Abstract
Previous studies found that inactivation of the central amygdala (CeA) severely impaired acquisition of cerebellum-dependent delay eye-blink conditioning (EBC) in male rats and rabbits. Sex differences in EBC and the effects of stress on EBC have been reported and might be related to sex differences in amygdala modulation of cerebellar learning. The current study examined the effects of CeA inactivation with muscimol on acquisition and retention of EBC in female rats. Like male rats, CeA inactivation in female rats severely impaired EBC acquisition and retention. Comparison of the female data with previously published data from males indicates no substantive sex differences in the effects of CeA inactivation on acquisition or retention of EBC. The results indicate that amygdala modulation of cerebellar learning is not sex-specific.
Keywords: female, rat, cerebellum, amygdala, conditioning, learning, memory
Cerebellum-dependent learning is modulated by the amygdala (Farley et al., 2016; Neufeld & Mintz, 2001; Taub & Mintz, 2010; Weisz et al., 1992). The central amygdala (CeA) projects to cerebellar sensory input nuclei and lesions or inactivation of the CeA slow acquisition of cerebellum-dependent eye-blink conditioning (EBC) in rabbits, rats, and mice (Blankenship et al., 2005; Boele et al., 2010; Burhans & Schreurs, 2008; Farley et al., 2016, 2018; Lee & Kim, 2004; Pochiro & Lindquist, 2016; Weisz et al., 1992). Inactivation of the CeA also causes a deficit in retention of EBC (Farley et al., 2016, 2018; Siegel et al., 2015). In addition to the behavioral effects of CeA inactivation, learning-specific neuronal firing in the cerebellum is also severely attenuated during acquisition and post-asymptotic retention (Farley et al., 2016). Although lesions or inactivation of the CeA cause severe deficits in EBC, the CeA is not essential for EBC since rats eventually learn with extensive training (Farley et al., 2018). Our current interpretation of these findings is that the CeA modulates EBC by increasing conditioned stimulus (CS) input to the cerebellum (Farley et al., 2016, 2018; Pochiro & Lindquist, 2016; Taub & Mintz, 2010). This sensory gating or CS facilitation hypothesis explains why the CeA is crucial for EBC during both acquisition and retention.
All of the data supporting the CS facilitation hypothesis come from male subjects. This is potentially problematic because sex differences in EBC and the effects of stress on EBC have been reported by Wood and Shors (Wood & Shors, 1998). Specifically, females learn EBC faster than males among unstressed rats. For rats given restraint stress, EBC is facilitated in males but impaired in females. It is therefore possible that CeA inactivation in female rats has no effect or even facilitates EBC. To examine possible sex differences in the role of the CeA in EBC, we replicated our first experiment with muscimol inactivation of the CeA during acquisition and retention of EBC in male rats (Farley et al., 2016) with female rats.
Methods
Subjects were 22 Long-Evans adult female rats (11/group), approximately three months old. Rats were pair housed until surgery and then housed singly in the Bowen Sciences Building at the University of Iowa, on a 12 h light/dark cycle with food and water available ad libitum. All experimental procedures were approved by the University of Iowa’s Institutional Animal Care and Use Committee.
Approximately one week before training, rats were anesthetized with isoflurane (2-5%) and given surgery to implant eyelid electromyography (EMG) electrodes, a bipolar stimulating electrode, and bilateral cannulae in the CeA. The skull was exposed and 0.7 mm diameter holes were burred over the CeA. Custom-fabricated 27-gauge guide cannulae were lowered to 0.5 mm dorsal to the CeA. Guide cannulae were plugged with a stylet that extended 0.5 mm beyond the tip of the guide. Coordinates relative to bregma were AP −2.2, ML ±4.1, and DV −7.1. A bipolar stimulating electrode (PlasticsOne # MS303/1-B/SPC) was implanted in the superficial fascia caudal to the left eye (for US delivery). Two stainless steel PFA insulated wires (AM-Systems # 791000) were implanted within the orbicularis oculi to measure differential EMG during eyelid closure. The EMG electrode wires were soldered to gold pins (AM-Systems # 520200) housed within a 3D printed connector strip. The electrode connectors and cannulae were affixed to the animal’s skull with dental acrylic (AM-Systems # 525000, 526000). The rats were given post-surgical analgesics for 48 hours.
