Abstract
Nitrogen heterocycle represents a ubiquitous skeleton in natural products and drugs. Late-stage C(sp3)-H bond functionalization of N-heterocycles with broad substrate scope remains a challenge and of particular significance to modern chemical synthesis and pharmaceutical chemistry. Here, we demonstrate copper-catalysed late-stage C(sp3)-H functionalizaion of N-heterocycles using commercially available catalysts under mild reaction conditions. We have investigated 8 types of N-heterocycles which are usually found as medicinally important skeletons. The scope and utility of this approach are demonstrated by late-stage C(sp3)-H modification of these heterocycles including a number of pharmaceuticals with a broad range of nucleophiles, e.g. methylation, arylation, azidination, mono-deuteration and glycoconjugation etc. Preliminary mechanistic studies reveal that the reaction undergoes a C-H fluorination process which is followed by a nucleophilic substitution.
Subject terms: Homogeneous catalysis, Synthetic chemistry methodology
Late-stage C(sp3)-H bond functionalization of N-heterocycles with broad substrate scope remains a challenge and of particular significance to modern chemical synthesis and pharmaceutical chemistry. Here the authors show copper-catalysed late-stage C(sp3)-H functionalizaion of N-heterocycles using commercially available catalysts under mild reaction conditions.
Introduction
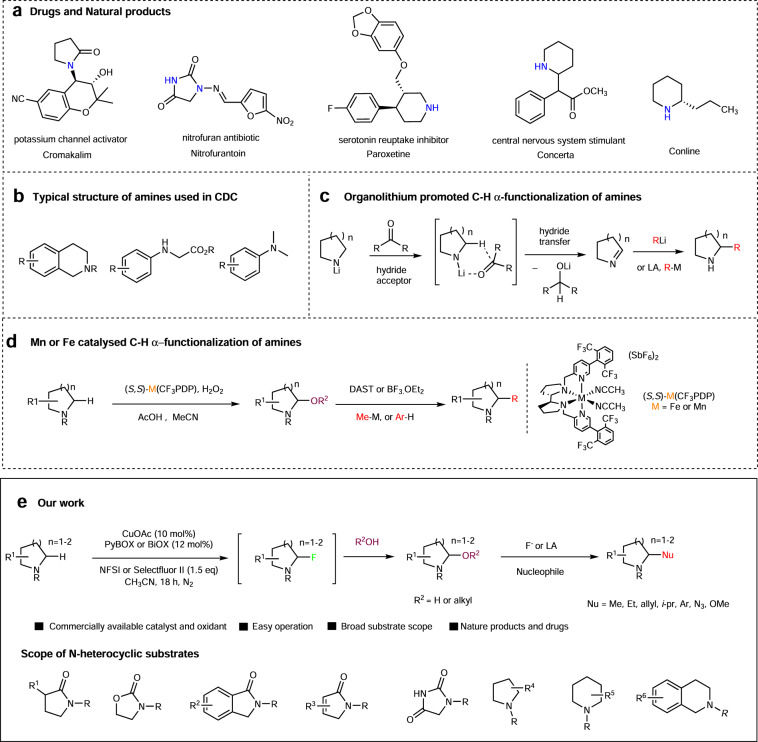
Nitrogen heterocycles (N-heterocycles) hold a special position in natural products chemistry and medicinal chemistry as evidenced by that ~60% of US Food and Drug Administration-approved drugs contain a N-heterocycle1, 2. Direct C(sp3)–H bond functionalization of N-heterocycles represents an attractive topic and is of paramount importance to drug development3–9. A number of approaches on C–H bond alkylation of N-heterocycle have been developed so far, e.g., C–H oxidation to iminium intermediate6–8, 10–13, lithiation of α-position, C(sp3)–H bond activation14–17, photoredox-based approaches, etc18–24. In particular, cross-dehydrogenative coupling (CDC), which proceeds via C–H oxidation to iminium intermediate, developed by Li25, 26 based on the oxidative activation of C(sp3)–H bonds of amines and ethers has been intensively studied (Fig. 1b)25, 26. However, the scope of the amine coupling partner is limited to a few electron-rich aromatic amines. An elegant C–H alkylation of cyclic amines was developed recently by Seidel and colleagues14, 15, 27 using organolithium as a strong base and ketones as a hydride acceptor to generate cyclic imine, which was subsequently captured by organolithium and Grignard reagent. However, the use of organolithium restricts its application in late-stage functionalization. Despite all these advances, most of these methods developed so far have focused on simple azacycles, and late-stage C(sp3)–H bond functionalization of N-heterocycles with broad substrate scope remains a challenge. Late-stage C(sp3)–H hydroxylation of N-heterocycles furnishing hemiaminal intermediate, which undergoes further iminium formation and alkylation, offers a general approach to access various functionalization. However, no general approach has been developed until recently. Traditionally, hemiaminal were prepared either from electrochemical oxidation of amine or from reduction of lactams13, 28–32. The challenge in direct C(sp3)–H hydroxylation probably lies in over-oxidation to carbonyl or elimination to olefin at high temperature or under forcing conditions33, 34. During the course of our research, White and colleagues8 reported a Mn-catalyzed C(sp3)–H hydroxylation of N-heterocycles, including many drugs and the hemiaminal intermediates, was transformed further to methylated products. The same group earlier reported an Fe-catalyzed C(sp3)–H hydroxylation of N-heterocycles35. However, in these two cases, the catalyst employed is not commercially available, which requires ten steps to complete the synthesis and great care should be taken to avoid over-oxidation, e.g., dropwise addition of H2O2 using a syringe pump and low reaction temperature.
Fig. 1. C(sp3)–H functionalization of N-heterocycles.
a Representative examples of N-heterocycles in pharmaceuticals and natural products. b Previously reported nitrogenous substrates of CDC reactions. c Previous work on organolithium promoted C–H α-functionalization of amines. d Previous work on Mn- or Fe-catalyzed C–H α-functionalization of amines. e This work: Cu-catalyzed C–H functionalization of N-heterocycles with NFSI or Selectflour II as oxidants and scope of N-heterocyclic substrates.
Herein, we show a Cu(I)-catalyzed late-stage C(sp3)–H functionalization of N-heterocycles via CDC approach using H2O or alcohol as coupling partners. The hydroxylation/alkoxylation of a variety of N-heterocycles proceeds in good yield under mild reaction conditions. The resulting hemiaminal or aminal intermediates were further functionalized with a broad range of nucleophiles promoted by Lewis acid or fluorination reagent. Compared with the previous work, our system has the following advantages: (1) the BOX type ligands employed in our approach are commercial; (2) common functional groups, such as C=C double bonds and secondary alcohols, which are not suitable for previous approach, are well compatible with our system; (3) a radical fluorination process is involved in this approach and the fluorinated intermediates have the potential to be transformed in situ to many other functional groups in a single step, whereas Mn/H2O2 chemistry is only a radical hydroxylation.
