Abstract
SEARCH for Diabetes in Youth (SEARCH) was initiated in 2000 as a multicenter study to address major gaps in the understanding of childhood diabetes in the United States. An active registry of youth diagnosed with diabetes at age <20 years since 2002 assessed prevalence, annual incidence, and trends by age, race/ethnicity, sex, and diabetes type. An observational cohort nested within the population-based registry was established to assess the natural history and risk factors for acute and chronic diabetes-related complications, as well as the quality of care and quality of life of children and adolescents with diabetes from diagnosis into young adulthood. SEARCH findings have contributed to a better understanding of the complex and heterogeneous nature of youth-onset diabetes. Continued surveillance of the burden and risk of type 1 and type 2 diabetes is important to track and monitor incidence and prevalence within the population. SEARCH reported evidence of early diabetes complications highlighting that continuing the long-term follow-up of youth with diabetes is necessary to further our understanding of its natural history and to develop the most appropriate approaches to primary, secondary, and tertiary prevention of diabetes and its complications. This review summarizes two decades of research and suggests avenues for further work.
Keywords: type 1 diabetes, type 2 diabetes, youth, surveillance, epidemiology, health disparities
Graphical abstract
We provide a summary of the design and methods used and an overview of major findings since the inception of the SEARCH for Diabetes in Youth Study in 2000. These include a summary of the risk, burden, and prognosis of diabetes diagnosed under the age of 20 years, with an emphasis on the disparities that were identified over these years. We also summarize morbidity and mortality information; patterns of, and barriers to, care of diabetes in youth; behavioral and social correlates; issues related to sustainable surveillance; and what the increase in young adults with diabetes who become parents implies.
Introduction
At the start of the 21st century, important gaps existed in the understanding of diabetes in youth under 20 years of age. Some of these gaps included sparse and incomplete incidence and prevalence data by type of diabetes, age, sex, and race/ethnicity; the uncertainty of whether the natural history of type 2 diabetes (T2D) was similar or different in youth compared with adults; whether an etiologic classification of childhood diabetes could be developed; the burden and risk factors for diabetes-related early complications; and the quality of health care and quality of life of youth with diabetes. In addition, data on demographics, social determinants of health, and patient-reported outcomes, including race and ethnicity, income, education, health insurance, geographic location, neighborhood characteristics, healthcare utilization, diabetes education, and others, were largely unknown on a population basis for youth with diabetes and their families.
The SEARCH for Diabetes in Youth (SEARCH) study was initiated in 2000 with funding from the Division of Diabetes Translation of the Centers for Disease Control and Prevention (CDC) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) to address these gaps and respond to emerging issues in the field of youth onset diabetes. The study was funded in 4–5-year grant cycles from 2000 ending in September 2020, a period of approximately 20 years.
We provide here a summary of the design and methods used and an overview of major findings since inception. These include a summary of the risk, burden, and prognosis of diabetes diagnosed under the age of 20 years, with an emphasis on the disparities that were identified over these years. It also summarizes morbidity and mortality information; patterns of, and barriers to, care of diabetes in youth; behavioral and social correlates; issues related to sustainable surveillance; and what the increase in young adults with diabetes who become parents implies. This paper provides a thorough update from a prior publication 1.
Design of SEARCH
Populations
One of the major goals of the study was to identify all youth with new-onset (incident) diabetes of any type (type 1 diabetes (T1D), T2D, and other types),2 except for gestational diabetes, diagnosed from 0 to 19 years of age, and to conduct periodic prevalence surveys to assess the burden of diabetes in the areas under study. Phase 1 (2000–2005) and 2 (2005–2010) included six recruitment centers: four geographic-based sites based in Ohio (eight counties, including Cincinnati, OH); the entire state of Colorado; five counties around Seattle, Washington; the entire state of South Carolina; and membership sites: two health plan–based sites in Hawaii and Southern California (health plan enrollees from one plan in seven counties); and under the direction of Colorado and in coordination with the Indian Health Service, American Indian reservation–based populations in Arizona and New Mexico. Phases 3 (2010–2015) and 4 (2015–2020) continued with five of the six original centers (excluding Hawaii). (Fig. 1)
Figure 1.

Locations of SEARCH sites in the United States. Colorado and South Carolina included all counties in the states. Seattle and Cincinnati included defined counties surrounding the cities. The Southern California Kaiser Permanente included members except from San Diego and Colorado coordinated Native American sites in Arizona and New Mexico. Hawaii was included through phase 3 of SEARCH.
Study components
SEARCH brought together multiple components relevant to youth-onset diabetes research: an active surveillance registry component (Fig. 2 top) to assess trends in incidence and prevalence. In addition, a separately funded observational cohort study was funded by NIDDK and CDC that provided for two additional follow-up visits on ~3000 youth with T1D and T2D3 (Fig. 2, bottom).
Figure 2.

The SEARCH study design summary. Prevalence was measured in the registry starting in 2001 and was repeated in 2009 and 2017. Incidence (new clinical diagnosis) was measured annually starting in 2002. Youth diagnosed in 2002–2006, 2008, 2012, and 2016 had a baseline in-person visit for measurement of diabetes autoantibodies, albuminuria, BMI, cardiovascular risk factors, and sociodemographic, quality of care, and quality of life questionnaires. Youth with baseline visits (incident cases in 2002–2005) were invited to return in 12, 24, and 60 months after their baseline visit for additional visits. Those with a baseline visit and at least 5 years of duration were asked to join the cohort study, from 2012 to 2020, which added measures of early complications (retinopathy, cardiac autonomic, and peripheral neuropathy, and arterial stiffness) in two visits.
Registry
In the geographic centers, the registries conducted active surveillance in the individual study areas by developing a network of endocrinologists (pediatric and adult), other high volume practices, hospitals, and health systems. These networks reported from one to four or more times per year to each geographic center registry all individuals with probable diabetes using administrative information. In the membership sites, individuals with diabetes were identified from electronic health records and reporting by endocrinologists along with chart reviews. All registries removed duplicate records, produced a listing of all unduplicated persons with diabetes, and conducted chart reviews to verify the presence of physician-diagnosed diabetes and other eligibility criteria (eligible residence in the study area, age <20 years at diagnosis, and noninstitutionalized) and to identify demographics and characteristics, such as diabetic ketoacidosis (DKA), within the first 6 months of diabetes. These activities were conducted under HIPAA waivers from the responsible institutional review boards.
To estimate incidence for a given year required collecting data from the network for longer periods of time, since an individual with newly diagnosed diabetes, especially T2D, may not first appear at a network members practice until well after diagnosis. An analysis of time from diagnosis to case registration showed that over 95% of all detected cases could be identified within 30 months of the end of the diagnosis year 4. Thus, even though SEARCH was funded through 2020, incidence results are incomplete for 2019–2020 at this writing.
During 2002–2015, among 69,457,475 youth at risk for diabetes, SEARCH identified 14,638 youths with incident T1D and 3916 with incident T2D 5. On the basis of the capture–recapture analysis 6, 7, there was a 98–99% completeness of ascertainment of cases of T1D and 92–97% for T2D.
With physician or health system permission, eligible persons were sent a brief questionnaire, and attendance was requested at a baseline in-person research visit to collect biochemical, genetic, and clinical data, which was conducted under informed consent or assent.
Population denominators
The geographic-based sites used race-bridged postcensal estimates of the nonmilitary, noninstitutionalized midyear populations in the center catchment areas as denominators to represent the population at risk. Health plans used end-of-year membership rolls, and Indian Health Service beneficiary rolls provide American Indian site denominators. The surveillance population was similar to the U.S. youth population with respect to the distribution of race/ethnicity, age, household income, and parental education 7. The population under yearly surveillance for incident cases was approximately 5.5 million children <20 years of age, comprising about 4.9% of the U.S. population <20 years 8. Approximately 3.5 million children <20 years of age were under surveillance in 2001, 2009, and 2017 for prevalence, owing to smaller areas of surveillance in Colorado and South Carolina than for incidence.
Cohort study
The cohort study was developed to understand risk factors for acute and chronic complications, to estimate the burden of such complications as they differed by duration, age, sex, and race/ethnicity, and to explore the processes of care and quality of care received by participants. Youth with a baseline in-person visit in 2002–2006, 2008, 2012, and 2016, and at least 5 years of duration by the time of the cohort visit were invited to participate 3. Of registered cases, 39.6% participated in the baseline in-person visit 3 and 64.2% of those with a baseline visit had a cohort visit 3. Those with a baseline visit were similar to all registered cases on multiple sociodemographic and biological parameters 7.
Study findings
The changing landscape of pediatric diabetes in the United States
Historical context.
From the 1960s through the mid-1990s, worldwide reports suggested that the incidence of T1D was increasing 2–4% each year, with earlier onset of diagnosis 9. T1D was primarily described as a condition occurring among non-Hispanic White youth. However, few population-based data existed in the United States to monitor trends in incidence, age at onset, or racial and ethnic distribution of incident T1D cases.
Initial case reports of youth-onset T2D cases emerged toward the end of the 20th century, coinciding with an increase in the prevalence of obesity in youth from 5% in the early 1970s to 14% by the end of the 1990s. Early studies monitoring trends in T2D in youth were limited to Native American communities 10, 11 or were clinic-based studies 12.
With the initiation of SEARCH, population-based estimates of incidence, prevalence, and trends in incidence and prevalence across time for youth-onset T1D and T2D, became possible. To date, nearly 30,000 cases of both incident and prevalent youth-onset diabetes have been registered. Data collected on these registered cases have informed our understanding of the changing landscape of youth-onset diabetes for nearly 20 years.
Disparities in the incidence of youth-onset T1D and T2D.
Initial (2002–2006) population-based estimates of youth-onset diabetes incidence (number of new cases per population) demonstrated significant racial and ethnic disparities. The incidence of T1D peaked among 10- to 14-year-olds. While few cases of T2D were identified among those less than 10 years, the incidence of T2D among non-Hispanic black, Asian and Pacific Islander, and American Indian populations for 10–14 years was greater than T1D. The higher incidence of T2D compared with T1D was observed among 15- to19-year-olds for every group except non-Hispanic Whites.3 This disparity has persisted across time, with most recent reports demonstrating an overall higher incidence of T2D as compared with T1D in youth from racial and ethnic groups of color across ages 10–19 years 5.
Within diabetes type, surveillance of trends has demonstrated a marked increase in the incidence of both T1D and T2D. Overall, across the period 2002–2015, a 1.9% annual increase in T1D and a 4.8% annual increase in T2D incidence was observed. However, this increase has been experienced disproportionately among certain population groups. In T1D, non-Hispanic Blacks, Hispanics, and Asian/Pacific Islanders experienced the highest annual percent change increase (2.7, 4.0, and 4.4%/year, respectively) as compared with non-Hispanic Whites (0.7% increase/year). For T2D, the highest increase in incidence was observed among Asian and Pacific Islander youth, at 7.7%/year, as compared with 6.5%/year for Hispanics, 6.0%/year for non-Hispanic Blacks, 3.7%/year for Native Americans, and no significant change for non-Hispanic White youth (Fig. 3) 5.