Conditioning chambers (12" L x 11" W x 10" H) were housed within sound attenuation boxes. One wall of the chamber was fitted with speakers for the tone CS. An EMG and bipolar electrode tether passed through a commutator and were connected to a DC amplifier and a stimulus isolator, respectively. The amplified (2000X) EMG was filtered (0.5-5.0 kHz) and integrated before being digitized by a desktop computer. The stimulus isolator was also connected to the computer which specified the timing of the unconditioned stimulus (US). All surfaces of the conditioning chamber were wiped with 70% ethanol prior to the beginning of each session.
At least 30 min prior to each of the first 5 sessions, and for retention tests, 0.2 μL of 2.0 mM muscimol (GABAA-R agonist,) or 0.2 μL of 0.1 M phosphate buffered saline (PBS; 6 μl/hr) was infused into the CeA bilaterally. A custom built 32-gauge infusion cannula was lowered into the guide and extended 0.5 mm ventrally beyond its tip. Infusion cannulae were removed 2 min after the end of the infusion.
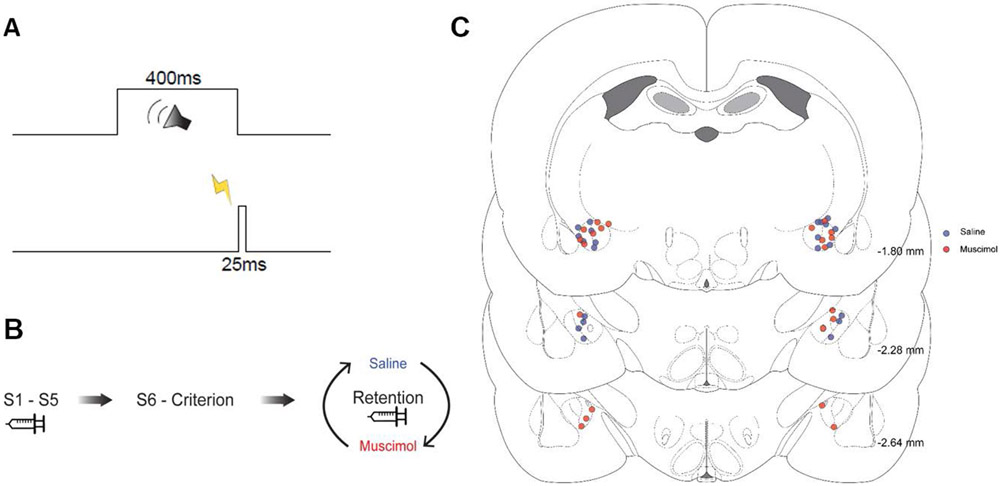
Figure 1 shows the onsets and offsets of the stimuli used in EBC. The sampling window for each trial was 1000 ms, consisting of a 300 ms pre-CS baseline period, 400 ms CS period, 25-ms US period, and 275-ms post-US period. The CS was a pure tone (2.0 kHz, 85 dB) and the US was periorbital electrical stimulation (2.0 mA). The mean inter-trial interval was 30 s ± 10 s. Each session consisted of ten 10-trial blocks including 9 paired CS-US trials and one CS-alone trial. Muscimol infusions were separated by 48 hrs to minimize tolerance (Farley et al., 2016; Freeman et al., 2005). No pre-session infusions occurred from session 6 through reaching a criterion of 2 consecutive sessions of 80% CRs or a clear asymptote. Rats from both groups were then given 2 retention sessions with infusions of PBS or muscimol in a counterbalanced order.
