Abstract
Exercise mobilizes angiogenic cells, which stimulate vascular repair. However, limited research suggests exercise-induced increase of endothelial progenitor cell (EPCs) is completely lacking in type 1 diabetes (T1D). Clarification, along with investigating how T1D influences exercise-induced increases of other angiogenic cells (hematopoietic progenitor cells; HPCs) and cell surface expression of chemokine receptor 4 (CXCR4) and 7 (CXCR7), is needed. Thirty T1D patients and 30 matched non-diabetes controls completed 45 min of incline walking. Circulating HPCs (CD34+, CD34+CD45dim) and EPCs (CD34+VEGFR2+, CD34+CD45dimVEGFR2+), and subsequent expression of CXCR4 and CXCR7, were enumerated by flow cytometry at rest and post-exercise. Counts of HPCs, EPCs and expression of CXCR4 and CXCR7 were significantly lower at rest in the T1D group. In both groups, exercise increased circulating angiogenic cells. However, increases was largely attenuated in the T1D group, up to 55% lower, with CD34+ (331 ± 437 Δcells/mL vs. 734 ± 876 Δcells/mL p = 0.048), CD34+VEGFR2+ (171 ± 342 Δcells/mL vs. 303 ± 267 Δcells/mL, p = 0.006) and CD34+VEGFR2+CXCR4+ (126 ± 242 Δcells/mL vs. 218 ± 217 Δcells/mL, p = 0.040) significantly lower. Exercise-induced increases of angiogenic cells is possible in T1D patients, albeit attenuated compared to controls. Decreased mobilization likely results in reduced migration to, and repair of, vascular damage, potentially limiting the cardiovascular benefits of exercise.
Trial registration: ISRCTN63739203.
Subject terms: Stem cells, Cardiology, Diseases, Endocrinology, Medical research, Risk factors
Introduction
Endothelial progenitor cells (EPCs), first discovered in 1997, are mononuclear cells which have the potential to stimulate vascular repair1. Evidence demonstrates that these cells can differentiate into endothelial cells in vitro1,2, incorporate into sites of angiogenesis in vivo3,4 and exert proangiogenic abilities via paracrine action2. First identified as cells in peripheral blood expressing CD34, a marker of haematopoiesis5, these precursor cells are now known as haematopoietic stem/progenitor cells (HPC). It is suggested that a more focused phenotype that includes endothelial markers, such as VEGFR2, identifies a sub-population that can differentiate into endothelial cells and therefor are true EPCs6.
The number and function of both HPCs and EPCs are clinically relevant, with lower concentrations associated with endothelial dysfunction7 and a greater risk of cardiovascular events and mortality8,9. Within individuals with type 1 diabetes, most10–13, but not all studies14, have found reduced circulating numbers of HPCs and EPCs compared to matched non-diabetes controls. In combination with hyperglycemia and glucose fluctuations, it is possible that dysfunctional HPCs and EPCs contribute to increased vascular damage15,16 and progression of micro and macrovascular complications17, with individuals with type 1 diabetes having a two- to eightfold increase in mortality rates compared with the general population largely due to cardiovascular diseases (CVD)18–20. Whilst improved glycemic control is associated with reduced development of CVD21, incidence remains elevated even in individuals who have successfully addressed modifiable risk factors18.
In healthy individuals, acute exercise can mobilize both HPCs and EPCs into circulation, and improve their angiogenic function22–24. However, exercise-induced increases of EPCs appears attenuated in those with chronic diseases25,26, which may partially explain the increased CVD risk in these populations. Indeed, increased pre-operative exercise-induced mobilization of EPCs is correlated with reduced post-operative complications after major thoracic surgery27, while HPCs response to exercise was a stronger predictor of myocardial ischemia and mortality than resting circulating count in patients with coronary artery disease over a subsequent 3 year period28. Insight into the ability of these cells to respond to a stimulus, such as exercise, migrating into circulation and homing to ischemic tissue can be measured by the surface expression of chemokine (C-X-C motif) receptor 4 (CXCR4) and 7 (CXCR7)29,30, although evidence on the influence of type 1 diabetes is lacking. Within other chronic diseases, diminished number of CD34+CXCR4+ cells may be a better predictor of mortality than CD34+ cells alone9, while the expression of CXCR7 has been linked to cell survival in diabetic condition in vitro, although limited evidence exists in human studies30.
While mobilisation of HPCs and EPCs appears attenuated to direct stimulation in both type 1 and 2 diabetes31,32, and exercise-induced increases appears attenuated in type 2 diabetes26, there is limited information in Type 1 diabetes, a vastly different disease. Type 1 diabetes patients are typically not obese, tend to be diagnosed at an early age (if not childhood), and generally live much more active lives with higher levels of cardio-respiratory fitness33, albeit slightly lower than the general non-diabetes general public34. At present, the two studies that have had investigated EPC mobilization with acute exercise in individuals with type 1 diabetes have found total lack of mobilisation35,36. However, as previous studies have measured EPCs as a percentage of circulating mononuclear cells, where any mobilization is likely masked by increases in overall leucocyte counts with exercise37, they failed to capture the expected post-exercise mobilization in the non-diabetes controls.
Thus, due to the increased risk of vascular complications in this disease, this study aimed to definitely explore whether exercise-induced increases of HPCs and EPCs is possible for people with type 1 diabetes. Additionally, this study aimed to explore how type 1 diabetes influences deeper phenotypes of angiogenic cells, including not previously measured cell surface expression of key chemotactic receptors CXCR4 and CXCR7, comparing to age-, sex-, fitness- and BMI- matched controls at rest and during exercise-induced mobilisation. We hypothesized that the type 1 diabetes group will have reduced resting and exercise-induced increases of HPCs and EPCs compared to healthy controls.
Methods
Participants
Participants were recruited from the Newcastle Diabetes Centre and Newcastle University. Participants with type 1 diabetes had a confirmed clinical diagnosis; age 18–65 years with a diabetes duration ≥ 3 years; HbA1c < 86 mmol/mol (10.0%); and absence of diabetes-related complications apart from non-proliferating retinopathy. Eligibility criteria for the non-diabetes participants comprised being aged between 18 and 65 years, non-smoker, and free from any history of chronic diseases.
All participants provided written informed consent and the study was approved by the NHS HRA North East Tyne & Wear South Research Ethics and Newcastle University Ethics Committees (code:16/NE/0192, registry:ISRCTN63739203). All methods were performed in accordance with the relevant guidelines and regulations.
Screening visit
All participants attended the Newcastle NIHR Clinical Research Facility (CRF) on two occasions. Firstly, a screening visit to determine eligibility, medical assessment and peak oxygen uptake (). Participant height, body mass (Seca 220 stadiometer / Seca 889 scales, Seca, Germany) and medical history were taken. Participants underwent a modified 12-lead resting and exercising electrocardiogram to screen for cardiac abnormalities. Eligible participants completed a maximal graded exercise treadmill (Valiant 2 CPET, Lode, Groningen, Netherlands) test using the Bruce protocol38 to determine . Glucose levels in participants with type 1 diabetes were managed as per the guidance of Riddell et al.39.