Figure 3.

Model-adjusted incidence rates of youth-onset T1D and T2D per 100,000 person-years overall and by race/ethnicity. SEARCH for Diabetes in Youth; 2002–2015. Persons who were AI were predominantly from one southwest tribe. Rates are 2-year moving averages. Overall model adjusted for age, sex, and race/ethnicity; race/ethnicity changes adjusted for age and sex. * P < 0.05. Modified from Ref. 5. AI, American Indian; APC, annual percent change in incidence based on a change model from 2020 – 2015; API, Asian Pacific Islander.
Disparities in the prevalence of youth-onset T1D and T2D.
Surveillance of population-based prevalence (number of total cases existing at a point in time) is critical to monitoring population burden and disparities and informing the allocation of public health resources. In 2001, when first estimates of population-based prevalence of youth T1D and T2D were reported from SEARCH, T1D accounted for 90% of all diabetes cases among non-Hispanic Whites. For Native American youth, T2D accounted for the highest proportion of diabetes cases observed. In 2001, accounting for the race/ethnicity and sex distribution in the United States, it was estimated that ~154,000 youth were living with diabetes in the United States 13.
Prevalence of both T1D and T2D has increased across all racial and ethnic groups from 2001 to 2017, with the greatest increases observed among non-Hispanic Whites and non-Hispanic Blacks for T1D and non-Hispanic Blacks, Asian/Pacific Islanders, and Hispanics for T2D (Fig. 4). While the overall prevalence of T1D remains higher than T2D under the age of 20 years, the change in prevalence across this period for T2D was nearly double that of T1D (0.3/1000 to 0.7/1000 for T2D and 1.5/1000 to 2.2/1000 for T1D)14
Figure 4.

Prevalence per 1000 Youth <20 years of age at onset by type (T1D and T2D) by Race/Ethnicity and year (2001, 2009, and 2017).14 Significant increases (P < 0.05) in T1D and T2D were observed from 2001 to 2017 for each race/ethnicity group except for T2D among Native Americans (P = 0.06). The greatest increases in T1D were among NHW and NHB and for T2D, among NHB, Hispanics, and Asian/PIs. Asian/PI, Asian Pacific Islander; NHB, non-Hispanic Black; NHW, non-Hispanic White.
Projections for youth-onset T1D and T2D burden.
The increasing trend in the incidence and prevalence of youth-onset diabetes is of significant concern, but perhaps most concerning is the disparity between racial/ethnic groups. With the trends noted above, coupled with the shift in the landscape of the demographic distribution of the U.S. population toward a more racially and ethnically diverse population, the public health burden of youth-onset diabetes will likely continue to grow. By 2050, it is estimated that as many as 600,000 youths <20 years of age will be living with T1D, at a prevalence of 5.2 cases/1000 youth <20 years of age. An estimated ~84,000 youth, aged 10–19 years, will have T2D, at a prevalence of 0.8 cases/1000 youth 15.
Disparities in morbidity and mortality associated with youth-onset diabetes.
Disparities in health outcomes by socioeconomic characteristics have been ingrained in U.S. society for centuries but recently have come to the forefront of public awareness in the past 40–50 years. These inequalities are reflected in all chronic illnesses, and despite attempts to level the playing field in pediatric health care, outcomes in youth onset diabetes are no exception. Moreover, while the incidence of diabetes-related complications has declined over the past 10 years in older adults, rates of major adverse cardiovascular events, amputation, and end-stage kidney disease have risen among younger age groups (18–44 years)16. Identifying disparities in morbidity and mortality associated with youth-onset diabetes has been a primary aim of the SEARCH Study since its inception.
Acute complications: diabetic ketoacidosis (DKA) and severe hypoglycemia.
SEARCH data were used in a multinational study of trends in DKA at diabetes diagnosis occurring from 2006–2016. While the combined prevalence of DKA was 29% (95% CI: 29–30%) for all nations, the United States fell into the higher range at 36% (95% CI: 35–38%), and was one of the few countries to have a rising trend in DKA at diagnosis 17. Sociodemographic factors associated with increased risk for DKA at diagnosis in the United States included minority race/ethnicity (RR 1.14; 95% CI: 1.06–1.22) and age <5 years compared with >15 years (RR 1.23, 95% CI: 1.13–1.35). Although HbA1c levels one year after diagnosis were similar, those with DKA at presentation had a worse glycemic trajectory, being 0.16% per year higher than those without DKA at presentation 18. Moreover, Black race, Hispanic ethnicity, and low-income families not only had a higher risk of DKA at diagnosis but also a higher risk for worsening glycemic status, independent of DKA at diagnosis 19.
Severe hypoglycemic events, defined as very low blood sugar requiring help from another person, occurred over a 6-month time period in approximately 7% of youth with T1D and 2.6% of youth with T2D 20. While there is a wide range in frequency of severe hypoglycemia, the median frequency was one event over 6 months for both T1D and T2D. Among individuals experiencing severe hypoglycemia, emergency medical services were contacted for 40% of youth with T1D and 14% of youth with T2D. Temporal trends were not examined.
Chronic early complications and comorbidities.
In the exploration of complications that occurred in persons with a short duration of diabetes (early complications), SEARCH characterized diabetes type according to likely etiology defined by the presence of diabetes autoantibodies and insulin resistance 21, 22. While the type of diabetes reported by the provider was highly correlated with this etiologic type, there was 15–20% misclassification in both T1D and T2D.7 The use of etiologic type is relevant in this era of increasing obesity, as autoimmunity and insulin resistance are likely to have differing pathogenic risk factors and mechanisms for the development of complications. For example, the magnitude of insulin sensitivity has been linked to albuminuria 23, 24 and arterial stiffness 25, 26.
The prevalence of early diabetes-related complications were reported among 1746 participants with T1D (mean age 18 years (SD 4); 76% non-Hispanic White) and 272 with T2D (mean age 22 years (SD 4); 26% non-Hispanic White) at a mean adjusted diabetes duration of 8 years and an average age of 21 years in both groups 3. The age-adjusted prevalence of microvascular complications and macrovascular risk factors are shown in Table 1. With the exception of cardiac autonomic neuropathy, all other complication rates were higher in youth and young adults with T2D versus T1D, even after adjustment for sociodemographic factors. Additional adjustments for differences in clinical factors (especially the waist–height ratio) attenuated to non-significance the increased odds of arterial stiffness and hypertension, but not the increased burden of albuminuria, retinopathy, and neuropathy among individuals with T2D compared with their peers with T1D 3. At an average age of 21 years and a mean of 8 years of disease duration, approximately 1 in 3 individuals with T1D and almost 3 out of 4 of those with T2D had at least one microvascular complication or cardiovascular comorbidity. 3 Early cardiovascular comorbidities were notably prevalent, and more so in youth-onset T2D: up to 14% of those with T1D had cardiovascular autonomic neuropathy, hypertension, or arterial stiffness, compared with almost half (47%) of those with T2D 3. Early evidence of cardiovascular disease was also apparent from increased carotid intima-media thickness relative to age-matched controls without diabetes 27, 28, particularly among those with worse glycemic control and/or higher BMI.
Table 1.
Age-adjusted prevalence of early diabetes–related chronic complications and comorbidities by diabetes type among 2018 youth and young adults with the duration of 8 years, SEARCH for Diabetes in Youth Study
| Complication and race/ethnicity category |
Type 1 diabetes N = 1746 |
Type 2 diabetes N = 272 |
Risk difference (Type 2 - Type 1) | P |
|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | ||
| Diabetic kidney disease | 5.8 (4.6 to 7.4) | 19.9 (15.1 to 25.7) | 14.0 (9.1 to 19.9) | <0.001 |
| Retinopathy | 5.6 (4.4 to 7.0) | 9.1 (6.3 to 12.9) | 3.5 (0.4 to 7.7) | 0.02 |
| Peripheral neuropathy | 8.5 (7.1 to 10.2) | 17.7 (13.6 to 22.7) | 9.2 (4.8 to 14.4) | <0.001 |
| Cardiovascular autonomic neuropathy |
14.4 (12.5 to 16.6) | 15.7 (11.7 to 20.6) | 1.2 (−3.1 to 6.5) | 0.6 |
| Arterial stiffness | 11.6 (9.8 to 13.6) | 47.4 (40.3 to 54.7) | 35.9 (29 to 42.9) | <0.001 |
| Hypertension | 10.1 (8.6 to 11.9) | 21.6 (17.1 to 26.9) | 11.5 (6.8 to 16.9) | <0.001 |
Rows in BOLD are statistically significant at P < 0.05
Prevalence is model derived and adjusted to a duration of 8 years and an age of 21 years.
Approximately 6% of youth and young adults with T1D included in the cohort had multiple complications 29. Specific types of vascular complications tended to cluster, particularly retinopathy with albuminuria and with arterial stiffness, suggesting shared etiologies. Cluster analysis of risk factors for multiple complications identified that T1D youth of non-White race/ethnicity, without health insurance, and with household income less than $50,000 per year were at the highest risk.
Mortality.
Among youth and young adults with an average diabetes duration of 5.3 years, crude mortality rates in T1D and T2D are 71 and 186 deaths per 100,000 person-years, respectively 30. Leading contributors to the 26 deaths among individuals with T1D include diabetes-related acute and chronic complications and accidents, whereas, in T2D, the majority of the 15 deaths were results of accidents or self-harm. Figure 5 depicts the overall and stratified standardized mortality ratios (SMR). Mortality ratios among individuals with T1D were comparable with the general population (SMR = 1.1, 95%: CI 0.7–1.6), while individuals with T2D had mortality over twice that of the general population (SMR = 2.4, 95% CI: 1.3–3.9). Females, non-Hispanic Blacks, and adolescents aged 15–19 years also had higher SMR.
Figure 5.
