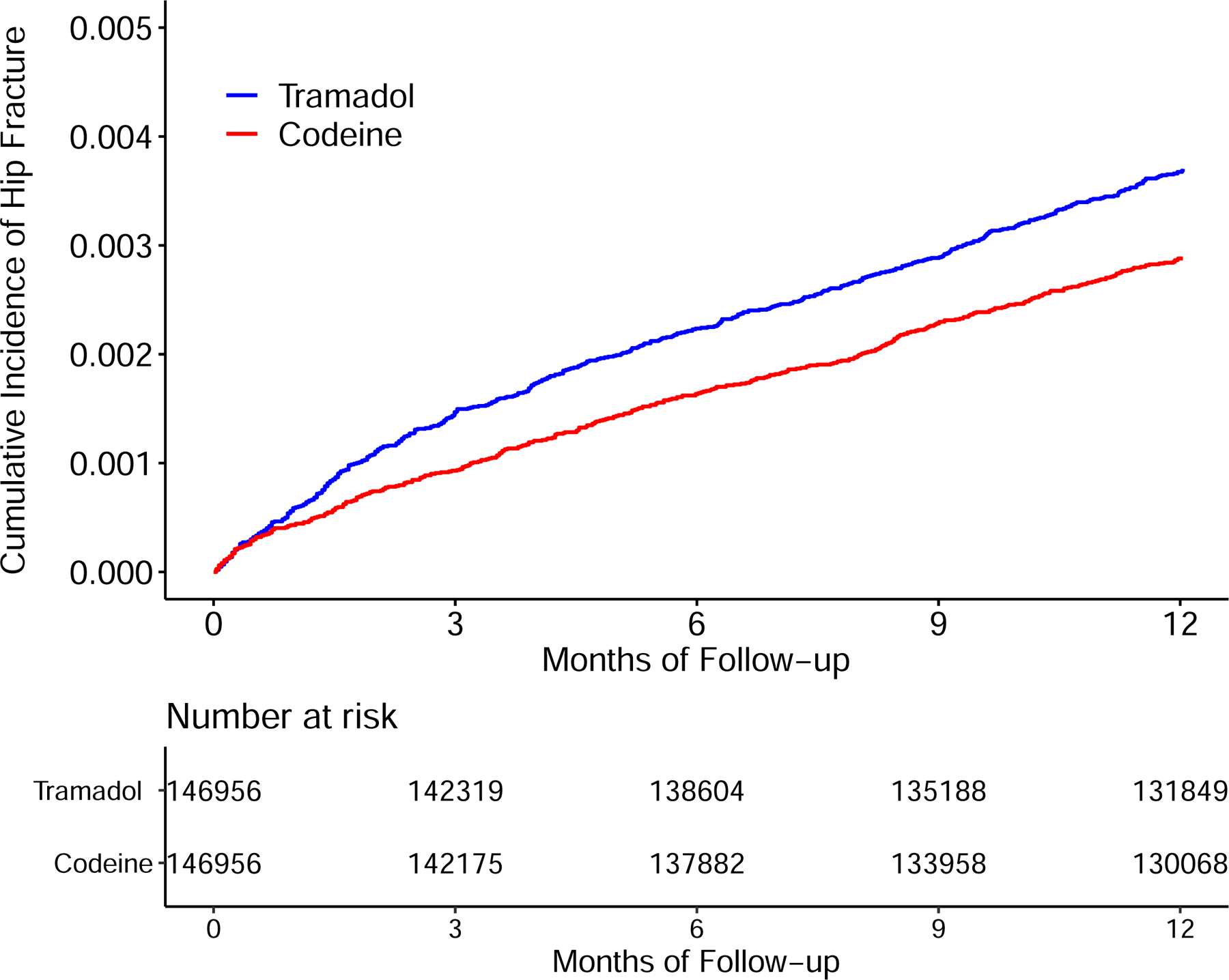
Time to Incident Hip Fracture for the Propensity-score Matched Cohorts of Patients with Noncancer Pain and Tramadol Initiation Comparing with Initiation of Codeine.
Table 2.
Incident Hip Fracture Within One Year Among Patients Initiating Tramadol Comparing with Initiation of another Commonly Used Weak Opioid (Codeine)
| Weak opioid |
||
|---|---|---|
| Tramadol vs. Codeine | ||
| Participants (n) | 146,956 | 146,956 |
| Incident hip fracture (n) | 518 | 401 |
| Mean follow-up (years) | 0.95 | 0.94 |
| Rate (/1000 person-years)* | 3.7 | 2.9 |
| RD (/1000 person-years, 95% CI) | 0.8 (0.4, 1.2) | 0.0 (reference) |
n, number; RD, rate difference; 95% CI, 95% confidence interval.
Number (rate) of competing event (i.e., death) in tramadol and codeine group was 5,449 (39.2/1000 person-years) and 4,984 (36.0/1000 person-years), respectively.
Figure 3.