Main trial visit
Participants attended the CRF at least 7 days after the initial screening. Individuals arrived at the exercise lab at ~ 8.30am after an overnight fast, having been instructed to avoid structured exercise in the 48 h preceding the visit.
The participants with type 1 diabetes maintained their normal basal insulin regimen. If they experienced a hypoglycemic event overnight prior to the study visit, the visit was reorganised. If blood glucose on waking was > 10 mmol/L, they were instructed to have a small corrective bolus of rapid-acting insulin (≤ 2 units).
Upon arrival, the non-dominant arm of each participant was cannulated and resting (baseline) blood samples were drawn. The initial 4 mL drawn was discarded to avoid contamination of mature circulating endothelial cells with cells released from the punctured vein during the cannulation. One 10 mL EDTA vacutainer (Becton, Dickinson and Company, New Jersey, USA) was collected at baseline and, immediately post-exercise. An additional 4 mL EDTA Vacutainer was drawn at baseline for analysis of HbA1c at the Newcastle Clinical Laboratory. Capillary blood was collected at all-time points and analysed by a HemoControl analyser (EKF, Cardiff, UK) to determine haematocrit and haemoglobin concentration.
Participants consumed a 30 g carbohydrate snack (Belvita, Mondelēz International, USA) immediately after baseline blood draws and remained rested for 20 min. Participants walked on an incline for 45 min at 60% at a comfortable stride length (8.06 ± 5.09% at 4.30 ± 0.47 kph). Participants’ treadmill velocity and gradient were calculated using , velocity, and gradient data from the preliminary test40. Breath-by-breath respiratory parameters (Metalyzer 3B-R3 CPET, Cortex, Leipzig, Germany) were continuously recorded throughout, with gradient adjusted at 10 and 30 min if was > 10% different than target . Participants with type 1 diabetes had a target capillary blood glucose > 7 mmol/L for the duration of the exercise with 6 individuals given 10 g of additional carbohydrates, administered via a glucose drink.
Upon completion of the exercise, venous blood samples were immediately drawn from the cannula. Participants rested for 60 min before another venous blood sample was drawn and being discharged from the CRF if capillary blood glucose concentration > 3.9 mmol/L (70 mg/dL).
Flow cytometry enumeration of hematopoietic and endothelial progenitor cells
HPCs and EPCs were quantified on a flow cytometer (BD LSRFortessa X20; BD Biosciences, USA) within 4 h of blood draw6. Briefly, 200 µL of whole peripheral blood collected in EDTA was incubated with 10 µL anti-CD34 FITC, 10 µL anti-VEGFR2 APC, 10 µL anti-CD45 BV421 (BioLegend, San Diego, CA, USA), 10 µL anti-CXCR4 APC Cy7, and 10 µL anti-CXCR7 PE (BioLegend, San Diego, CA, USA) in a BD Trucount (BD Biosciences, USA) tube at 4 °C for 30 min in the dark. Four mL of red blood cell lysis buffer (BD Pharm LyseTM, BD Biosciences, United Kingdom) was added and left to incubate for a further 30 min at 4 °C in the dark before enumeration by flow cytometry. The samples were vortexed at low speed to resuspend beads and reduce cell aggregation. Samples were analysed for 45 min or until 500,000 CD45+ events had been enumerated, whichever occurred first. The LSRFortessa was equipped with a blue, yellow/green, red, violet and ultra violet lasers (488 nm, 561 nm, 635 nm, 405 nm and 355 nm wavelengths, respectively).
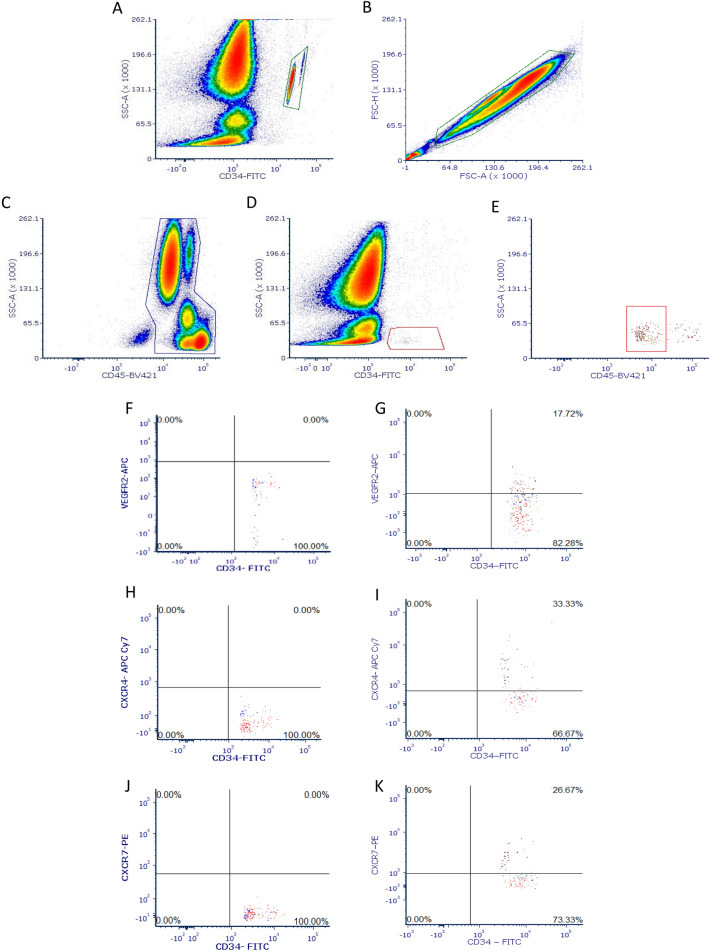
Compensation using BD CompBead (BD Biosciences, USA), was performed prior to collecting each participant’s data to correct for any spectral overlap. Due to highly unreliable nature of isotype controls in rare event analysis6, positive (VEGFR2) and negative (VEGFR2, CXCR4, CXCR7) control samples were used to help determine the gating of positive events by histogram and dot plot (Fig. 1F,H,J). Between samples, FACS clean (BD Biosciences, USA) and deionized water was used to decontaminate the flow cytometer for 5 min.
Figure 1.
Enumeration of HCPs and EPCs by flow cytometry. (A) Gating of the fluorescent beads from the Trucount Tubes to determine absolute cell count. (B) Forward scatter height versus forward scatter area density plot for gating doublet exclusion. (C) Gating of CD45+ mononuclear cells. (D) Identification of CD45+ cells expressing CD34+ with low side scatter (CD34+ cells). (E) Gating of low expression of CD45+ (CD34+CD45dim cells). (F) Negative controls for the identification the gating of positive VEGFR2+ events. (G) Identification of VEGFR2+ on CD34+ or CD34+CD45dim cells. (H) Negative controls for the identification the gating of positive CXCR4 events. (I) Identification of CXCR4 cell surface expression upon all HPC and EPCs phenotypes. (J) Negative controls for the identification the gating of positive CXCR7 events. (K) Identification of CXCR7 cell surface expression upon all HPC and EPCs phenotypes.
