Abstract
Ewing Sarcoma (ES) is the second most common primary bone tumor in children and adolescents. There are few known epidemiological or genetic risk factors for ES. Numerous reports describe incidence rates and trends within the United States, but international comparisons are sparse. We used the Cancer Incidence in Five Continents (CI5) data to estimate age standardized incidence rates (ASRs; cases per million) and 95% confidence intervals (95% CI), male-to-female incidence rate ratios (IRR; 95% CI), and the average annual percent change in incidence (AAPC; 95% CI) for ES by geographic region for children and adults aged 0–49 years. We also estimated the ASR for each country or country subpopulation among the 10–19-year-old age range; capturing the peak incidence of ES. In total, 15,874 ES cases ages 0–49 were reported in the CI5 series between 1988 and 2012. AAPC estimates varied by age group and geographic region. Most of the statistically significant AAPCs showed an increased incidence over time; the only statistically significant decreases in incidence were observed among 20– 29-year-olds and 30–39-year-olds in Southern Asia at −1.93 and −1.67 percent. When categorized by predominant ancestry, we observed countries and subpopulations with predominately African, East Asian, and South-east Asian ancestry had the lowest incidence rates, whereas Pacific Islanders and populations with predominantly European and North African/Middle Eastern ancestry had the highest. An excess incidence in males was observed in most regions. Our results highlight substantial variation in ES incidence across geographic populations, reflecting potential ancestral influence on disease risk.
Keywords: Ewing Sarcoma, Incidence, International
Introduction
Ewing sarcoma (ES) is a small, round blue cell tumor with a presumed mesenchymal or neuroectodermal origin.1 ES tumors most often present adjacent to bone and therefore is generally classified as a bone tumor, although histologically and cytogenetically similar tumors occur in soft tissue and will be included under the broader designation of Ewing Sarcoma Family of Tumors (ESFT).2 Despite being exceedingly rare the molecular biology of ES and ESFT has been well-described. The somatic genetics of ES are unusually homogenous, with over 90% of tumors featuring a translocation between the EWSR1 and FLI1 genes to form the EWS-FLI1 fusion oncoprotein.3,4 EWS-FLI1 is an aberrant transcription factor which enacts widespread alterations to DNA methylation and gene expression through its binding to GGAA repeats and high-affinity ETS domains.1 As binding of the fusion oncoprotein to GGAA repeats depends on their length, which is polymorphic, germline variation is widely thought to underlie risk for ES.5,6
Aside from its unusual genetic homogeneity the most notable feature of ES is its wide differences in incidence by ancestry with higher incidence noted in populations of European ancestry compared to African or Asian ancestry7 These differences are apparent within the United States Surveillance, Epidemiology and End Results (SEER) program, which reported ES incidence rates in non-Hispanic White individuals (1.5 cases per million) to be substantially higher than in African Americans (0.2 cases per million) and Asian/Pacific Islanders (0.8 cases per million).8 Additional SEER analyses indicate tumor size, proportion of soft-tissue tumors and survival also differ by race.7 However these categorizations of convention in the U.S. obscure considerable cultural and genetic diversity.
International comparisons of ES rates also can be informative for ancestral disease risk, but to our knowledge have not been systematically examined recently.9 The latest global comparisons assessed ASRs by country for 2003–2007 and temporal patterns for 12 countries only.10 Here we take advantage of the Cancer Incidence on Five Continents (CI5) database to examine trends in ES incidence over a 25-year time period (1988–2012) and make comparisons across regions and populations in the most recent eras (2003–2007 and 2008–2012).
Materials and Methods
Materials
ES cases and populations among children and adults aged 0–49 years old were extracted from all registries available for the last five volumes (VII: 1988–1992, VIII: 1993–1997, IX: 1998–2002 X: 2003–2007 and XI: 2008–2012) of the Cancer Incidence in Five Continents (CI5) series.11–15 The CI5 series requires registries adhere to strict quality standards for inclusion, resulting in high quality and comparable data.16 Starting in 1988, the CI5 included morphology codes in addition to cancer site, allowing for analysis of tumors predominant in the pediatric population.17
Cancer Classification
The data in the CI5 series are mainly organized by anatomical site using the International Classification of Disease (ICD) codes. However, starting with VII in 1988, the CI5 were further grouped according to histological codes based on the International Classification of Diseases for Oncology (ICD-O-2) allowing for analysis of tumors predominant in the pediatric population.15 Specifically, the CI5 uses the International Classification of Disease revision 10 (ICD-10) across the all time-periods within this analysis and uses the ICD-O-2 in volumes VII and VIII and ICD-O-3 in volumes IX-XI. In all volumes, the ES cancer classification is comprised of ICD-10 codes C40–41 with ICD-O code 9260.
Statistical Analysis
Initial analyses were conducted combining registries into 15 geographic regions due to the extreme rarity of ES. We used regions defined by the United Nations Statistics Division (UNSD) as a meaningful way to combine data for statistical analysis.18 Due to data availability we were not able to divide data perfectly into all subregions defined by UNSD, exceptions include Africa, Oceania, and Central Asia. Due to the heterogeneity and limited number of registries available, Africa was divided into Northern Africa and Sub-Saharan Africa, rather than breaking Sub-Saharan Africa into further regions as done in the UNSD classification. Oceania was not broken down further as registries outside of Australia and New Zealand were not consistently available. No data was available for Central Asia. Lastly, the countries and registries available in each volume changed over time and generally more data were available for later time periods.11–15 In order to assess trends in incidence with stable estimates, data were pooled for registries within regions despite changing availability of data, as in our previous epidemiology publications.19–21
Incidence rates and average annual percent change (AAPC) were reported by 0–9, 10–19, 20–29, 30–39 and 40–49 age ranges. When 5-year age categories were combined, age standardization was performed using the WHO 2000–2025 average population.22 Incidence rates were not calculated when there were fewer than 5 cases. Incidence trends over time were evaluated based on the estimated AAPC. AAPCs and 95% confidence intervals (CIs) were calculated by modeling the effect of time on cases using Poisson regression with a population offset for population heterogeneity and robust standard errors to correct for overdispersion in some models. AAPCs were not calculated when there were three or more time-periods with less than 5 cases.