Results
Reaction optimization
Inspired by recent advances in copper-catalyzed radical C–H activation using N–F reagents36–39, in particular C–H fluorination40, we reasoned if C–H functionalization of α-position of nitrogen could be achieved via copper-catalyzed C–H fluorination, due to the similar bond dissociation energy (BDE) of benzylic C–H bonds and α(C–H) bond of N-heterocycles41, 42. The resulting fluorinated intermediate is prone to hydrolysis under acidic conditions to give hemiaminal. This approach is advantageous over direct hydroxylation using OH radical, as hyperconjugative activation of hemiaminals often leads to over-oxidation to the carbonyl43–45.
To test our hypothesis, we began our study by the reaction of oxazolidinones 1a and H2O in the presence of 10 mol% of CuOTf/L1 as the catalyst and N-fluorobenzenesulfonimide (NFSI) (1.5 eq.) as the oxidant in CH3CN at 25 °C (Table 1, entry 1). Gratifyingly, the desired CDC product 2a was obtained in 26% yield albeit with low conversion. Encouraged by this result, we further systematically optimized the reaction conditions to improve the yield of the reaction. When the reaction was performed without a ligand, the reaction was sluggish and only trace amount of 2a was produced (entry 2). A survey of other solvents was then carried out (entries 3–5), but no one led to a higher yield than in CH3CN. When the reaction was performed at elevated temperature of 35 °C, a slightly higher yield of 2a was achieved without byproduct formation from elimination or over-oxidation (40% yield, entry 6). Then we turned our attention to Cu salts. Cu(II) catalyst generated from Cu(OTf)2 and L1 was less efficient, and failed to give a higher yield (entry 7). The yield was further improved to 73% by using CuBr in place of CuOTf (entry 8). To our delight, with the catalyst formed from CuOAc and L1, the yield of 2a was dramatically improved to 89% (entry 9). Screening of other PyBOX ligands showed that the substituent at the stereogenic center was crucial to the efficiency of the reaction (entries 9–12). Catalysts formed from L1 or L2 with aromatic rings gave significantly higher yields than L3 or L4 with aliphatic substituents. We also expanded the application of this C–H functionalization reaction toward the synthesis of aminals. When MeOH was used as the coupling partner, the desired product 2aa was successfully obtained in 95% yield (Table 1, entry 17). Controlled experiments were performed and indicated that the reaction did not proceed without Cu salts or NFSI (Supplementary Table 5).
Table 1.
Reaction optimizationa.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | [Cu] | Solvent | T (°C) | Ligand | Product | Yieldb |
| 1 | CuOTf | CH3CN | 25 | L1 | 2a | 26% |
| 2 | CuOTf | CH3CN | 25 | - | 2a | Trace |
| 3 | CuOTf | DCM | 25 | L1 | 2a | 5% |
| 4 | CuOTf | DCE | 25 | L1 | 2a | Trace |
| 5 | CuOTf | acetone | 25 | L1 | 2a | 11% |
| 6 | CuOTf | CH3CN | 35 | L1 | 2a | 40% |
| 7 | Cu(OTf)2 | CH3CN | 35 | L1 | 2a | 29% |
| 8 | CuBr | CH3CN | 35 | L1 | 2a | 73% |
| 9 | CuOAc | CH3CN | 35 | L1 | 2a | 89% |
| 10 | CuOAc | CH3CN | 35 | L2 | 2a | 78% |
| 11 | CuOAc | CH3CN | 35 | L3 | 2a | Trace |
| 12 | CuOAc | CH3CN | 35 | L4 | 2a | 31% |
| 14 | CuOAc | CH3CN | 35 | L5 | 2a | 78% |
| 15 | CuOAc | CH3CN | 35 | L6 | 2a | 48% |
| 16 | CuOAc | CH3CN | 35 | L7 | 2a | 40% |
| 17c | CuOAc | CH3CN | 35 | L1 | 2aa | 95% |
aReaction conditions: 1a (0.2 mmol), H2O (0.6 mmol), CuOAc (0.02 mmol), ligand (0.024 mmol), NFSI (0.3 mmol), and solvent (2.0 mL), 18 h.
bIsolated yield.
cReaction conditions: 1a (0.2 mmol), H2O (0.6 mmol), MeOH (0.6 mmol), CuOAc (0.02 mmol), L1 (0.024 mmol), NFSI (0.3 mmol), CH3CN (2.0 mL), 18 h.
Substrate scope evaluation
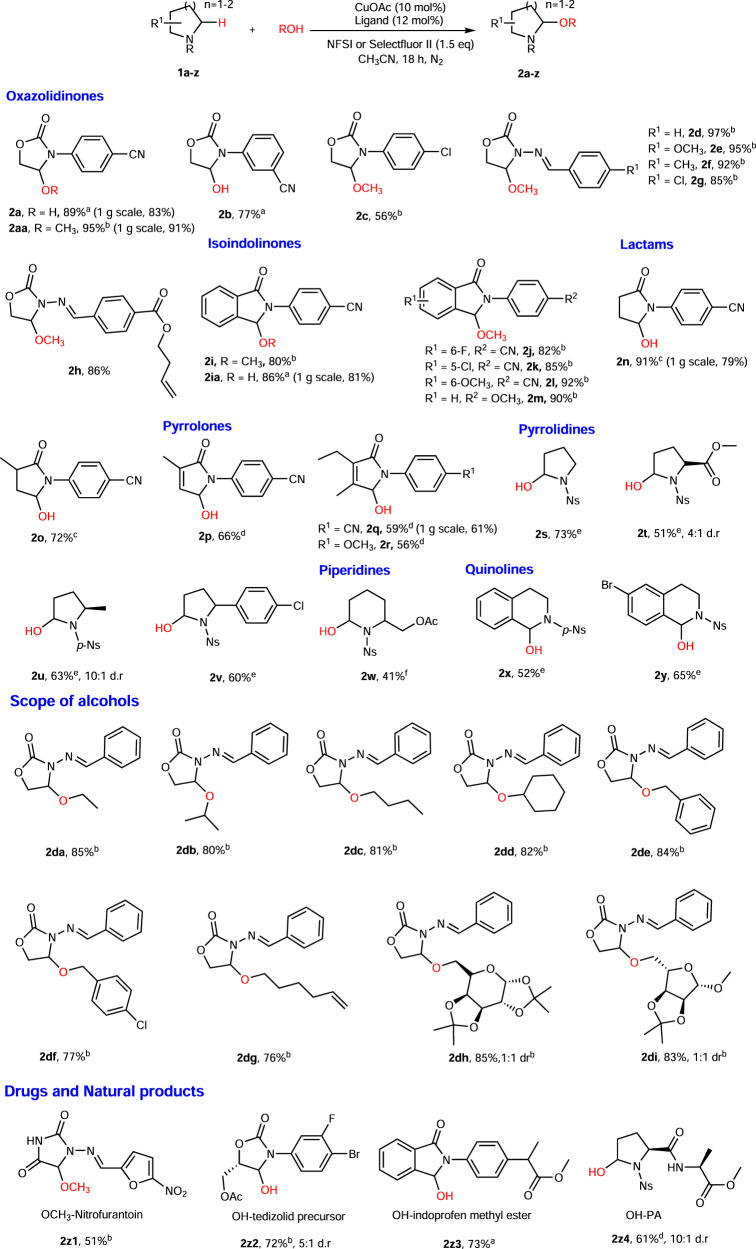
After optimizing the reaction conditions, we turned to explore the scope of N-heterocyclic substrates of this reaction (Fig. 2). Overall, despite the wide range of C–H BDEs of the N-heterocycles, we successfully applied this protocol to 34 compounds including 8 different types of N-heterocycles, which were usually found as important skeletons in pharmaceuticals and natural products. By changing different ligands and N–F reagents, all the N-heterocycles investigated were applicable to this strategy affording the desired products in moderate-to-good yields (41–97%). The reactions of oxazolidinones and isoindolinones derivatives proceeded smoothly under the standard reaction conditions with L1/CuOAc/NFSI. For