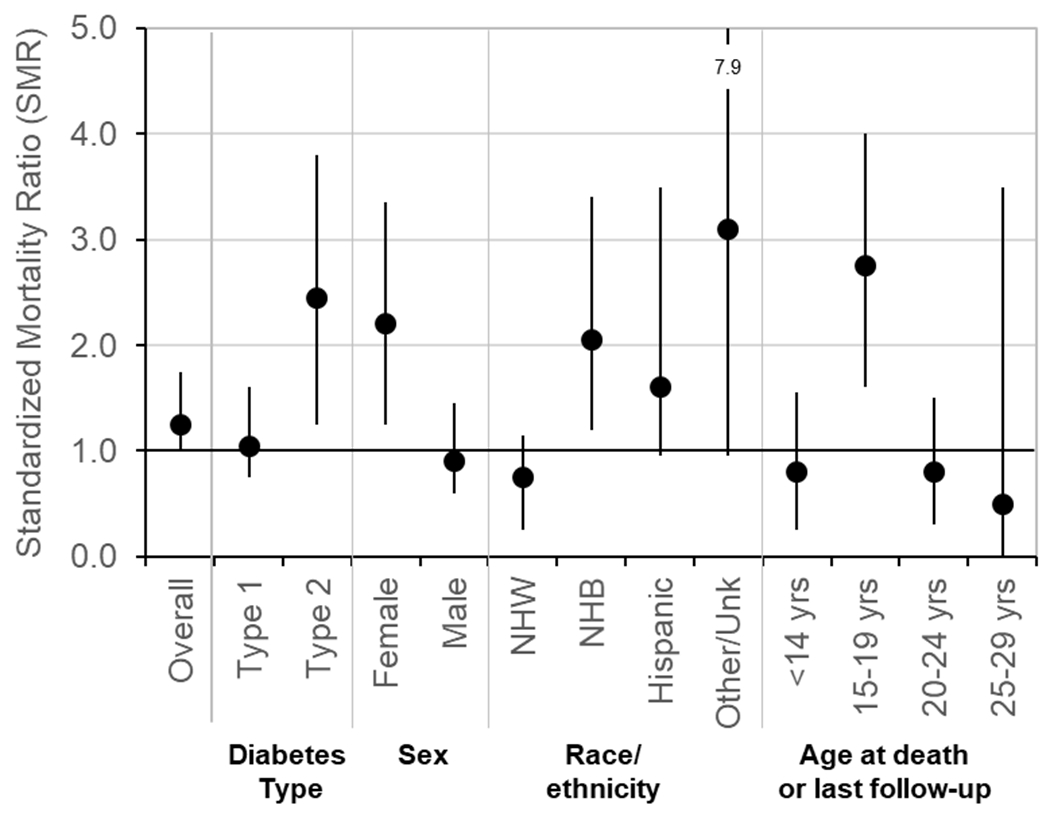
Age-, sex-, and race-standardized mortality ratios for 6840 youth with T1D and 1518 youth with T2D stratified by diabetes type, sex, race/ethnicity and age in the SEARCH For Diabetes in Youth Study. Mortality rates in populations in the same geographic areas were used as referents. NHB, non-Hispanic Black; NHW, non-Hispanic White. Modified from Ref. 30.
Evidence for significant, adverse long-term disease-associated complications and comorbidities for youth-onset T2D is continuing to build. Youth diagnosed with diabetes 10–15 years ago are well into their third and even fourth decade of life, adding to the burden of diabetes in adults. Surveillance of complications and comorbidities will be important to determine how to mitigate risks and the burden of T2D. Surveillance of T1D complications and comorbidities will also be important given the increase in incidence and prevalence of T1D among racial and ethnic youth groups.
Barriers to quality pediatric diabetes care
Over the 20-year duration of the study, there have been opportunities to identify barriers and disparities in care and factors that lead to a successful transition from pediatric to adult care.
Access and process barriers.
Using questions from the National Longitudinal Study of Adolescent Health and Consumer Assessment of Healthcare Providers and Systems (CAHPS) surveys, sociodemographic factors were found to be associated with both access and process barriers to care.31 Access barriers include having a regular provider, getting care thought to be needed, and cost of care. Processes of quality care include contextual care, that is, care that takes into account personal and family context, provider communication, and ability to obtain information. Greater barriers to accessing care and having a regular provider were seen in those with less parental education, utilizing public or health insurance, Black or Hispanic, and living in a single-parent household or ≥2 households.31 Cost of care was a major barrier for all, except those on Medicaid.31 Furthermore, Hispanic ethnicity and low parental education were associated with communication and contextual care barriers.31 More than 80% of participants reported ≥1 barrier to care, most commonly cost, followed by obtaining information, communication, and contextual care.31
Both direct and indirect costs can pose significant barriers. Direct costs were examined by asking families of youth with T1D to report out of pocket expenses (OOPE) for diabetes medicines and supplies; mean cost was $65/month; 60% spent >$50/month, and 40% spent >$100/month.32 Using an insulin pump or continuous glucose monitor was associated with higher OOPE.32 These technologies often improve outcomes, but the financial cost can be burdensome and make them inaccessible. Lower household income, lower parental educational attainment, and being on public health insurance were associated with lower OOPE, as was seeing a pediatric endocrinologist relative to an adult endocrinologist or family practice provider.32
Disparities in care.
Disparities in diabetes care are multifaceted, including access, outcomes, and treatment regimens. Early in SEARCH, we reported on the association of treatment regimen with sociodemographic measures, health insurance, and glycemic control in over 2,700 participants with T1D, mean age 13.2 years, and diabetes duration of 5 years. Insulin pump use was associated with better A1c in all but the youngest age group (Fig. 6).33 Participants were more likely to be on an insulin pump or the basal–bolus regimen than multiple injection regimens if they were non-Hispanic White, had higher household income, and private health insurance.33
Figure 6.
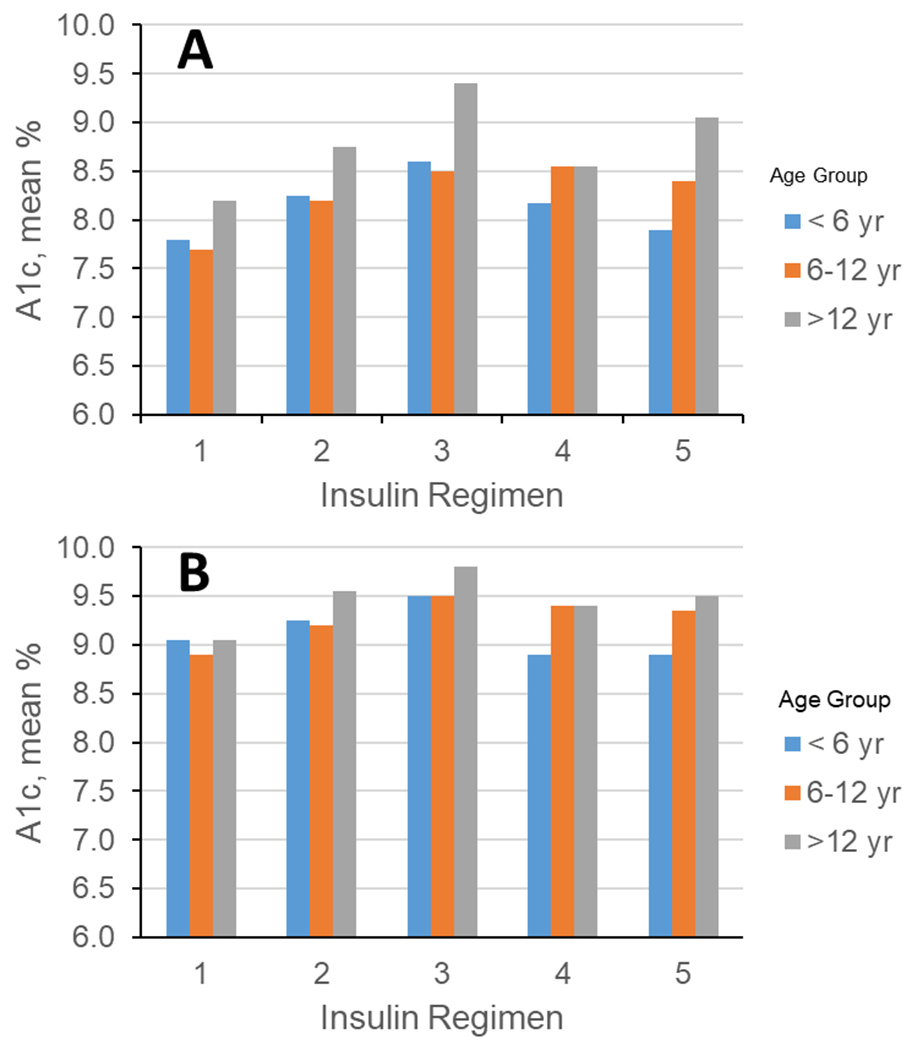
Mean A1C (%) by age group and insulin regimen. (A) Unadjusted and (B) adjusted for sex, race/ethnicity, income, education, insurance, center, DM duration, and frequency of glucose monitoring. Insulin regimens: (10) insulin pump; (2) glargine + rapid-acting insulin; (3) glargine + two or more insulins; (4) multiple injections w/o glargine; and (5) two or fewer insulin injections. Modified from Ref. 33.
We subsequently examined factors associated with glycemic control within treatment regimens.34 Those using an insulin pump were more likely to have good or intermediate control; however, the proportion with good control was very low at 14.4%, even for those on pump treatment (Fig. 7).34 For those on insulin pumps, non-White race/ethnicity, living in ≥2 households, and public health insurance were ≥2 times more likely to have poor glycemic control.34 For those using multiple daily injections (MDI), access, and process barriers (e.g., provider not spending enough time with family) were associated with poorer glycemic control.34
Figure 7.

Glycemic control (percent with 95% confidence intervals) within insulin regimen groups for youth onset T1D in the SEARCH for Diabetes Study. Poor A1c ≥9.5%; intermediate A1c 7.5% to ˂9.5%; and good A1c ˂7.5%. Insulin regimens were classified into three categories: (1) basal–bolus with continuous subcutaneous infusion (insulin pump therapy); (2) basal–bolus injections with glargine or detemir plus rapid-acting insulin (insulin lispro, insulin aspart, or insulin glulisine); and (3) mixed insulin regimen consisting of (a) multiple daily injections (≥3 injections) with glargine or detemir insulin plus NPH insulin plus regular or rapid-acting insulin, (b) multiple daily injections (≥3 injections) with any insulin types excluding basal insulin (glargine or detemir), or (c) one to two injections per day, excluding insulin glargine or detemir. Modified from Ref. 34.
SEARCH assessed quality of care through self-reported receipt of testing, following American Diabetes Association pediatric guidelines, and associations with satisfaction with care. “Meeting criteria” was defined as ≥3 A1c measurements in the last year, blood pressure checks at each visit, and lipid screening, urine albumin/creatinine, and foot and eye exams in the prior 1–2 years. About 60% of participants met the criteria for A1c, 93% for blood pressure, 71% for lipid screening, 63% for albuminuria, 81% for an eye exam, and 64% for a foot exam.35 Those meeting criteria had a mean A1c that was 0.28% lower than those not meeting screening criteria.35 For each one-step increase in satisfaction, there was a greater likelihood of meeting criteria.35 Most of these significant findings were attenuated after adjusting for race/ethnicity and socioeconomic factors, highlighting opportunities not only to improve the quality of care but also to address disparities in care.
Disparities in transition to adult care.
