Abstract
Background:
Lymph node metastasis (LNM) drastically reduces survival after resection of intrahepatic cholangiocarcinoma (IHC). Optimal treatment is ill-defined, and it is unclear if tumor mutational profiling can support treatment decisions.
Methods:
Patients with liver-limited IHC with or without LNM treated with resection (N=237), hepatic arterial infusion chemotherapy (HAIC) (N=196), or systemic chemotherapy alone (SYS) (N=140) at our institution between 2000–2018 were included. Genomic sequencing was analyzed to determine if genetic alterations could stratify outcomes for patients with LNM.
Results:
For node-negative patients, resection was associated with the longest median overall survival (OS) (59.9 months, 95% CI: 47.2–74.31), followed by HAIC (24.9 months, 95% CI: 20.3–29.6), and SYS (13.7 months, 95% CI: 8.9–15.9) (P <0.001). There was no difference in survival for node-positive patients treated with resection (median OS 19.7 months, 95% CI: 12.1–27.2) or HAIC (18.1 months, 95% CI: 14.1–26.6) (P=0.560), however, survival in both groups was greater than SYS (11.2 months, 95% CI: 14.1–26.6) (P=0.024). Node-positive patients with at least one high-risk genetic alteration (TP53 mutation, KRAS mutation, CDKN2A/B deletion) had worse survival compared to wild-type patients, (median OS 12.1 months, 95% CI: 5.7–21.5, P=0.002), regardless of treatment. Conversely, there was no difference in survival for node-positive patients with IDH1/2 mutations compared to wild-type patients.
Conclusions and Relevance:
There was no difference in OS for patients with node-positive IHC treated by resection vs. HAIC, and both treatments had better survival than SYS alone. The presence of high-risk genetic alterations provides valuable prognostic information that may help guide treatment.
Introduction
Intrahepatic cholangiocarcinoma (IHC) has seen a rising incidence over the last three decades1. Although resection provides the best potential for a long-term cure, disease-specific factors, such as multifocality (satellite nodules or intrahepatic metastases), tumor size, lymphovascular invasion, tumor burden, and lymph node metastases (LNM) are associated with worse survival2–8. In particular, LNM at the time of resection is associated with a median survival of 15–18 months, less than half that of patients with node-negative disease3,4. Similar to other gastrointestinal malignancies, induction chemotherapy for initially unresectable IHC may allow for resection in a subset of appropriately selected patients; however, data on this approach are limited, and its role in clinical management remains unclear9–11. Moreover, systemic chemotherapy (SYS) alone has limited efficacy, with an overall survival (OS) of 15–19 -months for patients with liver-limited IHC in some series12,13. Although tumor mutational profiling has led to the use of targeted therapies for patients with IDH1 mutations (mut) and FGFR2 fusions (fus), the role and impact of these agents remains to be determined, and a survival benefit over SYS has not yet been demonstrated14–16.
Hepatic arterial infusion chemotherapy (HAIC) with floxuridine (FUDR) is an attractive locoregional treatment for IHC. A recent single arm phase 2 clinical trial at our institution demonstrated both the safety and efficacy of this modality in combination with systemic gemcitabine and oxaliplatin for unresectable disease17. In this trial, median OS was 25.0 months, and 1-year survival was 89.5%. Of particular interest, there was no difference in 2-year survival between node-negative and node-positive patients (node negative 60% (95% C.I. 40-%–91% vs. node positive, 50% (95% C.I., 30%–83%), P=0.66).
Based on these data, the objective of this study was to compare survival outcomes in node-positive IHC patients treated with HAIC vs. resection. We hypothesized that there would be no difference in survival between the two cohorts. As a secondary objective, we analyzed targeted next-generation sequencing (NGS) data to determine if specific somatic genetic alterations could better stratify outcomes in node-positive patients and help guide treatment selection.