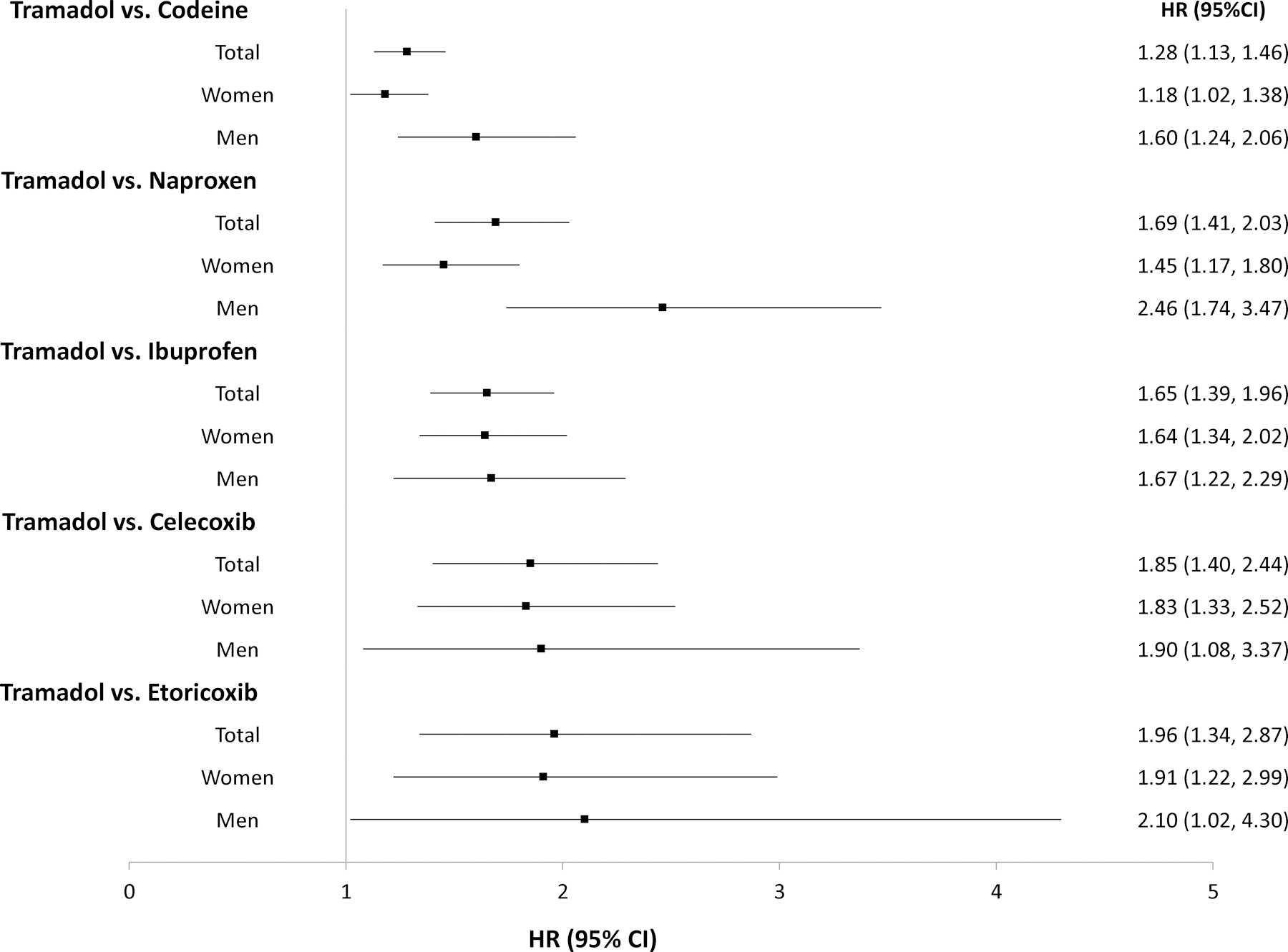
Forest Plot of Hazard Ratios and Related 95% Confidence Intervals of Hip Fracture for the Propensity-score Matched Cohorts of Patients with Noncancer Pain and Tramadol Initiation Comparing with Initiation of Codeine, Naproxen, Ibuprofen, Celecoxib, or Etoricoxib.
The tramadol cohort also had a higher risk of incident hip fracture than did either the naproxen (Figure 4A) or the ibuprofen (Figure 4B) cohort. As shown in Table 3, a total of 313 cases of incident hip fracture (2.9/1000 person-years) were reported in the tramadol cohort and 185 (1.7/1000 person-years) cases were reported in the naproxen cohort. Relative to naproxen initiation, the HR of hip fracture for initiation of tramadol was 1.69 (95% CI: 1.41 to 2.03) (Figure 3) and the corresponding RD was 1.2 (95% CI: 0.8 to 1.6) /1000 person-years (Table 3). Similarly, the risk of incident hip fracture was also higher in the tramadol cohort (3.4/1000 person-years) than in the ibuprofen cohort (2.0/1000 person-years) (HR = 1.65, 95% CI: 1.39 to 1.96) (Table 3 and Figure 3). Results from sex subgroup analyses (Figure 3, Appendix) and several sensitivity analyses did not change materially (Appendix).
Figure 4.