The transition from pediatric to adult diabetes care can be difficult for youth and young adults. In 185 youth with T1D who were ≥16 years old at the baseline visit, the mean age of transition from pediatric to adult diabetes care was 21.1 years36. Factors associated with leaving pediatric care were older age, less parental education, and higher A1c at baseline.36 Higher A1c at baseline was associated with a lower likelihood of leaving pediatric care, highlighting the tendency of pediatric providers to retain individuals with greater psychosocial needs. Poor glycemic control was more common in those who left pediatric care. Most striking was race/ethnicity, with minority racial/ethnic groups 3.5 times more likely to have poor glycemic control relative to non-Hispanic White participants.36
Similar results were observed in young adults with T2D regarding the transition from pediatric to adult care. In 182 young adults with T2D who were in pediatric care when ˂18 years of age, 56% transferred to adult care by 25 years of age while 15% were receiving no care for their diabetes.37 Older age, older age at diagnosis, and (unlike T1D) higher baseline A1c were all associated with leaving pediatric care. Males were more likely to have no care at follow up. Those with no provider or an adult provider were more than four times more likely to have poor glycemic control compared with those followed by a pediatric provider.37 Those with private health insurance were more likely to remain in pediatric care, whereas those on Medicaid or uninsured were more likely to have no care at follow up.37
SEARCH provided evidence that barriers to care exist, with >80% of SEARCH participants reporting at least one barrier.31 It is incumbent on diabetes providers to follow excellent existing guidelines, to enhance patient and family satisfaction, and to address disparities on all levels—barriers to care, treatment, and outcomes. Successful transition from pediatric to adult care that ensures continuity of care and sustained glycemic control is essential. While our findings may represent the best-case scenario, as participants were sufficiently motivated to attend a study visit, participants sometimes reported no recent care, provider, or health insurance; and worrisome complications were observed in these individuals. There is a clear need to identify better strategies to improve the delivery of care and outcomes so that all youth and young adults with diabetes can live full and healthy lives.
Behavioral, psychosocial, and social correlates of pediatric diabetes
Behavioral correlates—nutrition and dietary behaviors.
Nutrition and dietary behaviors are major correlates of age at onset, glycemic control, and cardiovascular risk factors in pediatric diabetes from early life onward.
Early life factors.
Intrauterine exposure to maternal gestational or T2D (i.e., fetal overnutrition) was associated with a younger age at diagnosis of T2D.38 Consumption of sugar-sweetened beverages or juice in infancy was associated with a younger age at diagnosis of T1D, particularly among those with the high-risk/susceptible genotype.39 Higher fatty acid and leucine intake in the year after the diagnosis of T1D was associated with higher fasting c-peptide levels 1–2 years later, suggesting a protective effect of these nutrients on sustained beta-cell function.40 Further, higher vitamin D levels in the first year post-diagnosis of T1D was associated with lower insulin resistance in cross-sectional analyses, even after adjustment for body mass index.41
Glycemic control.
Glycemic control and cardiovascular risk factors (obesity, dyslipidemia, hypertension) were improved with greater adherence to the Dietary Approaches to Stop Hypertension (DASH) diet;42–44 greater intake of leucine, protein, and fatty acid intake;45 and a lower intake of fructose,46 trans-fatty acids,47 diet beverages,48, 49 sweetened beverages, eggs, potatoes, and high-fat meat.50 Positive nutrition behaviors, such as counting carbohydrates51, 52 and eating <5 times/day,53 were associated with better glycemic control for both types of pediatric diabetes. All of these analyses were adjusted for insulin regimen and other key clinical and sociodemographic covariates, highlighting a potential benefit of dietary intake on HbA1c and cardiovascular risks above and beyond clinical treatment.
Populations at special risks.
Importantly, these findings highlighted subpopulations that were most likely to have suboptimal nutrition or dietary behaviors. Specifically, living farther from supermarkets, living closer to fast food outlets, having parent(s) with lower educational attainment, increasing age, and lower physical activity were associated with lower adherence to the DASH diet.42, 44, 54 Participants who were male, non-White, older, or from lower-income households had the highest intake of sugar-sweetened beverages,55, 56 and were less likely to have dietary consumption patterns associated with better glycemic and cardiovascular profiles.50 These data help explain some of the persistent disparities in pediatric diabetes management and specifically highlight the need to consider both individual- and community-level factors in dietary counseling for pediatric diabetes.
Screen time and smoking behaviors.
Other behaviors that are key to glycemic control and cardiovascular risk factors were screen time and smoking. Increases in daily screen time were associated with rising A1c levels for T1D and T2D and were associated with further elevations in triglycerides or LDL cholesterol for T1D.57 Males, non-Hispanic Blacks, and Hispanics reported watching significantly more television than females and non-Hispanic Whites,58 and thus may specifically warrant clinical attention and support for reducing screen time. Former and current tobacco users exhibited elevated cardiovascular risk factors compared with never users, with the most robust associations observed with dyslipidemia in T1D.26 Up to 15% of SEARCH participants reported current tobacco use, with the highest use observed among those with T2D, of American Indian heritage, or from lower-income households.59 Given the adverse impact of smoking on cardiometabolic health in this high-risk group, continued efforts to reduce smoking in pediatric diabetes patients is critical.
Psychological correlates.
Psychological correlates of glycemic control in youth-onset diabetes include depressive symptoms, quality of life, perceived weight status, unhealthy weight loss practices, and disordered eating behaviors. Among youth with T1D or T2D, elevated A1c levels were associated with more depressive symptoms and declining diabetes-related quality of life.60–62 These associations were also higher among those who engaged in unhealthy weight loss practices or disordered eating behaviors to address their weight.63, 64 Females with diabetes exhibited these adverse psychological states and behaviors more frequently than males, as did individuals who are older, are of non-White race/ethnicity, are overweight/obese, live in lower socioeconomic households, or have other comorbid conditions.60–64 Given the negative association with glycemic control, it is important to provide mental health screening, especially to individuals with these sociodemographic characteristics, and ensure ongoing support for improved psychological states. These data further demonstrate that subgroups with already known disparities are particularly at risk of having complex barriers to glycemic control and confirm the need for comprehensive and integrated support from a diverse clinical care team.
Social correlates.
The incidence of T1D was highest in neighborhoods with a medium population density and lowest in neighborhoods with lower affluence,65, 66 consistent with international studies,67–69 suggesting that socioeconomic status–related lifestyles may potentially influence the etiology of T1D. By contrast, the incidence of T2D was highest in areas with low population density and rural classification,70 which may be due to disparities in health-promoting built environments (e.g., limited access to healthy foods, fewer physical activity facilities, and reduced walkability).71, 72 Indeed, participants living in neighborhoods with relatively lower supermarket density had higher body mass indices and waist circumferences.54 Surprisingly, participants living farther from fast food outlets also had significantly higher BMIs,54 although this is likely due to the higher density of both supermarkets and fast food outlets in neighborhoods with greater population density. At the household level, 16% of SEARCH participants with T1D and 29% with T2D experienced food insecurity or limited availability of nutritionally adequate and safe food.73 Food insecurity is associated with a nearly 1% increase in A1c levels in T1D, even after adjustment for socioeconomic indicators and health insurance.74, 75 Participants with low or very low food security were more likely to be from households with lower affluence and more likely to be using insulin injections rather than an insulin pump.74, 75 These results indicate that youth and young adults from disadvantaged households face multiple barriers to achieving glycemic control, with food insecurity specifically accounting for a notable elevation in A1c.
SEARCH has identified multiple social, behavioral, and psychological correlates linked to pediatric diabetes incidence, progression, and management (Table 2), the number of which further demonstrate the complexity of this disease and the comprehensive support required to manage it well. For children and adolescents who are still developing cognitively and emotionally, the burden is substantial and appears to grow as they transition through adolescence to adulthood. Males and females struggle with different psychosocial and behavioral components of diabetes, and individuals of minority race/ethnicity or lower socioeconomic status appear to face the most barriers to optimal social, behavioral, and psychological conditions. The development of effective strategies that target the behavioral, psychological, and social correlates is needed for both types of pediatric diabetes.
Table 2.
Statistically significant behavioral, psychological, and social associations of pediatric onset T1D and T2D, the SEARCH for Diabetes in Youth Study
| Age at onset and progression | Glycemic control | Cardiovascular risk factors | ||||
|---|---|---|---|---|---|---|
| T1D | T2D | T1D | T2D | T1D | T2D | |
| Behavioral | ||||||
| ↓ Sugar-sweetened beverages |
↑ Age at onset | ↓ A1c | ↓ Dyslipidemia |
|||
| ↓ Juice | ↑ Age at onset | |||||
| ↓ Diet beverages | ↓ A1c | ↓ Dyslipidemia | ||||
| ↓ Eggs, potatoes, and high-fat meat | ↓ A1c | ↓ Obesity, dyslipidemia, blood pressure, and arterial stiffness |
||||
| ↑ DASH diet | ↓ A1c | ↓ blood pressure | ↓ Obesity, dyslipidemia, and blood pressure |
|||
| ↑ Protein | ↓ A1c | |||||
| ↓ Carbohydrates | ↓ A1c | |||||
| ↓ Fructose | ↓ Blood pressure | |||||
| ↑ Fatty acid | ↑ c-peptide | ↓ A1c | ||||
| ↑ Leucine | ↑ c-peptide | ↓ A1c | ||||
| ↑ Vitamin D | ↓ Insulin resistance | ↓ Obesity | ||||
| ↓ Trans-fatty acids | ↓ Blood pressure and dyslipidemia |
|||||
| ↑ Counting carbohydrates | ↓ A1c | ↓ A1c | ↓ Dyslipidemia | |||
| ↓ Daily eating episodes | ↓ A1c | ↓ A1c | ||||
| ↓ Screen time | ↓ A1c | ↓ A1c | ↓ Dyslipidemia | |||
| ↓ Smoking | ↓ Dyslipidemia | |||||
| Psychological | ||||||
| ↓ Depressive symptoms | ↓ A1c | ↓ A1c | ||||
| ↓ Diabetes-related quality of life |
↓ A1c | ↓ A1c | ||||
| ↓ Unhealthy weight loss practices |
↓ A1c | ↓ A1c | ||||
| ↓ Disordered eating behaviors | ↓ A1c | ↓ A1c | ||||
| Social | ||||||
| Medium population density | ↑ Incidence | |||||
| ↓ Neighborhood affluence | ↓ Incidence | |||||
| ↓ Rurality | ↓ Incidence | |||||
| ↑ Supermarket density | ↓ Obesity | ↓ Obesity | ||||
| ↑ Proximity to fast-food outlets | ↓ Obesity | ↓ Obesity | ||||
| ↓ Food insecurity | ↓ A1c | |||||
Note: All results are from SEARCH papers referenced in this article.
BP, blood pressure; T1D, T1D diabetes; T2D, T2D diabetes.