Following data acquisition, flow cytometry files were analysed using FCS Express 7 (De Novo, California, USA). Counts of HPC and EPC numbers were converted to cells/mL using BD Trucount, with the number of positive cell events divided by the number of Trucount bead event, and then multiplying by the known total BD Trucount bead concentration. Haematocrit and haemoglobin concentration measures were used to adjust absolute cell counts changes in blood volume using the Dill and Costill method41. Instead of presenting as a proportion of total events enumerated by flow cytometry, a valid methodology for the measuring of rare cells at rest6, the use of Trucount tubes permits the acquisition of absolute cell counts of cells, and allows the exact changes in response to a stimulus to be measured. As overall leucocyte counts acutely increase with exercise37, any changes in rare cells are likely masked or hidden when measure as a percentage of total events.
The gating strategies for enumeration of the HPCs (CD34+, CD34+CD45dim)5 and EPCs (CD34+VEGFR2+, CD34+CD45dimVEGFR2+)6,42 and subsequent cell surface expression of CXCR4 and CXCR7 are displayed in Fig. 1. Selected time-points were run in duplicate, with blood from a single vacutainer separated and fluorescent-labelled antibodies added before analysis by flow cytometry, with an intra-individual CV% of 8.68%.
Statistical analysis
Statistically significant differences between the type 1 diabetes and non-diabetes control group were determined by independent sample T-test. Data were assessed for normality and outliers by box-plots and Shapiro–Wilk test. Excessively skewed data were transformed using square root and logarithmic transformation. When transformation failed, group difference data were assessed by Mann–Whitney U Test. Time course change data (pre, immediately post and 1 h post exercise) was analysed by mixed-effects model. GraphPad Prism 8.0.1 (San Diego, USA) and IBM SPSS Statistics (version 24, IBM, Armonk NY) software packages were used to analyse the data. Statistical significance set at p ≤ 0.05. Data are presented as mean ± standard deviation throughout.
Ethics approval
All participants provided written informed consent and the study was approved by the NHS HRA North East Tyne & Wear South Research Ethics and Newcastle University Ethics Committees (code:16/NE/0192).
Results
Demographic data are shown in Table 1. Age, BMI and were comparable between the matched groups.
Table 1.
Participant demographic data.
| Type 1 diabetes group | Non-diabetes control group | p-value | |
|---|---|---|---|
| N | 30 | 30 | |
| Male/female | 16/14 | 16/14 | |
| Age (years) | 38.2 ± 12.0 | 37.6 ± 12.1 | 0.840 |
| HBA1C (mmol/mol) | 58.5 ± 9.1 | 33.5 ± 2.3 | < 0.001 |
| (%) | 7.5 ± 3.0 | 5.2 ± 2.4 | < 0.001 |
| BMI (kg/m2) | 25.2 ± 3.7 | 24.7 ± 4.6 | 0.656 |
| (ml/kg/min) | 38.8 ± 9.5 | 42.4 ± 12.4 | 0.205 |
| Age at diagnosis | 18.2 ± 8.6 | – | |
| Range (years) | 8 to 35 | ||
| Duration of diabetes | 20.0 ± 13.0 | – | |
| Range (years) | 3 to 47 | ||
| Method of control (MDI/CSII) | 15/15 | – |
Bold signifies p ≤ 0.05.
Data presented as mean ± SD. P value from independent samples t-test.
On average, participants exercised at 58.8% of their , with no differences between the groups (p = 0.907). There were no episodes of hypoglycemia (< 3.9 mmol/L) during the exercise bout.
Resting levels of circulating HPCs and EPCs are lower in the participants with type 1 diabetes than non-diabetes controls
Circulating numbers of HPCs CD34+ (type 1 diabetes; 1468 ± 611 cells/mL, CON; 2048 ± 768 cells/mL, p = 0.001) and CD34+CD45dim (type 1 diabetes; 1189 ± 536 cells/mL, CON; 1684 ± 765 cells/mL, p = 0.003) were significantly lower at rest in the type 1 diabetes group compared to the non-diabetes controls (Fig. 2A). Resting counts of EPCs CD34+VEGFR2+ (type 1 diabetes; 411 ± 159 cells/mL, CON; 664 ± 217 cells/mL, p < 0.001) and CD34+CD45dimVEGFR2+ (type 1 diabetes; 292 ± 121 cells/mL CON; 462 ± 177 cells/mL, p < 0.001) were also significantly lower at rest within the type 1 diabetes group compared to the non-diabetes controls (Fig. 2A). Additionally, circulating number of all HPCs and EPCs expressing CXCR4 and CXCR7 were significantly lower in the type 1 diabetes group than the matched non-diabetes controls (Fig. 2B,C).
Figure 2.
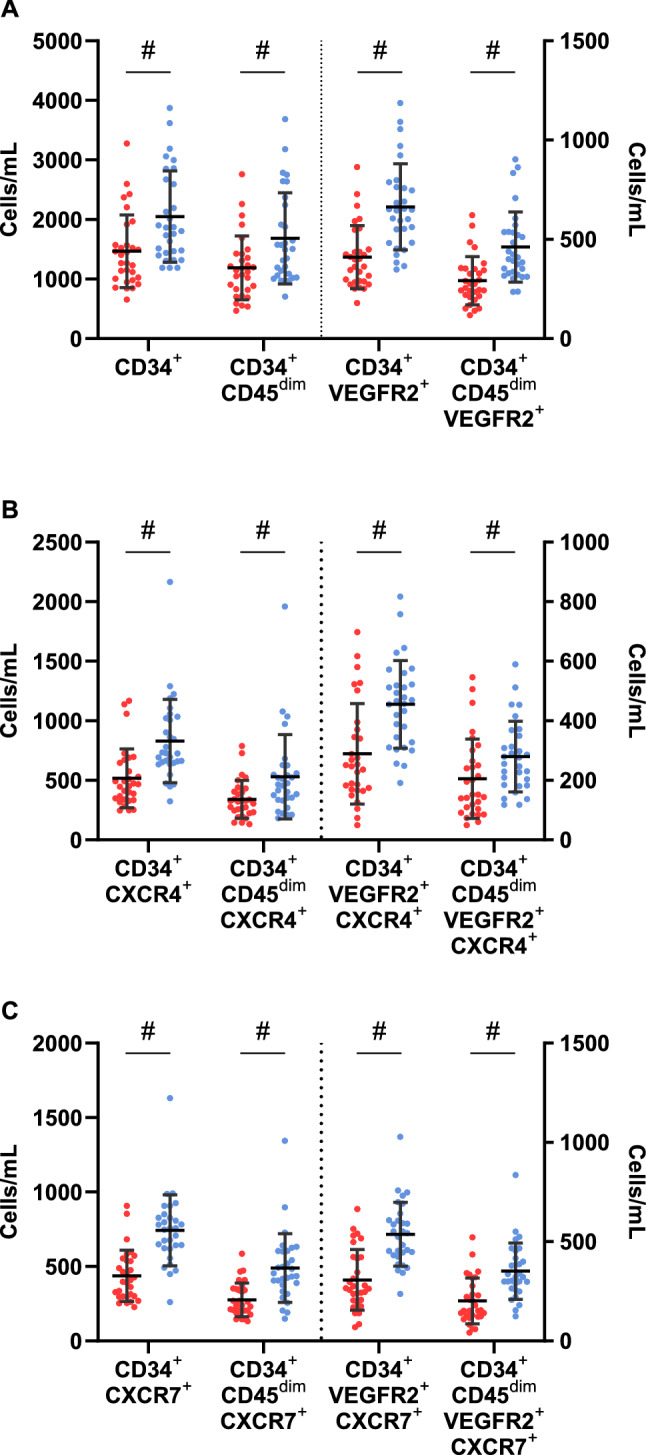
Resting circulating number of CD34+, CD34+CD45dim HPCs and CD34+VEGFR2+, CD34+CD45dimVEGFR2+ EPCs (A), and the number of these cells expressing CXCR4+ (B) and CXCR7+ (C) between the type 1 diabetes (red circles) and non-diabetes (blue circles) groups. #—signifies significant difference between the type 1 diabetes and non-diabetes groups. Data shown are mean ± SD.