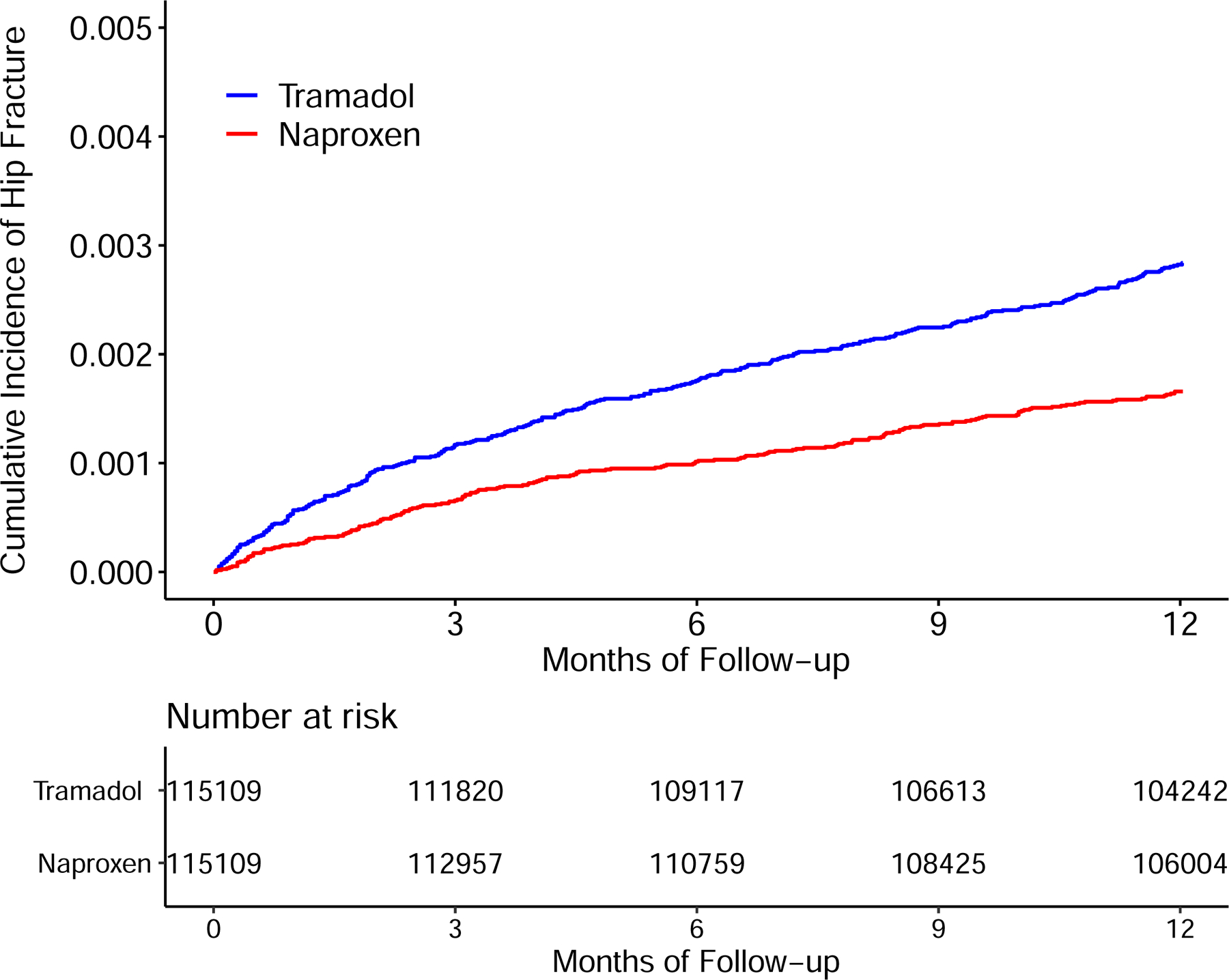
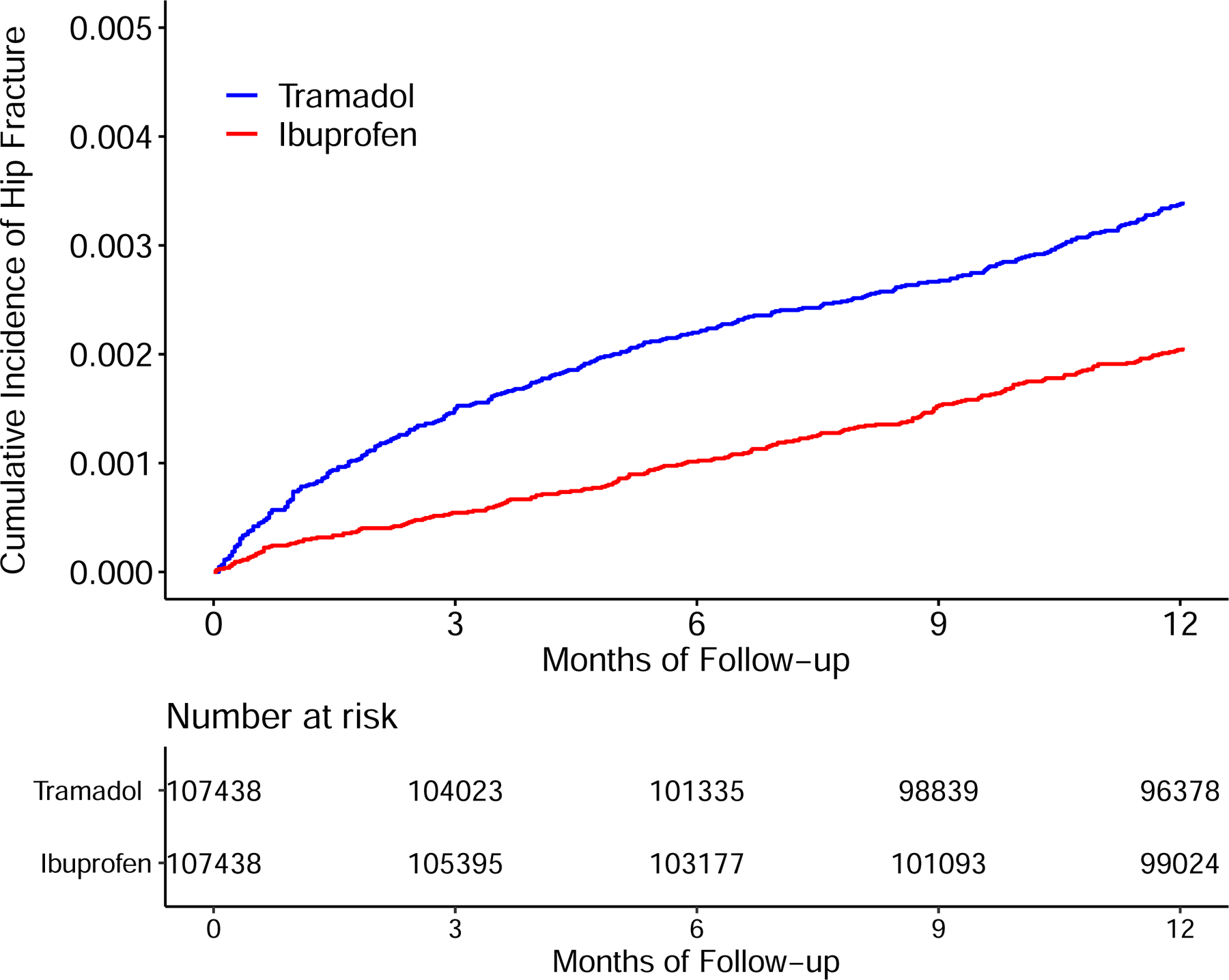
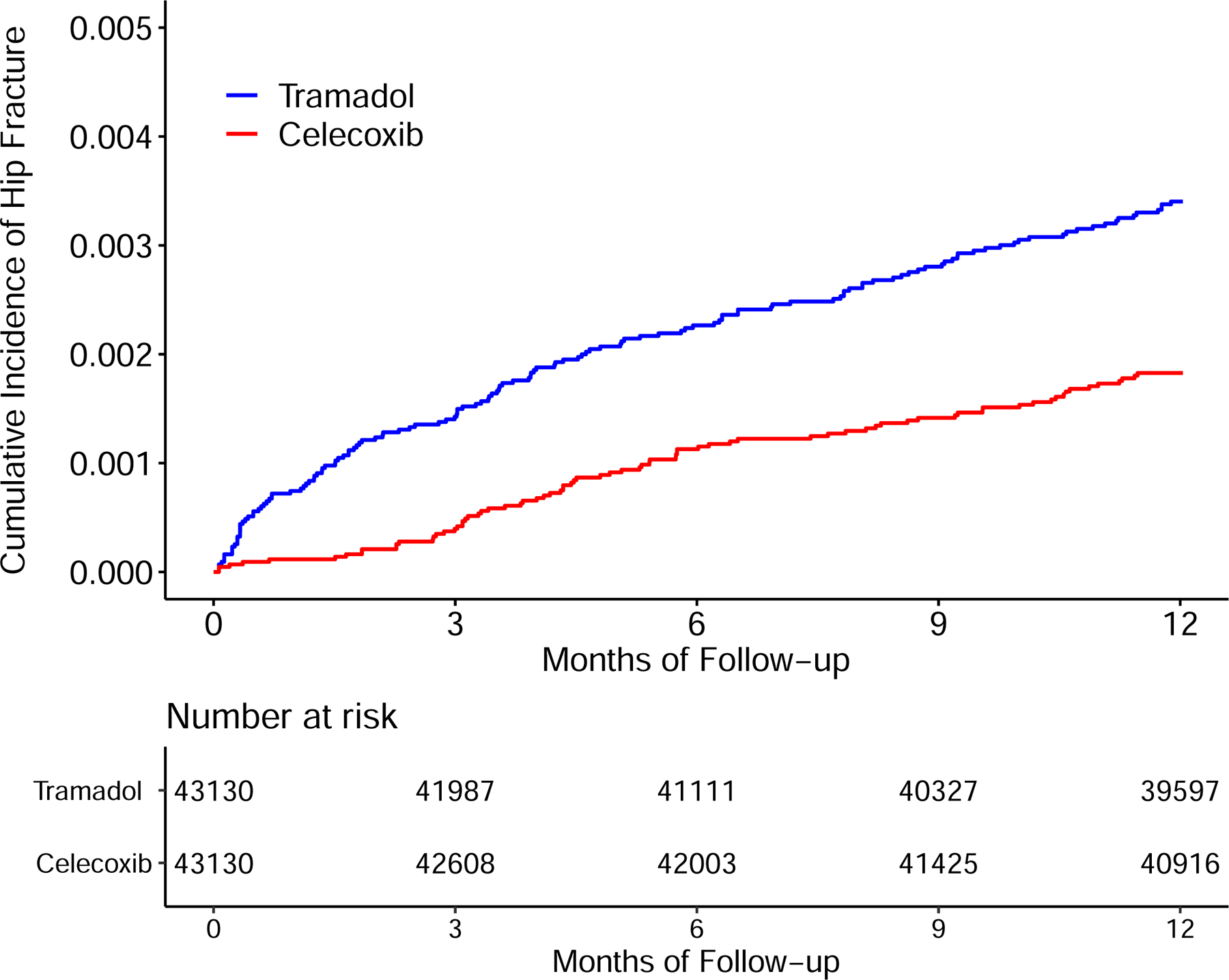
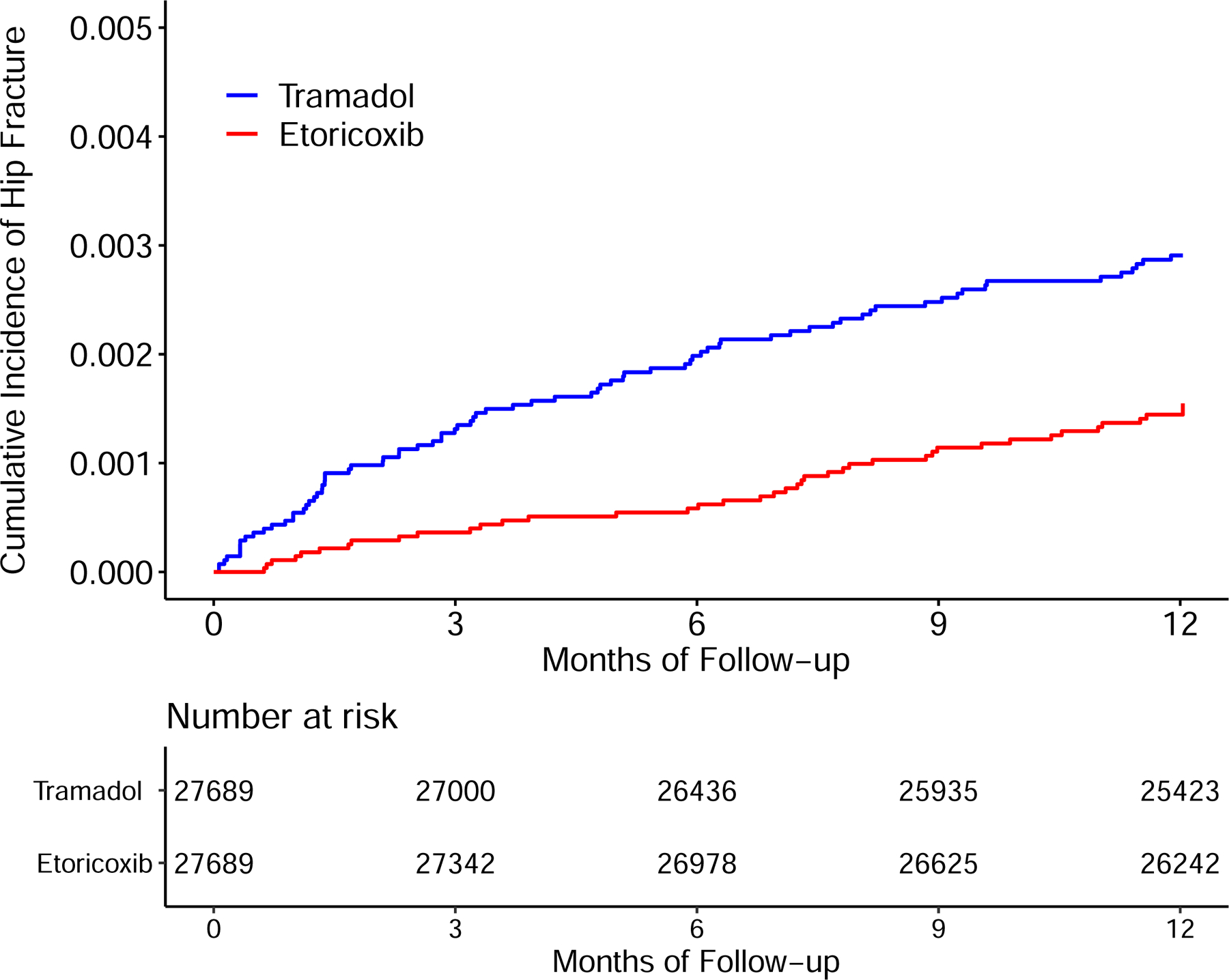
Time to Incident Hip Fracture for the Propensity-score Matched Cohorts of Patients with Noncancer Pain and Tramadol Initiation Comparing with Initiation of Naproxen (A), Ibuprofen (B), Celecoxib (C), or Etoricoxib (D).
Table 3.
Incident Hip Fracture Within One Year Among Patients Initiating Tramadol Comparing with Initiation of One of Two Commonly Used Nonselective NSAIDs (Naproxen, Ibuprofen) or One of Two Cyclooxygenase-2 Inhibitors (Celecoxib, Etoricoxib)
| Nonselective NSAIDs |
||||
|---|---|---|---|---|