Materials and Methods
Cohort Selection
This was a retrospective analysis of a prospectively maintained database of patients with IHC from a single institution and Institutional Review Board approval was obtained prior to data collection. For all patients enrolled in research, investigations were conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained. For this retrospective research protocol (#16–698), informed consent was waived. We identified patients with biopsy-proven, liver-limited IHC with or without regional LNM, diagnosed and treated between 2000–2018 with either curative-intent resection, HAIC (with SYS), or SYS alone (See Supplementary Figure 1 for CONSORT diagram). Our operative approach and outcomes in patients with resectable disease have been previously described1,18. HAIC is delivered via hepatic arterial infusion pump (HAIP), and the technical details and outcomes from this therapy have also been previously described17,19–21. For the majority of patients undergoing both resection and HAIP placement, assessment of locoregional lymph nodes was performed either as a formal portocaval/hepatoduodenal and peripancreatic lymphadenectomy (stations 12 and 13 according to the Japanese Society of Hepato-Biliary-Pancreatic Surgery classification22) or as a targeted excision-based lymphadenectomy. For patients without formal nodal basin sampling, no lymphadenectomy was performed based on the absence of suspicious imaging findings and/or intraoperative assessment. At Memorial Sloan Kettering Cancer Center (MSKCC), all patients are evaluated with contrast-enhanced, multi-phase computed tomography (CT) scans, often supplemented with contrast-enhanced magnetic resonance imaging (MRI) and/or positron emission tomography (PET) when clinically indicated. Additionally, all patients under consideration for HAIP placement undergo CT liver angiography. Patients who received HAIC and subsequently underwent resection due to favorable tumor response, as well as patients who never initiated HAIC after pump placement were analyzed within the HAIC cohort on an intention-to-treat basis. Lymphadenectomy of the regional nodal basin was performed and nodal staging was determined at the time of initial HAIP placement in patients who subsequently underwent resection after receiving liver-directed chemotherapy.
Definitions
For patients submitted to resection or HAIC, N1 disease was defined as ≥ 1 lymph node with metastatic disease in the pathological specimen, N0 was defined as no evidence of LNM, and NX was assigned to those individuals who did not have any lymph nodes recovered or sampled and were unable to be formally staged. Initial survival analysis demonstrated no statistically significant difference in survival between N0 and NX patients and they were combined into one cohort for remaining analyses and referred to collectively as N0 (see Supplementary Figure 2). In order to contextualize the findings, we also identified patients with a similar burden of disease treated with systemic chemotherapy alone (SYS). This cohort was stratified by the presence or absence of suspicious locoregional lymphadenopathy based on the aforementioned stations, as determined by cross-sectional imaging23. Multifocal disease was defined as the presence of intrahepatic metastases or satellite nodules, either radiographically, intraoperatively, or on final pathology. Tumor size was defined as the largest diameter of the tumor in the pathological specimen for resected patients, or the largest diameter seen on imaging for patients who were treated with HAIC or SYS alone.
Genomic Analysis
We performed a secondary analysis to determine how known driver gene alterations impact the survival of patients with N1 disease. Tumor and matched normal tissue from patients were profiled to identify somatic genomic alterations with MSK-IMPACT™ (Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets) (NCT01775072)24–26. Sequencing was performed predominantly on tissue from primary tumors (N=195, 91.5%); however, tissue from local recurrence (N=6, 2.8%), extrahepatic recurrence (N=4, 1.9%), and LNM (N=14, 6.6%) were also analyzed. In brief, MSK-IMPACT™ is a hybridization capture-based next-generation sequencing assay designed to sequence all exons and selected introns of oncogenes, tumor-suppressor genes, and other potentially actionable genes based on current therapies; the number of genes sequenced in the assay has increased from 341 to 468 since the initial publication of this method26. This assay reliably detects single nucleotide variations, insertion-deletion mutations (indels), copy number alterations, and fusions. We examined somatic mutations that were considered oncogenic drivers, based on OncoKB27. Genomic data from patients with N1 disease treated with HAIC and resection were analyzed together, given the similar survival observed. Of the subset of patients with genomic data available (N = 219), 64 (29.2%) were previously analyzed as part of a separate study28.