The remainder of analyses comparing incidence across regions and populations used data aggregated from the last two CI5 volumes, 2003–2007 and 2008–2012, due to low numbers for individual countries. Male to female incidence rate ratios (IRR) and 95% CIs for each geographic region were calculated for the 10–14 and 15–19 age groups, which represent the peak incidence for ES. We also calculated incidence rates for the peak incidence age group, 10–19, by country or ethnic subgroup when a country had data regarding subgroup-specific numerators and population denominators. 95% confidence intervals were calculated using Poisson exact methods to provide precision for zero case counts. We ranked the rates from largest to smallest and plotted them.
In addition, we categorized countries or sub-groups by predominant ancestry using CIA Factbook23 population breakdowns to classify populations as Predominantly European, North African/Middle Eastern, South American Admixed, Eastern Asian, Southern Asian, South-eastern Asian, Pacific Islander, or Predominantly African. Subpopulations for which ancestry was unclear such as “Kuwait: Non-Kuwaiti” were excluded from the analysis. Using these clusters defined by predominant ancestry, we performed a Kruskal Wallis test by ranks. This method is a nonparametric approach to determine whether two or more independent groups originate from the same distribution.24,25 We further explored the effect of each ancestry using Poisson regression modeling the effect of ancestry on cases with a log population offset. European Ancestry was used as the comparison group.
All analyses were conducted using SAS software version 9.4 (Cary, North Carolina, USA). Figures 1, 2, 4 and 5 were created in GraphPad Prism v8.0.0 (GraphPad Software, La Jolla, CA). Figure 3 was created in ArcGIS Desktop 10.5.1 (Environmental Systems Research Institute, Redlands, CA). Statistical significance was determined using two-sided hypothesis tests (alpha of 0.05).
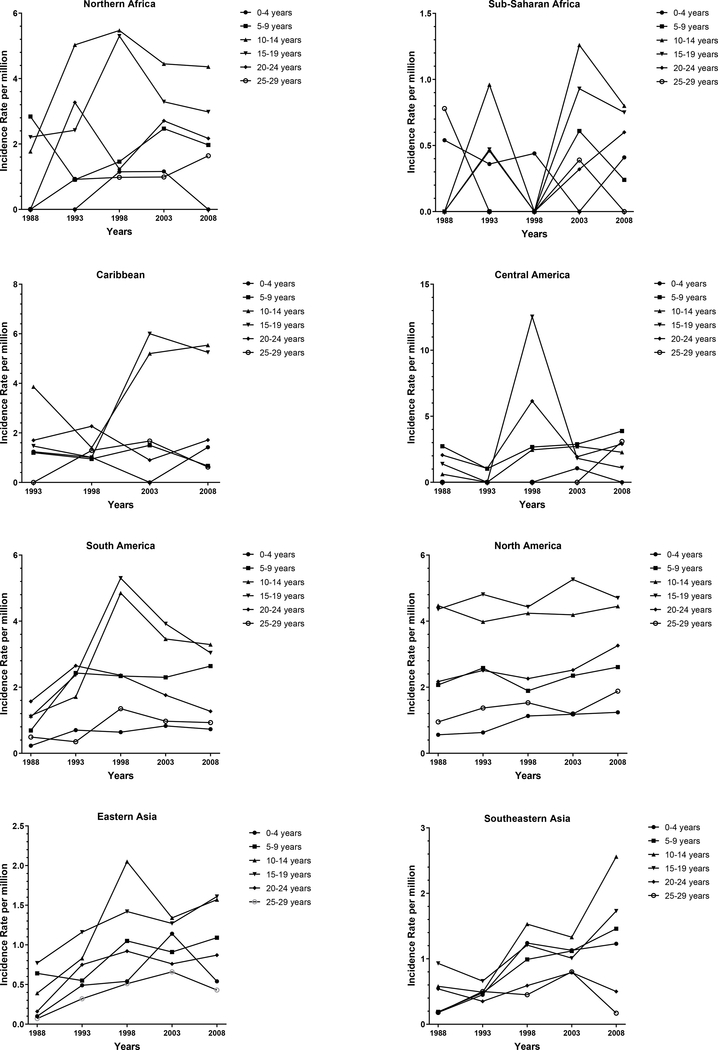
Figure 1:
Incidence rate (IR) per million of Ewing Sarcoma (ES) by time-period for each age group in each geographic region, Cancer Incidence in Five Continents (CI5) 1988–2012.
*No data were available for the Caribbean region in the 1988–1992 time-period.
Figure 2:
Male to female Ewing sarcoma (ES) incidence rate ratios (IRR) and 95% confidence intervals (CI) by geographic region in 10–14 and 15–19-year-old age groups, Cancer Incidence in Five Continents (CI5) 2003–2012.
Figure 4:
Incidence rate (IR) per million and 95% confidence intervals (CI) of Ewing Sarcoma (ES) among 10–19-year-olds by country and predominant ancestry, Cancer Incidence in Five Continents (CI5) 2003–2012. *Groups with a non-classifiable ancestry were not included.
Figure 5:
(A) Odds ratios (OR) and 95% confidence intervals (CI) for SNPs identified from published GWAS literature in Ewing Sarcoma (ES)29,30 and (B) SNP risk allele frequencies from 1000 Genomes in genes among European, Hispanic, East Asian and African populations.
Figure 3:
Ewing Sarcoma (ES) incidence rates (IR) per million among 10–19-year-olds among countries, Cancer Incidence in Five Continents (CI5) 2003–2012.
Results
During 1988 to 2012, there were 15,874 cases (9,589 male and 6,285 female) of ES in children and adults aged 0–49 years old reported in the CI5 series.