| Tramadol vs. Naproxen | Tramadol vs. Ibuprofen | |||
| Participants (n) | 115,109 | 115,109 | 107,438 | 107,438 |
| Incident hip fracture (n) | 313 | 185 | 349 | 212 |
| Mean follow-up (years) | 0.95 | 0.96 | 0.95 | 0.96 |
| Rate (/1000 person-years)* | 2.9 | 1.7 | 3.4 | 2.0 |
| RD (/1000 person-years, 95% CI) | 1.2 (0.8, 1.6) | 0.0 (reference) | 1.4 (0.9, 1.8) | 0.0 (reference) |
| Cyclooxygenase-2 Inhibitors |
||||
| Tramadol vs. Celecoxib | Tramadol vs. Etoricoxib | |||
| Participants (n) | 43,130 | 43,130 | 27,689 | 27,689 |
| Incident hip fracture (n) | 142 | 77 | 78 | 40 |
| Mean follow-up (years) | 0.96 | 0.98 | 0.96 | 0.98 |
| Rate (/1000 person-years) | 3.4 | 1.8 | 2.9 | 1.5 |
| RD (/1000 person-years, 95% CI) | 1.6 (0.9, 2.3) | 0.0 (reference) | 1.5 (0.7, 2.3) | 0.0 (reference) |
NSAIDs, non-steroidal anti-inflammatory drugs; n, number; RD, rate difference; 95% CI, 95% confidence interval.
Number (rate) of competing event (i.e., death) in comparison of tramadol and naproxen group was 3,418 (31.2/1000 person-years) and 1,431 (12.9/1000 person-years), in comparison of tramadol and ibuprofen group was 3,958 (38.9/1000 person-years) and 1,977 (19.1/1000 person-years), in comparison of tramadol and celecoxib group was 1,776 (43.3/1000 person-years) and 751 (17.8/1000 person-years), in comparison of tramadol and etoricoxib group was 877 (33.1/1000 person-years) and 366 (13.5/1000 person-years), respectively.