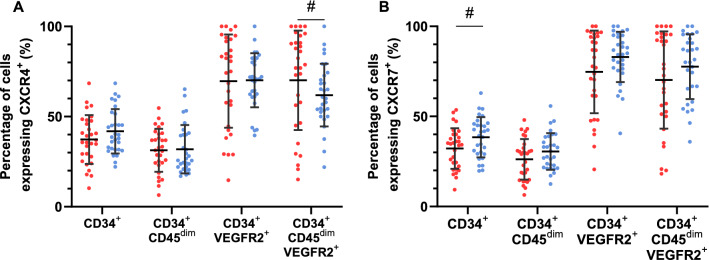
When expressed as a percentage of the HPC and EPC phenotypes, CXCR4 expression at rest tended to be similar between groups. However, percentage of CD34+CD45dimVEGFR2+ expressing CXCR4 was significantly higher in in the type 1 diabetes group (p = 0.050) (Fig. 3A). Percentage of cells expressing CXCR7 at rest tended to be lower in the type 1 diabetes group, with CD34+CXCR7+ significantly so (p = 0.035) (Fig. 3B).
Figure 3.
The percentage of CD34+, CD34+CD45dim HPCs and CD34+VEGFR2+, CD34+CD45dimVEGFR2+ EPCs expressing CXCR4+ (A) and CXCR7+ (B) between the type 1 diabetes (red circles) and non-diabetes (blue circles) groups. #—signifies significant difference between the type 1 diabetes and non-diabetes groups. Data shown are mean ± SD.
Type 1 diabetes patients display attenuated HPC and EPC mobilization in response to acute exercise
The mean delta change (Δ) in pre to post-exercise cell numbers is displayed in Fig. 4. The type 1 diabetes group had attenuated mobilization of HPCs and EPCs, ranging from 39 to 55% lower across the phenotypes when compare to the non-diabetes group, with CD34+ HPCs (331 ± 437 Δ cells/mL vs. 734 ± 876, Δ cells/mL p = 0.048) and CD34+VEGFR2+ EPCs (171 ± 342 Δ cells/mL vs. 303 ± 267 Δ cells/mL, p = 0.006) significantly lower.
Figure 4.
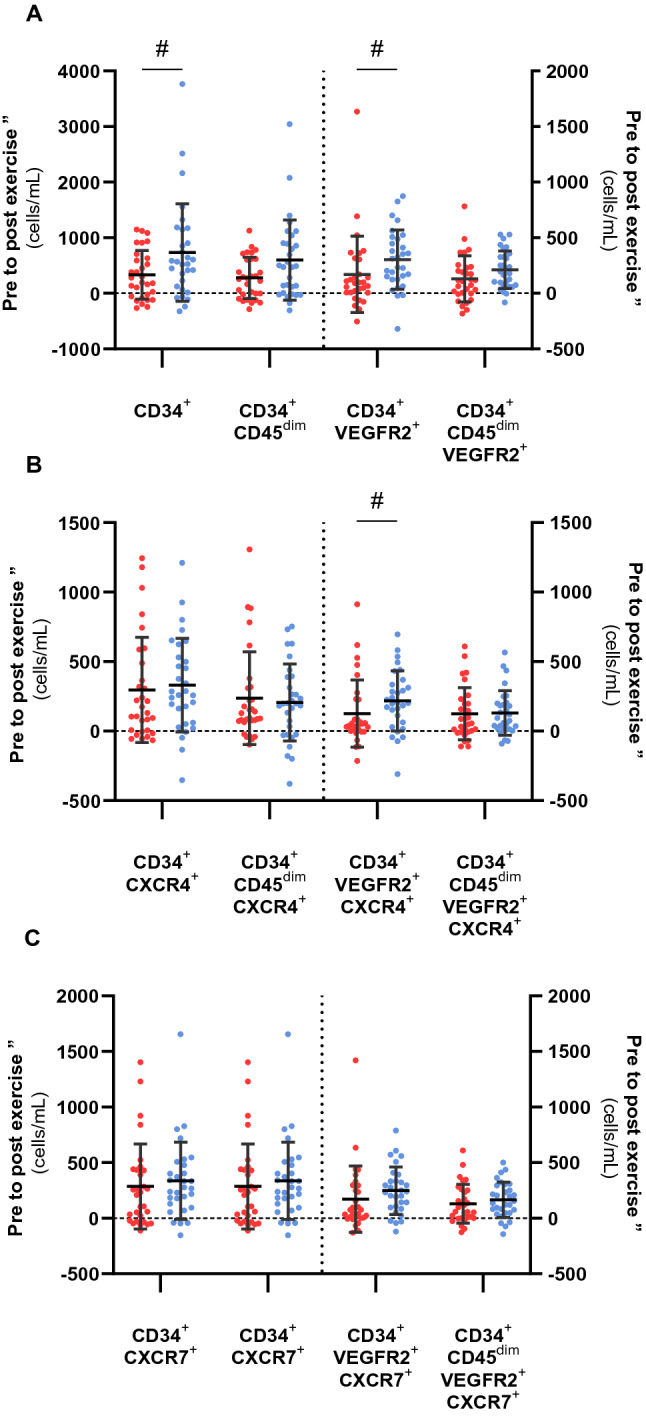
Pre to post exercise delta change (∆ cells, cells/mL) of HPCs and EPCs (A), HPCs and EPCs expressing CXCR4+ (B), HPCs and EPCs expressing CXCR7+ (C) in participants with type 1 diabetes (red circle) and non-diabetes controls (blue circle) in response to a single bout of moderate-intensity exercise. #—signifies significant difference between the type 1 diabetes and non-diabetes groups. Data shown are mean ± SD.