Research opportunities created by SEARCH
Continued surveillance of youth-onset diabetes
Continued surveillance of youth onset diabetes prevalence is critical to monitor and track disease burden and to inform public health resource allocation. Understanding trends in the incidence of disease can provide evidence of improvement in the environment or from policy changes aimed at improving risk once clinical trials have shown evidence of efficacy. With a high proportion of providers utilizing electronic health records for management of patient care, new opportunities have emerged for conducting surveillance of chronic disease. These approaches are not without limitations, as the data collected are for patient care, as opposed to research; thus misclassification and missing data are common challenges in working with these data. Still, newer methods for utilizing electronic health record data are emerging. Substudies at two sites showed that the use of search algorithms that used International Classification of Diseases - 9 (ICD-9) codes and limited chart review were potentially ways to use EHRs for surveillance 76–78. An additional report showed that the use of ICD-10 codes significantly improved the identification of the type of diabetes 79. SEARCH recently demonstrated the ability to ascertain cases of T1D and T2D through testing of a rule-based algorithm approach and multinomial regression approach applied to electronic health record data. For T1D, either approach yielded a sensitivity of >0.95 and specificity of >0.96 to detect true cases. For T2D, the rule-based method, combined with chart reviews, yielded a sensitivity and specificity of ≥0.91 80. The year of diagnosis, which is required to identify a new-onset case for annual incidence estimates is not generally available in administrative or structured EHR data. In the attempt to identify the year of onset, youth (<20 years) with potential evidence of diabetes in 2017 (ICD code, elevated glucose or HbA1c, or DM medication) were identified from the inpatient and outpatient EHR data of three participating Children’s Hospitals. All potential cases were chart reviewed to determine true diabetes status, type, and diagnosis date. Cases were restricted to those with diagnosis date and data post-2008 as a result of EHR limitations in earlier years. The use of the first ICD code identified in the EHR, and multi-criteria algorithms (using ICD code or elevated glucose, A1c, or medications) classified diagnosis year correctly 88.9% and 88.4% of the time, respectively, with significant improvement in later years of onset in the EHR.81 Thus, it appears that the use of EHRs with a limited additional chart review in specific instances may be a cost-effective way to conduct surveillance.
Surveillance for youth onset diabetes is part of a robust registry system over long periods of time in much of Europe and Australia, but not in the U.S. SEARCH was not designed to be geographically or demographically representative of the United States though it enrolled a similar population 7. In addition, record linkage is difficult in the United States with multiple health systems and payers. The SEARCH surveillance work over the past 20 years informed a new funding initiative, supported by the CDC and NIDDK, for surveillance of the burden of diabetes by type in children, adolescents, and young adults (Diabetes in Children And Young Adults (DiCAYA)). This new surveillance effort will be underway soon at several sites in the United States, including several prior SEARCH sites. However, the successor to SEARCH, DiCAYA, is also not designed to be representative. It is likely that significant geographic differences exist in both T1D and T2D in the United States, which could provide clues to the role of the environment in the etiology of diabetes. Whether every state needs a surveillance system depends on a number of factors: actual risk in the population; the prevalence of social and behavioral correlates summarized above; and the presence of enough evidence-based interventions that could reduce risks in the population such that surveillance could examine impact at a local, state, or regional level. We are clearly not at that stage for youth onset diabetes but identifying interventions that are scalable and linking population surveillance to areas that are introducing such interventions is an appropriate way to establish efficiency. There also has not been a tradition of linking population registries for youth onset diabetes to clinical care, yet many types of registries at hospitals or systems influence care regularly. Whether these two systems can converge with the advent of the EHR remains to be seen.
The future of offspring in the setting of parental diabetes
The rise in youth-onset T1D and T2D has resulted in a growing number of women and men entering their reproductive years with diabetes,8, 82 with potential adverse impacts on pregnancy, perinatal, and offspring health outcomes.83 Maternal diabetes triples the risk for major congenital anomalies,84 and increases the risk for fetal macrosomia, shoulder dystocia, and neonatal respiratory disorders.85–87 Poor glycemic control in early pregnancy is associated with spontaneous abortion and birth defects.84 In most studies, adverse effects are similarly observed in both T1D and T2D.88–92 Yet other maternal factors that contribute to adverse outcomes are more common among women with T2D, including overweight and obesity,87 tobacco use,93 chronic hypertension88, 93, lower use of preconception care services,94 and late entrance into prenatal care.95 Collectively, these data highlight the importance of managing risk factors for pregnancy complications before conception to facilitate optimal perinatal outcomes.
Offspring of women with diabetes may also exhibit impaired health trajectories from infancy onward. Many have elevated weight for length in infancy and childhood, especially when born large for gestational age.96, 97 Others have relatively slower growth in infancy followed by accelerated growth in childhood, resulting in higher body weight as early as 5–7 years.98, 99 Throughout childhood and adolescence, offspring of women with diabetes exhibit increased body size,100, 101 more glucose intolerance,101 and a higher prevalence of T2D102 compared with unexposed offspring. They also have increased blood pressure,103–106 inflammation and endothelial dysfunction,105 dyslipidemia,107 and a higher prevalence of metabolic syndrome.108 While the postnatal environment and modeled health behaviors do contribute to transgenerational diabetes,65, 66, 70, 109 intrauterine exposure to maternal diabetes is a major risk factor. Compared with siblings born before mothers developed T2D, siblings born after the mothers diagnosis had a greater BMI (2.6 kg/m2) and were 3.6 times more likely to have T2D themselves by their early 20s.110
Paternal diabetes may also confer increased risk to offspring, although effects are less robust and not as clear as for maternal diabetes.111 Offspring of fathers with diabetes may be smaller at birth,112 but later exhibit increased body size, adiposity, metabolic disturbance, and T2D.112–116 Genetic transmission of high-risk alleles for T1D, epigenetic effects on offspring gene expression, and the fathers influence on the postnatal environment and family practices are all potential mechanisms. Given the growing number of males with diabetes during their reproductive years, a better understanding of the specific influences of paternal diabetes on offspring health is warranted.
Notably, poor outcomes are not observed among all offspring of parents with diabetes, and there is substantial variability in the magnitude of risk conferred by diabetes status100, 101, 117. Neither genetics nor intrauterine environmental indicators (e.g., prenatal glycemic control or birthweight) fully explain the individual variability in offspring outcomes 118, 119. Other postnatal factors are likely involved, but supporting evidence is currently limited. Among children of mothers with T1D in the Netherlands, the prevalence of overweight/obesity among offspring at 6–8 years was nearly three-fold higher in those whose mothers were currently overweight, 118 highlighting the potential for maternal health and modeled behaviors to modify the effect of intrauterine exposures. Similarly, among offspring exposed to maternal T1D or T2D, breastfeeding is associated with reduced obesity,120, 121 and thus may be a strategy for improving outcomes in this high-risk population. Better diet and activity behaviors in offspring exposed to gestational diabetes have been associated with reduced body size and adiposity in childhood and adolescence;122, 123 yet, similar evidence is not currently available for pregestational diabetes. Further research into the modifiable, postnatal factors that could mitigate risk could develop targeted interventions for the growing number of offspring born to parents with diabetes.
Aging with diabetes
The SEARCH cohort participants are now in their 20s and are exhibiting an increasing burden of cardiovascular-related complications and comorbidities. These trends are likely to worsen as participants transition into their 3rd and 4th decades of life, in parallel with the increasing prevalence of overweight/obesity in both T1D (~35%) and T2D (~90%) diabetes,124 as well as dyslipidemia and smoking. 59, 124, 125. Recent National Health and Nutrition Examination Survey (NHANES) data suggest that young adulthood is a vulnerable period for persons with diabetes: at a mean age of 36.4 years, with an average of 7.7 years of clinical diabetes duration, 29% had no health insurance, 22% had Medicaid-like plans, and only 65% reported having a usual place to receive care 126. Moreover, young adults with diabetes had significantly elevated lipids and adiposity, poorer diet, and less physical activity, and were less likely to be treated for high cholesterol or hypertension than older adults with diabetes. The reasons for these troubling trends regarding diabetes in young adulthood are not clear. They may result from a more aggressive disease process among people with a younger age at onset of diabetes, for which increasing data are available, at least with respect to youth-onset T2D. 127 Alternatively, a combination of systemic, socioeconomic, healthcare access, transition from pediatric to adult care, limited health insurance, and other factors may be responsible, as suggested by SEARCH. 36 This is an area for further research since youth and young adults with diabetes face a longer lifetime exposure to hyperglycemia, hypertension, and obesity, which will likely result in increased risks of end-organ damage at an earlier age than among prior generations of patients with diabetes.
Summary and conclusions
SEARCH has provided substantial data on the current state of youth-onset diabetes in multiple race/ethnic groups:128 American Indians 129, Asian Pacific Islanders 130, Hispanics 131, non-Hispanic Blacks 132, and non-Hispanic Whites 133. These studies have included descriptive epidemiology of prevalence and incidence 7, 8, projections of future impact 15, metabolic investigations, such as the longitudinal changes in β cell function over time 134, 135, the role of glycemic control 136, 137, metabolic and genetic characteristics 22, types of treatment regimens and population differences in utilization 138, studies of out of pocket expenses 32, transition from pediatric to adult care 36, development of early microvascular complications by type within race/ethnic groups 3, and mortality compared with the U.S. age-appropriate population 30.
SEARCH has identified significant trends in youth onset diabetes. Both T1D and T2D are becoming more common in the U.S. youth5. Cardiovascular and microvascular risk factors are common and subclinical complications occur early, especially among youth with T2D and those of non-White race/ethnic backgrounds, 3 indicating a need for active intervention for detection and treatment of early complications at an earlier duration of diabetes than might have been expected. Multiple barriers to care have been identified, especially among persons of color and those with lower education, health insurance, and income. In adults, studies have shown improvements in diagnosis and care when insurance has become available 139–142, but even in managed care organizations, ethnic/racial differences remain 143, 144. Thus, future research may identify additional factors, perhaps partially embedded in systemic racism, that are operating. What is urgently needed is the identification of interventions that are efficacious, multifaceted, scalable, and affordable to begin to reduce these disparities.
SEARCH has shown that as youth with diabetes age, they transition from pediatric to adult care (or no care) at rates that differ by type of diabetes, insurance status, race/ethnicity, income, and other factors. Such transitions are points in the clinical course of illness that often result in poorer quality of care and poorer glycemic status 36, among other outcomes. Ways to improve transitions of care are needed.
SEARCH has provided population-wide estimates of burden and prognosis of youth-onset diabetes in the United States, very much like more established chronic disease registries, such as cancer. It is crucial to continue to invest in youth-onset diabetes surveillance and longitudinal studies focused on contemporary cohorts since these populations will bear the consequences of chronic diseases for much of their life.
Acknowledgements
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their healthcare providers, whose participation made this study possible.
The results do not reflect the official positions of the NIDDK, NIH, or CDC.
SEARCH 3/4: The authors wish to acknowledge the involvement of the Kaiser Permanente Southern California Clinical Research Center (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group); the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina, the NIH/National Center for Advancing Translational Sciences (NCATS) Grant number UL1 TR000062, UL1 Tr001450; Seattle Children’s Hospital and the University of Washington, NIH/NCATS Grant number UL1 TR00423; the University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS Grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH Grant number P30 DK57516); the University of Cincinnati, NIH/NCATS Grant number UL1 TR000077, UL1 TR001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions.