Statistical Analysis
Continuous variables were described with median and range and compared using Wilcoxon rank sum test. Categorical variables were described with count and percentage and compared using Fischer’s exact test. Overall survival (OS) was defined as time from initial treatment initiation (resection, HAIP placement, or initiation of SYS) to the date of death or censored at the last follow-up. OS was analyzed using Kaplan Meier methods, and the log-rank test was used to assess differences between treatment groups. Cox proportional hazards models were used to test the association of demographic, clinicopathologic, and genomic features, as well as the interaction between lymph node status and treatment on mortality. Because genomic testing was performed on an increasing number of genes over time, data for some genes were not available for all patients. Only genes that were altered in ≥5% of patients were included in genomic statistical analyses. Tissue derived from primary and non-primary tissue were analyzed together based on previous literature demonstrating no significant difference in alterations between primary and metastatic sites for IHC28. A P value of <0.05 was considered statistically significant. Data were analyzed using SAS Version 9.4 (SAS institute, Cary, North Carolina), and R 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
During the study period, 573 patients with IHC were eligible for inclusion (Table 1). Two hundred and thirty-seven patients (41.4%) underwent curative intent resection, 196 patients (34.2%) received HAIC, and 140 patients (24.4%) received SYS alone. Seventy-three of 237 resected patients (30.8%) received adjuvant chemotherapy. Of the patients treated with SYS alone, 89/140 (63.6%) received gemcitabine plus oxaliplatin or cisplatin and 21/140 (15%) received other gemcitabine-based regimens. In the HAIC cohort, 14/196 patients (7.2%) later underwent a curative-intent hepatic resection, 194/196 (99.0%) received at least one cycle of liver-directed chemotherapy, and 72.4% (142/196) received SYS in addition to HAIC FUDR. In this latter group, 58/142 patients (40.8%) received gemcitabine plus oxaliplatin, 41/142 (28.9%) received irinotecan, and 27/142 (19.0%) gemcitabine alone. Among patients who received HAIC or underwent resection and later progressed or recurred in the liver, there was no difference in the proportion who received additional locoregional therapies (23.1% HAIC vs. 35.5% resection, P = 0.311).
Table 1.
Demographic and Pathological Features of Patients Undergoing Resection or Receiving HAIC or SYS.
| Variable | Resection (N=237) |
HAIC (N=196) |
SYS (N=140) |
P Value |
|---|---|---|---|---|
| Age, years (median, range) | 67.6 (19.0–88.6) | 62.0 (30.1–85.7) | 66.1 (32.2–92.3) | <0.001* |
| Female Gender, N (%) | 105 (44.3) | 80 (40.8) | 67 (47.9) | 0.434 |
| Race, N (%) | 0.122 | |||
| White | 202 (87.1) | 172 (90.5) | 111 (82.8) | |
| Non-white | 30 (12.9) | 18 (9.5) | 23 (17.2) | |
| Missing | 5 | 6 | 6 | |
| Multifocal liver disease, N (%) | 53 (22.4) | 145 (74.0) | 105 (75.0) | <0.001* |
| Tumor size, cm, (median, range) | 5.6 (1.3–24.0) | 8.6 (1.0–19.5) | 8.0 (2.7–19.6) | <0.001* |
| Grade | 0.003* | |||
| Well differentiated | 2 (0.9) | 5 (2.9) | 3 (2.6) | |
| Moderately differentiated | 167 (72.9) | 97 (55.4) | 70 (60.3) | |
| Poorly differentiated | 60 (26.2) | 73 (41.7) | 43 (37.1) | |
| Missing | 8 | 21 | 24 | |
| Lymphadenectomy, N (%) | 136 (57.4) | 137 (69.9) | N/A | 0.009* |
| Treatment Era, N (%) | <0.001* | |||
| 1999–2009a | 102 (43.0) | 74 (37.8) | 22 (15.7) | |
| 2010–2019 | 135 (57.0) | 122 (62.2) | 118 (84.3) | |
| Lymph Nodes Resected | 0.401 | |||
| Median (interquartile range) | 2 (1–4) | 2 (1–4) | ||
| Mean (range) | 3.30 (1–16) | 3.02 (0–26) | ||
| Nodal status, N (%) | 0.002* | |||
| N1 | 38 (16.0) | 56 (28.6) | ||
| N0 | 98 (41.4) | 81 (41.3) | ||
| NX | 101 (42.6) | 59 (30.1) | ||
| Locoregional Lymphadenopathy, N (%) | 45 (19.1) | 48 (24.5) | 57 (40.7) | <0.001* |
P<0.05
First HAIP was placed in 2001
HAIC = hepatic arterial infusion chemotherapy, HAIP = hepatic arterial infusion pump, SYS = systemic chemotherapy
Patients who received HAIC were younger than both resected patients and patients receiving SYS alone [HAIC 62.0 years (range 30.1–85.7) vs. SYS 66.1 years (range 32.2–92.3) and resected 67.6 years (range 19.0–88.6), P<0.001] (Table 1). Compared to patients submitted to resection, HAIC and SYS patients were more likely to have multifocal liver disease (HAIC 74.0% and SYS 75.0% vs. resected 22.4%; P<0.001), larger median tumor size [HAIC 8.6 cm (range 1.0–19.5) and SYS 8.0 cm (range 2.7–19.6) vs. resected, 5.6 cm (range 1.3–24.0); P<0.001], and poorly differentiated tumors (HAIC 41.7% and SYS 37.1% vs. resected, 26.2% P = 0.003). Among resected patients, 136/237 (57.4%) underwent a lymphadenectomy, compared to 137/196 (69.9%) of those who underwent HAIP placement (P=0.009). The median number of lymph nodes harvested was 2 for both cohorts (IQR 1–4 for resection, 1–4 for HAIP placement), whereas the mean was 3.3 for resection and 3.01 for HAIC, however both ranged widely (1–16 for resection and 1–26 for HAIP) (P=0.401). HAIC patients were also more likely than resected patients to have N1 disease (28.6% HAIP vs. 16.0% resected, P=0.002). Of resected patients with N1 disease, 29/38 (76.3%) received adjuvant chemotherapy.