Figure 1.
A, Duration and timing of the tone conditioned stimulus (400 ms) and periorbital stimulation unconditioned stimulus (25 ms) used for training and testing. B, Infusion schedule during acquisition with separate groups receiving saline or muscimol infusions into the central amygdala (S1-S5), after acquisition with no infusions (S6-criterion), and during retention tests where both groups received saline and muscimol on separate days (order counterbalanced). C, Histology reconstructions with the tip of the infusion cannula depicted by blue dots for the saline group and red dots for the muscimol group. Numbers indicate AP stereotaxic coordinates.
Integrated EMG activity for each trial was analyzed with a custom MATLAB-based application. CRs were defined as EMG activity that exceeded a threshold of 0.4 units (amplified and integrated units) above the baseline mean during the CS period, 80 ms after its onset. EMG activity that exceeded the threshold during the first 80 ms of the CS period was defined as a startle (alpha) response. Unconditioned responses (UR) were defined as activity that crossed the threshold after the offset of the US. Any trial where the pre-CS baseline signal crossed threshold was removed from analysis.
All dependent variables were analyzed across sessions in the acquisition phase (sessions 1-5) with linear mixed effects modeling (R, version 3.6.0), which circumvents some of the limitations of repeated measures ANOVA for learning data, including the assumption of independent observations and intolerance to missing data (Boisgontier & Cheval, 2016). Failure to account for the non-independence of trial, block, or session data in a learning experiment can increase the probability of a Type I error (false positive). Mixed effects modeling accounts for the non-independence of the learning data, yielding a lower Type I error rate. Models included fixed effects for group (PBS, Muscimol) and session (acquisition sessions 1-5). Random effects included intercept and session for each rat. The CR percentage data were best fit by a quadratic function, which was added to the models. Satterthwaite approximations to degrees of freedom were used for the mixed effects analyses. The t statistic is presented for comparisons of learning curves for two groups because there are only two data points (slope or quadratic function). Data from the retention sessions were analyzed separately by ANOVA rather than mixed effects modeling because each rat had a single data point for the PBS and Muscimol tests. Data from CS-US paired trials and CS-alone probe trials were included in all CR analyses.
At completion of training, animals were anesthetized with sodium pentobarbital and perfused with 120 ml 0.1M PBS and 4% paraformaldehyde. Brains post-fixed for 24h and were cryoprotected for 48h, then sliced in 50 μm thick coronal sections. Sections were mounted and stained with thionin and analyzed for cannula placement.
Results
The rats in the muscimol and PBS groups had bilateral cannula placements within the CeA or within .5 mm of the CeA (Figure 1B). Cannulae placements were consistent with previous experiments using male rats (Farley et al., 2016, 2018).
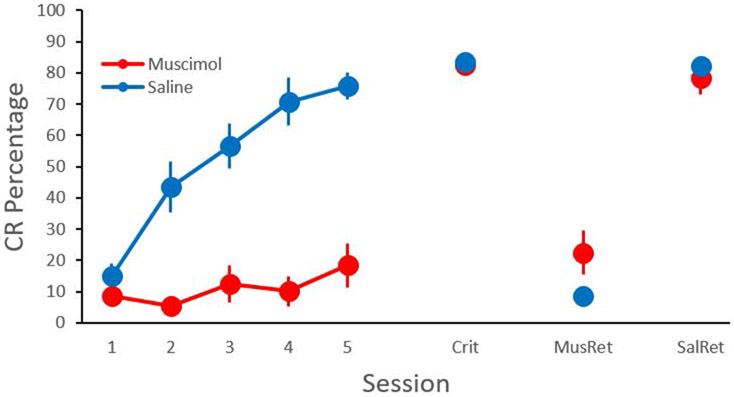
Female rats given muscimol inactivation of the CeA were severely impaired relative to the PBS control group during acquisition of EBC (Figure 2, Sessions 1-5). Rats in the muscimol group were given additional training after the cessation of infusions, learning to the criterion of 80% CRs on two consecutive sessions, indicating that the repeated infusions did not produce lesions that impaired EBC. After reaching the criterion, both groups were given retention tests with PBS or muscimol infusions into the CeA on separate testing days (order of infusions counterbalanced). Both training groups were severely impaired during retention tests with muscimol infusions into the CeA relative to tests with PBS infusions (Figure 2). The percentage, amplitude, and timing of the UR were not affected by CeA inactivation. The results are consistent with previous studies with male rats (Farley et al., 2016, 2018).