Funding
Grant Support (SEARCH 1, 2, 3):
SEARCH for Diabetes in Youth is funded by the CDC (PA numbers 00097, DP-05–069, and DP-10–001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
Grant Support (SEARCH 4):
The SEARCH for Diabetes in Youth Cohort Study (1UC4DK108173) is funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, and supported by the CDC.
The Population-Based Registry of Diabetes in Youth Study (1U18DP006131, U18DP006133, U18DP006134, U18DP006136, U18DP006138, U18DP006139) is funded by the CDC and supported by the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
Sites (SEARCH 1 through 4):
Kaiser Permanente Southern California (U18DP006133, U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U18DP006139, U48/CCU819241–3, U01 DP000247, and U18DP000247–06A1), Cincinnati Children’s Hospital Medical Center (U18DP006134, U48/CCU519239, U01 DP000248, and 1U18DP002709), the University of North Carolina at Chapel Hill (U18DP006138, U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U18DP006136, U58/CCU019235–4, U01 DP000244, and U18DP002710–01), and Wake Forest University School of Medicine (U18DP006131, U48/CCU919219, U01 DP000250, and 200–2010-35171)
SEARCH authorship list
Listed within each site/center: PI(s) and then alphabetically within each institution/facility.
The writing group for this manuscript wishes to acknowledge the contributions of the following individuals to the SEARCH for Diabetes in Youth Study:
SEARCH sites:
California: (PI) Jean M. Lawrence, ScD, MPH, MSSA; Peggy Hung, MPH; Corinna Koebnick, PhD, MSc; Xia Li, MS; Eva Lustigova, MPH; Kristi Reynolds, PhD, MPH for the Department of Research & Evaluation, Kaiser Permanente Southern California, Pasadena California, and David J. Pettitt, MD, Santa Barbara, California.
Carolinas: (PI) Elizabeth J. Mayer-Davis, PhD; Amy Mottl, MD, MPH; Joan Thomas MS, RD for the University of North Carolina, Chapel Hill. Malaka Jackson, MD; Lisa Knight, MD; Angela D. Liese, PhD, MPH; Christine Turley, MD for the University of South Carolina. Deborah Bowlby, MD for the Medical University of South Carolina. James Amrhein, MD; Elaine Apperson, MD; Bryce Nelson, MD for Greenville Health System and Eau Claire Cooperative Health Center.
Colorado: (PI) Dana Dabelea, MD, PhD; Anna Bellatorre, PhD; Tessa Crume, PhD, MSPH; Richard F. Hamman, MD, DrPH; Katherine A. Sauder, PhD; Allison Shapiro, PhD, MPH; Lisa Testaverde, MS for the LEAD Center in the Department of Epidemiology, Colorado School of Public Health, University of Colorado Denver. Georgeanna J. Klingensmith, MD; David Maahs, MD; Marian J. Rewers, MD, PhD; Paul Wadwa, MD for the Barbara Davis Center for Childhood Diabetes. Stephen Daniels, MD, PhD; Michael G. Kahn, MD, PhD; Greta Wilkening, PsyD for the Department of Pediatrics and Children’s Hospital. Clifford A. Bloch, MD for the Pediatric Endocrine Associates. Jeffrey Powell, MD, MPH for the Shiprock Service Unit, Navajo Area Indian Health Service. Kathy Love-Osborne, MD for the Denver Health and Hospital Authority. Diana C. Hu, MD for the Pediatrics Department, Tuba City Regional Health Care Center, Tuba City, AZ.
Ohio: (PI) Lawrence M. Dolan, MD; Amy S. Shah, MD, MS; Debra A. Standiford, MSN, CNP; Elaine M. Urbina, MD, MS for the Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati.
Washington: (PI) Catherine Pihoker, MD; Irl Hirsch, MD; Grace Kim, MD; Faisal Malik, MD, MSHS; Lina Merjaneh, MD; Alissa Roberts, MD; Craig Taplin, MD; Joyce Yi-Frazier, PhD for the University of Washington. Natalie Beauregard, BA; Cordelia Franklin, BS; Carlo Gangan, BA; Sue Kearns, RN; Mary Klingsheim, RN; Beth Loots, MPH, MSW; Michael Pascual, BA for Seattle Children’s Hospital. Carla Greenbaum, MD for Benaroya Research Institute.
Centers and laboratory:
The CDC: Giuseppina Imperatore, MD, PhD and Sharon H. Saydah, PhD.
The National Institute of Diabetes and Digestive and Kidney Diseases, NIH: Barbara Linder, MD, PhD.
The Central Laboratory: (PI) Santica M. Marcovina, PhD, ScD (PI); Alan Chait, MD; Noemie Clouet-Foraison, PhD; Jessica Harting; Greg Strylewicz, PhD for the University of Washington Northwest Lipid Metabolism and Diabetes Research Research Laboratories.
The coordinating center: (Co-PIs) Ralph D’Agostino, Jr., PhD, Elizabeth T. Jensen, MPH, PhD; Lynne E. Wagenknecht, DrPH; Ramon Casanova, PhD; Jasmin Divers, PhD; Maureen T. Goldstein, BA; Leora Henkin, MPH, M.Ed; Scott Isom, MS; Kristin Lenoir, MPH; June Pierce, AB; Beth Reboussin, PhD; Joseph Rigdon, PhD; Andrew Michael South, MD, MS; Jeanette Stafford, MS; Cynthia Suerken, MS; Brian Wells, MD, PhD; and Carrie Williams, MA, CCRP for Wake Forest School of Medicine.
Footnotes
Competing interests
The authors declare no competing interests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC and the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Hamman RF, Bell RA, Dabelea D, et al. 2014. The SEARCH for Diabetes in Youth Study: Rationale, Findings, and Future Directions. Diabetes Care. 37: 3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 2019. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 42: S13–s28. [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. 2017. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. 317: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crume TL, Hamman RF, Isom S, et al. 2016. Factors influencing time to case registration for youth with type 1 and type 2 diabetes: SEARCH for Diabetes in Youth Study. Ann Epidemiol. 26: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divers J, Mayer-Davis EJ, Lawrence JM, et al. 2020. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths - Selected Counties and Indian Reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 69: 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verlato G & Muggeo M. 2000. Capture-recapture method in the epidemiology of type 2 diabetes: a contribution from the Verona Diabetes Study. Diabetes Care. 23: 759–764. [DOI] [PubMed] [Google Scholar]
- 7.Dabelea D, Mayer-Davis EJ, Saydah S, et al. 2014. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 311: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. 2017. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 376: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gale EA 2002. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 51: 3353–3361. [DOI] [PubMed] [Google Scholar]
- 10.2003. Diabetes among young American Indians--Montana and Wyoming, 2000–2002. MMWR Morb Mortal Wkly Rep. 52: 1127–1129. [PubMed] [Google Scholar]
- 11.Harwell TS, McDowall JM, Moore K, et al. 2001. Establishing surveillance for diabetes in American Indian youth. Diabetes Care. 24: 1029–1032. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D, Hamman RF & Knowler WC. 2017. "Diabetes in youth". In Diabetes in America, Vol. NIH Pub No. 17–1468. Cowie CC, Casagrande SS, Menke A, et al. , Eds.: 15.11–15.54. Bethesda, MD: National Institutes of Health. [Google Scholar]
- 13.Liese AD, D'Agostino RB Jr., Hamman RF, et al. 2006. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 118: 1510–1518. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence JM, Liese AD, Saydah S, et al. 2020. 1464-P: Trends in Prevalence of Youth-Onset Type 1 and Type 2 Diabetes, 2001–2017: The SEARCH for Diabetes in Youth Study. Diabetes. 69: 1464-P. [Google Scholar]
- 15.Imperatore G, Boyle JP, Thompson TJ, et al. 2012. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 35: 2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregg EW, Hora I & Benoit SR. 2019. Resurgence in Diabetes-Related Complications. JAMA. 321: 1867–1868. [DOI] [PubMed] [Google Scholar]
- 17.Cherubini V, Grimsmann JM, Åkesson K, et al. 2020. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia. 63: 1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duca LM, Reboussin BA, Pihoker C, et al. 2019. Diabetic ketoacidosis at diagnosis of type 1 diabetes and glycemic control over time: The SEARCH for diabetes in youth study. Pediatr Diabetes. 20: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahkoska AR, Shay CM, Crandell J, et al. 2018. Association of Race and Ethnicity With Glycemic Control and Hemoglobin A(1c) Levels in Youth With Type 1 Diabetes. JAMA Netw Open. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saydah S, Imperatore G, Divers J, et al. 2019. Occurrence of severe hypoglycaemic events among US youth and young adults with type 1 or type 2 diabetes. Endocrinology, diabetes & metabolism. 2: e00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabelea D, D'Agostino RB Jr., Mason CC, et al. 2011. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 54: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabelea D, Pihoker C, Talton JW, et al. 2011. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 34: 1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mottl AK, Divers J, Dabelea D, et al. 2016. The dose-response effect of insulin sensitivity on albuminuria in children according to diabetes type. Pediatr Nephrol. 31: 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mottl AK, Lauer A, Dabelea D, et al. 2013. Albuminuria according to status of autoimmunity and insulin sensitivity among youth with type 1 and type 2 diabetes. Diabetes Care. 36: 3633–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gourgari E, Stafford JM, D'Agostino R Jr., et al. 2020. The association of low-density lipoprotein cholesterol with elevated arterial stiffness in adolescents and young adults with type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth study. Pediatr Diabetes. 21: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AS, Dabelea D, Talton JW, et al. 2014. Smoking and arterial stiffness in youth with type 1 diabetes: the SEARCH Cardiovascular Disease Study. J Pediatr. 165: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbina EM, Dabelea D, D'Agostino RB Jr., et al. 2013. Effect of Type 1 Diabetes Mellitus on Carotid Structure and Function in Adolescents and Young Adults: The SEARCH CVD Study. Diabetes Care. 36: 2597–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah AS, Dabelea D, Fino NF, et al. 2016. Predictors of Increased Carotid Intima-Media Thickness in Youth With Type 1 Diabetes: The SEARCH CVD Study. Diabetes Care. 39: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauder KA, Stafford JM, Mayer-Davis EJ, et al. 2019. Co-occurrence of early diabetes-related complications in adolescents and young adults with type 1 diabetes: an observational cohort study. Lancet Child Adolesc Health. 3: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds K, Saydah SH, Isom S, et al. 2018. Mortality in youth-onset type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth study. J Diabetes Complications. 32: 545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valenzuela JM, Seid M, Waitzfelder B, et al. 2014. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 164: 1369-1375 e1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merjaneh L, Pihoker C, Divers J, et al. 2019. Out of Pocket Diabetes-Related Medical Expenses for Adolescents and Young Adults With Type 1 Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care. 42: e172–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paris CA, Imperatore G, Klingensmith G, et al. 2009. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 155: 183-189 e181. [DOI] [PubMed] [Google Scholar]