After combining N0 and NX patients, the new N0 cohort was compared to N1 patients, and there was no difference in age, gender, race, or tumor size. However, N1 patients were more likely than N0 patients to have multifocal disease (70.2% vs. 38.9%, P <0.001) and poorly differentiated tumors (51.1% vs. 27.7%, P <0.001) (Supplementary Table 1). Importantly, these findings were not different than the three-way comparison of the original N0 vs. NX vs. N1 cohorts.
N0 patients treated with resection had the longest survival median OS (59.9 months, 95% CI: 47.2–74.31), followed by HAIC (24.9 months, 95% CI: 20.3–29.6) (Figure 1a). Patients with liver-limited IHC without suspicious locoregional adenopathy on imaging treated with SYS alone (83/140 without adenopathy, 59.3%) had the shortest survival in this comparison, (median OS 13.7 months, 95% CI: 8.7–19.4; P<0.001). In contrast, for patients with N1 disease, there was no difference in survival, regardless of whether they were treated with curative-intent resection (median OS 19.7 months, 95% CI: 12.1–27.2) or HAIC (18.1 months, 95% C.I. 14.1–26.6) (P=0.560) (Figure 1b); however, median OS in patients with liver-limited IHC and locoregional adenopathy treated with SYS (N = 57/140, 40.7%) was the shortest (11.2 months, 95% CI: 8.9–15.9) (P=0.024). We performed a supplementary analysis looking at survival stratified by suspicious adenopathy on imaging alone and found a nearly identical survival for the HAIC cohort, however an improved survival in resected patients (Supplementary Figure 3).
Figure 1.
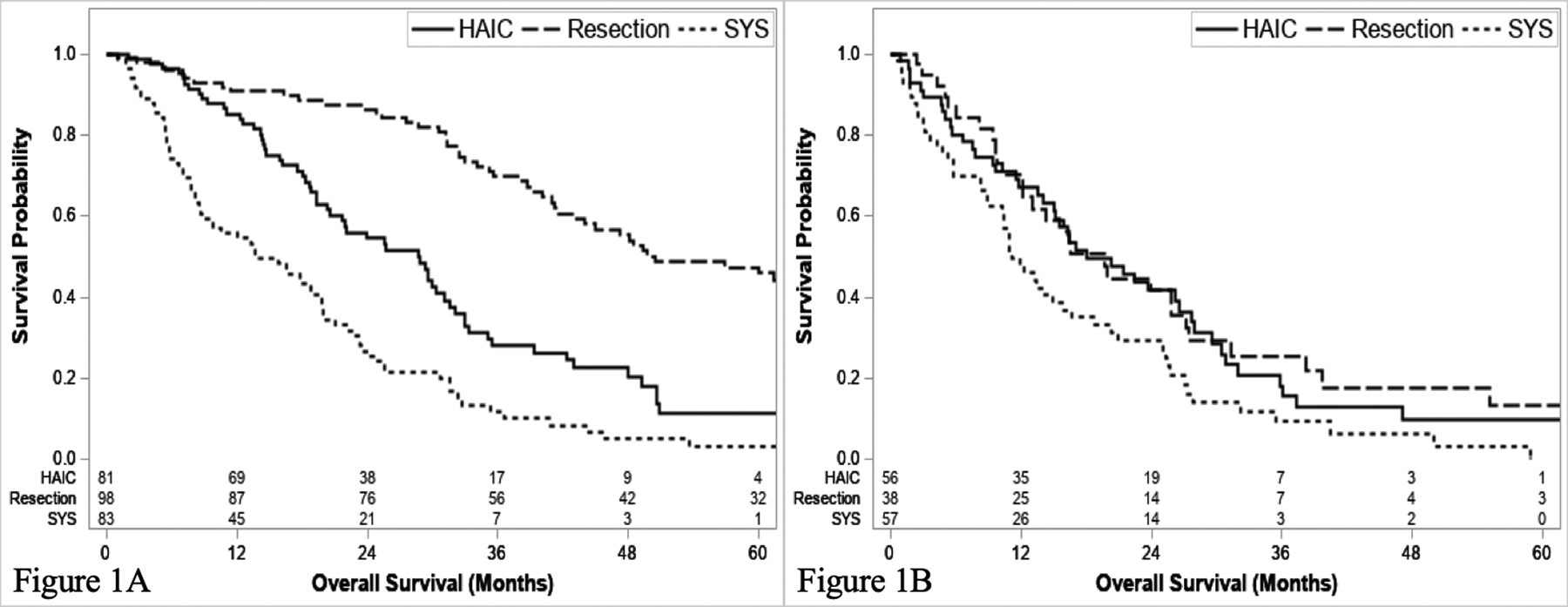
Overall survival of N0 (a) and N1 patients (b) stratified by treatment.