Figure 2.
Mean (± SEM) percentage of eyeblink conditioned responses (CR) across training and testing sessions in female rats. Separate groups received saline or muscimol infusions into the central amygdala during acquisition sessions 1-5. Infusions were discontinued until rats reached a criterion of 80% CRs for two consecutive days (Crit). Both groups were then given retention tests where they received saline (SalRet) or muscimol (MusRet) on separate days (order counterbalanced).
The CR percentage data for sessions 1-5 were analyzed using linear mixed effects modeling with fixed effects of group, session, and a quadratic function (across sessions) and random effects for intercept, slope, and the quadratic function. There was a significant Group X Quadratic Session interaction, t(22.85) = 2.238, P = 0.035301. The interaction indicates that the PBS group showed a significantly greater increase in CR percentage across sessions with a negatively accelerated curve relative to the muscimol group (Figure 1).
Testing data were analyzed using a repeated measures ANOVA with session (Criterion, PBS, Muscimol) as a within subjects variable and group as the between subjects variable (Figure 2). The group effect was not significant, indicating that prior acquisition training with PBS or muscimol did not affect testing after criterion. However, an effect of session indicated that muscimol reduced CR% during retention testing in both groups F(1.502, 30.036) = 175.354, p = 0.000 (Greenhouse-Geisser adjusted degrees of freedom). The interaction of the group and session factors was not significant.
Discussion
Inactivation of the CeA with muscimol severely impaired acquisition of EBC in female rats. The magnitude of the acquisition impairment was very similar to the deficit found in previous experiments using male rats (Farley et al., 2016, 2018). Additional training without drug infusions resulted in learning in the group originally trained with muscimol, reaching the same asymptote as the controls. Subsequent infusions of muscimol into the CeA resulted in a severe but reversible deficit in retention in both groups, which also replicates the findings from male rats (Farley et al., 2016, 2018).
A comparison of the data from the current study and a nearly identical study conducted with male rats indicates that the effects of CeA inactivation are very similar (Farley et al., 2016). In contrast to a previous study (Wood & Shors, 1998), we did not find faster learning in the female rats relative the male rats; however, our assessment compares different studies conducted several years apart, whereas the Wood and Shors (1998) study compared sexes within the same experiment. It is also possible that procedural differences or strain differences (Wood and Shors (1998) used Sprague–Dawley rats) could account for the different findings. Additional studies with both sexes will help to pinpoint the factors that reveal sex differences in EBC.
The results of the current study indicate that the CeA plays a crucial role in acquisition and retention of EBC in female rats. Based on inactivation data, neuroanatomy, and neurophysiology in male rats, we hypothesized that the CeA modulates the strength of CS input to the cerebellum (Farley et al., 2016). The CS facilitation by the CeA is thought to function as an increase in attention to the CS (Gallagher et al., 1990; Holland & Gallagher, 1993b, 1993a). The finding by Wood and Shors (1998) of sex differences in the effects of restraint stress on EBC suggests that the CeA does not modulate EBC by a non-specific increase in vigilance or by emotional conditioning in female rats. Rather, this finding is consistent with the CS facilitation hypothesis in which female rats increase attention to the CS because it provides predictive information.
Acknowledgments
This research was supported by the National Institutes of Health grant NS088567 to JHF.
