Abstract
Background:
Cannabis use and cannabis use disorders are increasing in prevalence, including among pregnant women. The objective was to evaluate the association of a cannabis-related diagnosis (CRD) in pregnancy and adverse maternal and infant outcomes.
Methods:
We queried an administrative birth cohort of singleton deliveries in California between 2011–2017 linked to maternal and infant hospital discharge records. We classified pregnancies with CRD from International Classification of Disease codes. We identified nicotine and other substance-related diagnoses (SRD) in the same manner. Outcomes of interest included maternal (hypertensive disorders) and infant (prematurity, small for gestational age, NICU admission, major structural malformations) adverse outcomes.
Results:
From 3,067,069 pregnancies resulting in live births, 29,112 (1.0%) had a CRD. CRD was associated with an increased risk of all outcomes studied; the strongest risks observed were for very preterm birth (aRR 1.4, 95% CI 1.3, 1.6) and small for gestational age (aRR 1.4, 95% CI 1.3, 1.4). When analyzed with or without co-exposure diagnoses, CRD alone conferred increased risk for all outcomes compared to no use. The strongest effects were seen for CRD with other SRD (preterm birth aRR 2.3, 95% CI 2.2, 2.5; very preterm birth aRR 2.6, 95% CI 2.3, 3.0; gastrointestinal malformations aRR 2.0, 95% CI 1.6, 2.6). The findings were generally robust to unmeasured confounding and misclassification analyses.
Conclusions:
CRD in pregnancy was associated with increased risk of adverse maternal and infant outcomes. Providing education and effective treatment for women with a CRD during prenatal care may improve maternal and infant health.
Keywords: cannabis related diagnosis, epidemiology, adverse maternal outcomes, adverse birth outcomes
1. Introduction
Presently, over half of the states in the United States have passed laws to legalize cannabis for medical or recreational purposes. From 2002 to 2013 the prevalence of cannabis use more than doubled to 9.5% among individuals 18 and older, with significant increases observed across demographic subgroups (Hasin et al., 2015). Further, one-third of cannabis users met DSM-IV criteria for a cannabis use disorder, the behavioral disorder that can occur with chronic cannabis use (Hasin et al., 2015). The prevalence and frequency of self-reported past-month cannabis use among women of reproductive age and of pregnant women has seen parallel increases. In the 2019 National Survey on Drug Use and Health, 17% of women surveyed between the ages of 15 to 44, and 6% of pregnant women reported cannabis use (“National Survey on Drug use and Health,” 2019). The self-report of heavy cannabis use in this sample has also increased. The adjusted prevalence of past-month daily/near daily cannabis among pregnant women increased from 0.9% to 3.4% between 2002–2017 (Volkow et al., 2019). International Classification of Diseases (ICD) codes have also been used to capture prenatal cannabis exposure. From ICD10, cannabis-related diagnoses include cannabis abuse with or without withdrawal, cannabis dependence, and cannabis use unspecified. Between 1999–2013, pregnancies with an ICD code for a cannabis-related diagnosis rose from 3.2 to 8.5 per 1,000 births (Petrangelo et al., 2019). Historically, cannabis users in pregnancy were more likely to report concomitant substance use, including alcohol, tobacco and illicit substances (Ko et al., 2015; Michalski et al., 2020), many of which confer independent risks for negative birth outcomes. It is unclear whether the propensity for concomitant substance use will change as cannabis becomes increasingly legal.
Tetrahydrocannabinol (THC), the psychoactive component of cannabis, acts on the cannabinoid receptors that are expressed in the central nervous system and peripheral tissues (Metz and Borgelt, 2018). THC readily crosses the placenta, and the endocannabinoid system of the fetus is present from at least gestational day 16 (Volkow et al., 2017). The endocannabinoid system plays an important role in implementation and maintenance of the pregnancy, and it is plausible that disruption of endocannabinoid signaling could compromise placentation leading to adverse pregnancy outcomes (Metz and Stickrath, 2015; Richardson et al., 2016). Animal models dating back to the 1970s have demonstrated that early stages of mammalian development are sensitive to cannabis-induced birth defects, with consistent, reproducible array of structural abnormalities following relatively high doses of THC (Gilbert et al., 2016; Joneja, 1976). Recently, several ecologic analyses have reported higher prevalence of structural malformations in areas with greater cannabis consumption (Reece and Hulse, 2020a, 2020b, 2019a); however, individual level data are necessary to further interrogate these findings and make assertions about possible causal mechanisms. In 2018, the National Academies of Sciences reported substantial evidence of an association between prenatal cannabis exposure, lower birthweight and infant admission to the neonatal intensive care unit (NICU), and some evidence of maternal anemia (Committee on the Health Effects of Marijuana: National Academies of Sciences, 2018). However, the inconsistent literature was not sufficient to support associations with other adverse outcomes, including prematurity and major malformations in infants. Others have reviewed the evidence with similar conclusions (Conner et al., 2016; Gunn et al., 2016; Metz and Stickrath, 2015; Singh et al., 2020), although notably, newer studies have offered more support for an association with preterm birth and small for gestational age offspring (Corsi et al., 2019; Luke et al., 2019; Michalski et al., 2020; Petrangelo et al., 2019).
Medical and public health experts are widely opposed to efforts to criminalize substance use by pregnant women (American Medical Association’s Board of Trustees, 1990; Angelotta and Appelbaum, 2017; Committee on Substance Abuse, 1995; The American College of Obstetricians and Gynecologists, 2011), and maintain that punitive measures taken toward pregnant women have no proven benefit and are contrary to the welfare of the mother and fetus (American Medical Association’s Board of Trustees, 1990; Committee on Substance Abuse, 1995; Faherty et al., 2020). The medical model of addiction views substance use disorders as chronic, relapsing diseases, and promotes treatment to reduce consumption of substances during pregnancy. Given the increasing prevalence of cannabis use and cannabis use disorders, it is essential that we continue to estimate the risks that prenatal exposure has on the pregnant woman and the developing offspring; enabling women to make informed choices and supporting treatment provision for those who would benefit from that healthcare.
We queried an administrative birth cohort in the state of California to investigate the association between a cannabis-related diagnosis (CRD) and adverse maternal and infant outcomes. Specifically, we sought to 1) characterize prevalence of CRD, both as a stand-alone exposure and concomitant with other substance-related diagnoses (SRD), over the period of 2011–2017; and 2) estimate the association between CRD and adverse maternal (hypertensive disorders) and infant (prematurity, small for gestational age, NICU admission, and major structural malformations) outcomes.