In a univariable Cox proportional hazards model including patients treated with resection and HAIC, ≥ 1 LNM (hazard ratio [HR] 2.4, 95% CI: 1.8–3.2; P<0.001), multifocal liver disease (HR 2.3, 95% CI: 1.9–3.0; P<0.001), and tumor size (HR 1.1, 95% CI: 1.06–1.12) were all associated with worse survival (Table 2). There was a differential impact of HAIC on the N0 and N1 cohorts, as assessed by a statistically significant interaction effect (P = 0.009). Analogous to our survival models, the HAIC cohort was associated with worse survival compared to resection, only in the context of N0 disease (HR 2.9, 95% CI:.2.2–3.8); whereas, there was no association for patients with N1 disease (HR 1.2, 95% CI: 0.7–1.9). On multivariable analysis, multifocal liver disease (HR 1.6, 95% CI: 1.2–2.1; P <0.001), tumor size (HR 1.05, 95% CI: 1.01–1.1, P=0.009), and the interaction between treatment and lymph node status was independently associated with worse overall survival (P <0.001). As expected, treatment with HAIC compared to resection remained associated with worse survival for patients with N0 disease (HR 2.1, 95% C.I. 1.6–2.8), but not those with N1 disease (HR 0.8, 95% C.I. 0.5–1.3). Thus, in patients with N1 disease, treatment (resection vs. HAIC) did not impact survival, even after accounting for multiple demographic and disease-specific variables.
Table 2.
Cox proportional hazards and Wald test for univariable and multivariable survival models.
| Variable | Univariable Model | Multivariable Model | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% C.I. | P-Value | Hazard Ratio | 95% C.I. | P-Value | |
| Age | 1.00 | 1.0–1.0 | >0.95 | |||
| Female Sex (Ref. male) |
0.91 | 0.72–1.14 | 0.407 | |||
| Nonwhite race (Ref. white) |
0.95 | 0.7–1.4 | 0.792 | |||
| Well differentiated (Ref. poorly differentiated) |
0.64 | 0.23–1.73 | 0.374 | |||
| Moderately differentiated (Ref. poorly differentiated) |
0.71 | 0.55–0.90 | 0.005* | |||
| Tumor size | 1.09 | 1.06–1.12 | <0.001* | 1.05 | 1.01–1.08 | 0.009* |
| ≥ 1 lymph node with metastasis | 2.41 | 1.8–3.2 | <0.001* | |||
| Multifocal liver disease | 2.35 | 1.9–3.0 | <0.001* | 1.61 | 1.2–2.1 | <0.001* |
| Interaction Terms | Univariable | Multivariable | ||||
| HAIC when N0 (Ref. resection) |
2.88 | 2.2–3.8 | <0.001* | 2.09 | 1.6–2.8 | <0.001* |
| HAIC when N1 (Ref. resection) |
1.17 | 0.7–1.9 | 0.81 | 0.5–1.3 | ||
P<0.05
HAIC = hepatic arterial infusion chemotherapy, SYS = systemic chemotherapy
Genomic Analysis
Of the 433 patients treated with either HAIC or resection, 219 (50.6%) had genomic profiling data available for analysis (N=139 resected, N=80 HAIC) (MSK-IMPACT 341 gene panel [N=26], 410 gene panel [N=93], and 468 gene panel [N=100]). Fifty-two of these patients (23.7%) had LNM at the time of locoregional basin sampling (N=31 HAIC and N=20 resected). Eleven individual genes had somatic alterations in ≥ 5% of patients, with IDH1 (18.7% prevalence), ARID1A (15.5%), FGFR2 (15.5%), TP53 (14.6%), and BAP1 (13.2%) as the five most prevalent (Supplementary Table 2).