There were no significant differences between the groups in the Δ of CXCR4+ or CXCR7+ HPC and EPC phenotypes (p > 0.05), other than for CD34+VEGFR2+CXCR4+ EPCs, where the mobilization in the type 1 diabetes group was 42% lower compared to controls (126 ± 242 Δ cells/mL vs. 218 ± 217 Δ cells/mL, p = 0.040).
Within the type 1 diabetes group, exercised-induced increases of the HPCs and EPCs was significantly greater in the cells that also expressed CXCR4 or CXCR7, with the progenitor cells negative for a chemokine receptor having between 64 and 101% less mobilization (Table 2). In comparison, within the controls, only the CD34+VEGFR2+ EPCs positive for CXCR4 had significantly higher mobilization than the CXCR4 negative cells (218 ± 217 Δ cell/mL vs. 85 ± 143 Δ cell/mL, p = 0.007). Additionally, the CD34+VEGFR2+ and CD34+CD45dimVEGFR2+ EPCs positive for CXCR7 also had significantly greater mobilization than those negative for CXCR7 (248 ± 213 Δ cell/mL vs. 55 ± 132 Δ cell/mL, p < 0.001 and 166 ± 158 Δ cell/mL vs. 46 ± 112 Δ cell/mL, p = 0.005, respectively).
Table 2.
Mean delta change (Δ) in pre to post-exercise cell numbers of HPCs and EPCs expressing CXCR4 and CXCR7 versus those negative for CXCR4 and CXCR7 for the type 1 diabetes and control groups.
| CXCR4+ | CXCR4− | p | CXCR7+ | CXCR7− | p | |
|---|---|---|---|---|---|---|
| Type 1 diabetes group | ||||||
| CD34+ | 297 ± 378 | 34 ± 268 | 0.006 | 286 ± 383 | 45 ± 293 | 0.018 |
| CD34+CD45dim | 237 ± 333 | 40 ± 267 | 0.031 | 203 ± 283 | 74 ± 279 | 0.105 |
| CD34+VEGFR2+ | 126 ± 242 | 44 ± 178 | 0.084 | 171 ± 298 | − 1 ± 85 | 0.002 |
| CD34+CD45dimVEGFR2+ | 124 ± 186 | 5 ± 75 | 0.003 | 130 ± 175 | − 1 ± 75 | < 0.001 |
| Control group | ||||||
| CD34+ | 332 ± 337 | 403 ± 641 | 0.468 | 337 ± 348 | 397 ± 766 | 0.686 |
| CD34+CD45dim | 206 ± 278 | 391 ± 631 | 0.173 | 227 ± 243 | 380 ± 631 | 0.311 |
| CD34+VEGFR2+ | 218 ± 217 | 85 ± 143 | 0.007 | 248 ± 213 | 55 ± 132 | < 0.001 |
| CD34+CD45dimVEGFR2+ | 130 ± 161 | 82 ± 131 | 0.276 | 166 ± 158 | 46 ± 112 | 0.005 |
Bold signifies p ≤ 0.05.
Data presented as mean ± SD. P value from dependent samples t-test.
Time course kinetics and association between clinical variables and resting and exercise-induced progenitor cell number
All HPC and EPC phenotypes, and their cell surface expression of CXCR4 and CXCR7, had a main effect of time with immediately post-exercise sample significantly higher than the baseline samples (p < 0.002). Additionally, CD34+, CD34+CXCR4+, CD34+CXCR7+ HPCs had a significantly higher count 1-h post-exercise compared to pre-exercise levels (p = 0.042, p = 0.010 and p = 0.013, respectively). There was a group x time interaction for the CD34+ HPCs, remaining elevated at 1 h post exercise in the type 1 diabetes group but not the healthy controls (Supplementary Fig. 1).
Clinical variables (HbA1c, BMI, age, , age at diagnosis and duration of diabetes) were assessed for correlations with resting concentrations and Δ from pre- to post-exercise (cells/mL) (Supplementary Table 1, 2). HbA1c was negatively correlated with HPC and EPC concentration at rest for all participants (n = 60, r > − 0.272, p < 0.036). However, when split into the type 1 diabetes (n = 30) and non-diabetes control (n = 30) groups, the relationships were no longer significant, except for HbA1c and CD34+CD45dimVEGFR2+CXCR7+ EPCs (r = − 0.364, p = 0.048) in the type 1 diabetes group. An older age of type 1 diabetes diagnosis positively correlated with CD34+CD45dim cells (r = 0.361, p = 0.050). Within the type 1 diabetes group, no clinical variable correlated with Δ in HPCs or EPCs from pre- to post-exercise.
Discussion
We investigated the influence of type 1 diabetes on circulating HPC and EPC numbers, and the cell surface expression of CXCR4 and CXCR7 on these cells, at rest and in response to a submaximal exercise bout. For the first time, we demonstrate that individuals with type 1 diabetes are able to increase HPCs and EPCs into circulation in response to exercise. However, mobilization of these angiogenic cells is attenuated in comparison to matched non-diabetes controls, which may play a role in the increased risk of vascular complications seen in type 1 diabetes.
Our primary finding that individuals with type 1 diabetes can mobilize HPCs and EPCs in response to exercise is of interest, as exercise-induced mobilization has been shown to be a more powerful predictor of complications and mortality than basal circulating count in thoracic surgery and coronary artery disease patients27,28, and contrasts previous research which found no mobilisation of EPCs in type 135,36 or 2 diabetes26. Differences between our study and those previously exploring exercise-induced mobilisation in people with type 1 diabetes likely arose due to alternative ways of quantifying circulating angiogenic cell numbers. While our study used BD Trucount tubes to calculate absolute cell counts and adjusted these results for changes in blood volume41, accurately determining cell changes in response to an exercise stressor, previous studies have only measured circulating EPCs as a percentage of circulating mononuclear cells, where any exercise-induced mobilization was likely concealed by increases in overall leucocyte counts around exercise37. Additionally, we included a much deeper examination of angiogenic cell phenotypes, demonstrating that both HPCs and EPCs are mobilised by individuals with type 1 diabetes.