Incidence Rates and Trends by Geographic Region (1988–2012)
The peak age of ES incidence was between 10–19 for all regions (Figure 1). Significant increases in incidence from 1988–2012 (Table 1) were seen in the 0–9 age group in North America (AAPC: 1.79), South-eastern Asia (AAPC: 7.20), Southern Asia (AAPC:0.89), Western Asia (AAPC: 2.75) and Southern Europe at (AAPC: 2.84). In the 10–19 age group, significant increases were observed in South-eastern Asia, Northern Europe and Western Europe at 6.85, 2.05 and 2.49 percent respectively. We also observed significant increases in 20–29-year-olds in North America (AAPC: 2.98), Eastern Europe (AAPC: 1.84), Northern Europe (AAPC: 2.28), Southern Europe (AAPC: 2.28) and Western Europe (AAPC: 1.71). Significant increases in the 30–39 age group occurred in North America (AAPC: 1.94), Northern Europe (AAPC: 3.41), and Western Europe (AAPC: 2.8). There were also significant increases in Southern Europe and Northern America in the 40–49 age category at 5.79 and 3.98 percent respectively. The only significant decreases in incidence were observed in the 20–29 and 30–39 age category in Southern Asia at-1.93 and −1.67 percent. There were no regions that showed statistically significant increases across all age categories. However, incidence increased in most age groups in North America, Northern Europe, Southern Europe and Western Europe.
Table 1.
Ewing Sarcoma (ES) age standardized incidence rates(ASR) per million by time period and age category and the average annual percent change (AAPC) in incidence over 1988–2012 from the Cancer Incidence in Five Continents (CI5) data
| Age Standardized Incidence Rates1 | |||||||
|---|---|---|---|---|---|---|---|
| Age group | 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | 2008–2012 | AAPC (95% CI)2,3 | |
| Northern Africa | 0–9 yrs | - | - | 1.31 | 1.82 | - | - |
| 10–19 yrs | - | 3.70 | 5.38 | 3.85 | 3.62 | 0.07 (−4.57, 4.92) | |
| 20–29 yrs | - | 2.17 | 1.12 | 1.92 | 1.92 | 3.13 (−4.61, 11.49) | |
| 30–39 yrs | - | - | - | - | - | - | |
| 40–49 yrs | - | - | - | - | - | - | |
| Sub-Saharan Africa | 0–9 yrs | - | - | - | - | - | - |
| 10–19 yrs | - | - | - | 1.09 | 0.77 | - | |
| 20–29 yrs | - | - | - | - | - | - | |
| 30–39 yrs | - | - | - | - | - | - | |
| 40–49 yrs | - | - | - | - | - | - | |
| Caribbean | 0–9 yrs | - | - | - | 0.78 | - | - |
| 10–19 yrs | - | - | 1.21 | 5.60 | 5.39 | 9.05 (−3.10, 22.73) | |
| 20–29 yrs | - | - | 1.81 | 1.31 | - | - | |
| 30–39 yrs | - | - | - | - | - | - | |
| 40–49 yrs | - | - | - | - | - | - | |
| Central America | 0–9 yrs | 1.32 | - | - | 2.02 | 2.02 | 3.40 (−0.53, 7.49) |
| 10–19 yrs | - | - | - | 2.27 | 1.67 | - | |
| 20–29 yrs | - | - | - | - | 3.00 | - | |
| 30–39 yrs | - | - | - | - | - | - | |
| 40–49 yrs | - | - | - | - | - | - | |
| South America | 0–9 yrs | - | 1.57 | 1.48 | 1.57 | 1.70 | 3.51 (−0.17, 7.33) |
| 10–19 yrs | 1.12 | 2.04 | 5.08 | 3.70 | 3.16 | 1.95 (−4.13, 2.54) | |
| 20–29 yrs | 1.06 | 1.54 | 1.88 | 1.38 | 1.11 | −0.99 (−4.39, 2.54) | |
| 30–39 yrs | - | - | 0.77 | 0.91 | 0.62 | 4.85 (−4.58, 15.22) | |
| 40–49 yrs | - | - | 0.54 | 0.34 | 0.27 | −2.47 (−7.37, 2.69) | |
| North America | 0–9 yrs | 1.31 | 1.60 | 1.52 | 1.76 | 1.93 | 1.79 (1.07, 2.51) |
| 10–19 yrs | 4.42 | 4.39 | 4.33 | 4.72 | 4.58 | 0.34 (−0.13, 0.80) | |
| 20–29 yrs | 1.51 | 1.92 | 1.89 | 1.86 | 2.57 | 2.28 (0.67, 3.92) | |
| 30–39 yrs | 0.70 | 0.97 | 0.91 | 1.02 | 1.13 | 1.94 (0.84, 3.05) | |
| 40–49 yrs | 0.22 | 0.47 | 0.74 | 0.61 | 0.80 | 3.98 (0.31, 7.79) | |