2. Materials and methods
This retrospective cohort is a population based administrative cohort comprised of over 3 million pregnancies in California. All births in the state of California with a resulting birth certificate were eligible for inclusion in the administrative cohort. Birth certificates were linked to hospital discharge, emergency department, and/or ambulatory surgery record(s) (referred to here as health records) maintained by the California Office of Statewide Health Planning and Development. Health records provided diagnostic codes based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) and 10th Revision, Clinical Modification (ICD-10). Records were linked for one year before the infant’s birth (pregnant women only) through one year after birth (pregnant woman and infant). Our analytic sample was limited to live-born, singleton deliveries between 2011–2017 (Supplemental Figure 1), which is the latest year that linkage has been performed. The study was approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California.
2.1. Exposure, outcomes and covariates
Exposures, outcomes and covariates of interest were identified from health records made during pregnancy or the delivery episode, or from birth record variables where applicable (data source and specific ICD codes are in Supplemental Table 1). Maternal diagnoses from health records were identified from any visit in pregnancy or the delivery episode. Infant diagnoses were identified from delivery or any encounter in the first year of life. CRD was identified from ICD-9 (304.3: cannabis dependence, 305.2: non-dependent cannabis abuse) and ICD-10 codes (F12: cannabis-related disorders). We further identified the use of nicotine and other substance-related diagnoses (opioids, sedatives, hypnotic or anxiolytics, cocaine or other stimulants, and hallucinogens). Maternal outcomes included hypertensive disorders of pregnancy (preeclampsia or gestational hypertension). Infant outcomes included preterm birth (<37 weeks of gestation) and very preterm birth (<32 weeks of gestation), small for gestational age (<10th centile birthweight), NICU admission (yes/no), and major structural malformations (present/absent). Malformations were identified from previous human and animal literature to include oral clefts (Gilbert et al., 2016; Van Gelder et al., 2014), critical cardiac malformations (Reece and Hulse, 2019b; Williams et al., 2004), eye malformations (Gilbert et al., 2016), central nervous system (CNS) malformations (van Gelder et al., 2009; Warshak et al., 2015), and gastrointestinal malformations (Forrester and Merz, 2007; Torfs et al., 1994; Van Gelder et al., 2014). Potential confounders were identified a priori and included maternal race and ethnicity, age, payer source, education, pre-pregnancy BMI, anxiety disorder, major depressive disorder, bipolar disorder, preexisting hypertension, preexisting diabetes, and alcohol-related diagnosis. Given the strong and well-documented relationship between prenatal alcohol exposure and these outcomes (Jones et al., 2010; Nykjaer et al., 2014; O’Leary et al., 2009), alcohol was deliberately separated from other substances and adjusted for in multivariable analysis.
2.2. Statistical analyses
CRD was first operationalized as any CRD in pregnancy, irrespective of co-exposures. In subsequent models, exposure was stratified into CRD 1) without nicotine or other SRD, 2) concomitant use of nicotine only, and 3) concomitant SRD (with or without nicotine). To characterize CRD across the study period, we first quantified the rate of CRD per 100,000 deliveries by delivery date calendar year, including a linear test for trend. Additionally, within each calendar year we quantified the proportion of cannabis-related diagnoses that included concomitant use of nicotine or other SRD. We then summarized maternal demographic and pregnancy characteristics by diagnosis of CRD, which was further stratified by co-exposures. All outcomes were analyzed as binary outcomes, for which we performed multivariable Poisson regression with robust standard errors (Zou, 2004) to estimate risk ratios for pregnancy and birth outcomes. Termed a ‘modified Poisson’ regression, these generalized linear models estimate relative risk and confidence intervals for binary dependent variables using robust error variance. Models of CRD were adjusted for previously listed potential confounders, in addition to nicotine and SRD. Separately, we regressed each outcome on a four-level variable of no CRD, CRD alone, CRD and nicotine, and CRD and SRD. These models were adjusted for the same covariates with the exception of nicotine and SRD. For models of major structural malformations, we assessed each malformation separately, and subsequently created a variable to include the presence of any of the select major malformations. All multivariable analyses used complete case analysis.
Administrative databases may have sub-adequate capture of important confounders such as nicotine, other substance use and obesity (Andrade et al., 2017; Tawfik et al., 2019). Further, there may be bias in who receives diagnoses in pregnancy, particularly surrounding substance use diagnoses. To assess biases arising from these limitations, we performed two bias analyses to assess unmeasured confounding and exposure misclassification (R package episensr). First, we calculated the E-value, or the strength of an unmeasured confounder necessary to negate the observed exposure-outcome association. E-values were computed for each outcome in the ‘any CRD’ models. To assess exposure misclassification, we performed a probabilistic misclassification analysis. In 2012–2013, the estimated prevalence of DSM-IV cannabis use disorder was 3–8% among respondents 18–34 years of age (Hasin et al., 2015). From those estimates, assuming a true rate of 5% (in contrast to the observed 1%), we considered the effects of nondifferential misclassification on each outcome, varying the sensitivity in those with and without each outcome from 0.2 through 0.8 over 50,000 replications. Specificity was effectively set at 1.0 as we did not anticipate false positives being of concern. It is also possible that women without an adverse birth outcome are more likely to have undiagnosed cannabis use than women who have an adverse birth outcome. Therefore, we performed an analysis varying only the sensitivity of exposure classification among pregnancies without the outcome to determine how low sensitivity would need to be to negate our original findings. Sensitivity in pregnancies with the outcome, and specificity in all pregnancies was set at 1.0.
All analyses were performed in SAS 9.4 with the exception of the bias analysis, which was performed in R 3.6.2.
3. Results
Of the 3,067,069 pregnancies resulting in singleton live births, women were most likely to identify as Hispanic (49%) followed by non-Hispanic White (27%). Most women were between 18–34 years of age, and were equally split (48% each) between public and private insurance. Slightly under half of women were underweight or normal weight, and 17% had less than a high school education. From the cohort, 29,112 (1.0%) had a CRD diagnosis. Of pregnancies with a cannabis-related diagnosis, 53% had a CRD only, 23% had a CRD and nicotine, and 24% had a CRD and another SRD (Table 1). CRD in pregnancy increased from 696 to 1,208 per 100,000 singleton live births over the study period (8.2% per year, Ptrend <0.0001). Among women with a cannabis-related diagnosis, the proportion with CRD without nicotine or SRD (CRD alone) increased from 50% to 59% (Figure 1).
Table 1.