the oxazolidinones, a higher yield was found when para-CN (2a, 89%) rather than meta-CN (2b, 77%) was used as a substituent group on the phenyl ring. The yield of para-Cl substituted 2c is lower than that of para-CN substituted 2a (56% vs. 89%). Due to the fact that hemiaminal products and the substrates with N-imine substituent were difficult to separate, the corresponding aminal products 2d–2h were reported instead (85–97% yield). In the case of isoindolinone, aminal products were given instead as well, due to solubility issue of the corresponding hemiaminals. The CDC products 2i–2m were all successfully formed in high yields (80–92%). Isindolinone 2l with electron-rich substituent on the benzene ring gave a higher yield than other isoindolinone substrates (92% vs. 80–85%). It is worth mentioning that lactams gave low yields under the standard reaction conditions. Slightly modified conditions were applied to lactams, e.g., CuOAc/L2/20 °C, to avoid the formation of C–C double bond by elimination or the carbonyl by over-oxidation (2n–2o, 72–79% yield). Pyrrolones N-heterocycle, in which low reaction efficiency was observed using CuOAc/L1, also proved to be good substrates with CuOAc/L2, affording products 2p–2r in good yields (66% and 59%, respectively).
Fig. 2. Scope of N-heterocyclic substrates.
Reaction conditions: N-heterocyclic substrates (0.2 mmol), H2O (0.6 mmol), CuOAc (0.02 mmol), ligand (0.024 mmol), NFSI (0.3 mmol), CH3CN (2.0 mL), under N2; isolated yields reported. aL1, temperature: 35 °C. bL1, ROH (0.6 mmol). cL2, temperature: 20 °C. dLigand L2, temperature: 30 °C. eL6, Selectflour II instead of NFSI, temperature: 20 °C. fCuOAc (0.2 eq.), L6 (0.22 eq), Selectflour II (2.0 eq.) instead of NFSI, temperature: 20 °C.
For the common N-heterocycle pyrrolidines, initially, we studied the N-protecting groups (Supplementary Table 1) and nitrobenzenesulfonyl (Ns) was eventually identified as the optimal N-protecting group for this hydroxylation reaction, which was in accordance with previous reports8, 35. A survey of ligands (Supplementary Table 2), F-reagent (Supplementary Table 4), and temperature indicated that the optimal reaction conditions were CuOAc/L6/Selectfluor II/20 °C. Under these optimized conditions, Ns-pyrrolidine 1s and Ns-pyrrolidines bearing more activated tertiary C–H bonds could be tolerated to afford the target products in good yields with high regioselectivities (2s–2v, 51–73% yield). In the case of Ns-protected piperidine, relatively harsh conditions were required (CuOAc (0.2 eq), ligand L6 (0.22 eq), Selectflour II (2.0 eq)) to achieve reasonable yield (2w, 41%). Moreover, the scope of this protocol was successfully extended to Ns-tetrahydroisoquinolines delivering desired products 2x and 2y in moderate yields (52% and 65%, respectively).
Next, we evaluated the performance of this C–H functionalization reaction with various alcohols. Oxazolidinone 1d was selected as a representative for these studies. Several alcohols, including ethanol, isopropanol, n-butyl alcohol, and cyclohexanol were applicable to this new protocol affording the CDC products in excellent yields (2da–2dd, 76–85%). It should be mentioned that benzyl alcohol with benzylic C–H bond and 5-hexen-1-ol with a terminal double bond were compatible to the reaction conditions furnishing the CDC products in good yields (2de–2dg, 76–84%).
In view of the importance of glycoconjugation of drugs in pharmaceutical chemistry, which has been extensively used to improve the cancer cell selectivity and solubility of drugs in aqueous solution46–48, two monosaccharide derivatives were tested for C–H glycoconjugation. Both hexose derivative and pentose derivative were suitable substrates for this transformation and excellent yields were observed (2dh, 85% and 2di, 83%). The C–H functionalization reaction also proceeded effectively in generating structural diversity for late-stage functionalization of a number of pharmaceuticals, including the nitrofuran antibiotic-nitrofurantoin (2z1, 51%), the precursor of antibiotic-tedizolid (2z2, 72%), anti-inflammatory drug-methyl ester of indoprofen (2z3, 73%), and a natural product proline-based dipeptide 2z4 (61%). To demonstrate the scalability of this method, gram-scale reactions of 2a, 2aa, 2ia, 2n, and 2q were performed and comparable yields were observed with those of the small-scale reactions (95% vs. 91%, 89% vs. 83%, 86% vs. 81%, 91% vs. 79, and 59% vs. 61%, respectively).