| Eastern Asia | 0–9 yrs | 0.38 | 0.53 | 0.81 | 1.01 | 0.82 | 3.19 (−0.06, 6.55) |
| 10–19 yrs | 0.59 | 1.00 | 1.71 | 1.30 | 1.59 | 2.91 (−0.54, 6.48) | |
| 20–29 yrs | - | 0.53 | 0.70 | 0.71 | 0.64 | 2.89 (−1.84, 7.85) | |
| 30–39 yrs | - | - | 0.40 | 0.40 | 0.31 | 4.46 (−3.00, 12.49) | |
| 40–49 yrs | - | 0.20 | 0.16 | 0.19 | 0.27 | 4.59 (−0.59, 8.75) | |
| Southeastern Asia | 0–9 yrs | - | 0.46 | 1.12 | 1.12 | 1.35 | 7.20 (3.05, 11.51) |
| 10–19 yrs | 0.76 | 0.58 | 1.37 | 1.16 | 2.11 | 6.85 (3.12, 10.72) | |
| 20–29 yrs | - | 0.42 | 0.52 | 0.80 | 0.34 | 0.47 (−5.13, 6.40) | |
| 30–39 yrs | - | 0.30 | - | 0.48 | 0.23 | 1.57 (−4.66, 8.21) | |
| 40–49yrs | - | - | - | 0.25 | 0.40 | - | |
| Southern Asia | 0–9 yrs | 1.31 | 1.29 | 1.46 | 1.45 | 1.53 | 0.89 (0.41, 1.38) |
| 10–19 yrs | 3.28 | 3.72 | 3.65 | 3.50 | 2.47 | −0.57 (−3.38, 0.48) | |
| 20–29 yrs | 1.65 | 1.59 | 1.50 | 1.38 | 1.11 | −1.93 (−2.72, −1.13) | |
| 30–39 yrs | 0.58 | 0.60 | 0.51 | 0.55 | 0.42 | −1.67 (−2.95, −0.37) | |
| 40–49 yrs | 0.62 | 0.38 | 0.50 | 0.23 | 0.43 | −1.82 (−6.55, 3.16) | |
| Western Asia | 0–9 yrs | 1.49 | 1.62 | 2.00 | 1.75 | 2.56 | 2.75 (0.87, 4.66) |
| 10–19 yrs | 4.48 | 6.50 | 6.51 | 6.61 | 6.51 | 0.80 (−0.50, 2.12) | |
| 20–29 yrs | 2.18 | 2.88 | 3.27 | 2.88 | 2.77 | 0.40 (−1.60, 1.71) | |
| 30–39 yrs | - | 0.88 | 0.84 | 1.02 | 0.97 | 0.85 (−0.12, 1.82) | |
| 40–49 yrs | - | 0.94 | 1.08 | 0.56 | 0.93 | −0.67 (−4.48, 3.30) | |
| Eastern Europe | 0–9 yrs | 1.22 | 1.19 | 1.99 | 1.36 | 1.45 | 0.62 (−1.87, 3.19) |
| 10–19 yrs | 3.48 | 2.78 | 4.46 | 3.90 | 4.42 | 1.56 (−0.29, 3.44) | |
| 20–29 yrs | 1.25 | 1.06 | 1.61 | 1.41 | 1.70 | 1.84 (0.16, 3.56) | |
| 30–39 yrs | 0.57 | 0.30 | 0.24 | 0.59 | 0.61 | 2.46 (−2.22, 7.36) | |
| 40–49 yrs | 0.29 | - | 0.29 | 0.23 | 0.26 | 0.88 (−3.24, 5.18) | |
| Northern Europe | 0–9 yrs | 1.31 | 1.19 | 1.61 | 1.84 | 1.54 | 1.59 (−0.35, 3.56) |
| 10–19 yrs | 3.57 | 3.36 | 4.68 | 4.64 | 5.04 | 2.05 (0.77, 3.35) | |
| 20–29 yrs | 1.90 | 1.70 | 2.19 | 2.43 | 3.13 | 2.98 (1.60, 4.37) | |
| 30–39 yrs | 0.68 | 0.66 | 0.85 | 0.97 | 1.24 | 3.41 (2.33, 4.51) | |
| 40–49 yrs | 0.52 | 0.52 | 0.50 | 0.53 | 0.54 | 0.15 (−0.25, 0.55) | |
| Southern Europe | 0–9 yrs | 1.34 | 2.26 | 2.02 | 2.72 | 2.69 | 2.84 (0.85, 4.86) |
| 10–19 yrs | 4.60 | 5.72 | 6.08 | 5.20 | 7.75 | 1.97 (−0.02, 3.99) | |
| 20–29 yrs | 1.80 | 1.79 | 2.29 | 2.29 | 2.77 | 2.28 (1.36, 3.20) | |
| 30–39 yrs | 1.14 | 0.94 | 0.76 | 1.17 | 1.07 | 0.51 (−2.12, 3.21) | |
| 40–49 yrs | - | 0.33 | 0.35 | 0.34 | 0.70 | 5.79 (2.02, 9.71) | |
| Western Europe | 0–9 yrs | 1.30 | 2.44 | 1.98 | 2.69 | 2.25 | 1.66 (−1.08, 4.47) |
| 10–19 yrs | 4.78 | 4.35 | 5.17 | 7.08 | 6.93 | 2.49 (1.00, 4.00) | |
| 20–29 yrs | 1.90 | 2.21 | 2.42 | 2.62 | 2.75 | 1.71 (1.30, 2.12) | |
| 30–39 yrs | 0.80 | 0.66 | 0.99 | 1.40 | 1.22 | 2.80 (0.28, 5.39) | |
| 40–49 yrs | - | 0.37 | 0.62 | 0.44 | 0.69 | 4.87 (−0.06, 10.04) | |
| Oceania | 0–9 yrs | 1.56 | 1.90 | 1.35 | 2.52 | 2.38 | 2.41 (−0.39, 5.28) |
| 10–19 yrs | 3.89 | 6.12 | 5.71 | 5.58 | 5.95 | 1.20 (−0.69, 3.12) | |
| 20–29 yrs | 1.56 | 2.44 | 3.17 | 3.08 | 2.40 | 1.57 (−1.58, 4.82) | |
| 30–39 yrs | 1.03 | 0.64 | 0.95 | 0.86 | 0.98 | 0.41 (−2.14, 3.02) | |
| 40–49 yrs | - | 0.59 | 0.36 | 0.67 | 0.75 | 3.21 (−0.22, 6.77) | |
Rates were not calculated for strata with less than 5 cases are denoted by a dash (−).
Estimates in bold are statistically significant.
AAPCs were not calculated for categories with less than 5 cases for three or more time periods, as denoted by a dash (−).