We created a univariable Cox proportional hazards model for patients with N1 disease, based previously described mutational profiles and somatic alterations, which included IDH1/2mutations (IDH1/2mut), FGFR2 fusions (FGFR2fus), CDKN2A/B deletions (CDKN2A/Bdel), and a high-risk cohort which was comprised of any TP53 mutation, KRAS mutation, or CDKN2A/B deletion17,29–33. Mutations in IDH1/2 had no impact on survival (IDH1/2mut HR 0.73, 95% CI: 0.3–1.6; P=0.429) nor did FGFR2fus (HR 0.64, 95% CI: 0.15–2.7; P = 0.545). However, CDKN2Adel (HR 3.3, 95% CI 1.1–9.5, P = 0.030), CDKN2Bdel (HR 6.0, 95% CI 2.5–14.6, P <0.001), and combined CDKN2A/Bdel (HR 5.0, 95% CI 2.1–11.6, P <0.001) were all associated with worse survival.
The median OS for N1 IDH1/2mut patients was not significantly different compared to IDH1/2wt patients (27.7 [95% CI 5.0–36.0] vs. 18.1 months [95% CI 12.1–30.9], P = 0.427) (Figure 2). Among all patients with IDH1/2mut, 10/49 patients (20.4%) received treatment with an IDH-inhibitor (either ivosidenib or vorasidenib) as part of a clinical trial (8 HAIC, 2 resection). Similarly, there was no difference in survival for N1 FGFR2fus (N=4) compared to FGFR2wt patients (N=48) (median OS 31.4 months [95% CI 5.7–31.4] vs. 21.5 months [95% CI 13.1–27.7]; P=0.542).