Synthetic utilities
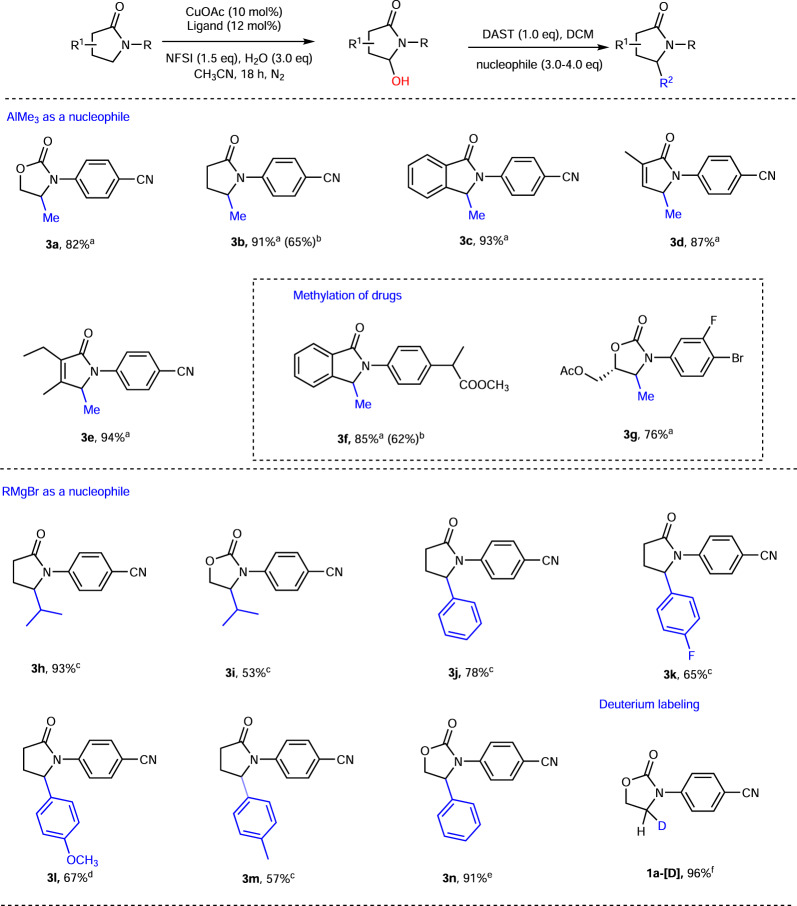
Our subsequent studies focused on late-stage alkylation of these heterocycles. The late-stage alkylation includes two sequences of reactions: (1) CDC approach using H2O as coupling partner to give hemiaminal and (2) Lewis acid or fluorination reagent promoted nucleophilic substitution. As shown in Fig. 3, alkylation reagents such as organoaluminum reagents and Grignard reagents were tested. All these organometalic nucleophiles reacted well with the fluoride intermediates, obtained by treating hemiaminals with diethylaminosulfur trifluoride (DAST), to produce the desired products in good yields (53–94%). Given the importance of “magic methyl” effect in drugs49, 50, we also performed methylation reactions of drug 1z3 and a drug precursor 1z2, and both of the methylated products 3f and 3g were obtained in excellent yields (85% and 76%, respectively). Remarkably, late-stage methylation can also be successfully performed in a one-pot manner without purification of hemiaminals. For example, methylated products 3b and anti-inflammatory drug 3g can be accessed via a one-pot procedure in 65% and 62% yield, respectively, which are comparable with those obtained from two steps with purification of intermediates. With Grignard reagents, iPr and substituted phenyl Grignard reagents were used to demonstrate the late-stage alkylation reaction and the desired products were successfully obtained (53–93% yield). Given the importance of deuterated drugs, we also successfully applied this protocol to mono-deuteration of drugs using 1a as an example (96% yield).
Fig. 3. Late-stage alkylations.
Reaction conditions: aHemiaminal 2 (0.1 mmol), CH2Cl2 (1.0 mL), DAST (0.1 mmol) added at −78 °C; rt for 1 h; cooled to −78 °C, AlMe3 (0.3 mmol) added, stirred 2 h; rt for 1 h, yield of the second step. b1l or 1y (0.2 mmol), H2O (0.3 mmol), CuOAc (0.02 mmol), L2 or L1 (0.022 mmol), NFSI (0.3 mmol), CH3CN (2.0 ml), under N2, followed by CH2Cl2 (1.0 mL), DAST (0.1 mmol) at −78 °C; rt for 1 h; cooled to −78 °C, AlMe3 (0.3 mmol) added, stirred 2 h; rt for 1 h, overall yield of two steps. cHemiaminal 2 (0.1 mmol), CH2Cl2 (1.0 mL), DAST (0.1 mmol) added at −78 °C; rt for 1 h; cooled to −78 °C, RMgBr (0.4 mmol) added, stirred for 5 h; rt for 1 h. d(A) bpy (0.4 mmol), THF (0.5 mL), RMgBr (0.4 mmol) added at −40 °C for 0.5 h; rt for 0.5 h; (B) 1l (0.1 mmol), CH2Cl2 (1.0 mL), DAST (0.1 mmol) added at −78 °C; rt for 1 h; (B) added to (A), stirred 5 h at −40 °C; rt for 1 h. e(A) CuBr·Me2S (0.4 mmol), THF (0.5 mL), RMgBr (0.4 mmol) added at −40 °C for 0.5 h; rt for 0.5 h; (B) 1a (0.1 mmol), CH2Cl2 (1.0 mL), DAST (0.1 mmol) added at −78 °C; rt for 1 h; (B) added to (A), stirred for 5 h at −40 °C; rt for 1 h. f1a (1.0 mmol), TFA (2.0 mL), NaBD4 (4.0 mmol) added at 0 °C; rt for 2 h.
Late-stage functionalization of pyrrolidine derivatives was carried out next, as pyrrolidine is one of the most common N-heterocycle in drugs and peptides1. As shown in Fig.4, 2s as an example, treatment of 2s with BF3·OEt2 and various organic nucleophiles provided a diverse set of functionalized pyrrolidine derivatives in generally moderate-to-good yields (59–90%). Similarly, the OH group can be successfully replaced by organoaluminum reagents and organozinc reagents to produce 2-Me and 2-Et-substituted pyrrolidines 3p and 3q in 90% and 78% yield, respectively. Moreover, 2s can also be successfully converted to 2-allyl product 3s and 2-N3 product 3t by organotin reagent and organosilicon reagents (85% and 76% yield, respectively). In addition, Friedel–Crafts reactions of 1,2-dimethylindole and 2-naphthol with 2s were also successful affording 3o and 3r in 59% and 71% yields, respectively.
Fig. 4. Synthetic applications of pyrrolidine derivative 2s.
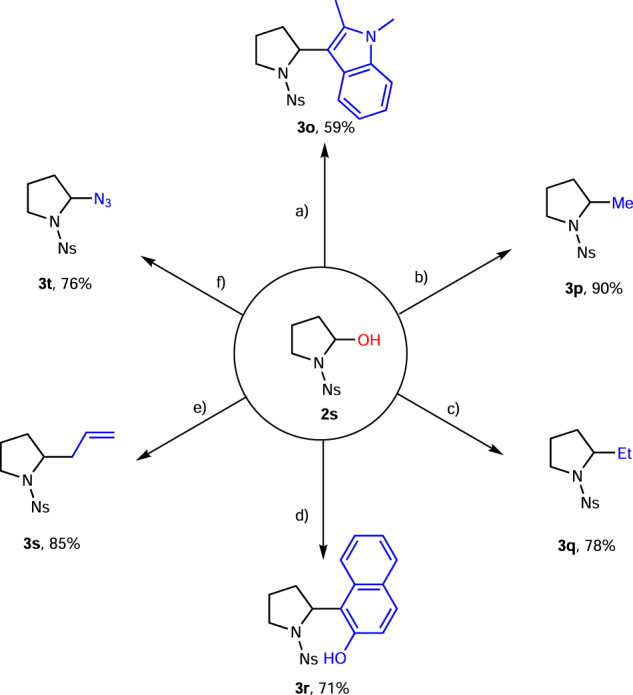
Reaction conditions: 2s (0.1 mmol), CH2Cl2 (1.0 mL), BF3·OEt2 (0.2 mmol), and nucleophile (0.3 mmol) added at −78 °C for 1 h; rt for 3 h. (a) 1,2-Dimethylindole as a nucleophile and the reaction was performed at −78 °C for 3 h. (b) AlMe3 as a nucleophile. (c) Et2Zn as a nucleophile. (d) 2-Naphthol as a nucleophile. (e) Allyltributylstannane as a nucleophile. (f) TMSN3 as a nucleophile.