Male to Female Incidence Rate Ratios in 10–19-year age category (2003–2012)
We observed a male excess in all geographic regions, except the Caribbean, for both 10–14 and 15–19 age groups (Figure 2). There were no significant incidence rate ratios (IRRs) that showed a female excess. Significantly more males were diagnosed with ES than females in the 10–14 age group in Southern Europe (IRR:1.54), Western Asia (IRR: 1.44), and South-eastern Asia (IRR: 1.54) (Figure 2, Supplementary Table 1). IRR estimates in 10–14-year-olds ranged from 0.64 in the Caribbean to 2.21 in Northern Africa. IRRs were more commonly observed in the 15–19-year-olds with Oceania, Southern Europe, Northern Europe, Eastern Europe, Western Asia, Southern Asia, South-eastern Asia, Eastern Asia, North America, South America, and Northern Africa all having significantly more male diagnoses (Figure 2, Supplementary Table 1). IRRs in 15–19-year-olds ranged from 1.24 in Sub-Saharan Africa to 4.04 in Northern Africa, although both estimates were relatively imprecise.
Incidence Rates by Country and Subpopulations in 10–19-year age category (2003–2012)
Incidence Rates by country during 2003–2012 ranged from 0 to 9.97 cases per million in the 10–19-year age category (Figure 3). The highest rate was observed in New Caledonia. Brunei, Cuba, French Guiana, Martinique, Seychelles, South Africa and Zimbabwe all observed zero cases of ES with population ranges from 5,3818 in the Seychelles to 2,348,661 in South Africa. High incidence rates were seen in countries in Oceania, Southern Europe, Western Europe and Western Asia (Figure 3). Low incidence was observed in countries in Africa, the Caribbean and Eastern Asia.
Incidence Rates by Predominant Ancestry in 10–19-year age category (2003–2012)
We also graphed the incidence rate during 2003–2012 by predominant ancestry (Figure 4), which demonstrates that countries and subpopulations with predominantly African, East Asian, and South-east Asian ancestry had the lowest incidence rates. Pacific Islanders and populations with predominantly European and North African/Middle Eastern ancestry groupings had the highest incidence rates. However, many populations with North African/Middle Eastern ancestry had lower incidence rates as well, so no clear pattern of incidence emerged among North African/Middle Eastern ancestry. South American Admixed and Southern Asian ancestry categories also did not demonstrate a clear pattern of incidence by ancestry. The results of Kruskal-Wallis indicated ancestry was a significant indicator of incidence (p<0.0001). Compared to Predominantly European, Northern African/Middle eastern ancestry did not demonstrate a statistically significant difference (IRR:1.08, 95%CI: 0.84 – 1.39), Pacific Islanders had a greater, although imprecisely estimated, incidence (IRR: 2.18, 95%CI: 0.99 – 4.81)), South American Admixed, South Asian, Southeast Asian, East Asian, and Predominantly African had a statistically significant lower incidence with IRRs of 0.66, 0.58, 0.33, 0.30, 0.16, respectively (Supplementary Table 2).
Discussion
In this study, we used CI5 data to compare ES incidence rates over time and across populations. Overall, we observed trends in ES incidence that differed by age group and geographic region, with most of the statistically significant AAPCs showing an increased incidence over time. Additionally, a male excess of ES appeared to be consistent across most regions but substantial variation in incidence was observed across populations. Predominately African, East Asian, and Southeast Asian populations had the lowest rates of ES based on both regional groupings and individual registry data. Pacific islanders appeared to have the highest incidence rates, albeit this conclusion is based on three registries with imprecise estimates. A nearly 15-fold difference in rates was observed between highest and lowest incidence registries with non-zero rates; a 5-fold difference in rates was observed within predominantly European populations.
It is unclear whether the predominantly increasing trends in ES incidence are real or reflect changes in diagnostic criteria or improvements in diagnostic technology. In the CI5, many unspecified bone tumors or sarcomas are grouped together so it is unclear how diagnostic substitution may influence our results. We evaluated the number of cases, incidence rates and trends over time of unspecified bone tumors (Supplementary Table 3). We did not observe corresponding decreases in unspecified tumors over time for many of our significant increases. Many of the case counts were also too low to significantly affect our estimates without being comprised entirely of ES tumors. Prior studies have reported ES incidence rates to have increased, decreased, or remain unchanged over time, depending on the geographic region studied.9,26–28 We also considered the possibility that our findings may be due to changing demographic factors. Increasing parental age,29 low gestational age,30 and high birth weight31 have all been associated with increased ES risk in children. However, it remains unclear whether these factors contribute to incidence trends that we observed in older age groups, and few other extrinsic factors have been associated with this disease.9, 29–31 More recent analyses indicate that germline genetic susceptibility contributes substantially to ES development and may be a stronger risk factor than locally varying extrinsic factors.32–34 However, changes to the frequency of genetic risk variants within a population are unlikely to have occurred during a 25-year timespan. Therefore, the possibility that at least some of the trends we observed in ES incidence are a consequence of changing case detection and recognition over time merits consideration.
In more recent years of available data (2003 – 2012), we observed ES to occur less frequently in African and East Asian populations as compared to predominately European populations. Our findings closely align with the racial differences in ES incidence observed in the U.S. and elsewhere.7–8,35–38 We note that some estimates in our study are remarkably precise due to very large populations (e.g. China, Uganda); even rates near 0 are informative in instances of large denominators because they indicate with confidence that ES is nearly absent in these populations. Some countries in our study have been multiethnic for some time. However, many are monoethnic or nearly so (e.g. East Asian countries), which lends credence to the possibility that disparities in ES incidence are driven predominantly by differences in genomic ancestry rather than lifestyle factors. This finding is further supported by results from diaspora populations in our study. For example, populations with predominately sub-Saharan African ancestry had similarly low incidence rates both in Africa (i.e. Kenya and Uganda) and outside the continent (e.g. U.S. Blacks). Similar incidence rates were also observed in India and among Indian populations in Malaysia and Singapore.
Molecular data suggests that differences in risk may be influenced by genomic ancestry. The ES transcriptional program involves interactions between somatically acquired EWSR1-FLI1 fusion proteins and inherited germline variation,6,32,33 of which the frequency of germline variants could differ based on ancestral origin.5,39 Comparisons in somatic genetics of ES between ancestries with disparate rates are few, but one comparison between tumors in Japanese and European patients found frequent losses of 19p and 19q in Japanese patients, suggesting the possibility that germline variation that leads to differing rates of occurrence may also lead to differing patterns of alterations.40 Interestingly, the frequency of known ES susceptibility alleles are not consistently higher in the population with the highest risk of disease (i.e. Europeans; illustrated in Figure 5).32,33,41 Coupled with limited extrinsic risk factors for ES, this unexpected lack of increased risk allele frequencies in high risk populations indicates that yet-to-be discovered, ancestry-specific risk alleles may exist and should be the focus of future discovery.