The risk of incident hip fracture was higher in the tramadol cohort than in either the celecoxib cohort (3.4/1000 person-years vs. 1.8/1000 person-years) (Figure 4C) or the etoricoxib cohort (2.9/1000 person-years vs. 1.5/1000 person-years) (Figure 4D). The RDs of incident hip fracture for the tramadol cohort were 1.6 (95% CI: 0.9 to 2.3) and 1.5 (95% CI: 0.7 to 2.3) /1000 person-years, compared with the celecoxib and the etoricoxib cohorts, respectively (Table 3). The corresponding HRs were 1.85 (95% CI: 1.40 to 2.44) and 1.96 (95% CI: 1.34 to 2.87), respectively (Figure 3). The results of sex subgroup analyses (Figure 3, Appendix) and sensitivity analyses (Appendix) remained similar.
In addition, according to the quantitative sensitivity analyses the relation (i.e., HR) of potential residual confounder(s) to both tramadol initiation and incident hip fracture need to be≥2.69 in order to completely explain away the weakest association observed in our primary analyses of comparison of tramadol initiators with NSAIDs initiators (i.e., HR = 1.65 for tramadol initiators vs. ibuprofen initiators).
DISCUSSION
This population-based cohort study, utilizing a relatively large sample, found that the initiation of tramadol involved a higher risk of incident hip fracture than did the initiation of either a commonly-used weak opioid (i.e., codeine) or commonly-used NSAIDs (i.e., naproxen, ibuprofen, celecoxib, and etoricoxib). The sensitivity analyses had similar results, indicating that the observed associations were robust and raising a concern on the potential risk of hip fracture among initiators of tramadol use.
Comparison with Previous Studies
To date, tramadol has become one of the most commonly used pain-relief medications around the world; however, to our knowledge, its safety profile, such as risk of fracture, remains unclear. Several studies have examined the association between tramadol use and the risk of fracture in various settings, but the results are conflicting.(23–25) One case-control study based on the data retrieved from the Denmark national registry reported that tramadol users had an approximately 55% higher risk of fracture at the hip, forearm, or spine than non-users; however, the corresponding association with codeine users was much weaker (odds ratio [OR] = 1.16).(23) Similarly, a study from the UK General Practice Research Database suggested that the current use of tramadol (OR = 1.25) or codeine (OR = 1.20) vs. non-use was associated with an increased risk of fracture at either hip, humerus, or wrist.(25) Unfortunately, these findings are likely to be susceptible to the potential confounding by indication because both studies used non-users as a comparison group.(23,25) In a propensity-score matched cohort study using the US Medicare database the authors claimed that the incidence of fracture at hip, pelvis, wrist, and humerus was lower in tramadol initiators (7/100 person-years) than that in codeine initiators (27/100 person-years) during the 180-day follow-up period.(24) However, the study was unable to adjust for BMI, smoking, and alcohol use due to lack of the information from the database.(24) Second, the two important demographic factors for fracture are substantially different in these two studies. In the previously published study,(24) the average age of subjects was approximately 80 years and 80% were women. In our study, the average age was 65 years and 66% were women. Furthermore, the study did not specifically evaluate the association of tramadol initiation with the risk of hip fracture, a disease that is often associated with the worse consequence, such as disability and death.(26,27) As a result, (i.e., different study population, different outcome variable), the incidence rate of fracture in their study was much higher than ours (270 per 1000 person-years vs. 2.9 per 1000 person-years in codeine cohort; 70 per 1000 person-years vs. 3.7 per 1000 person-years in tramadol cohort).(24) Our study demonstrated that the risk of hip fracture among tramadol initiators is not only higher than that among NSAIDs initiators, but also higher than that among codeine (another weak opioid) initiators. Further studies that evaluate the potential mechanisms, such as whether tramadol use increases the risk of osteoporosis or risk of fall, will help us better elaborate the association between tramadol use and the risk of hip fracture.
Possible Explanations
Previous studies have found that tramadol could activate μ opioid receptors and suppress central serotonin and norepinephrine reuptake, resulting in seizures,(18) dizziness,(42,43) and/or delirium.(44) Subsequently, such side effects may cause an increased risk of fall. In fact, several studies have reported that tramadol use was indeed associated with a higher risk of fall, which is a critical risk factors for fracture.(19–22) All these studies appear to suggest that relation of tramadol to the risk of hip fracture may be, at least partly, through its effect on fall.