Maternal characteristics and demographics by cannabis-related diagnosis among women in the state of California with deliveries between 2011–2017
| No cannabis-related diagnosis (n=3,037,957) | Any cannabis-related diagnosis (n=29,112) | Cannabis-related diagnosis alone (n=15,321) | Cannabis-related diagnosis and nicotine (n=6,705) | Cannabis-related diagnosis and substance-related diagnosis (with or without nicotine) (n=7,086) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 804386 | 26.5 | 9870 | 33.9 | 4132 | 27.0 | 3049 | 45.5 | 2689 | 37.9 |
| Hispanic | 1494841 | 49.2 | 9540 | 32.8 | 5832 | 38.1 | 1227 | 18.3 | 2481 | 35.0 |
| Non-Hispanic Black | 145134 | 4.8 | 6113 | 21.0 | 3490 | 22.8 | 1540 | 23.0 | 1083 | 15.3 |
| Asian | 445027 | 14.6 | 331 | 1.1 | 225 | 1.5 | 47 | 0.7 | 59 | 0.8 |
| Multiple/other | 148569 | 4.9 | 3258 | 11.2 | 1642 | 10.7 | 842 | 12.6 | 774 | 10.9 |
| Maternal age | ||||||||||
| Less than 18 years | 50750 | 1.7 | 925 | 3.2 | 636 | 4.2 | 137 | 2.0 | 152 | 2.1 |
| 18–34 years | 2367034 | 77.9 | 25845 | 88.8 | 13648 | 89.1 | 6039 | 90.1 | 6158 | 86.9 |
| Greater than 34 years | 620062 | 20.4 | 2340 | 8.0 | 1037 | 6.8 | 528 | 7.9 | 775 | 10.9 |
| missing | 11 | 0.0 | 2 | 0.0 | 0 | 0.0 | 1 | 0.0 | 1 | 0.0 |
| Source of payment | ||||||||||
| Private insurance | 1453172 | 47.8 | 6849 | 23.5 | 4588 | 29.9 | 1162 | 17.3 | 1099 | 15.5 |
| Public insurance | 1438576 | 47.4 | 21499 | 73.8 | 10456 | 68.2 | 5408 | 80.7 | 5635 | 79.5 |
| Other payment | 146209 | 4.8 | 764 | 2.6 | 277 | 1.8 | 135 | 2.0 | 352 | 5.0 |
| Maternal education | ||||||||||
| Less than 12 years | 519005 | 17.1 | 7389 | 25.4 | 3276 | 21.4 | 1821 | 27.2 | 2292 | 32.3 |
| missing | 128919 | 4.2 | 1590 | 5.5 | 797 | 5.2 | 332 | 5.0 | 461 | 6.5 |
| Pre-pregnancy BMI | ||||||||||
| Underweight/normal weight | 1491324 | 49.1 | 14056 | 48.3 | 7127 | 46.5 | 3380 | 50.4 | 3549 | 50.1 |
| Overweight | 762791 | 25.1 | 6665 | 22.9 | 3651 | 23.8 | 1439 | 21.5 | 1575 | 22.2 |
| Obese | 657707 | 21.6 | 6853 | 23.5 | 3945 | 25.7 | 1564 | 23.3 | 1344 | 19.0 |
| Missing | 126135 | 4.2 | 1538 | 5.3 | 598 | 3.9 | 32.2 | 4.8 | 618 | 8.7 |
| Anxiety disorder | 66001 | 2.2 | 3232 | 11.1 | 1438 | 9.4 | 765 | 11.4 | 1029 | 14.5 |
| Major depressive disorder | 57756 | 1.9 | 3256 | 11.2 | 1433 | 9.4 | 725 | 10.8 | 1098 | 15.5 |
| Bipolar disorder | 19810 | 0.7 | 2244 | 7.7 | 671 | 4.4 | 559 | 8.3 | 1014 | 14.3 |
| Preexisting diabetes | 350947 | 11.6 | 2738 | 9.4 | 1366 | 8.9 | 666 | 9.9 | 706 | 10.0 |
| Preexisting hypertension | 65941 | 2.2 | 1361 | 4.7 | 583 | 3.8 | 300 | 4.4 | 478 | 6.7 |
| Nicotine | 82645 | 2.7 | 10721 | 36.8 | 0 | 0.0 | 6705 | 100.0 | 4016 | 56.7 |
| Substance-related diagnosisa | 27192 | 0.9 | 7086 | 24.3 | 0 | 0.0 | 0 | 0.0 | 7086 | 100.0 |
| Alcohol-related diagnosis | 4732 | 0.2 | 1499 | 5.1 | 125 | 0.8 | 88 | 1.3 | 1286 | 18.1 |
excluding alcohol or cannabis-related diagnoses
Figure 1.
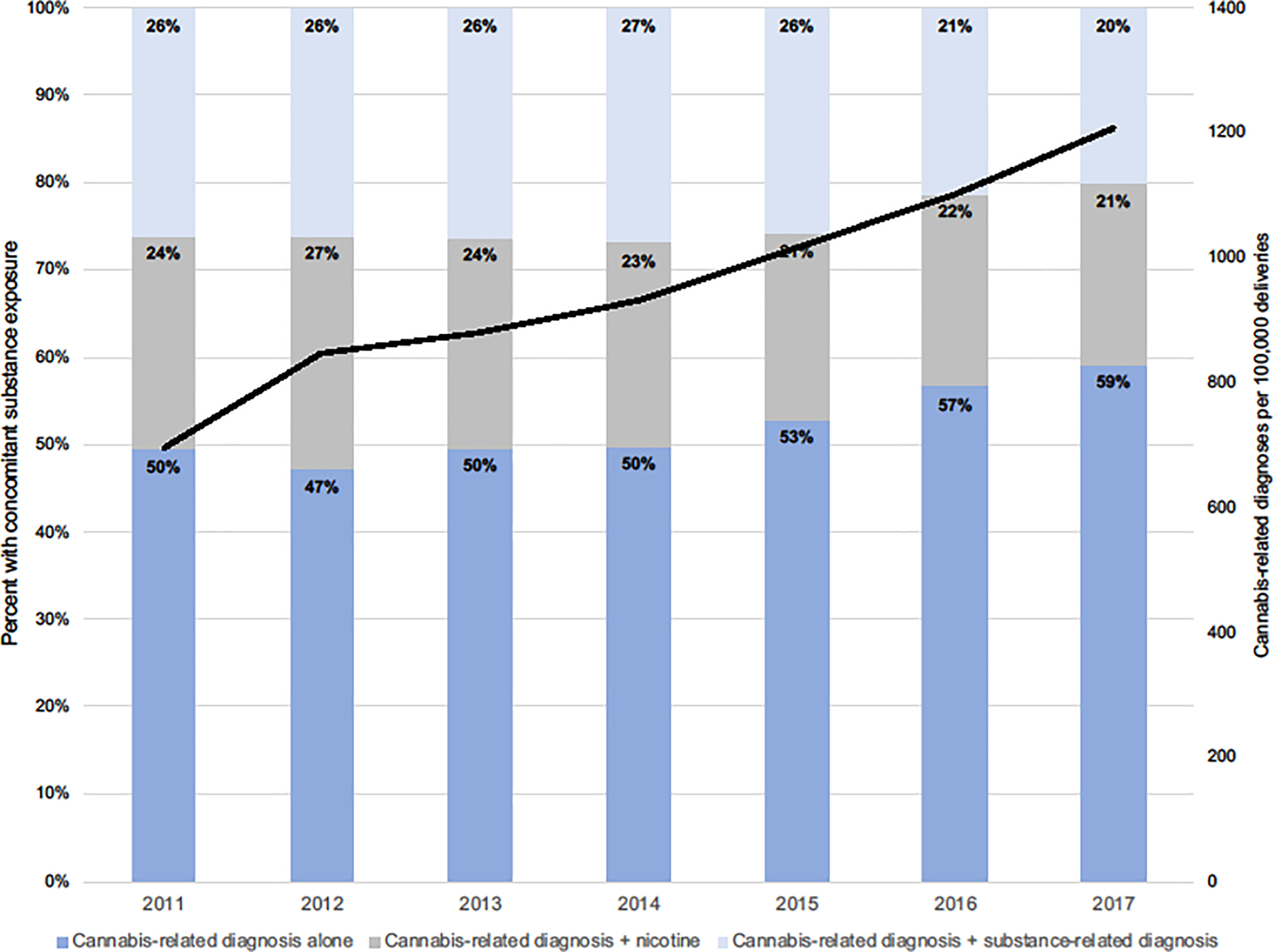
Prevalence of cannabis-related diagnosis, with or without concomitant exposures from 2011–2017. Black line denotes the prevalence of cannabis-related diagnoses per 100,000 deliveries.