Previous studies also report an excess risk of ES among males compared to females.42 We observed the similar excess sex risk to be more pronounced in the 15–19-year-old age group compared to the 10–14-year-old age group. The cause of sex differences in ES is poorly understood. Males tend to have higher birth weights compared to females and this has been posited as one explanation. However the association with birth weight on ES is not consistent between studies and a recent study showed that birth weight does not mediate sex differences for most childhood cancers.31,43 Skeletal growth differences between males and females, particularly in pubertal stages, have also been suggested as an explanation and may explain why the excess risk is highest in the age group following male adolescent puberty.44 Yet, the few studies to evaluate this hypothesis found no relationship between growth and development factors in early adolescence and risk of ES.31 Previous studies have described a cancer registration sex disparity in low and middle income countries, particularly in the CI5.45,46 While most of our sex ratios are similar in effect size, the differences in the magnitude of male excess risk between Northern Africa and other geographic regions may be due to under registration of girls in cancer registries within the region.
The strengths of our study include the large database of high-quality registry data from international sources spanning over two decades. However, we note that the data are still susceptible to coding inaccuracies that may have resulted in misclassified ES cases and this misclassification may differ across registry.47 Further, restriction to the morphological categories available within the CI5 and the inability to assess the burden of unspecified tumors relevant to ES is an important limitation. Additionally, small case counts and population estimates precluded us from including all registries in the analyses and in some instances resulted in imprecise estimates. Further, relatively few registries in multiethnic countries report rates by race or ethnicity, thus we relied on estimates of the “predominant” ancestry which were necessarily imprecise categorizations. The predominant ancestry of some populations also was unclear, including “South American Admixed,” which originates from large-scale interbreeding between previously isolated and genomically diverse populations.
Our study confirms in an international database spanning the most recent 25 years that ES incidence rates vary substantially across populations. We also observed rising rates of ES that were not consistent across geographic regions or age groups. Known environmental or non-ancestral genetic risk factors are unlikely to account for the substantial differences in ES incidence that we observed. Therefore, future analyses are needed to determine the extent to which genomic ancestry influences ES development.
Supplementary Material
Novelty and Impact:
Racial and ethnic disparities in Ewing sarcoma (ES) incidence are observed in the United States and elsewhere, but international incidence rates have not been systematically evaluated recently. We used data from the Cancer Incidence in Five Continents database to describe trends in ES incidence across time, geographic regions, and race/ethnicity. The findings highlight a need for future research on the association of genomic ancestry with ES risk.
Acknowledgments
Funding
Research reported in this publication was supported by NIH grant P30CA077598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1-TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was also supported by grants from the National Institutes of Health (T32 CA099936 to AKH and T32 AR050938 to BD) ) as well as the Intramural Research Program of the National Cancer Institute.
Abbreviations:
- AAPC
average annual percent change
- ASR
age-standardized incidence rates
- CI
confidence interval
- CI5
Cancer Incidence in Five Continents
- ES
Ewing sarcoma
Footnotes
Conflict of Interest
The authors have no conflict of interest relevant to this article to disclose.
Ethics statement
This study used publicly-available, group-level data which did not require ethical approval.
Data Availability Statement
The Cancer Incidence in Five Continents data is publicly available at: https://ci5.iarc.fr/Default.aspx and the CIA factbook is publicly available at: https://www.cia.gov/the-world-factbook. Processed datasets used in the analysis are available from the corresponding author upon reasonable request.
References
- 1.Grünewald TGP,, Cidre-Aranaz F, Surdex D, Tomazou EM, de Alava E, Kovar H, Sorensen PH, Delattre O, Dirksen U.Ewing sarcoma. Nat Rev Dis Primers 4, 5 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Campbell K, Shulman D, Janeway KA, DuBois SG. Comparison of Epidemiology, Clinical Features, and Outcomes of Patients with Reported Ewing Sarcoma and PNET over 40 Years Justifies Current WHO Classification and Treatment Approaches. Sarcoma. 2018;2018:1712964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aurias A, Rimbaut C, Buffe D, Zucker JM, Mazabraud A. Translocation involving chromosome 22 in Ewing’s sarcoma. A cytogenetic study of four fresh tumors. Cancer Genet Cytogenet. 1984;12(1):21–25. [DOI] [PubMed] [Google Scholar]