Strengths and Limitations
Several characteristics of the present study deserve comment. First, using a population-based cohort study we found that the risk of incident hip fracture among tramadol initiators was not only higher than that among NSAIDs initiators, but also higher than that among codeine initiators, suggesting that the confounding by indication may not substantially account for an increased risk of hip fracture for tramadol. This was further supported by the evidence that risk factor profiles between initial prescription of tramadol and that of codeine were similar even before propensity-matching, except a few (e.g., BMI was higher among tramadol than codeine prescriptions) that may lower the risk of fracture for tramadol. Nevertheless, as in all observational studies, we can’t rule out the impact of potential residual confounders when comparing the risk of hip fracture between initial prescription of tramadol and other pain-relief medications. Second, we adopted a new-user design to compare the risk of hip fracture among tramadol initiators with initiators of several commonly used pain-relief medications. This design minimizes the potential selection bias. Third, because THIN does not include bone density or any frailty measurements, these two potential confounders could not be adjusted for in our analysis. Fourth, administrative data are often lacking in information of over-the-counter medications use (e.g., NSAIDs); thus, the exposure assessment is susceptible to misclassification bias. Such bias, if occurs, would affect the observed association either towards the null (i.e., stop taking tramadol but taking the over-the-counter NSAIDs) or away the null (i.e., taking tramadol and over-the-counter NSAIDs at the same time). Since the National Health Service England provides free healthcare for most services, including medications, ordered by GPs to individuals aged 60 years or older, it is unlikely that most patients would purchase these drugs over-the-counter without a prescription. In a sensitivity analysis restricted to individuals aged 60 years or older, we found that the relation of tramadol initiation to the risk of hip fracture did not change materially when compared with other pain-relief medications (tramadol vs. codeine: HR=1.28 (95% CI: (1.12 to 1.47); tramadol vs. naproxen: HR=1.68 (95% CI: 1.39 to 2.04); tramadol vs. ibuprofen: HR=1.67 (95% CI: 1.39 to 1.99); tramadol vs. celecoxib: HR=1.75 (95% CI: 1.32 to 2.32); tramadol vs. etoricoxib: HR=1.91 (95% CI: 1.28 to 2.84)), suggesting the impact of over-the-counter NSAIDs use may not be substantial. In addition, most patients who took pain-relief medication often change their initiated treatment; thus, hip fracture could occur after subjects stopped or changed their medication. Thus, estimates would be larger from “as-treated” analysis than “intention-to-treat analysis” due to minimizing misclassification, likely to be non-differential, of exposure. Finally, the biological mechanisms accounting for the association between tramadol use and the risk of hip fracture have not been fully understood; thus, future studies are warranted to elucidate such an association.
Clinical Implications
Pain is highly prevalent among the elderly population. In parallel to the aging process of the society, both frailty and chronic diseases involving pain are likely to increase. Owing to the adverse effects of commonly used NSAIDs (i.e., their cardiovascular, gastrointestinal, or renal risks) and safety concerns of traditional opioids (i.e., dependence and increased mortality), tramadol has been considered as an alternative pain relief medication.(15–18) Several professional organizations have strongly or conditionally recommended tramadol as the first-line therapy for the treatment of osteoarthritis,(45,46) Grade A for management of pain in patients with fibromyalgia,(47,48) or the second-line therapy for chronic low back pain patients with an inadequate response to non-pharmacologic treatments,(49) and its use has been increasing rapidly over the past decades.(12,13,50,51) Although the HR value of tramadol vs. naproxen in men (2.46) is larger than that in women (1.45), the rate difference in men (1.38/1000 person-years) is closer to that observed in women (1.05/1000 person-years). The large difference in HRs observed in men and women is likely due to relatively low risk of hip fracture in men who were initially prescribed naproxen. Considering the significant impact of hip fracture on morbidity, mortality, and healthcare cost,(52) our results point to the need to consider tramadol’s associated risk of fracture in clinical practice and treatment guidelines.
CONCLUSION
In this population-based cohort study we found that the initiation of tramadol was associated with a higher risk of hip fracture than the initiation of codeine and commonly used NSAIDs.
Supplementary Material
Acknowledgements
Everyone who contributed significantly to the work has been listed.
Funding
This work was supported by the National Institutes of Health (K23 AR069127, P60 AR047785), National Natural Science Foundation of China (81772413, 81702207, 81702206, 81930071) and the the Postdoctoral Science Foundation of Central South University (182130). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental data
Appendix
Disclosures
No conflict of interest for any of the authors.
Ethical approval
This study received approval from the medical ethical committee of Xiangya Hospital (2018091077), with waiver of informed consent.
Patient Consent
Not required.
Scientific approval
The protocol of this study was approved by the THIN Scientific Review Committee (18THIN078).
References
- 1.van den Bergh JP, van Geel TA, Geusens PP. Osteoporosis, frailty and fracture: implications for case finding and therapy. Nat Rev Rheumatol. 2012;8(3):163–72. [DOI] [PubMed] [Google Scholar]
- 2.Robbins J, Aragaki AK, Kooperberg C, Watts N, Wactawski-Wende J, Jackson RD, et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA. 2007;298(20):2389–98. [DOI] [PubMed] [Google Scholar]
- 3.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517–22. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Johnell O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int. 2005;16(3):229–38. [DOI] [PubMed] [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. [DOI] [PubMed] [Google Scholar]
- 6.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947–53. [DOI] [PubMed] [Google Scholar]
- 7.Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–60. [DOI] [PubMed] [Google Scholar]
- 8.Khalili H, Huang ES, Jacobson BC, Camargo CA Jr., Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry SD, Kiel DP, Colon-Emeric C. Hip Fractures in Older Adults in 2019. JAMA. 2019. [DOI] [PMC free article] [PubMed]