Compared to women without a CRD, women with a CRD were more likely to identify as non-Hispanic White, Black or other/multiple races, be less than 34 years of age, use public insurance, have less than 12 years of education, have a mental health diagnosis, have preexisting hypertension, use nicotine, and have an alcohol and other substance-related diagnosis (Table 1). We were also interested in understanding whether these factors differed by the presence or absence of other concomitant exposures. Compared to women with a cannabis-related diagnosis plus another SRD, women with CRD alone were more likely to be less than 18 years of age, more likely to have private insurance, more likely to have at least 12 years of education, less likely to have a mental health diagnosis, and less likely to have an alcohol-related diagnosis in pregnancy.
3.1. Maternal and infant outcomes
All adjusted risk ratios are displayed in figures 2–3; frequencies and percentages of each outcome along with the crude and adjusted risk estimates are in the supplemental tables 2–3.
Figure 2.
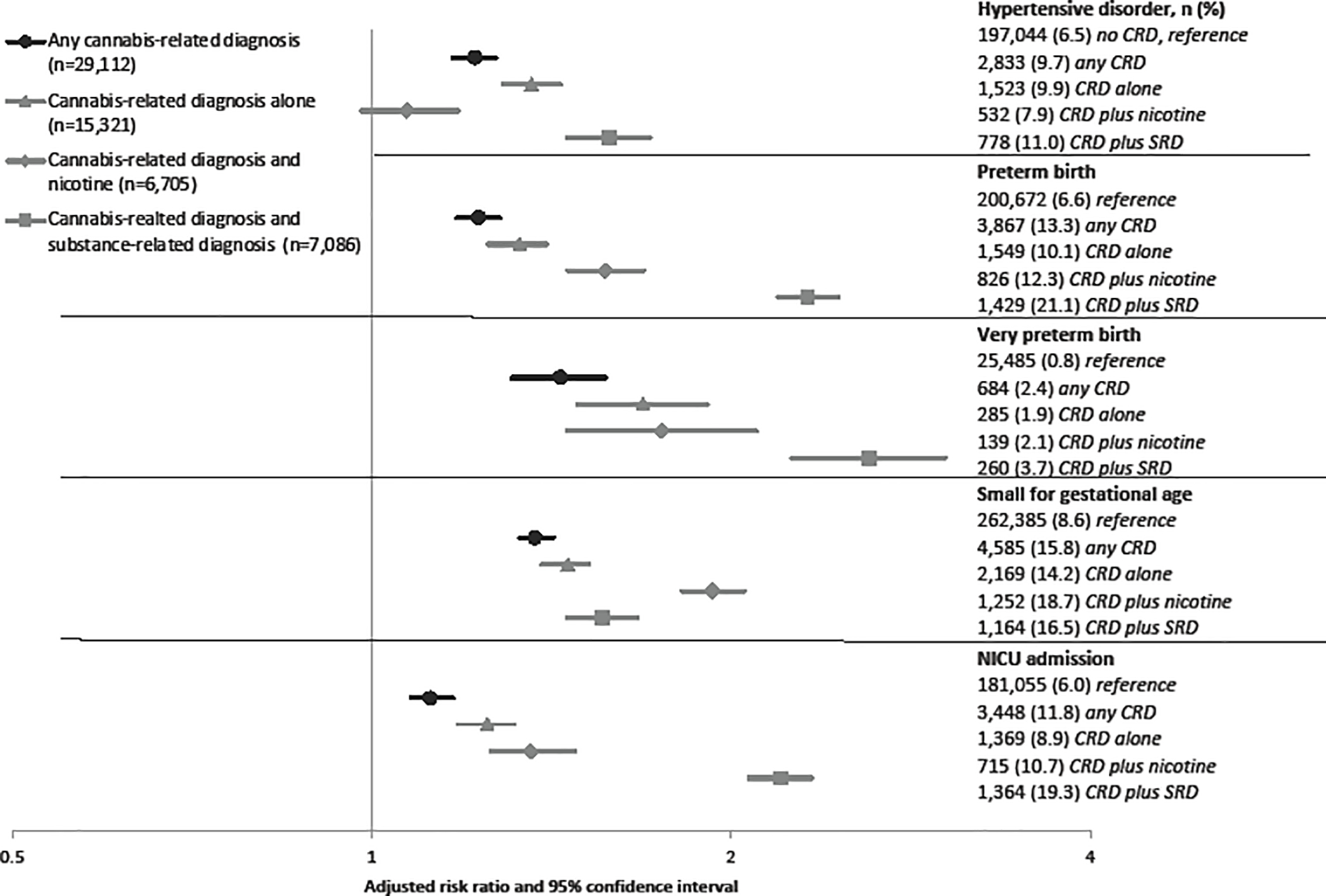
Multivariable risk ratio estimates and 95% confidence intervals. All models adjusted for pre-pregnancy BMI, race and ethnicity, payer source, anxiety, depression, bipolar disorder, preexisting hypertension, preexisting diabetes, maternal age and education and alcohol use. Models of any cannabis-related diagnoses (in black) further adjusted for nicotine use and other substance-related diagnoses.
Figure 3.