These results again demonstrate that type 1 diabetes has a detrimental impact on circulating EPCs and HPCs, with previous research demonstrating a reduced resting count10–12 and impaired angiogenic function including: impaired ability to differentiate into endothelial cells, reduced migration to areas of ischemia, reduced angiogenic paracrine secretion, and increased apoptosis43. As these circulating cells play an important role in maintaining endothelial integrity7, the reduced circulating numbers seen in this study may play an important causative role in the development of diabetic complications and increased CVD through reduced endothelial repair8, with lower levels of both HPCs and EPCs counts associated with extensive multi-site atherosclerosis44. Our study is the first to demonstrate that circulating numbers of these angiogenic cells expressing CXCR4 and CXCR7 are also significantly lower in individuals with type 1 diabetes, findings similar to those seen in people with type 2 diabetes45. The reduced number of cells expressing CXCR4 and CXCR7 likely results in the reduced ability to migrate into circulation and to ischemic tissue within diabetes29,30,46, which may further exacerbate endothelial dysfunction and microvascular abnormalities and increase the risk of mortality47.
Within our study, both groups were well matched, the participants were not obese or old, and had moderate cardiorespiratory fitness (38.8 ± 9.5 mL/min/kg), which contrasts enormously to work conducted exploring exercise-induced mobilisation of EPCs in type 2 diabetes26. Additionally, our participants with type 1 diabetes had no major diabetes-related complications. Despite this, we showed that the increased circulating HPCs and EPCs from pre- to post-exercise in the type 1 diabetes group, CD34+ HPCs, CD34+VEGFR2+, CD34+VEGFR2+CXCR4+ EPC counts were significantly attenuated compared to the non-diabetes controls. Strikingly, mean post-exercise concentrations of most the phenotypes were lower in the type 1 diabetes group than the resting concentrations of the controls. The reduced exercise-induced mobilization is similar to previous studies that found no mobilisation of HPCs and EPCs to indirect CXCR4+ stimulation31 and slightly attenuated mobilisation to direct CXCR4+ antagonists in a mixed group of type 1 and 2 diabetes participants32. It is unclear why a direct CXCR4+ antagonist can mobilise angiogenic cells from the bone marrow while an indirect cannot. As exercise mobilised HPCs and EPCs negative for CXCR4 and CXCR7 in the controls, but not the type 1 diabetes group, this suggests pathways other than stromal cell–derived factor-1α (SDF-1α)/CXCR4 are also impaired by deregulated glucose control seen in diabetes.
While the exact mechanism for mobilising angiogenic cells in response to exercise has not been fully elucidated, mobilization is dependent on both duration and intensity, with a higher intensity potentially needed in this study in order for all participants to achieve mobilisation23. Post-exercise counts have been shown to positively correlate with increased circulating levels of SDF-1α, VEGF, erythropoietin and tissue expression of hypoxia-inducible factor 1-α. Suppressed release of VEGF and SDF-1α, key for the mobilization and homing of progenitor cells from the bone marrow to areas of ischemia, have been demonstrated in a murine model of diabetes48, and may explain the reduced increase in HPCs and EPCs seen in this study. Additionally, high glucose conditions have been shown to reduce the angiogenic function of HPCs and EPCs49, as well as increasing senescence and apoptosis43. Within type 1 diabetes mouse models, it has been demonstrated that increased vascular damage ultimately results in the exhaustion and depletion of progenitor cells stored within the bone marrow. Moreover, dysfunctional osteoblastic niches and microangiopathy damage to the blood vessels in the bone resulting in an impaired ability to egress these cells into circulation in response to ischemia50. Microvascular dysfunction and altered blood flow can occur in the early stages of Type 1 diabetes, with hyperglycaemia and oxidative stress reducing the bioavailabilty of nitrix oxide51,52. Endothelial nitrix oxide synthase induces smooth muscle relaxition and blood vessel dilation, and is strong modulator of circulating angiogenic cells funtion and homing53. It has been demonstrated that pancreas transplants improves endothelial function in conjunction with the normalisation of glucose metabolism by restoring endothelial nitric oxide synthase54, which likely explains the post islet-transplant improvements in circulating angiogenic cell function55. This raises the intriguing possibility that exercise, improvements in glycemic control and vasodilatory dietary supplements could increase endothelial nitric oxide synthase, improving endothelial and angiogenic cells functions within people with type 1 diabetes, warranting further study56.
There is growing evidence for separate and important functions of CXCR7, promoting endothelial proliferation and angiogenesis, and plying a critical role in the survival of EPCs. Our results are supported by the observations by Dai et al.30 demonstrating that the percentage of EPCs expressing CXCR7 but not CXCR4 was reduced in a diabetes mouse and in vitro model, but not Vigorelli et al.57 who showed reduced CXCR4 protein expression when exposing CD34+ cells to a high glucose environment in vitro. As knockdown of CXCR7 impairs vascular tube formation and upregulation rescues angiogenic function of diabetic EPCs30, the reduction in CXCR7 angiogenic cells within this study is of clinical significance, highlighting the dysfunctional nature of these cells in people with type 1 diabetes. The effect of glucose upon CXCR4 is controversial, with high glucose reported to both increase58 and inhibit expression59.
Limitations of this study include the lack of an apoptosis marker, making it likely that non-viable cells were quantified. This especially true of EPCs within the type 1 diabetes group, where increased apoptosis is likely due to hyperglycemia43, and mean fluorescence intensity of VEGFR2 staining is slightly greater in dead versus live cells60. Measuring progenitor cell mobilising stimuli would also have been beneficial, especially as the number of HPCs negative for a chemokine receptor mobilized with exercise was substantially lower in the type 1 diabetes group suggesting impairment of an additional pathway other than the SDF-1α/CXCR4 axis. Future research needs to explore whether different methods of diabetes management and improving glycemic control results in improvement in exercise-induced mobilization of these cells within individuals with type 1 diabetes. Improving HbA1c, and reducing glycemic variability (by switching diabetes management to continuous subcutaneous insulin infusion) both increase basal concentrations of EPCs13,61, while severe hypoglycemia is associated with a marked depletion of circulating HPCs and EPCs in individuals with type 2 diabetes62. Potentially, they also influence exercise-induced mobilization. Regular exercise training, in both healthy and diseased populations, has also been shown to increase basal concentration of EPCs and HPCs62. Therefore, determining if exercise training could increase basal concentration and restore exercise-induced mobilization in individuals with type 1 diabetes, with the aim of improving vascular repair and reducing both micro and macrovascular diabetes complications merits further study.
Conclusion
In conclusion, people with type 1 diabetes have reduced resting and attenuated mobilization of EPCs and HPCs with exercise compared to matched controls. Reduced mobilization of HPCs and EPCs with exercise may play a role in the increased cardiovascular risk in individuals with type 1 diabetes.
Supplementary Information
Acknowledgements
The authors thank the study participants for their time, effort, and commitment, as well as the research teams at the Newcastle National Institute for Health Research Clinical Research Facility, Newcastle-upon-Tyne, for their assistance with data collection, and the Newcastle University Flow Cytometry Core Facility (FCCF) for assistance with the generation of Flow Cytometry data.