- 4.May WA, Gishizky ML, Lessnick SL, Lunsford LB, Lewis BC, Delattre O, Zucman J, Thomas G, Denny CT. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci U S A. 1993;90(12):5752–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck R, Monument MJ, Watkins WS, Smith R, Boucher KM, Schiffman JD, Jorde LB, Randall RL, Lessnick SL. EWS/FLI-responsive GGAA microsatellites exhibit polymorphic differences between European and African populations. Cancer Genet. 2012;205(6):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grünewald TGP, Bernard V, Gilardi-Hebenstreit P, Raynal V, Surdex D, Aynaud M-M, Mirabeau O, Cidre-Aranaz F, Tirode F, Zaidi S, Perot G, Jonker AH, Lucchesi C, Le Deley MC, Oberlin O, Marec-Berard P, Veron AS, Reyanud S, Lapouble E, Boeva V, Frio TR, Alonso J, Bhatia S, Pierron G, Cancel-Tassin G, Cussenot O, Cox DG, Morton LM, Machiela MJ, Chanock SJ, Charnay P, Delattre O. Chimeric EWSR1-FLI1 regulates the Ewing sarcoma susceptibility gene EGR2 via a GGAA microsatellite. Nat Genet. 2015;47(9):1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worch J, Matthay KK, Neuhaus J, Goldsby R, Dubois SG. Ethnic and racial differences in patients with Ewing sarcoma. Cancer. 2010;116(4):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jawad MU, Cheung MC, Min ES, Schneiderbauer MM, Koniaris LG, Scully SP. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome. Cancer. 2009;115(15):3526–3536. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM, Stiller CA, Nectoux J. International variations in the incidence of childhood bone tumours. Int J Cancer. 1993;53(3):371–376. [DOI] [PubMed] [Google Scholar]
- 10.Valery PC, Laversanne M, Bray F. Bone cancer incidence by morphological subtype: a global assessment. Cancer Causes & Control. 2015;26:1127–1139. [DOI] [PubMed] [Google Scholar]
- 11.Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J, editors. Cancer Incidence in Five Continents, Vol. VII. IARC Scientific Publications, 1997; No. 143, Lyon, IARC. [Google Scholar]
- 12.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer Incidence in Five Continents, Vol VIII. IARC Scientific Publications, 2002; No. 155, Lyon, IARC. [Google Scholar]
- 13.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P, editors. Cancer Incidence in Five Continents, Vol. IX. IARC Scientific Publications, 2007; No. 160, Lyon, IARC. [Google Scholar]
- 14.Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R and Ferlay J editors, Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Ferlay J editors. Cancer Incidence in Five Continents, Vol. X. IARC Scientific Publications, 2014; No. 164, Lyon, IARC. [Google Scholar]
- 15.Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J, editors. Cancer Incidence in Five Continents, Vol. XI. IARC Scientific Publications, 2017; No. 166, Lyon, IARC. [Google Scholar]
- 16.Parkin DM, Ferlay J, Curado M-P, Bray F, Edwards B, Shin HR, Forman D. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. 2010;127(12):2918–2927. [DOI] [PubMed] [Google Scholar]
- 17.Parkin DM, Shanmugaratnam K, Sobin L, Ferlay J, Whelan SL. Chapter 4. Histological groups. In: Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J, editors. Cancer Incidence in Five Continents, Vol VIII. IARC Scientific Publications, 1997; No. 143:34–44. Lyon, IARC. [Google Scholar]
- 18.United Nations Statistics Division. UNSD — Methodology. Series M, No. 49. Accessed February 25, 2018. https://unstats.un.org/unsd/methodology/m49/
- 19.Hubbard AK, Spector LG, Fortuna G, Marcotte EL, Poynter JN. Trends in International Incidence of Pediatric Cancers in Children Under 5 Years of Age: 1988–2012. JNCI Cancer Spectr. 2019;3(1):pkz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubbard AK, Poynter JN. Global incidence comparisons and trends in ovarian germ cell tumors by geographic region in girls, adolescents and young women: 1988–2012. Gynecol Oncol. 2019;154(3):608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams LA, Hubbard AK, Scheurer ME, Spector LG, Poynter JN. Trends in paediatric central nervous system tumour incidence by global region from 1988 to 2012. Int J Epidemiol. 2020: dyaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad OB, Boschi-Pinto C, Lopez Christopher AD, Murray JL, Lozano R, Inoue M. Age Standardization of Rates: A new WHO Standard. 2001;No 31. [Google Scholar]
- 23.The World Factbook - Central Intelligence Agency. Accessed January 26, 2020. https://www.cia.gov/library/publications/the-world-factbook/
- 24.Kruskal WH, Wallis WA. Use of Ranks in One-Criterion Variance Analysis. J Am Stat Assoc. 1952;47(260):583–621. [Google Scholar]
- 25.Dunn OJ. Multiple Comparisons Using Rank Sums. Technometrics. 1964;6(3):241–252. [Google Scholar]
- 26.Chakraborty D, Rangamani S, Kulothungan V, Chaturvedi M, Stephen S, Das P, Sudarshan KL, Surya RJ, Kumar KS, John A, Manoharan N, Koyande SS, Swaminathan R, Ramesh C, Shrivastava A, Ganesh B, Mathur P, Nandakumar A. Trends in incidence of Ewing sarcoma of bone in India – Evidence from the National Cancer Registry Programme (1982–2011). J Bone Oncol. 2018;12:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esiashvili N, Goodman M, Marcus RB. Changes in Incidence and Survival of Ewing Sarcoma Patients Over the Past 3 Decades: Surveillance Epidemiology and End Results Data. J Pediatr Hematol Oncol. 2008;30(6):425–430. [DOI] [PubMed] [Google Scholar]