- 34.Snyder LL, Stafford JM, Dabelea D, et al. 2019. Socio-economic, demographic, and clinical correlates of poor glycaemic control within insulin regimens among children with Type 1 diabetes: the SEARCH for Diabetes in Youth Study. Diabet Med. 36: 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik FS, Stafford JM, Reboussin BA, et al. 2020. Receipt of recommended complications and comorbidities screening in youth and young adults with type 1 diabetes: Associations with metabolic status and satisfaction with care. Pediatr Diabetes. 21: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lotstein DS, Seid M, Klingensmith G, et al. 2013. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics. 131: e1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal S, Raymond JK, Isom S, et al. 2018. Transfer from paediatric to adult care for young adults with Type 2 diabetes: the SEARCH for Diabetes in Youth Study. Diabet Med. 35: 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettitt DJ, Lawrence JM, Beyer J, et al. 2008. Association between maternal diabetes in utero and age at offsprings diagnosis of type 2 diabetes. Diabetes Care. 31: 2126–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crume TL, Crandell J, Norris JM, et al. 2014. Timing of complementary food introduction and age at diagnosis of type 1 diabetes: the SEARCH nutrition ancillary study (SNAS). Eur J Clin Nutr. 68: 1258–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer-Davis EJ, Dabelea D, Crandell JL, et al. 2013. Nutritional factors and preservation of C-peptide in youth with recently diagnosed type 1 diabetes: SEARCH Nutrition Ancillary Study. Diabetes Care. 36: 1842–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The NS, Crandell JL, Lawrence JM, et al. 2013. Vitamin D in youth with Type 1 diabetes: prevalence of insufficiency and association with insulin resistance in the SEARCH Nutrition Ancillary Study. Diabet Med. 30: 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liese AD, Bortsov A, Gunther AL, et al. 2011. Association of DASH diet with cardiovascular risk factors in youth with diabetes mellitus: the SEARCH for Diabetes in Youth study. Circulation. 123: 1410–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes TL, Crandell JL, Bell RA, et al. 2013. Change in DASH diet score and cardiovascular risk factors in youth with type 1 and type 2 diabetes mellitus: The SEARCH for Diabetes in Youth Study. Nutr Diabetes. 3: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunther AL, Liese AD, Bell RA, et al. 2009. Association between the dietary approaches to hypertension diet and hypertension in youth with diabetes mellitus. Hypertension. 53: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamichhane AP, Crandell JL, Jaacks LM, et al. 2015. Longitudinal associations of nutritional factors with glycated hemoglobin in youth with type 1 diabetes: the SEARCH Nutrition Ancillary Study. Am J Clin Nutr. 101: 1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couch SC, Crandell JL, Shah AS, et al. 2013. Fructose intake and cardiovascular risk factors in youth with type 1 diabetes: SEARCH for diabetes in youth study. Diabetes Res Clin Pract. 100: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The NS, King IB, Couch SC, et al. 2018. Plasma trans-palmitoleic acid is associated with cardio-metabolic risk factors in youth with type 1 diabetes. Diabetes Metab. 44: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bortsov AV, Liese AD, Bell RA, et al. 2011. Sugar-sweetened and diet beverage consumption is associated with cardiovascular risk factor profile in youth with type 1 diabetes. Acta Diabetol. 48: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liese AD, Crandell JL, Tooze JA, et al. 2015. Sugar-sweetened beverage intake and cardiovascular risk factor profile in youth with type 1 diabetes: application of measurement error methodology in the SEARCH Nutrition Ancillary Study. Br J Nutr. 114: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamichhane AP, Liese AD, Urbina EM, et al. 2014. Associations of dietary intake patterns identified using reduced rank regression with markers of arterial stiffness among youth with type 1 diabetes. Eur J Clin Nutr. 68: 1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The NS, Crandell JL, Thomas J, et al. 2013. Correlates of medical nutrition therapy and cardiovascular outcomes in youth with type 1 diabetes. J Nutr Educ Behav. 45: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauder KA, Stafford JM, The NS, et al. 2020. Dietary strategies to manage diabetes and glycemic control in youth and young adults with youth-onset type 1 and type 2 diabetes: The SEARCH for diabetes in youth study. Pediatr Diabetes. Jul 31. doi: 10.1111/pedi.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li C, D'Agostino RB Jr., Dabelea D, et al. 2018. Longitudinal association between eating frequency and hemoglobin A1c and serum lipids in diabetes in the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2015;16:382–391 PMC4291304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamichhane AP, Mayer-Davis EJ, Puett R, et al. 2012. Associations of built food environment with dietary intake among youth with diabetes. J Nutr Educ Behav. 44: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bortsov A, Liese AD, Bell RA, et al. 2011. Correlates of dietary intake in youth with diabetes: results from the SEARCH for diabetes in youth study. J Nutr Educ Behav. 43: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayer-Davis EJ, Nichols M, Liese AD, et al. 2006. Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc. 106: 689–697. [DOI] [PubMed] [Google Scholar]
- 57.Li C, Beech B, Crume T, et al. 2015. Longitudinal association between television watching and computer use and risk markers in diabetes in the SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 16: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lobelo F, Liese AD, Liu J, et al. 2010. Physical activity and electronic media use in the SEARCH for diabetes in youth case-control study. Pediatrics. 125: e1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reynolds K, Liese AD, Anderson AM, et al. 2011. Prevalence of tobacco use and association between cardiometabolic risk factors and cigarette smoking in youth with type 1 or type 2 diabetes mellitus. J Pediatr. 158: 594-601 e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawrence JM, Standiford DA, Loots B, et al. 2006. Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics. 117: 1348–1358. [DOI] [PubMed] [Google Scholar]
- 61.Hood KK, Beavers DP, Yi-Frazier J, et al. 2014. Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health. 55: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hilliard ME, Lawrence JM, Modi AC, et al. 2013. Identification of minimal clinically important difference scores of the PedsQL in children, adolescents, and young adults with type 1 and type 2 diabetes. Diabetes Care. 36: 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence JM, Liese AD, Liu L, et al. 2008. Weight-loss practices and weight-related issues among youth with type 1 or type 2 diabetes. Diabetes Care. 31: 2251–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nip ASY, Reboussin BA, Dabelea D, et al. 2019. Disordered Eating Behaviors in Youth and Young Adults With Type 1 or Type 2 Diabetes Receiving Insulin Therapy: The SEARCH for Diabetes in Youth Study. Diabetes Care. 42: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puett RC, Lamichhane AP, M DN, et al. 2012. Neighborhood context and incidence of type 1 diabetes: the SEARCH for Diabetes in Youth study. Health Place. 18: 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liese AD, Puett RC, Lamichhane AP, et al. 2012. Neighborhood level risk factors for type 1 diabetes in youth: the SEARCH case-control study. Int J Health Geogr. 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haynes A, Bulsara MK, Bower C, et al. 2006. Independent effects of socioeconomic status and place of residence on the incidence of childhood type 1 diabetes in Western Australia. Pediatr Diabetes. 7: 94–100. [DOI] [PubMed] [Google Scholar]
- 68.Holmqvist BM, Lofman O & Samuelsson U. 2008. A low incidence of Type 1 diabetes between 1977 and 2001 in south-eastern Sweden in areas with high population density and which are more deprived. Diabet Med. 25: 255–260. [DOI] [PubMed] [Google Scholar]
- 69.Cardwell CR, Carson DJ & Patterson CC. 2007. Secular trends, disease maps and ecological analyses of the incidence of childhood onset Type 1 diabetes in Northern Ireland, 1989–2003. Diabet Med. 24: 289–295. [DOI] [PubMed] [Google Scholar]
- 70.Liese AD, Lamichhane AP, Garzia SCA, et al. 2018. Neighborhood characteristics, food deserts, rurality, and type 2 diabetes in youth: Findings from a case-control study. Health Place. 50: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Findholt NE, Michael YL, Davis MM, et al. 2010. Environmental Influences on Childrens Physical Activity and Diets in Rural Oregon: Results of a Youth Photovoice Project. Online J Rural Nurs Health Care. 10: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X, Holt JB, Lu H, et al. 2014. Neighborhood commuting environment and obesity in the United States: an urban-rural stratified multilevel analysis. Prev Med. 59: 31–36. [DOI] [PubMed] [Google Scholar]
- 73.Malik F, Liese A, Reboussin B, et al. 2019. Prevalence and sociodemographic correlates of household food insecurity in youth and young adults with diabetes: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 20: O53. [Google Scholar]
- 74.Mendoza JA, Haaland W, D'Agostino RB, et al. 2018. Food insecurity is associated with high risk glycemic control and higher health care utilization among youth and young adults with type 1 diabetes. Diabetes Res Clin Pract. 138: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malik F, Saydah S, Jensen E, et al. 2020. Household Food Insecurity and its Associations with Glycemic Control and Acute Diabetes-Related Complications in Youth and Young Adults with Type 1 Diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 69: 2020-LB-6829-Diabetes [Google Scholar]
- 76.Lawrence JM, Black MH, Zhang JL, et al. 2014. Validation of Pediatric Diabetes Case Identification Approaches for Diagnosed Cases Using Information in the Electronic Health Records of a Large Integrated Managed Health Care Organization. American Journal of Epidemiology. 179: 27–38. [DOI] [PubMed] [Google Scholar]
- 77.Zhong VW, Obeid JS, Craig JB, et al. 2016. An efficient approach for surveillance of childhood diabetes by type derived from electronic health record data: the SEARCH for Diabetes in Youth Study. J Am Med Inform Assoc. 23: 1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong VW, Pfaff ER, Beavers DP, et al. 2014. Use of administrative and electronic health record data for development of automated algorithms for childhood diabetes case ascertainment and type classification: the SEARCH for Diabetes in Youth Study. Pediatric Diabetes. 15: 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chi GC, Li X, Tartof SY, et al. 2019. Validity of ICD-10-CM codes for determination of diabetes type for persons with youth-onset type 1 and type 2 diabetes. BMJ Open Diabetes Res Care. 7: e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wells BJ, Lenoir KM, Wagenknecht LE, et al. 2020. Detection of Diabetes Status and Type in Youth Using Electronic Health Records: The SEARCH for Diabetes in Youth Study. Diabetes Care. 43: 2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lenoir KM, Wagenknecht LE, Divers J, et al. 2020. 1441-P: Predicting Diabetes (DM) Diagnoses Dates Using the Electronic Health Record (EHR). Diabetes. 69: 1441-P. [Google Scholar]
- 82.Skinner AC, Ravanbakht SN, Skelton JA, et al. 2018. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tieu J, Middleton P, Crowther CA, et al. 2017. Preconception care for diabetic women for improving maternal and infant health. Cochrane Database Syst Rev. 8: CD007776. [DOI] [PubMed] [Google Scholar]
- 84.Leguizamon G, Igarzabal M & Reece E. 2007. Periconceptional care of women with diabetes mellitus. Obstet Gynecol Clin N Am. 34: 225–239. [DOI] [PubMed] [Google Scholar]