Mechanistic investigations
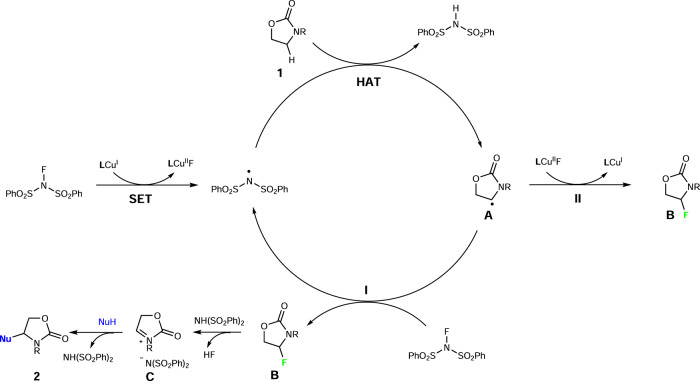
Concerning the reaction mechanism, we proposed two possible pathways for this C–H functionalization reaction as shown in Fig. 5. Initially, L/Cu(I) complex undergoes single electron transfer to NFSI, to produce a bis(phenyl)sulfonamide radical36–38. The bis(phenyl)sulfonamide radical then abstracts a hydrogen atom from C(sp3)–H adjacent to nitrogen of compound 1 to generate the corresponding radical A. In Path I, a radical chain propagation process is proposed, which is supported by recent work on alkyl radical fluorination51, 52, especially the work on fluorine transfer to alkyl radical from N–F reagents51. The radical A undergoes radical fluorination with NFSI to generate the corresponding fluorination intermediate B with regeneration of the bis(phenyl)sulfonamide radical to enter a radical chain process. In Path II, a Cu(I)/Cu(II) cycle is proposed. The radical A undergoes radical fluorination with CuII-F species by bimolecular homolytic substitution (SH2) reaction to generate the fluorination intermediate B53, 54 with regeneration of L/Cu(I) complex. Finally, the fluorinated intermediate B undergoes a nucleophilic substitution promoted by NH(SO2Ph)2 via the iminium intermediate C to afford the final CDC product 2 with elimination of HF54.
Fig. 5. Proposed reaction mechanism.
Two pathways proposed for this C–H functionalization reaction: fluorination via radical chain mechanism (Path I) and fluorination through a Cu(I)/Cu(II) catalytic cycle (Path II).
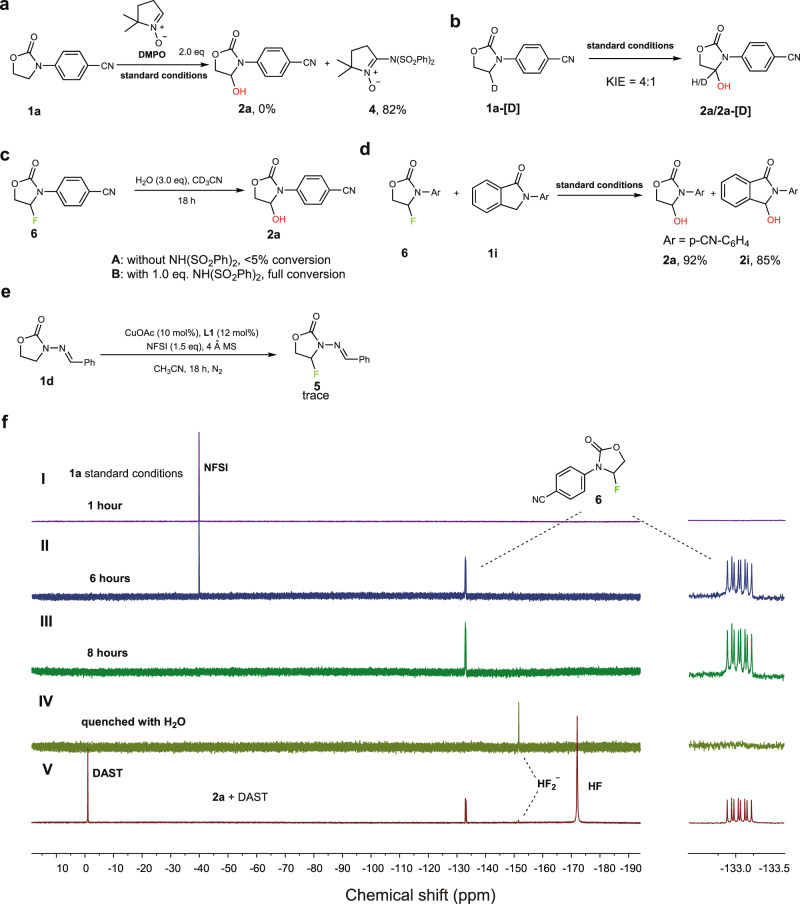
To verify our hypothesis, free-radical scavenging experiments with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was performed (Fig. 6a). The result showed that by adding 2.0 equiv. of DMPO into the reaction of 1a under standard reaction conditions, the C–H hydroxylation reaction was completely inhibited and no trapped adduct from DMPO and radical A was observed. Instead, the initially formed bis(phenyl)sulfonamide radical was successfully trapped by DMPO giving nitroxide radical derivative, which was further oxidized to more stable nitrone 455, 56. In addition, kinetic isotope effect (KIE) studies were also performed to test the key hydrogen atom transfer (HAT) step (Fig. 6b). The intramolecular H/D competition experiment (KIE: 4 : 1) indicated that HAT is the product-determining step. These results suggested a free-radical process was likely involved in this reaction. To further test our hypothesis, 19F nuclear magnetic resonance (NMR) spectroscopy experiments were performed to verify the existence of fluorination intermediate53 and the results were shown in Fig. 6f. When L1 (0.012 mmol), CuOAc (0.01 mmol), 1a (0.1 mmol), and NFSI (0.1 mmol) were mixed in CD3CN (0.5 mL) at room temperature under N2 and kept for 1 h, only the signal of NFSI (−39.4 p.p.m.) was found. After 6 h, a new peak at −133.1 p.p.m. (ddd, J = 70.0, 35.2, 23.3 Hz) emerged, which was attributed to fluorination intermediate 6. The structure of the intermediate was unambiguously confirmed by an authentic sample prepared from 2a and DAST in CD3CN (Fig. 6f, V). Identical chemical shifts, peak patterns, and coupling constants in both 19F and 1H NMR spectroscopy were observed in these samples (Fig. 6f, II, III, and V, and Supplementary Fig. 2). After 8 h, full consumption of NFSI was achieved and the signal of HF2− (−151.6 p.p.m.)57 was observed after the reaction was quenched by adding excess H2O. The hydroxylated product 2a and fluorination intermediate were both clearly seen in 1H NMR spectrum and ~50% conversion was achieved (Supplementary Fig. 2). To further confirm the role of NH(SO2Ph)2, the hydrolysis of 6 was studied and the conversion is followed by 1H NMR. When the hydrolysis of 6 with H2O (3.0 eq.) was performed in CD3CN under neutral conditions, the reaction is very slow and only <5% conversion was observed after 18 h (Fig. 6c). However, in the presence of NH(SO2Ph)2 (1.0 eq.), 6 was completely converted into 2a after 18 h (Fig. 6c). Next, to further confirm that the fluorination intermediate can be transformed into the final product under the reaction conditions, a cross-over control experiment was performed. The result showed that adding fluorination intermediate 6 prepared individually to the reaction of 1i under the standard reaction conditions did not affect the reaction and the corresponding hydroxylated product 2a and 2i were both obtained in good yield. (Fig. 6d). Altogether, these experiments clearly proved the existence of fluorination intermediate during the reaction process.