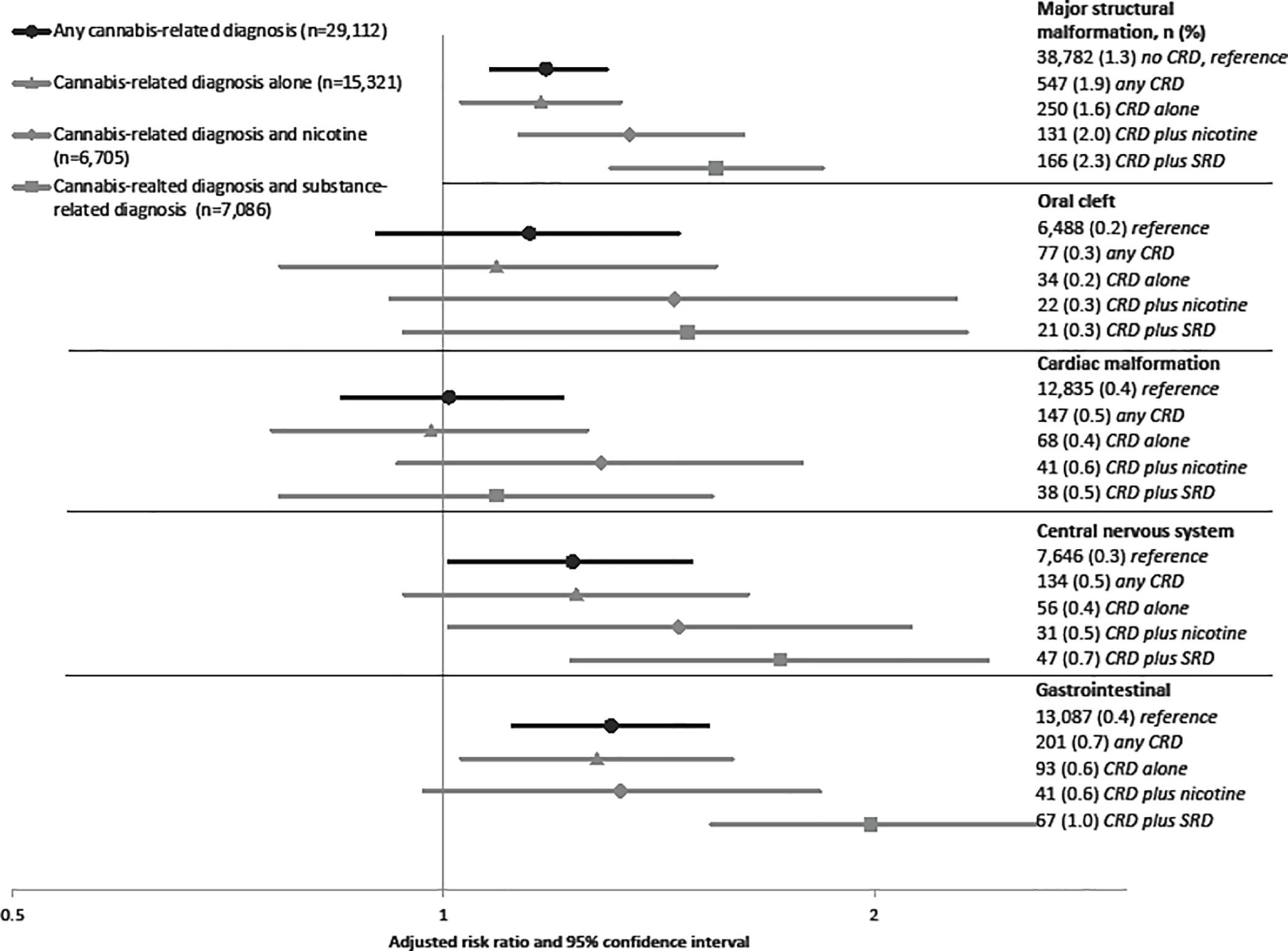
Multivariable risk ratio estimates and 95% confidence intervals. All models adjusted for pre-pregnancy BMI, race and ethnicity, payer source, anxiety, depression, bipolar disorder, preexisting hypertension, preexisting diabetes, maternal age and education and alcohol use. Models of any cannabis-related diagnoses (in black) further adjusted for nicotine use and other substance-related diagnoses. Eye malformations are not graphed due to scaling differences, but are displayed in Supplemental Table 3.
3.1.1. Hypertensive disorders of pregnancy
Prenatal hypertensive disorders were more common among women with CRD compared to women without a diagnosis (9.7% vs. 6.5%) (Supplemental Table 2). In multivariable analyses (Figure 2), women with CRD were 20% more likely to have a hypertensive disorder (1.2, 95% CI 1.2, 1.3). When CRD was analyzed with or without concomitant exposures, there was a 40% increased risk of a hypertensive disorder associated with having a CRD alone and a 60% increased risk of a hypertensive disorder associated with CRD and another SRD, compared to having no CRD. Effect estimates attenuated when assessing a CRD with nicotine use and hypertensive disorders.
3.1.2. Preterm birth and very preterm birth
The prevalence of preterm birth and very preterm birth was higher among women with any CRD compared to women with no CRD (13.3% vs. 6.6%; 2.4% vs. 0.8%; Supplemental Table 2). In multivariable analyses (Figure 2), CRD alone, CRD plus nicotine, and CRD plus SRD monotonically increased the risk of preterm birth relative to no cannabis-related diagnosis. CRD plus other SRD had a 2.3-fold increased risk of preterm birth (aRR 2.3, 95% CI 2.2, 2.5). A very similar pattern was observed with very preterm birth, with a 2.6-fold risk estimate of CRD and SRD, albeit with wider confidence intervals.
3.1.3. Small for gestational age
Having an infant small for gestational age occurred with greater frequency among women with any CRD relative to women without a diagnosis (15.8% vs. 8.6%) (Supplemental Table 2). In multivariable analyses, effect estimates for CRD alone and CRD with concomitant SRD were each associated with a modest increased risk of small for gestational age; CRD with nicotine conferred the greatest risk (aRR 1.9, 95% CI 1.8, 2.0) (Figure 2).
3.1.4. NICU admission
Effect estimates for NICU admission were similar to the effect estimates (in magnitude and pattern) to those of preterm birth (Supplemental Table 2, Figure 2).
3.1.5. Major structural malformations
In univariate analyses, there was a 50% increased risk of the offspring having a major malformation in women with any CRD compared to women without a diagnosis (1.9% vs. 1.3%), which remained significant in multivariable analysis (aRR 1.2, 95% CI 1.1, 1.3) (Supplemental Table 3). A monotonic increase in risk estimates was observed from CRD alone to CRD with nicotine and CRD with SRD, none of which had confidence intervals that included the null (Figure 3). Although numbers became increasingly small, we also assessed each individual malformation. All confidence intervals for oral clefts crossed the null, although only slightly in estimates for any CRD, CRD with nicotine and CRD with other SRD. The risk of cardiac malformations was also modestly elevated, although all estimates included the null. Eye malformations, which have been noted in animal literature (Gilbert et al., 2016), were rare and were not statistically significant (only shown in Supplemental Table 3). Conversely, CNS malformations and gastrointestinal malformations were associated with CRD, both alone and with concomitant exposures. The strongest risk was observed for CRD plus SRD in the risk for gastrointestinal malformations (aRR 2.0, 95% CI 1.6, 2.6).