- 28.Eyre R, Feltbower RG, Mubwandarikwa E, Jenkinson HC, Parkes S, Birch JM, Eden TOB, James PW, McKinney PA, Pearce MS, McNally RJQ. Incidence and survival of childhood bone cancer in northern England and the West Midlands, 1981–2002. Br J Cancer. 2009;100(1):188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson KJ, Carozza SE, Chow EJ, Fox EE, Horel S, McLaughlin CC, Mueller BA, Puumala SE, Reynolds P, Von Behren J, Spector LG. Parental age and risk of childhood cancer: A pooled analysis. Epidemiol Camb Mass. 2009;20(4):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spector LG, Puumala SE, Carozza SE, Chow EJ, Fox EE, Horel S, Johnson KJ, McLaughlin PR, Von Behren J, Mueller BA. Cancer Risk Among Children With Very Low Birth Weights. Pediatrics. 2009;124(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burningham Z, Hashibe M, Spector L, Schiffman JD. The Epidemiology of Sarcoma. Clin Sarcoma Res. 2012;2(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machiela MJ, Grünewald TGP, Surdez D, Reynaud S, Mirabeau O, Karlins E, Rubio RA, Zaidi S, Grossetete-Lalami S, Ballet S, Lapouble E, Laurence V, Michon J, Pierron G, Kovar H, Gaspar N, Kontny U, Gonzalez-Neira A, Picci P, Alonso J, Patino-Garcia A, Corradini N, Berard PM, Freedman ND, Rothman N, Dagnall CL, Burdett L, Jones K, Manning M, Wyatt K, Zhou W, Yeager M, Cox DG, Hoover RN, Khan J, Armstrong GT, Leisenring WM, Bhatia S, Robison LL, Kulozik AE, Kriebel J, Meitinger T, Metzler M, Hartmann W, Strauch K, Kirchner T, Kirksen U, Morton LM, Mirabella L, Tucker MA, Tirode F, Chanock SJ, Delattre O. Genome-wide association study identifies multiple new loci associated with Ewing sarcoma susceptibility. Nat Commun. 2018;9(1):3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Postel-Vinay S, Véron AS, Tirode F, Pierron G, Reynaud S, Kovar H, Oberlin O, Lapouble E, Ballet S, Lucchesi C, Dontny U, Gonzalez-Neira A, Picci P, Alonso J, Patino-Garcia A, de Paillerets BB, Laud K, Dina C, Froguel P, Clavel-Chapelon F, Dox F, Michon J, Chanock SJ, Thomas G, Cox DG, Delattre O. Common variants near TARDBP and EGR2 are associated with susceptibility to Ewing sarcoma. Nat Genet. 2012;44(3):323–327. [DOI] [PubMed] [Google Scholar]
- 34.Lin S-H, Sampson JN, Grünewald TGP, Surdez D, Reynaud S, Mirabeau O, Karlins E, Rubio RA, Zaidi S, Grossetete-Lalami S, Ballet S, Lapouble E, Laurence V, Michon J, Pierron G, Kovar H, Kontny U, Gonzalez-Neira A, Alonso J, Patino-Garcia A, Corradini N, Berard PM, Miller J, Freedman ND, Rothman N, Carter BD, Dagnall CL, Burdett L, Jones K, Manning M, Wyatt K, Zhou W, Yeager M, Cox DG, Hoover RN, Khan J, Armstrong GT, Leisenring WM, Bhatia S, Robison LL, Kulozik AE, Kriebel J, Meitinger T, Metzler M, Krumbholz M, Hartmann W, Strauch K, Kirchner T, Dirksen U, Mirabello L, Tuker MA, Tirode F, Morton LM, Chanock SJ, Delattre O, Machiela MJ. Low-frequency variation near common germline susceptibility loci are associated with risk of Ewing sarcoma. PLOS ONE. 2020;15(9):e0237792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worch J, Cyrus J, Goldsby R, Matthay KK, Neuhaus J, DuBois SG. Racial differences in the incidence of mesenchymal tumors associated with EWSR1 translocation. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2011;20(3):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linden G, Dunn JE. Ewing’s sarcoma in Negroes. Lancet Lond Engl. 1970;1(7657):1171. [PubMed] [Google Scholar]
- 37.Jensen RD, Drake RM. Rarity of Ewing’s tumour in Negroes. Lancet Lond Engl. 1970;1(7650):777. doi: 10.1016/s0140-6736(70)91002-0 [DOI] [PubMed] [Google Scholar]
- 38.Li FP, Tu JT, Liu FS, Shiang EL. Rarity of Ewing’s sarcoma in China. Lancet Lond Engl. 1980;1(8180):1255. doi: 10.1016/s0140-6736(80)91719-5 [DOI] [PubMed] [Google Scholar]
- 39.Riggi N, Knoechel B, Gillespie SM, Rheinbay E, Boulay G, Suva ML, Rossetti NE, Boonseng WE, Oksuz O, Cook EB, Formey A, Patel A, Gymrek M, Thapar V, Deshpande V, Ting DT, Hornicek FJ, Nielsen GP, Stamenkovic I, Aryee MJ, Bernstein BE, Rivera MN. EWS-FLI1 utilizes divergent chromatin remodeling mechanisms to directly activate or repress enhancer elements in Ewing sarcoma. Cancer Cell. 2014;26(5):668–681. doi: 10.1016/j.ccell.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozaki T, Schaefer KL, Wai D, Yokoyama R, Ahrens S, Diallo R, Hasegawa T, Shimoda T, Hirohashi S, Kawai A, Naito N, Morimoto Y, Inoue H, Boecker W, Juergens H, Winkelmann W, Dockhorn-Dworniczak B, Poremba C. Population-based genetic alterations in Ewing’s tumors from Japanese and European Caucasian patients. Ann Oncol. 2002;13(10:1656–64. [DOI] [PubMed] [Google Scholar]
- 41.Alexander TA, Machiela MJ. LDpop: an interactive online tool to calculate and visualize geographic LD patterns. BMC Bioinformatics. 2020;21(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaatsch P Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–285. [DOI] [PubMed] [Google Scholar]
- 43.Williams LA, Richardson M, Kehm RD, McLaughlin CC, Mueller BA, Chow EJ, Spector LG. The association between sex and most childhood cancers is not mediated by birthweight. Cancer Epidemiol. 2018;57:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva I dos S, Swerdlow AJ. Sex Differences in the Risks of Hormone-dependent Cancers. Am J Epidemiol. 1993;138(1):10–28. [DOI] [PubMed] [Google Scholar]
- 45.Bhopal SS, Mann KD, Pearce MS. Registration of cancer in girls remains lower than expected in countries with low/middle incomes and low female education rates. Br J Cancer. 2012;107(1):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce MS and Parker L. Childhood cancer registrations in the developing world: still more boys than girls. Int. J. Cancer. 2001;91:402–406. [DOI] [PubMed] [Google Scholar]
- 47.Lyu HG, Stein LA, Saadat LV, Phicil SN, Haider A, Raut CP; Dana-Farber/Brigham and Women’s Cancer Center Sarcoma Surgery Group. Assessment of the Accuracy of Disease Coding Among Patients Diagnosed With Sarcoma. JAMA Oncol. 2018;4(9):1293–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Cancer Incidence in Five Continents data is publicly available at: https://ci5.iarc.fr/Default.aspx and the CIA factbook is publicly available at: https://www.cia.gov/the-world-factbook. Processed datasets used in the analysis are available from the corresponding author upon reasonable request.