References
- Blankenship MR, Huckfeldt R, Steinmetz JJ, & Steinmetz JE (2005). The effects of amygdala lesions on hippocampal activity and classical eyeblink conditioning in rats. Brain Research, 1035, 120–130. 10.1016/j.brainres.2004.11.061 [DOI] [PubMed] [Google Scholar]
- Boele H-J, Koekkoek SKE, & De Zeeuw CI (2010). Cerebellar and Extracerebellar Involvement in Mouse Eyeblink Conditioning: The ACDC Model. Frontiers in Cellular Neuroscience, 3. 10.3389/neuro.03.019.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, & Cheval B (2016). The anova to mixed model transition. Neuroscience and Biobehavioral Reviews, 68, 1004–1005. 10.1016/j.neubiorev.2016.05.034 [DOI] [PubMed] [Google Scholar]
- Burhans LB, & Schreurs BG (2008). Inactivation of the central nucleus of the amygdala abolishes conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response and delays classical conditioning. Behavioral Neuroscience, 122, 75–88. 10.1037/0735-7044.122.1.75; 10.1037/0735-7044.122.1.75 [DOI] [PubMed] [Google Scholar]
- Farley SJ, Albazboz H, De Corte BJ, Radley JJ, & Freeman JH (2018). Amygdala central nucleus modulation of cerebellar learning with a visual conditioned stimulus. Neurobiology Learning and Memory, 150, 84–92. 10.1016/j.nlm.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley SJ, Radley JJ, & Freeman JH (2016). Amygdala Modulation of Cerebellar Learning. The Journal of Neuroscience, 36, 2190–2201. 10.1523/JNEUROSCI.3361-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Halverson HE, & Poremba A (2005). Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. The Journal of Neuroscience, 25, 889–895. 10.1523/jneurosci.4534-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, & Holland PC (1990). The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. The Journal of Neuroscience, 10, 1906–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, & Gallagher M (1993a). Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behavioral Neuroscience, 107, 246–253. [DOI] [PubMed] [Google Scholar]
- Holland PC, & Gallagher M (1993b). Effects of amygdala central nucleus lesions on blocking and unblocking. Behavioral Neuroscience, 107, 235–245. [DOI] [PubMed] [Google Scholar]
- Lee T, & Kim JJ (2004). Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. The Journal of Neuroscience, 24, 3242–3250. 10.1523/jneurosci.5382-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld M, & Mintz M (2001). Involvement of the amygdala in classical conditioning of eyeblink response in the rat. Brain Research, 889, 112–117. [DOI] [PubMed] [Google Scholar]
- Pochiro JM, & Lindquist DH (2016). Central amygdala lesions inhibit pontine nuclei acoustic reactivity and retard delay eyeblink conditioning acquisition in adult rats. Learning & Behavior, 44, 191–201. 10.3758/s13420-015-0199-5 [DOI] [PubMed] [Google Scholar]
- Siegel JJ, Taylor W, Gray R, Kalmbach B, Zemelman BV, Desai NS, Johnston D, & Chitwood RA (2015). Trace eyeblink conditioning in mice is dependent upon the dorsal medial prefrontal cortex, cerebellum, and amygdala: behavioral characterization and functional circuitry. ENeuro, 2. 10.1523/ENEURO.0051-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub AH, & Mintz M (2010). Amygdala conditioning modulates sensory input to the cerebellum. Neurobiology of Learning and Memory, 94, 521–529. 10.1016/j.nlm.2010.09.004; 10.1016/j.nlm.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Weisz DJ, Harden DG, & Xiang Z (1992). Effects of amygdala lesions on reflex facilitation and conditioned response acquisition during nictitating membrane response conditioning in rabbit. Behavioral Neuroscience, 106, 262–273. [DOI] [PubMed] [Google Scholar]
- Wood GE, & Shors TJ (1998). Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Sciences of the United States of America, 95, 4066–4071. 10.1073/pnas.95.7.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]