3.2. Bias analysis
In a bias analysis (Supplemental Table 4) we found that for most outcomes, unmeasured confounders would need at minimum to have RRs of 1.4 to 2.1, with both having a CRD and the outcome, to explain our findings in the CRD models. To illustrate using the model of preterm birth, an unmeasured variable would need to increase both the likelihood of having a CRD and the likelihood of preterm birth by 70% to negate the observed adjusted risk ratio of 1.2. When we performed a non-differential misclassification analysis, the resulting point estimates (Supplemental Table 4) compared to our original results (unadjusted ‘any diagnosis’ RRs in Supplemental Tables 2–3) were essentially unchanged. When we modeled differential exposure misclassification by outcome, we found that sensitivity would need to vary among pregnancies without the outcome of interest from 0.3 to 0.7 to negate our original findings.
4. Discussion
In this large, administrative birth cohort that included over 29,000 pregnancies with a CRD, we found an increase in the prevalence of having a CRD in pregnancy over the time period, most notably among women without other concomitant exposures to nicotine or another SRD. The prevalence of having a CRD increased from 0.7% in 2011 to 1.2% in 2017. CRD was independently associated with an increased risk of every outcome assessed. These results were robust to unmeasured confounders weak to moderate in strength, as well as differential misclassification of having a CRD.
Given the differences in exposure assessment (clinician diagnosis, self-report via surveys, molecular testing), it is challenging to directly compare our findings to previous studies. A study of births in the National Inpatient Sample in the United States between 1999–2013 is likely the most directly comparable (Petrangelo et al., 2019). ICD9 codes were used to identify CRD, which rose from 3.2 to 8.5 per 1,000 births over the study period. Having a CRD was associated with a 40% increased odds of preterm birth, and 35% increased odds of intrauterine growth restriction (Petrangelo et al., 2019). Both the prevalence estimates and findings for the two outcomes are quite similar to our own. Despite the limitations to direct comparisons with studies that did not rely on diagnostic codes, our findings do confirm some of the previous findings, particularly with respect to an increased risk of fetal growth restriction (here examined as small for gestational age), preterm birth, low birth weight and NICU admission (Conner et al., 2016; Corsi et al., 2019; Crume et al., 2018; Luke et al., 2019; Metz and Borgelt, 2018; Michalski et al., 2020; Nykjaer et al., 2014; O’Leary et al., 2009; Paul et al., 2020; Prince et al., 2018; Van Gelder et al., 2014; Warshak et al., 2015; Young-Wolff et al., 2017). Further, our findings of increased prevalence of select structural malformations are not without precedent. In a study from the National Birth Defects Prevention Study, self-reported cannabis use was associated with gastroschisis (analogous to our results of gastrointestinal malformations) while risk measures of oral clefts and cardiac malformations (like in our study) were not statistically significant (Van Gelder et al., 2014). Further, CNS malformations have been reported in individual (van Gelder et al., 2009; Warshak et al., 2015) and ecologic level analyses (Reece and Hulse, 2019a), which our findings supported. To our knowledge, few have reported on prenatal cannabis and hypertensive disorders, with results of cannabis conferring both risk and protective effects (Chabarria et al., 2016; Corsi et al., 2019; Warshak et al., 2015). There is biologic plausibility of a deleterious effect of cannabis on hypertensive disorders (Bondarenko, 2019), and our findings of an increased risk with CRD with or without other SRD warrant additional study.
As cannabis use and cannabis use disorders become more prevalent across the United States, including among pregnant women, understanding the impact of cannabis on the health of both pregnant women and their offspring is of increasing importance. This study adds to a growing body of literature demonstrating deleterious effects of cannabis in pregnancy, and supports the message by the American College of Obstetricians and Gynecologists that pregnant women should be encouraged to discontinue cannabis use (“Committee Opinion No. 722: Marijuana Use During Pregnancy and Lactation.,” 2017). However, women with cannabis-related diagnoses, particularly those with a cannabis use disorder, very likely require additional support beyond education. To date, there are few treatments aimed at prenatal cannabis use, although motivational interviewing, cognitive behavioral therapy and contingency management therapies have been used in non-pregnant women (Forray, 2016). Pharmacotherapy is not recommended for cannabis use disorders, thus prioritizing access to specialized health care services, respecting patient autonomy, providing comprehensive care that is responsive to comorbid mental and medical conditions, housing or economic insecurity or household dysfunction, and safeguarding against discrimination and stigmatization (World Health Organization, 2014) of women using cannabis in pregnancy is essential.
Strengths of this study include the California population based administrative dataset, a large state with tremendous economic and sociodemographic diversity. The dataset had over 29,000 pregnancies with a CRD, allowing for the study of relatively rare birth outcomes. Although reliance on diagnostic codes results in a narrow capture of cannabis exposure, our administrative cohort accurately reflected population trends of an increase of cannabis exposure. Additionally, we performed multiple sensitivity analyses to better understand the vulnerability of our findings to unmeasured confounding and misclassification of exposure. Our findings should also be viewed considering the limitations. First, our exposed cohort only reflects cannabis use either known to the provider or of significant enough concern to a provider to make a diagnosis, potentially resulting in stronger risk estimates when compared to use that did not present with use or rise to the level of concern of receiving a diagnosis. However, many providers do not ask and may not include a diagnosis even if known, resulting in misclassified individuals in the unexposed cohort who may be using equal or greater amounts of cannabis, which could attenuate findings. Our 2017 prevalence of CRD (1.2%) is approximately half of what was self-reported in pregnant women from Kaiser Permanente Northern California in 2016 (Young-Wolff et al., 2017). In addition to missed cases, there could be over-representation due to assumptions, implicit bias, or racism in asking about and documenting cannabis use in economic and racial or ethnic minorities. Given this uncertainty, these findings only generalize to individuals with a cannabis-related diagnosis. This limitation extends to the classification of the other substances assessed in this study. Second, if exposure misclassification was differential by the outcome (e.g. women with preeclampsia were more likely to receive a CRD than women without preeclampsia), effect estimates would be biased, most likely away from the null. Our differential misclassification analysis demonstrated that the sensitivity of the diagnosis among those with adverse outcomes would need to be between 0.3 to 0.7 (outcome dependent) to negate our findings. Future analysis of who receives a diagnosis, and how this differs by outcome or by other covariates is strongly warranted. Third, based on the reliance of administrative records, temporality of exposure with some outcomes is ambiguous, particularly with outcomes which occur in a narrow, critical window (e.g. malformations). This misclassification would likely bias results towards finding no effect, as women classified as exposed may have no longer been at risk for the outcome(s). Fourth, as with any observational study, confounding is always of concern. We selected potential confounders a priori to reflect the documented relationship between maternal sociodemographic and prenatal factors and adverse birth outcomes. Although the level of confounding necessary to fully explain our findings gives confidence in our results, the true magnitude of the association may differ, particularly as potential confounders may have biased results away from the null.