Figure 2.
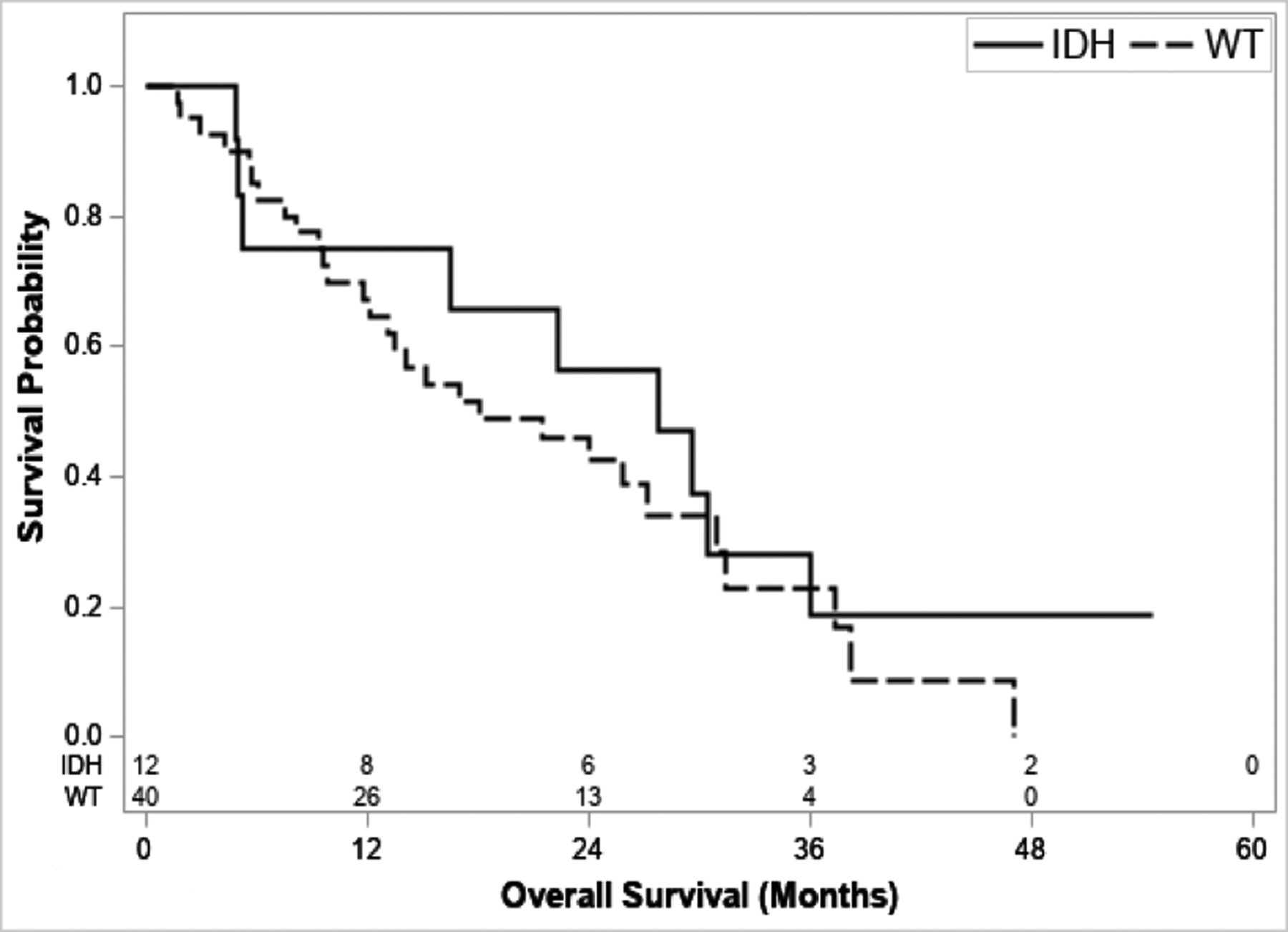
Survival stratified by IDH1/2mut status for N1 patients
Patients with any high-risk alteration and N1 disease had a poor outcome, with a median OS of 12.1 months (95% CI: 5.7–21.5) which was significantly worse compared to their wild-type counterparts (median OS 30.9 months [95% CI 18.1–47.0]) (P = 0.002) (Figure 3a). Similar findings were demonstrated for N1 patients with CDKN2Adel (median OS 9.4 months, 95% CI: 1.7–22.4) compared to CDKN2Awt (median OS 23.0, 95% CI 13.5–30.4) (P = 0.0219) (Supplementary Figure 4a), CDKN2Bdel (6.1 months [95% CI: 1.7–9.] vs.25.8 months [95% CI 15.2–30.9], P <0.001) (Supplementary Figure 4b), and combined CDKN2A/Bdel (7.1 months [95% CI 1.7–17.0] vs. 27.2 [95% CI 15.2–30.9], P <0.001) (Figure 3b).
Figure 3.
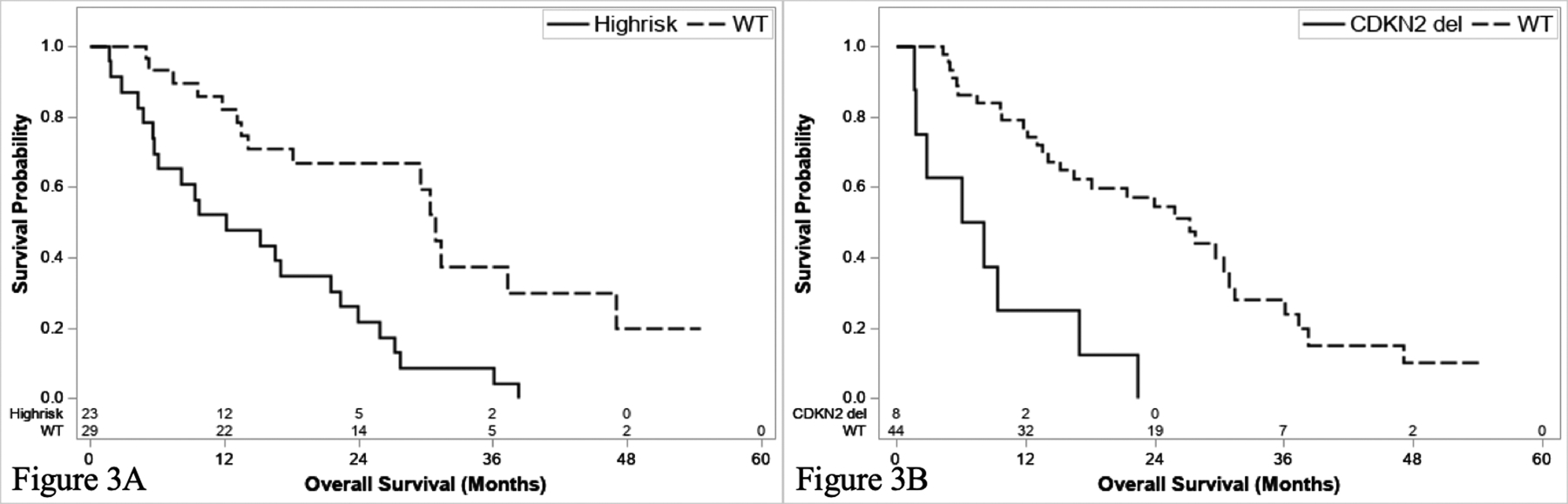
Survival stratified by high risk alteration (KRASmut, TP53mut, CDKN2A/Bdel) (a) and CDKN2A/Bdel for N1 patients.