- 85.Gabbe S & Graves C. 2003. Management of diabetes mellitus complicating pregnancy. Obstet Gynecol. 102: 857–868. [DOI] [PubMed] [Google Scholar]
- 86.Persson M, Norman M & Hanson U. 2009. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care. 32: 2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cundy T, Gamble G, Neale L, et al. 2007. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care. 30: 2603–2607. [DOI] [PubMed] [Google Scholar]
- 88.Balsells M, García-Patterson A, Gich I, et al. 2009. Maternal and Fetal Outcome in Women with Type 2 Versus Type 1 Diabetes Mellitus: A Systematic Review and Metaanalysis. Journal of Clinical Endocrinology & Metabolism. 94: 4284–4291. [DOI] [PubMed] [Google Scholar]
- 89.Klingensmith GJ, Pyle L, Nadeau KJ, et al. 2016. Pregnancy Outcomes in Youth With Type 2 Diabetes: The TODAY Study Experience. Diabetes Care. 39: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ballard JL, Holroyde J, Tsang RC, et al. 1984. High malformation rates and decreased mortality in infants of diabetic mothers managed after the first trimester of pregnancy (1956–1978). Am J Obstet Gynecol. 148: 1111–1118. [DOI] [PubMed] [Google Scholar]
- 91.Murphy HR, Steel SA, Roland JM, et al. 2011. Obstetric and perinatal outcomes in pregnancies complicated by Type 1 and Type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med. 28: 1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knight KM, Thornburg LL & Pressman EK. 2012. Pregnancy outcomes in type 2 diabetic patients as compared with type 1 diabetic patients and nondiabetic controls. J Reprod Med. 57: 397–404. [PubMed] [Google Scholar]
- 93.Knight KM, Pressman EK, Hackney DN, et al. 2012. Perinatal outcomes in type 2 diabetic patients compared with non-diabetic patients matched by body mass index. J Matern Fetal Neonatal Med. 25: 611–615. [DOI] [PubMed] [Google Scholar]
- 94.Steel A, Lucke J & Adams J. 2015. The prevalence and nature of the use of preconception services by women with chronic health conditions: an integrative review. BMC Womens Health. 15: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kinsley B 2007. Achieving better outcomes in pregnancies complicated by type 1 and type 2 diabetes mellitus. Clin Ther. 29 Suppl D: S153–160. [DOI] [PubMed] [Google Scholar]
- 96.Hammoud NM, de Valk HW, van Rossem L, et al. 2017. Growth and BMI during the first 14 y of life in offspring from women with type 1 or type 2 diabetes mellitus. Pediatr Res. 81: 342–348. [DOI] [PubMed] [Google Scholar]
- 97.Vohr BR, Lipsitt LP & Oh W. 1980. Somatic growth of children of diabetic mothers with reference to birth size. J Pediatr. 97: 196–199. [DOI] [PubMed] [Google Scholar]
- 98.Silverman BL, Rizzo TA, Cho NH, et al. 1998. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 21 Suppl 2: B142–149. [PubMed] [Google Scholar]
- 99.Touger L, Looker HC, Krakoff J, et al. 2005. Early growth in offspring of diabetic mothers. Diabetes Care. 28: 585–589. [DOI] [PubMed] [Google Scholar]
- 100.Pettitt DJ, Baird HR, Aleck KA, et al. 1983. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 308: 242–245. [DOI] [PubMed] [Google Scholar]
- 101.Silverman BL, Metzger BE, Cho NH, et al. 1995. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care. 18: 611–617. [DOI] [PubMed] [Google Scholar]
- 102.Dabelea D, Hanson RL, Lindsay RS, et al. 2000. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 49: 2208–2211. [DOI] [PubMed] [Google Scholar]
- 103.Cho NH, Silverman BL, Rizzo TA, et al. 2000. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J Pediatr. 136: 587–592. [DOI] [PubMed] [Google Scholar]
- 104.Bunt JC, Tataranni PA & Salbe AD. 2005. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 90: 3225–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.West NA, Crume TL, Maligie MA, et al. 2011. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 54: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tam WH, Ma RCW, Ozaki R, et al. 2017. In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes Care. 40: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manderson JG, Mullan B, Patterson CC, et al. 2002. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia. 45: 991–996. [DOI] [PubMed] [Google Scholar]
- 108.Boney CM, Verma A, Tucker R, et al. 2005. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 115: e290–296. [DOI] [PubMed] [Google Scholar]
- 109.Pulgaron ER & Delamater AM. 2014. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. 14: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dabelea D, Knowler WC & Pettitt DJ. 2000. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 9: 83–88. [DOI] [PubMed] [Google Scholar]
- 111.Sharp GC & Lawlor DA. 2019. Paternal impact on the life course development of obesity and type 2 diabetes in the offspring. Diabetologia. 62: 1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lindsay RS, Dabelea D, Roumain J, et al. 2000. Type 2 diabetes and low birth weight: the role of paternal inheritance in the association of low birth weight and diabetes. Diabetes. 49: 445–449. [DOI] [PubMed] [Google Scholar]
- 113.Abbasi A, Corpeleijn E, van der Schouw YT, et al. 2011. Maternal and paternal transmission of type 2 diabetes: influence of diet, lifestyle and adiposity. J Intern Med. 270: 388–396. [DOI] [PubMed] [Google Scholar]
- 114.Hypponen E, Smith GD & Power C. 2003. Parental diabetes and birth weight of offspring: intergenerational cohort study. BMJ. 326: 19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Linares Segovia B, Gutierrez Tinoco M, Izquierdo Arrizon A, et al. 2012. Long-term consequences for offspring of paternal diabetes and metabolic syndrome. Exp Diabetes Res. 2012: 684562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Penesova A, Bunt JC, Bogardus C, et al. 2010. Effect of paternal diabetes on pre-diabetic phenotypes in adult offspring. Diabetes Care. 33: 1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pettitt DJ, Nelson RG, Saad MF, et al. 1993. Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care. 16: 310–314. [DOI] [PubMed] [Google Scholar]
- 118.Rijpert M, Evers IM, de Vroede MA, et al. 2009. Risk factors for childhood overweight in offspring of type 1 diabetic women with adequate glycemic control during pregnancy: Nationwide follow-up study in the Netherlands. Diabetes Care. 32: 2099–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pettitt DJ, Knowler WC, Bennett PH, et al. 1987. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 10: 76–80. [DOI] [PubMed] [Google Scholar]
- 120.Hummel S, Pfluger M, Kreichauf S, et al. 2009. Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care. 32: 921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pettitt DJ & Knowler WC. 1998. Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care. 21 Suppl 2: B138–141. [PubMed] [Google Scholar]
- 122.Sauder KA, Bekelman TA, Harrall KK, et al. 2019. Gestational diabetes exposure and adiposity outcomes in childhood and adolescence: An analysis of effect modification by breastfeeding, diet quality, and physical activity in the EPOCH study. Pediatr Obes. 14: e12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang T, Wang P, Liu H, et al. 2017. Physical Activity, TV Watching Time, Sleeping, and Risk of Obesity and Hyperglycemia in the Offspring of Mothers with Gestational Diabetes Mellitus. Sci Rep. 7: 41115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu LL, Lawrence JM, Davis C, et al. 2009. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth Study. Pediatric Diabetes. 11: 4–11. [DOI] [PubMed] [Google Scholar]
- 125.Kim G, Divers J, Fino NF, et al. 2019. Trends in prevalence of cardiovascular risk factors from 2002 to 2012 among youth early in the course of type 1 and type 2 diabetes. The SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 20: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saydah S, Imperatore G, Mercado C, et al. 2019. The cardiometabolic risk profile of young adults with diabetes in the United States. Diabetes Care;42:1895–1902 PMID: 31221678 . [DOI] [PubMed] [Google Scholar]
- 127.Nadeau KJ, Anderson BJ, Berg EG, et al. 2016. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care. 39: 1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mayer-Davis EJ, Bell RA, Dabelea D, et al. 2009. The many faces of diabetes in American youth: type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for Diabetes in Youth Study. Diabetes Care. 32 Suppl 2: S99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dabelea D, DeGroat J, Sorrelman C, et al. 2009. Diabetes in Navajo youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 32 Suppl 2: S141–S147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu LL, Yi JP, Beyer J, et al. 2009. Type 1 and Type 2 diabetes in Asian and Pacific Islander U.S. youth: the SEARCH for Diabetes in Youth Study. Diabetes Care. 32 Suppl 2: S133–S140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lawrence JM, Mayer-Davis EJ, Reynolds K, et al. 2009. Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 32 Suppl 2: S123–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mayer-Davis EJ, Beyer J, Bell RA, et al. 2009. Diabetes in African American youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 32 Suppl 2: S112–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bell RA, Mayer-Davis EJ, Beyer JW, et al. 2009. Diabetes in non-Hispanic White youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 32 Suppl 2: S102–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dabelea D, Mayer-Davis E, Andrews J, et al. 2012. Clinical evolution of beta cell function in youth with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 55: 2443–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greenbaum CJ, Anderson AM, Dolan LM, et al. 2009. Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care. 32: 1839–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maahs DM, Dabelea D, D'Agostino J, et al. 2013. Glucose Control Predicts 2-Year Change in Lipid Profile in Youth with Type 1 Diabetes. J. Pediatrics. 162: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maahs DM, Nadeau K, Snell-Bergeon JK, et al. 2011. Association of insulin sensitivity to lipids across the lifespan in people with Type 1 diabetes. Diabet. Med. 28: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pihoker C, Badaru A, Anderson A, et al. 2013. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study. Diabetes Care. 36: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brown DS & McBride TD. 2015. Impact of the Affordable Care Act on access to care for US adults with diabetes, 2011–2012. Prev Chronic Dis. 12: E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kaufman HW, Chen Z, Fonseca VA, et al. 2015. Surge in newly identified diabetes among medicaid patients in 2014 within medicaid expansion States under the affordable care act. Diabetes Care. 38: 833–837. [DOI] [PubMed] [Google Scholar]
- 141.Myerson R & Laiteerapong N. 2016. The Affordable Care Act and Diabetes Diagnosis and Care: Exploring the Potential Impacts. Curr Diab Rep. 16: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wherry LR & Miller S. 2016. Early Coverage, Access, Utilization, and Health Effects Associated With the Affordable Care Act Medicaid Expansions: A Quasi-experimental Study. Ann Intern Med. 164: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trivedi AN, Zaslavsky AM, Schneider EC, et al. 2005. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med. 353: 692–700. [DOI] [PubMed] [Google Scholar]
- 144.Brown AF, Gregg EW, Stevens MR, et al. 2005. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 28: 2864–2870. [DOI] [PubMed] [Google Scholar]