5. Conclusions
In summary, in our study of over 29,000 exposed pregnancies, CRD was associated with an increased risk of hypertensive disorders in the mother, and prematurity, small for gestational age, NICU admission and select major malformations in the offspring. Effects were typically stronger when cannabis-related diagnosis was comorbid with nicotine or other SRDs, but were also seen when diagnosed alone. While our findings cannot generalize to all cannabis use in pregnancy, they support the importance of providing education and treatment options to women with a cannabis-related diagnosis and who are pregnant or could become pregnant.
Supplementary Material
Highlights.
Increasing prevalence of cannabis-related diagnosis (CRD) from 2011–2017.
Increased risk of all maternal and infant outcomes assessed from CRD.
Strongest effects for prematurity and gastrointestinal malformations.
Stronger effects when another substance-related diagnosis accompanied CRD.
Acknowledgments
Role of funding source
Study supported by the University of California San Francisco (UCSF) California Preterm Birth Initiative and the San Diego Study of Mothers and Infants at the University of California San Diego. Gretchen Bandoli is supported by a NIH award (K01 AA027811). No specific funding was received for this project, and the funders for the parent study had no input into the design, analysis, interpretations, or preparation of the manuscript.
Footnotes
Conflict of interest
The authors report no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Medical Association’s Board of Trustees, 1990. Legal interventions during pregnancy. Court-ordered medical treatments and legal penalties for potentially harmful behavior by pregnant women. JAMA 264, 2663–2670. [PubMed] [Google Scholar]
- Andrade SE, Bérard A, Nordeng HME, Wood ME, van Gelder MMHJ, Toh S, 2017. Administrative Claims Data Versus Augmented Pregnancy Data for the Study of Pharmaceutical Treatments in Pregnancy. Curr. Epidemiol. reports 4, 106–116. 10.1007/s40471-017-0104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotta C, Appelbaum PS, 2017. Criminal Charges for Child Harm from Substance Use in Pregnancy. J. Am. Acad. Psychiatry Law 45, 193–203. [PubMed] [Google Scholar]
- Bondarenko AI, 2019. Cannabinoids and Cardiovascular System, in: Bukiya AN (Ed.), Recent Advances in Cannabinoid Physiology and Pathology. Springer International Publishing, Cham, pp. 63–87. 10.1007/978-3-030-21737-2_5 [DOI] [Google Scholar]
- Chabarria KC, Racusin DA, Antony KM, Kahr M, Suter MA, Mastrobattista JM, Aagaard KM, 2016. Marijuana use and its effects in pregnancy. Am. J. Obstet. Gynecol 215, 506.e1–506.e7. 10.1016/j.ajog.2016.05.044 [DOI] [PubMed] [Google Scholar]
- Committee on Substance Abuse, 1995. Drug-Exposed Infants. Pediatrics 96, 364 LP–367. [PubMed] [Google Scholar]
- Committee on the Health Effects of Marijuana: National Academies of Sciences, 2018. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. THE NATIONAL ACADEMIES PRESS, Washington, DC. [PubMed] [Google Scholar]
- Committee Opinion No. 722: Marijuana Use During Pregnancy and Lactation., 2017.. Obstet. Gynecol 130, e205–e209. 10.1097/AOG.0000000000002354 [DOI] [PubMed] [Google Scholar]
- Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG, 2016. Maternal Marijuana Use and Adverse Neonatal Outcomes: A Systematic Review and Meta-analysis. Obstet. Gynecol 128. [DOI] [PubMed] [Google Scholar]
- Corsi DJ, Walsh L, Weiss D, Hsu H, El-Chaar D, Hawken S, Fell DB, Walker M, 2019. Association Between Self-reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes. JAMA 322, 145–152. 10.1001/jama.2019.8734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crume TL, Juhl AL, Brooks-Russell A, Hall KE, Wymore E, Borgelt LM, 2018. Cannabis Use During the Perinatal Period in a State With Legalized Recreational and Medical Marijuana: The Association Between Maternal Characteristics, Breastfeeding Patterns, and Neonatal Outcomes. J. Pediatr 197, 90–96. 10.1016/j.jpeds.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Faherty LJ, Stein BD, Terplan M, 2020. Consensus Guidelines and State Policies: The Gap Between Principle and Practice at the Intersection of Substance Use and Pregnancy. Am. J. Obstet. Gynecol. MFM 2. 10.1016/j.ajogmf.2020.100137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A, 2016. Substance use during pregnancy. F1000Research 5, F1000 Faculty Rev–887. 10.12688/f1000research.7645.1 [DOI] [Google Scholar]
- Forrester MB, Merz RD, 2007. Risk of selected birth defects with prenatal illicit drug use, Hawaii, 1986–2002. J. Toxicol. Environ. Health. A 70, 7–18. 10.1080/15287390600748799 [DOI] [PubMed] [Google Scholar]
- Gilbert M, Sulik K, Fish EW, Baker L., Dehart D., Parnell S., 2016. Dose-Dependent Teratogenicity of the Synthetic Cannabinoid CP-55,940 in Mice. Neurotoxicol. Teratol 58, 15–22. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JKL, Rosales CB, Center KE, Nuñez A, Gibson SJ, Christ C, Ehiri JE, 2016. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 6. 10.1136/bmjopen-2015-009986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF, 2015. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA psychiatry 72, 1235–1242. 10.1001/jamapsychiatry.2015.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joneja MG, 1976. A study of teratological effects of intravenous, subcutaneous, and intragastric administration of delta9-tetrahydrocannabinol in mice. Toxicol. Appl. Pharmacol 36, 151–162. 10.1016/0041-008x(76)90035-1 [DOI] [PubMed] [Google Scholar]
- Jones KL, Hoyme HE, Robinson LK, del Campo M, Manning MA, Prewitt LM, Chambers CD, 2010. Fetal Alcohol Spectrum Disorders: Extending the Range of Structural Defects. Am. J. Med. Genet. A 152A, 2731–2735. 10.1002/ajmg.a.33675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM, 2015. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am. J. Obstet. Gynecol 213, 201.e1–201.e10. 10.1016/j.ajog.2015.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke S, Hutcheon J, Kendall T, 2019. Cannabis Use in Pregnancy in British Columbia and Selected Birth Outcomes. J. Obstet. Gynaecol. Can 41, 1311–1317. 10.1016/j.jogc.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Metz TD, Borgelt LM, 2018. Marijuana use in pregnancy and while breastfeeding. Obstet. Gynecol 132, 1198–1210. 10.1097/AOG.0000000000002878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz TD, Stickrath EH, 2015. Marijuana use in pregnancy and lactation: A review of the evidence. Am. J. Obstet. Gynecol 213, 761–778. 10.1016/j.ajog.2015.05.025 [DOI] [PubMed] [Google Scholar]
- Michalski CA, Hung RJ, Seeto RA, Dennis C-L, Brooks JD, Henderson J, Levitan R, Lye SJ, Matthews SG, Knight JA, 2020. Association between maternal cannabis use and birth outcomes: an observational study. BMC Pregnancy Childbirth 20, 771. 10.1186/s12884-020-03371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Survey on Drug use and Health [WWW Document], 2019.. Subst. Abus. Ment. Heal. Serv. Adm URL https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables (accessed 9.17.20).