Abbreviations
- CVD
Cardiovascular diseases
- CXCR4
Chemokine receptor 4
- CXCR7
Chemokine receptor 7
- EPCs
Endothelial progenitor cells
- HPCs
Haematopoietic progenitor cells
- CRF
Newcastle NIHR Clinical Research Facility
Author contributions
G.S.T. recruited participants, designed study, researched data, wrote the manuscript. D.J.W. and M.R. designed study, researched data, wrote the manuscript. J.A.S. recruited participants, designed study, provided clinical cover and reviewed/edited the manuscript. A.S. and M.C. processed data, reviewed/edited the manuscript. A.B. and A.F. recruited participants, provided clinical cover and reviewed/edited the manuscript. E.S. and M.D.C. reviewed/edited the manuscript. K.S., J.H.S. and T.C. contributed to data collection and reviewed/edited the manuscript.
Funding
This study was funded by the Diabetes Research and Wellness Foundation (SCA/OF/12/15) award to DW. Funding was also provided by philanthropic award to DW from the Francis James Bell Endowment Fund, Country Durham Community Foundation.
Data availability
The datasets used during the current study are available from the corresponding author (Daniel J West; Email: daniel.west@newcastle.ac.uk, telephone: + 44 (0)191 20 87076) on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-93886-2.
References
- 1.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Hur J, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 3.Ananthaseshan S, et al. Locally Transplanted CD34+ Bone Marrow-Derived Cells Contribute to Vascular Healing After Vascular Injury. Elsevier; 2017. [DOI] [PubMed] [Google Scholar]
- 4.Leor J, et al. Human umbilical cord blood–derived CD133+ cells enhance function and repair of the infarcted myocardium. Stem Cells. 2006;24:772–780. doi: 10.1634/stemcells.2005-0212. [DOI] [PubMed] [Google Scholar]
- 5.Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–1389. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Craenenbroeck EM, et al. Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: Analytical considerations. Int. J. Cardiol. 2013;167:1688–1695. doi: 10.1016/j.ijcard.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Bruyndonckx L, et al. Endothelial progenitor cells and endothelial microparticles are independent predictors of endothelial function. J. Pediatr. 2014;165:300–305. doi: 10.1016/j.jpeds.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Rigato M, Fadini GP. Circulating stem/progenitor cells as prognostic biomarkers in macro-and microvascular disease: A narrative review of prospective observational studies. Curr. Med. Chem. 2018;25:4507–4517. doi: 10.2174/0929867324666170920154020. [DOI] [PubMed] [Google Scholar]
- 9.Samman Tahhan A, et al. Progenitor cells and clinical outcomes in patients with heart failure. Circ. Heart Fail. 2017;10:e004106. doi: 10.1161/CIRCHEARTFAILURE.117.004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palombo C, et al. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated type 1 diabetes. Cardiovasc. Diabetol. 2011;10:88. doi: 10.1186/1475-2840-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibal L, et al. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52:1464–1473. doi: 10.1007/s00125-009-1401-0. [DOI] [PubMed] [Google Scholar]
- 12.Loomans CJ, et al. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 13.Hörtenhuber T, et al. Endothelial progenitor cells are related to glycemic control in children with type 1 diabetes over time. Diabetes Care. 2013;36:1647–1653. doi: 10.2337/dc12-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arcangeli A, et al. Circulating endothelial progenitor cells in type 1 diabetic patients: Relation with patients’ age and disease duration. Front. Endocrinol. 2017;8:278. doi: 10.3389/fendo.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James S, Gallagher R, Dunbabin J, Perry L. Prevalence of vascular complications and factors predictive of their development in young adults with type 1 diabetes: Systematic literature review. BMC Res. Notes. 2014;7:593. doi: 10.1186/1756-0500-7-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadini GP, Albiero M, Bonora BM, Avogaro A. Angiogenic abnormalities in diabetes mellitus: Mechanistic and clinical aspects. J. Clin. Endocrinol. Metab. 2019;104:5431–5444. doi: 10.1210/jc.2019-00980. [DOI] [PubMed] [Google Scholar]
- 17.Yu CG, et al. Endothelial progenitor cells in diabetic microvascular complications: Friends or foes? Stem Cells Int. 2016;2016:1803989. doi: 10.1155/2016/1803989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawshani A, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N. Engl. J. Med. 2017;376:1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 19.Lind M, et al. Glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 20.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59:3216–3222. doi: 10.2337/db10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bebu I, et al. Risk factors for first and subsequent CVD events in type 1 diabetes: The DCCT/EDIC study. Diabetes Care. 2020;43:867–874. doi: 10.2337/dc19-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross MD, Wekesa AL, Phelan JP, Harrison M. Resistance exercise increases endothelial progenitor cells and angiogenic factors. Med. Sci. Sports Exerc. 2014;46:16–23. doi: 10.1249/MSS.0b013e3182a142da. [DOI] [PubMed] [Google Scholar]
- 23.Laufs U, et al. Running exercise of different duration and intensity: Effect on endothelial progenitor cells in healthy subjects. Eur. J. Cardiovasc. Prev. Rehabil. 2005;12:407–414. doi: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 24.Emmons R, Niemiro GM, Owolabi O, De Lisio M. Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J. Appl. Physiol. 2016;120:624–632. doi: 10.1152/japplphysiol.00925.2015. [DOI] [PubMed] [Google Scholar]
- 25.Van Craenenbroeck EM, et al. The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur. J. Appl. Physiol. 2011;111:2375–2379. doi: 10.1007/s00421-011-1843-1. [DOI] [PubMed] [Google Scholar]
- 26.Lutz AH, Blumenthal JB, Landers-Ramos RQ, Prior SJ. Exercise-induced endothelial progenitor cell mobilization is attenuated in impaired glucose tolerance and type 2 diabetes. J. Appl. Physiol. 2016;121:36–41. doi: 10.1152/japplphysiol.00349.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schier R, et al. Endothelial progenitor cell mobilization by preoperative exercise: A bone marrow response associated with postoperative outcome. Br. J. Anaesth. 2014;113:652–660. doi: 10.1093/bja/aeu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moazzami K, et al. Association between change in circulating progenitor cells during exercise stress and risk of adverse cardiovascular events in patients with coronary artery disease. JAMA Cardiol. 2020;5:147–155. doi: 10.1001/jamacardio.2019.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu TC, et al. A chemokine receptor, CXCR4, which is regulated by hypoxia-inducible factor 2α, is crucial for functional endothelial progenitor cells migration to ischemic tissue and wound repair. Stem Cells Dev. 2016;25:266–276. doi: 10.1089/scd.2015.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X, et al. Elevating CXCR7 improves angiogenic function of EPCs via Akt/GSK-3β/Fyn-mediated Nrf2 activation in diabetic limb ischemia. Circ. Res. 2017;120:e7–e23. doi: 10.1161/CIRCRESAHA.117.310619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fadini GP, et al. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care. 2013;36:943–949. doi: 10.2337/dc12-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadini GP, et al. Diabetes limits stem cell mobilization following G-CSF but not plerixafor. Diabetes. 2015;64:2969–2977. doi: 10.2337/db15-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Röhling M, et al. Differential patterns of impaired cardiorespiratory fitness and cardiac autonomic dysfunction in recently diagnosed type 1 and type 2 diabetes. Diabetes Care. 2017;40:246–252. doi: 10.2337/dc16-1898. [DOI] [PubMed] [Google Scholar]