- 10.Bigal LM, Bibeau K, Dunbar S. Tramadol Prescription over a 4-Year Period in the USA. Curr Pain Headache Rep. 2019;23(10):76. [DOI] [PubMed] [Google Scholar]
- 11.Fischer B, Kurdyak P, Jones W. Tramadol dispensing patterns and trends in Canada, 2007–2016. Pharmacoepidemiol Drug Saf. 2019;28(3):396–400. [DOI] [PubMed] [Google Scholar]
- 12.Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186–93. [DOI] [PubMed] [Google Scholar]
- 13.Patterson E Tramadol History and Statistics. Accessed 10 Jan 2019 from: http://drugabuse.com/library/tramadol-history-and-statistics/
- 14.Zeng C, Dubreuil M, LaRochelle MR, Lu N, Wei J, Choi HK, et al. Association of Tramadol With All-Cause Mortality Among Patients With Osteoarthritis. JAMA. 2019;321(10):969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz WA. Pharmacology and clinical experience with tramadol in osteoarthritis. Drugs. 1996;52 Suppl 3:39–47. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin JL, Kraemer JJ, Bajwa ZH. The use of opioids in the treatment of osteoarthritis: when, why, and how? Curr Rheumatol Rep. 2009;11(1):5–14. [DOI] [PubMed] [Google Scholar]
- 17.Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth Analg. 2017;124(1):44–51. [DOI] [PubMed] [Google Scholar]
- 18.Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: Understanding the Risk of Serotonin Syndrome and Seizures. Am J Med. 2018. [DOI] [PubMed]
- 19.Soderberg KC, Laflamme L, Moller J. Newly initiated opioid treatment and the risk of fall-related injuries. A nationwide, register-based, case-crossover study in Sweden. CNS Drugs. 2013;27(2):155–61. [DOI] [PubMed] [Google Scholar]
- 20.Costa-Dias MJ, Oliveira AS, Martins T, Araujo F, Santos AS, Moreira CN, et al. Medication fall risk in old hospitalized patients: a retrospective study. Nurse Educ Today. 2014;34(2):171–6. [DOI] [PubMed] [Google Scholar]
- 21.Moller J, Laflamme L, Soderberg Lofdal K. CYP2D6-inhibiting drugs and the increased risk of fall-related injuries due to newly initiated opioid treatment--a Swedish, register-based case-crossover study. Basic Clin Pharmacol Toxicol. 2015;116(2):134–9. [DOI] [PubMed] [Google Scholar]
- 22.Harstedt M, Rogmark C, Sutton R, Melander O, Fedorowski A. Polypharmacy and adverse outcomes after hip fracture surgery. J Orthop Surg Res. 2016;11(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260(1):76–87. [DOI] [PubMed] [Google Scholar]
- 24.Solomon DH, Rassen JA, Glynn RJ, Garneau K, Levin R, Lee J, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170(22):1979–86. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of fracture in adults: a nested case-control study using the general practice research database. Am J Epidemiol. 2013;178(4):559–69. [DOI] [PubMed] [Google Scholar]
- 26.Bhandari M, Swiontkowski M. Management of Acute Hip Fracture. N Engl J Med. 2017;377(21):2053–62. [DOI] [PubMed] [Google Scholar]
- 27.Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2(6):285–9. [DOI] [PubMed] [Google Scholar]
- 28.Stuart-Buttle CD, Read JD, Sanderson HF, Sutton Y. A language of health in action: Read Codes, classifications and groupings. Proc AMIA Annu Fall Symp. 1996:75–9. [PMC free article] [PubMed]
- 29.First Databank. Multilex drug data file. Accessed 10 Jan 2019 from: http://www.firstdatabank.co.uk/8/multilex-drug-data-file
- 30.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393–401. [DOI] [PubMed] [Google Scholar]
- 31.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14(7):465–76. [DOI] [PubMed] [Google Scholar]
- 32.Morris R, Carstairs V. Which deprivation? A comparison of selected deprivation indexes. J Public Health Med. 1991;13(4):318–26. [PubMed] [Google Scholar]
- 33.Collins GS, Mallett S, Altman DG. Predicting risk of osteoporotic and hip fracture in the United Kingdom: prospective independent and external validation of QFractureScores. BMJ. 2011;342:d3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misra D, Zhang Y, Peloquin C, Choi HK, Kiel DP, Neogi T. Incident long-term warfarin use and risk of osteoporotic fractures: propensity-score matched cohort of elders with new onset atrial fibrillation. Osteoporos Int. 2014;25(6):1677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misra D, Peloquin C, Kiel DP, Neogi T, Lu N, Zhang Y. Intermittent Nitrate Use and Risk of Hip Fracture. Am J Med. 2017;130(2):229 e15- e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study. Am J Epidemiol. 2010;172(7):843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 39.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365(9458):475–81. [DOI] [PubMed] [Google Scholar]
- 40.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 41.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med. 2014;33(10):1685–99. [DOI] [PubMed] [Google Scholar]
- 42.Fricke JR Jr., Hewitt DJ, Jordan DM, Fisher A, Rosenthal NR. A double-blind placebo-controlled comparison of tramadol/acetaminophen and tramadol in patients with postoperative dental pain. Pain. 2004;109(3):250–7. [DOI] [PubMed] [Google Scholar]
- 43.Altis K, Schmidtko A, Angioni C, Kuczka K, Schmidt H, Geisslinger G, et al. Analgesic efficacy of tramadol, pregabalin and ibuprofen in menthol-evoked cold hyperalgesia. Pain. 2009;147(1–3):116–21. [DOI] [PubMed] [Google Scholar]
- 44.Brouquet A, Cudennec T, Benoist S, Moulias S, Beauchet A, Penna C, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251(4):759–65. [DOI] [PubMed] [Google Scholar]
- 45.American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee:Evidence-Based Guideline, 2nd Edition. Accessed 10 Jan 2019 from: https://www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf [DOI] [PubMed]
- 46.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–74. [DOI] [PubMed] [Google Scholar]
- 47.Carville SF, Arendt-Nielsen L, Bliddal H, Blotman F, Branco JC, Buskila D, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67(4):536–41. [DOI] [PubMed] [Google Scholar]
- 48.Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluss E, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318–28. [DOI] [PubMed] [Google Scholar]
- 49.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of P. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514–30. [DOI] [PubMed] [Google Scholar]
- 50.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2014;66(10):1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson N, Sanchez-Riera L, Morros R, Diez-Perez A, Javaid MK, Cooper C, et al. Drug utilization in patients with OA: a population-based study. Rheumatology (Oxford). 2015;54(5):860–7. [DOI] [PubMed] [Google Scholar]
- 52.Leslie WD, O’Donnell S, Jean S, Lagace C, Walsh P, Bancej C, et al. Trends in hip fracture rates in Canada. JAMA. 2009;302(8):883–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