- Nykjaer C, Alwan NA, Greenwood DC, Simpson NAB, Hay AWM, White KLM, Cade JE, 2014. Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: evidence from a British cohort. J. Epidemiol. Community Health 68, 542 LP–549. 10.1136/jech-2013-202934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary CM, Nassar N, Kurinczuk JJ, Bower C, 2009. The effect of maternal alcohol consumption on fetal growth and preterm birth. BJOG 116, 390–400. 10.1111/j.1471-0528.2008.02058.x [DOI] [PubMed] [Google Scholar]
- Paul SE, Hatoum AS, Fine JD, Johnson EC, Hansen I, Karcher NR, Moreau AL, Bondy E, Qu Y, Carter EB, Rogers CE, Agrawal A, Barch DM, Bogdan R, 2020. Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results From the ABCD Study. JAMA Psychiatry. 10.1001/jamapsychiatry.2020.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrangelo A, Czuzoj-Shulman N, Balayla J, Abenhaim HA, 2019. Cannabis Abuse or Dependence During Pregnancy: A Population-Based Cohort Study on 12 Million Births. J. Obstet. Gynaecol. Can 41, 623–630. 10.1016/j.jogc.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Prince MA, Conner BT, Pearson MR, 2018. Quantifying Cannabis: A field study of marijuana quantity estimation. Psychol. Addict. Behav 32, 426–433. 10.1037/adb0000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK, 2020a. Broad Spectrum epidemiological contribution of cannabis and other substances to the teratological profile of northern New South Wales: geospatial and causal inference analysis. BMC Pharmacol. Toxicol 21, 75. 10.1186/s40360-020-00450-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK, 2020b. Canadian Cannabis Consumption and Patterns of Congenital Anomalies: An Ecological Geospatial Analysis. J. Addict. Med 14, e195–e210. 10.1097/ADM.0000000000000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK, 2019a. Cannabis Consumption Patterns Explain the East-West Gradient in Canadian Neural Tube Defect Incidence: An Ecological Study. Glob. Pediatr. Heal 6, 2333794X19894798. 10.1177/2333794X19894798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK, 2019b. Cannabis Teratology Explains Current Patterns of Coloradan Congenital Defects: The Contribution of Increased Cannabinoid Exposure to Rising Teratological Trends. Clin. Pediatr. (Phila). 58, 1085–1123. 10.1177/0009922819861281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KA, Hester AK, McLemore GL, 2016. Prenatal cannabis exposure - The “first hit” to the endocannabinoid system. Neurotoxicol. Teratol 58, 5–14. 10.1016/j.ntt.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Singh S, Filion KB, Abenhaim HA, Eisenberg MJ, 2020. Prevalence and outcomes of prenatal recreational cannabis use in high-income countries: a scoping review. BJOG An Int. J. Obstet. Gynaecol. 127, 8–16. 10.1111/1471-0528.15946 [DOI] [PubMed] [Google Scholar]
- Tawfik DS, Gould JB, Profit J, 2019. Perinatal Risk Factors and Outcome Coding in Clinical and Administrative Databases. Pediatrics 143, e20181487. 10.1542/peds.2018-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The American College of Obstetricians and Gynecologists, 2011. Substance abuse reporting and pregnancy: The role of the obstetrician-gynecologist. Obstet. Gynecol 117, 200–201. 10.1097/AOG.0b013e31820a6216 [DOI] [PubMed] [Google Scholar]
- Torfs CP, Velie EM, Oechsli FW, Bateson TF, Curry CJ, 1994. A population-based study of gastroschisis: demographic, pregnancy, and lifestyle risk factors. Teratology 50, 44–53. 10.1002/tera.1420500107 [DOI] [PubMed] [Google Scholar]
- Van Gelder MMHJ, Donders ART, Devine O, Roeleveld N, Reefhuis J, 2014. Using bayesian models to assess the effects of under-reporting of cannabis use on the association with birth defects, national birth defects prevention study, 1997–2005. Paediatr. Perinat. Epidemiol 28, 424–433. 10.1111/ppe.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder MMHJ, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N, 2009. Maternal periconceptional illicit drug use and the risk of congenital malformations. Epidemiology 20, 60–66. 10.1097/EDE.0b013e31818e5930 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Compton WM, Wargo EM, 2017. The Risks ofMarijuana Use During Pregnancy. JAMA - J. Am. Med. Assoc. 317, 129–130. 10.1001/jama.2016 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Han B, Compton WM, McCance-Katz EF, 2019. Self-reported Medical and Nonmedical Cannabis Use Among Pregnant Women in the United States. JAMA 322, 167–169. 10.1001/jama.2019.7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshak CR, Regan J, Moore B, Magner K, Kritzer S, Van Hook J, 2015. Association between marijuana use and adverse obstetrical and neonatal outcomes. J. Perinatol 35, 991–995. 10.1038/jp.2015.120 [DOI] [PubMed] [Google Scholar]
- Williams LJ, Correa A, Rasmussen S, 2004. Maternal lifestyle factors and risk for ventricular septal defects. Birth Defects Res. A. Clin. Mol. Teratol 70, 59–64. 10.1002/bdra.10145 [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2014. Guidelines for the identification and management of substance use and substance use disorders in pregnancy. Geneva, Switzerland. [PubMed] [Google Scholar]
- Young-Wolff KC, Tucker LY, Alexeeff S, Armstrong MA, Conway A, Weisner C, Goler N, 2017. Trends in self-reported and biochemically tested Marijuana use among pregnant females in California from 2009–2016. JAMA - J. Am. Med. Assoc 318, 2490–2491. 10.1001/jama.2017.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G, 2004. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am. J. Epidemiol 159, 702–706. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