Fig. 6. Mechanistic experiments.
a Free-radical scavenging experiment. b KIE experiment. c Conditions: CD3CN (0.5 mL), 6 (0.1 mmol), and H2O (0.3 mmol). d Cross-over experiment of the intermediate 6 and 1i under the standard conditions. e Conditions: in glovebox, 4 Å molecular sieve (0.5 g), CH3CN (1.0 mL), 1d (0.1 mmol), CuOAc (0.01 mmol), ligand 1 (0.012 mmol), and NFSI (0.15 mmol). f 19F (376 MHz) NMR spectra of the N-heterocyclic C–H functionalization reaction. Conditions: CD3CN (0.5 mL), 1a (0.1 mmol), CuOAc (0.01 mmol), ligand 1 (0.012 mmol), and NFSI (0.15 mmol). (I) 19F (376 MHz) NMR spectrum of the N-heterocyclic C–H functionalization reaction after 1 h. (II) 19F (376 MHz) NMR spectrum of the reaction after 6 h. (III) 19F (376 MHz) NMR spectrum of the reaction after 8 h. (IV) 19F (376 MHz) NMR spectrum of the reaction after adding 0.01 mL H2O. (V) Fluorinated intermediate 6 prepared from 2a and DAST: CD3CN (0.5 mL), 2a (0.1 mmol), and DAST (0.1 mmol) .19F (376 MHz) NMR spectrum of the reaction after 1 h.
Further investigations revealed an interesting finding for the mechanism of this reaction: specifically, the presence of H2O can substantially improve the rate of fluorination process. When the reaction of 1d was carried out under dry conditions in the presence of 4 Å molecular sieves, low conversion of the reaction was observed and only trace amount of fluorinated intermediate 6 was detected by 19F NMR (Fig. 6e). Furthermore, when the reaction of 1d and MeOH (3.0 eq.) was performed under dry conditions in the presence of activated 4 Å molecular sieves, low conversion was observed as well. It is likely that H2O molecules interact with fluorine by H-bonding interactions, which possibly facilitates the radical fluorination process58, 59.
Discussion
In summary, we have developed a Cu(I)-catalyzed late-stage C(sp3) α-functionalization of N-heterocycles. A series of N-heterocycles, including oxazolidinones, isoindolinones, lactams, pyrrolones, pyrrolidines, piperidines, quinolones, and imidazolidine-2,4-diones, was applicable to the present methodology. The protocol includes two sequences of reactions as follows: CDC reaction using H2O or alcohol as a coupling partner and subsequent Lewis acid or fluorination reagent promoted nucleophilic substitution. The intermediates hemiaminal or aminal are stable and can be isolated and used for various synthetic purposes. A broad range of nucleophiles were suitable for the present late-stage functionalization of these heterocycles including four drugs. Furthermore, this protocol is also applicable to glycoconjugation of drugs as exemplified by two monosaccharides derivatives, as well as mono-deuteration of drugs. Preliminary mechanistic studies reveal that the reaction undergoes a C–H fluorination process, which is followed by a substitution by H2O or alcohol to give stable hemiaminal or aminal products. Besides, it was found that H2O molecules could substantially accelerate the rate of fluorination process by H-bonding interactions with fluorine. This protocol features mild reaction conditions, high chemoselectivity, broad scope of both substrates and nucleophiles, and commercially available catalysts, which ensure its practical application in late-stage α-functionalization of N-heterocycles in natural products and drugs.
Methods
General procedure for C(sp3)–H functionalization of N-heterocyclics
In a Schlenk tube, N-heterocyclic compounds 1a (0.2 mmol), NFSI (0.3 mmol), L1 (0.024 mmol, 12 mol%), and CuOAc (0.02 mmol, 10 mol%) were dissolved in CH3CN (1.0 mL) under a N2 atmosphere, then H2O (0.6 mmol) was added. The reaction mixture was stirred at 35 °C for 24 h. Upon completion, saturated sodium bicarbonate (30 mL) was added and the reaction was extracted with dichloromethane (DCM) (2 × 30 mL), washed with brine (30 mL), dried over Na2SO4, and concentrated in v acuo. The residue was purified by column chromatography on silica gel with a gradient eluent of petroleum ether and ethyl acetate to provide the desired product 2a in a yield of 89%.
Supplementary information
Acknowledgements
We are grateful for the support of this work by the National Natural Science Foundation of China (21801260 and 21971267), Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery (2019B030301005), the 1000-Talent Youth Program of China, and the program for Guangdong Introducing Innovative and Entrepreneurial Teams (2017ZT07C069).
Author contributions
D.Z. and Z.C. conceived and designed the experiments and mechanism studies. D.Z. and R.W. guided the project. Z.C., J.H., G.C., H.Z., and W.S. performed the experiments. Z.C. and D.Z. wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file. The experimental procedures and characterization of all new compounds are provided in Supplementary Information. Extra data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Aiwen Lei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui Wang, Email: wangrui@lzu.edu.cn.
Depeng Zhao, Email: zhaodp5@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24671-y.