- 34.Komatsu WR, et al. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr. Diabetes. 2005;6:145–149. doi: 10.1111/j.1399-543X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 35.West DJ, et al. The inflammation, vascular repair and injury responses to exercise in fit males with and without Type 1 diabetes: An observational study. Cardiovasc. Diabetol. 2015;14:71. doi: 10.1186/s12933-015-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waclawovsky G, et al. Exercise on progenitor cells in healthy subjects and patients with type 1 diabetes. Med. Sci. Sports Exerc. 2015;48:190–199. doi: 10.1249/MSS.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 37.Peake JM, Neubauer O, Walsh NP, Simpson RJ. Recovery of the immune system after exercise. J. Appl. Physiol. 2017;122:1077–1087. doi: 10.1152/japplphysiol.00622.2016. [DOI] [PubMed] [Google Scholar]
- 38.Bruce R, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 39.Riddell MC, et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017;5:377–390. doi: 10.1016/S2213-8587(17)30014-1. [DOI] [PubMed] [Google Scholar]
- 40.Glass S, Dwyer G.B, American College of Sports Medicine . ACSM’S metabolic calculations handbook. Baltimore: Lippincott Williams &amp;amp; Wilkins; 2007. [Google Scholar]
- 41.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 42.Ross MD, et al. Lower resting and exercise-induced circulating angiogenic progenitors and angiogenic T cells in older men. Am. J. Physiol.-Heart Circ. Physiol. 2018;314:H392–H402. doi: 10.1152/ajpheart.00592.2017. [DOI] [PubMed] [Google Scholar]
- 43.Kang H, Ma X, Liu J, Fan Y, Deng X. High glucose-induced endothelial progenitor cell dysfunction. Diab. Vasc. Dis. Res. 2017;14:381–394. doi: 10.1177/1479164117719058. [DOI] [PubMed] [Google Scholar]
- 44.Hayek SS, et al. Circulating progenitor cells identify peripheral arterial disease in patients with coronary artery disease. Circ. Res. 2016;119:564–571. doi: 10.1161/CIRCRESAHA.116.308802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egan CG, et al. Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia. 2008;51:1296–1305. doi: 10.1007/s00125-008-0939-6. [DOI] [PubMed] [Google Scholar]
- 46.Fadini GP, Avogaro A. Diabetes impairs mobilization of stem cells for the treatment of cardiovascular disease: A meta-regression analysis. Int. J. Cardiol. 2013;168:892–897. doi: 10.1016/j.ijcard.2012.10.089. [DOI] [PubMed] [Google Scholar]
- 47.Samman Tahhan A, et al. Progenitor cells and clinical outcomes in patients with acute coronary syndromes. Circ. Res. 2018;122:1565–1575. doi: 10.1161/CIRCRESAHA.118.312821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong L, et al. Insulin modulates ischemia-induced endothelial progenitor cell mobilization and neovascularization in diabetic mice. Microvasc. Res. 2011;82:227–236. doi: 10.1016/j.mvr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Witkowski S, Guhanarayan G, Burgess R. Glucose and acute exercise influence factors secreted by circulating angiogenic cells in vitro. Physiol. Rep. 2016;4:e12649. doi: 10.14814/phy2.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oikawa A, et al. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler. Thromb. Vasc. Biol. 2010;30:498–508. doi: 10.1161/ATVBAHA.109.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng W, et al. Comparison of cerebral and cutaneous microvascular dysfunction with the development of type 1 diabetes. Theranostics. 2019;9:5854–5868. doi: 10.7150/thno.33738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lespagnol E, et al. Early endothelial dysfunction in type 1 diabetes is accompanied by an impairment of vascular smooth muscle function: A meta-analysis. Front. Endocrinol. 2020;11:203. doi: 10.3389/fendo.2020.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heiss C, et al. Nitric oxide synthase expression and functional response to nitric oxide are both important modulators of circulating angiogenic cell response to angiogenic stimuli. Arterioscler. Thromb. Vasc. Biol. 2010;30:2212–2218. doi: 10.1161/ATVBAHA.110.211581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziaja J, et al. Type 1 diabetic patients have better endothelial function after simultaneous pancreas-kidney transplantation than after kidney transplantation with continued insulin therapy. Diab. Vasc. Dis. Res. 2018;15:122–130. doi: 10.1177/1479164117744423. [DOI] [PubMed] [Google Scholar]
- 55.Petrelli A, et al. Improved function of circulating angiogenic cells is evident in type 1 diabetic islet-transplanted patients. Am. J. Transplant. 2010;10:2690–2700. doi: 10.1111/j.1600-6143.2010.03309.x. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy, O. et al. Supplementary nitric oxide donors and exercise as potential means to improve vascular health in people with type 1 diabetes: Yes to no? Nutrients11 (2019). [DOI] [PMC free article] [PubMed]
- 57.Vigorelli V, et al. Abnormal DNA methylation induced by hyperglycemia reduces CXCR 4 gene expression in CD 34+ stem cells. J. Am. Heart Assoc. 2019;8:e010012. doi: 10.1161/JAHA.118.010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jie W, et al. SDF-1α/CXCR4 axis is involved in glucose-potentiated proliferation and chemotaxis in rat vascular smooth muscle cells. Int. J. Exp. Pathol. 2010;91:436–444. doi: 10.1111/j.1365-2613.2010.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zafar N, et al. Circulating angiogenic stem cells in type 2 diabetes are associated with glycemic control and endothelial dysfunction. PLoS ONE. 2018;13:e0205851. doi: 10.1371/journal.pone.0205851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cappellari R, D’Anna M, Avogaro A, Fadini GP. Plerixafor improves the endothelial health balance: The effect of diabetes analysed by polychromatic flow cytometry. Atherosclerosis. 2016;251:373–380. doi: 10.1016/j.atherosclerosis.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 61.Maiorino MI, et al. Reducing glucose variability with continuous subcutaneous insulin infusion increases endothelial progenitor cells in type 1 diabetes: An observational study. Endocrine. 2016;52:244–252. doi: 10.1007/s12020-015-0686-7. [DOI] [PubMed] [Google Scholar]
- 62.Volaklis KA, Tokmakidis SP, Halle M. Acute and chronic effects of exercise on circulating endothelial progenitor cells in healthy and diseased patients. Clin. Res. Cardiol. 2013;102:249–257. doi: 10.1007/s00392-012-0517-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding author (Daniel J West; Email: daniel.west@newcastle.ac.uk, telephone: + 44 (0)191 20 87076) on reasonable request.