References
- 1.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 2.Taylor RD, MacCoss M, Lawson AD. Rings in drugs. J. Med. Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 3.Campos KR. Direct sp3 C-H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 2007;36:1069–1084. doi: 10.1039/B607547A. [DOI] [PubMed] [Google Scholar]
- 4.Topczewski JJ, Cabrera PJ, Saper NI, Sanford MS. Palladium-catalysed transannular C-H functionalization of alicyclic amines. Nature. 2016;531:220–224. doi: 10.1038/nature16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry IB, et al. Direct arylation of strong aliphatic C-H bonds. Nature. 2018;560:70–75. doi: 10.1038/s41586-018-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JZ, et al. Direct conversion of N-alkylamines to N-propargylamines through C-H activation promoted by Lewis acid/organocopper catalysis: application to late-stage functionalization of bioactive molecules. J. Am. Chem. Soc. 2020;142:16493–16505. doi: 10.1021/jacs.0c08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin W, et al. Enantioselective double manipulation of tetrahydroisoquinolines with terminal alkynes and aldehydes under copper(I) catalysis. Angew. Chem. Int. Ed. 2014;53:277–281. doi: 10.1002/anie.201308699. [DOI] [PubMed] [Google Scholar]
- 8.Feng K, et al. Late-stage oxidative C(sp3)-H methylation. Nature. 2020;580:621–627. doi: 10.1038/s41586-020-2137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blakemore DC, et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018;10:383–394. doi: 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- 10.Girard SA, Knauber T, Li C-J. The Cross-dehydrogenative coupling of Csp3-H bonds: a versatile strategy for C-C bond formations. Angew. Chem. Int. Ed. 2014;53:74–100. doi: 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]
- 11.Niu L, Liu J, Liang XA, Wang S, Lei A. Visible light-induced direct α C-H functionalization of alcohols. Nat. Commun. 2019;10:467. doi: 10.1038/s41467-019-08413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long H, et al. Regio- and diastereoselective cross-dehydrogenative coupling of tetrahydropyridines with 1,3-dicarbonyl compounds. Org. Lett. 2017;19:2146–2149. doi: 10.1021/acs.orglett.7b00787. [DOI] [PubMed] [Google Scholar]
- 13.Shono T, Matsumura Y, Tsubata K. Electroorganic chemistry. 46. A new carbon-carbon bond forming reaction at the α-position of amines utilizing anodic oxidation as a key step. J. Am. Chem. Soc. 1981;103:1172–1176. doi: 10.1021/ja00395a029. [DOI] [Google Scholar]
- 14.Chen W, Paul A, Abboud KA, Seidel D. Rapid functionalization of multiple C-H bonds in unprotected alicyclic amines. Nat. Chem. 2020;12:545–550. doi: 10.1038/s41557-020-0438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Ma L, Paul A, Seidel D. Direct α-C-H bond functionalization of unprotected cyclic amines. Nat. Chem. 2018;10:165–169. doi: 10.1038/nchem.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos KR, Klapars A, Waldman JH, Dormer PG, Chen C-y. Enantioselective, palladium-catalyzed α-arylation of N-Boc-pyrrolidine. J. Am. Chem. Soc. 2006;128:3538–3539. doi: 10.1021/ja0605265. [DOI] [PubMed] [Google Scholar]
- 17.Cordier CJ, Lundgren RJ, Fu GC. Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α-alkylations of N-Boc-pyrrolidine. J. Am. Chem. Soc. 2013;135:10946–10949. doi: 10.1021/ja4054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNally A, Prier CK, MacMillan DWC. Discovery of an α-amino C–H arylation reaction using the strategy of accelerated serendipity. Science. 2011;334:1114–1117. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw MH, Shurtleff VW, Terrett JA, Cuthbertson JD, MacMillan DWC. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science. 2016;352:1304–1308. doi: 10.1126/science.aaf6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty JW, Stephenson CR. Amine functionalization via oxidative photoredox catalysis: methodology development and complex molecule synthesis. Acc. Chem. Res. 2015;48:1474–1484. doi: 10.1021/acs.accounts.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain P, Verma P, Xia G, Yu J-Q. Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides. Nat. Chem. 2016;9:140–144. doi: 10.1038/nchem.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan Z, et al. Electrochemical oxidative C(sp3)-H azolation of lactams under mild conditions. Green. Chem. 2020;22:3742–3747. doi: 10.1039/D0GC00687D. [DOI] [Google Scholar]
- 23.Niu L, et al. Manganese-catalyzed oxidative azidation of C(sp3)-H bonds under electrophotocatalytic conditions. J. Am. Chem. Soc. 2020;142:17693–17702. doi: 10.1021/jacs.0c08437. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Lei A. Electrochemical/photochemical aminations based on oxidative cross-coupling between C-H and N-H. Synthesis. 2018;51:83–96. [Google Scholar]
- 25.Li C-J. Cross-dehydrogenative coupling (CDC): exploring C−C bond formations beyond functional group transformations. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Li C-J. CuBr-catalyzed efficient alkynylation of sp3 C−H bonds adjacent to a nitrogen atom. J. Am. Chem. Soc. 2004;126:11810–11811. doi: 10.1021/ja0460763. [DOI] [PubMed] [Google Scholar]
- 27.Paul A, Seidel D. α-Functionalization of cyclic secondary amines: Lewis acid promoted addition of organometallics to transient imines. J. Am. Chem. Soc. 2019;141:8778–8782. doi: 10.1021/jacs.9b04325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida JI, Shimizu A, Hayashi R. Electrogenerated cationic reactive intermediates: the pool method and further advances. Chem. Rev. 2018;118:4702–4730. doi: 10.1021/acs.chemrev.7b00475. [DOI] [PubMed] [Google Scholar]
- 29.Guan W, Santana SO, Liao J, Henninger K, Watson MP. Enantioselective alkynylation of unstabilized cyclic iminium ions. ACS Catal. 2020;10:13820–13824. doi: 10.1021/acscatal.0c04223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrero-Antonino JR, Sorribes I, Junge K, Beller M. Selective ruthenium-catalyzed reductive alkoxylation and amination of cyclic imides. Angew. Chem. Int. Ed. 2016;55:387–391. doi: 10.1002/anie.201508575. [DOI] [PubMed] [Google Scholar]
- 31.Takebayashi S, John JM, Bergens SH. Desymmetrization of meso-cyclic imides via enantioselective monohydrogenation. J. Am. Chem. Soc. 2010;132:12832–12834. doi: 10.1021/ja105783u. [DOI] [PubMed] [Google Scholar]
- 32.Peixoto S, Nguyen TM, Crich D, Delpech B, Marazano C. One-pot formation of piperidine- and pyrrolidine-substituted pyridinium salts via addition of 5-alkylaminopenta-2,4-dienals to N-acyliminium ions: application to the synthesis of (±)-nicotine and analogs. Org. Lett. 2010;12:4760–4763. doi: 10.1021/ol101783c. [DOI] [PubMed] [Google Scholar]
- 33.Roque JB, Kuroda Y, Göttemann LT, Sarpong R. Deconstructive fluorination of cyclic amines by carbon-carbon cleavage. Science. 2018;361:171–174. doi: 10.1126/science.aat6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, et al. Manganese-catalyzed desaturation of N-acyl amines and ethers. ACS Catal. 2019;9:9513–9517. doi: 10.1021/acscatal.9b03457. [DOI] [Google Scholar]
- 35.Osberger TJ, Rogness DC, Kohrt JT, Stepan AF, White MC. Oxidative diversification of amino acids and peptides by small-molecule iron catalysis. Nature. 2016;537:214–219. doi: 10.1038/nature18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science. 2016;353:1014–1018. doi: 10.1126/science.aaf7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao H, et al. Copper-catalyzed late-stage benzylic C(sp3)-H trifluoromethylation. Chem. 2019;5:940–949. doi: 10.1016/j.chempr.2019.02.006. [DOI] [Google Scholar]
- 38.Hu H, et al. Copper-catalysed benzylic C-H coupling with alcohols via radical relay enabled by redox buffering. Nat. Catal. 2020;3:358–367. doi: 10.1038/s41929-020-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez PJ, Belderrain TR, Muñoz-Molina JM. Recent advances in copper-catalyzed radical C-H bond activation using N-F reagents. Synthesis. 2021;53:51–64. doi: 10.1055/s-0040-1707234. [DOI] [Google Scholar]
- 40.Szpera R, Moseley DFJ, Smith LB, Sterling AJ, Gouverneur V. The fluorination of C-H bonds: developments and perspectives. Angew. Chem. Int. Ed. 2019;58:14824–14848. doi: 10.1002/anie.201814457. [DOI] [PubMed] [Google Scholar]
- 41.Muralha VSF, Borges dos Santos RM, Martinho Simões JA. Energetics of alkylbenzyl radicals: a time-resolved photoacoustic calorimetry study. J. Phys. Chem. A. 2004;108:936–942. doi: 10.1021/jp036293a. [DOI] [Google Scholar]
- 42.Wayner DDM, Clark KB, Rauk A, Yu D, Armstrong DA. C-H bond dissociation energies of alkyl amines: radical structures and stabilization energies. J. Am. Chem. Soc. 1997;119:8925–8932. doi: 10.1021/ja971365v. [DOI] [Google Scholar]
- 43.Ito R, Umezawa N, Higuchi T. Unique oxidation reaction of amides with pyridine-N-oxide catalyzed by ruthenium porphyrin: direct oxidative conversion of N-acyl-l-proline to N-acyl-l-glutamate. J. Am. Chem. Soc. 2005;127:834–835. doi: 10.1021/ja045603f. [DOI] [PubMed] [Google Scholar]
- 44.Mudithanapelli C, Dhorma LP, Kim MH. PIFA-promoted, solvent-csontrolled selective functionalization of C(sp2)-H or C(sp3)-H: nitration via C-N bond cleavage of CH3NO2, cyanation, or oxygenation in Water. Org. Lett. 2019;21:3098–3102. doi: 10.1021/acs.orglett.9b00751. [DOI] [PubMed] [Google Scholar]
- 45.Jin X, Kataoka K, Yatabe T, Yamaguchi K, Mizuno N. Supported gold nanoparticles for efficient α-oxygenation of secondary and tertiary amines into amides. Angew. Chem. Int. Ed. 2016;55:7212–7217. doi: 10.1002/anie.201602695. [DOI] [PubMed] [Google Scholar]
- 46.Mikuni K, et al. In vivo antitumor activity of novel water-soluble taxoids. Biol. Pharm. Bull. 2008;31:1155–1158. doi: 10.1248/bpb.31.1155. [DOI] [PubMed] [Google Scholar]
- 47.Pohl J, et al. D-19575-a sugar-linked isophosphoramide mustard derivative exploiting transmembrane glucose transport. Cancer Chemother. Pharmacol. 1995;35:364–370. doi: 10.1007/s002800050248. [DOI] [PubMed] [Google Scholar]
- 48.Stüben J, et al. Stüben, J. et al. Pharmacokinetics and whole-body distribution of the new chemotherapeutic agent β-D-glucosylisophosphoramide mustard and its effects on the incorporation of [methyl-3H]-thymidine in various tissues of the rat. Cancer Chemother. Pharmacol. 1996;38:355–365. doi: 10.1007/s002800050495. [DOI] [PubMed] [Google Scholar]
- 49.Barreiro EJ, Kummerle AE, Fraga CA. The methylation effect in medicinal chemistry. Chem. Rev. 2011;111:5215–5246. doi: 10.1021/cr200060g. [DOI] [PubMed] [Google Scholar]
- 50.Leung CS, Leung SS, Tirado-Rives J, Jorgensen WL. Methyl effects on protein-ligand binding. J. Med. Chem. 2012;55:4489–4500. doi: 10.1021/jm3003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rueda-Becerril M, et al. Fluorine transfer to alkyl radicals. J. Am. Chem. Soc. 2012;134:4026–4029. doi: 10.1021/ja211679v. [DOI] [PubMed] [Google Scholar]
- 52.Pitts CR, et al. Direct, catalytic monofluorination of sp3 C-H bonds: a radical-based mechanism with ionic selectivity. J. Am. Chem. Soc. 2014;136:9780–9791. doi: 10.1021/ja505136j. [DOI] [PubMed] [Google Scholar]
- 53.Bower JK, Cypcar AD, Henriquez B, Stieber SCE, Zhang S. C(sp3)-H fluorination with a copper(II)/(III) redox couple. J. Am. Chem. Soc. 2020;142:8514–8521. doi: 10.1021/jacs.0c02583. [DOI] [PubMed] [Google Scholar]
- 54.Buss JA, Vasilopoulos A, Golden DL, Stahl SS. Copper-catalyzed functionalization of benzylic C-H bonds with N-fluorobenzenesulfonimide: switch from C-N to C-F bond formation promoted by a redox buffer and Brønsted base. Org. Lett. 2020;22:5749–5752. doi: 10.1021/acs.orglett.0c02239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maurel V, Ravanat JL, Gambarelli S. Detection of reactive free radicals derived from nucleosides by liquid chromatography coupled to tandem mass spectrometry of DMPO spin trapping adducts. Rapid Commun. Mass Spectrom. 2006;20:2235–2242. doi: 10.1002/rcm.2579. [DOI] [PubMed] [Google Scholar]
- 56.Li J, et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature. 2019;574:516–521. doi: 10.1038/s41586-019-1655-8. [DOI] [PubMed] [Google Scholar]
- 57.Hudlicky M. Chemical shifts of fluorine in hydrogen fluoride and fluoride ion. J. Fluor. Chem. 1985;28:461–472. doi: 10.1016/S0022-1139(00)81136-7. [DOI] [Google Scholar]
- 58.Liang S, Hammond GB, Xu B. Hydrogen bonding: regulator for nucleophilic fluorination. Chem. Eur. J. 2017;23:17850–17861. doi: 10.1002/chem.201702664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pupo G, et al. Asymmetric nucleophilic fluorination under hydrogen bonding phase-transfer catalysis. Science. 2018;360:638–642. doi: 10.1126/science.aar7941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file. The experimental procedures and characterization of all new compounds are provided in Supplementary Information. Extra data are available from the corresponding author upon request.