Discussion
For patients with lymph node-positive IHC (N1), HAIC and resection were associated with similar survival in this retrospective series (18.1 months vs. 19.7 months, respectively). The results are even more striking given the greater disease burden in the HAIC cohort. Our results suggest that HAIC is a promising alternative treatment modality in the setting of LNM. Additionally, these data reaffirm the adverse impact of LNM on survival after curative-intent resection; patients with N1 disease had a median OS that was less than half that of patients without LNM (N0) (19.7 months vs. 50.5 months). Of note, while survival of N1 patients was similar in resected and HAIC-treated patients, survival in both subgroups was better than that seen in patients treated with SYS alone. Lastly, in hypothesis-generating subgroup comparisons based on genomic analysis, we found that both IDH1/2mut and FGFR2fus had little effect on prognosis in the presence of LNM. Notably, patients with N0 disease and IDH1mut had overlapping confidence intervals with node-positive patients, despite a 9-month survival difference. Conversely, our data suggest that patients with node-positive disease and high-risk alterations, such as KRASmut, TP53mut, and CDKN2A/Bdel may have a drastically worse prognosis compared to wild-type patients, with a median survival ≤1-year, regardless of treatment. These data demonstrate the importance of understanding the tumor mutational profile prior to making treatment decisions in patients with N1 disease, as the presence of high-risk alterations are associated with a uniformly poor prognosis.
Multiple reports have corroborated the adverse association of LNM on post-resection survival, including a multi-institutional retrospective series of 449 patients by de Jong et al., which reported a median OS of 22.9 months in resected patients with N1 disease7, similar to the findings reported after both resection and HAIC in our study. Martin et al. queried the National Cancer Database (NCDB) and identified patients with clinically node-positive IHC treated with either resection, SYS alone, or a combination of resection and SYS, and determined that resection and SYS is the optimal treatment strategy, with a median OS of 22.5 months vs. 11.9 months for SYS alone and 12.4 months for resection alone34. Furthermore, the phase 2 clinical trial investigating the efficacy of HAIC FUDR and systemic gemcitabine and oxaliplatin in unresectable IHC from our institution found no difference in survival between patients with node-negative and node-positive disease17. Taken together, these data suggest that control of liver disease is an important determinant of OS, even in the presence of LNM. Along with our findings, these data further suggest that HAIC may provide adequate hepatic disease control compared to resection. Major hepatectomy carries a morbidity rate that approaches 50% and includes both infectious complications and post-hepatectomy liver failure35,36. Moreover, the 90-day mortality may approach 5%37. HAIP placement can be performed with less morbidity and mortality than major liver resection, with a minimally invasive approach in some patients38. The utility of liver-directed chemotherapy for patients with LNM merits further prospective study39.
Lowery et al. profiled 152 IHCs and found a similar pattern of mutation prevalence as demonstrated herein, as well as an association between CDKN2A/B alterations and both reduced survival time and faster time to progression in the setting of advanced disease28. Although this report did not find any association between IDH1mut and survival, there was a tendency toward mutual exclusivity for IDH1:TP53 and IDH1:KRAS mutations found: both TP53 and KRAS were associated with worse survival in our series. IDH1/2 mutations have been associated with improved disease-free survival and OS previously, although the mechanism underlying this favorable prognosis is unkown29. Given the high prevalence of IDH mutations in IHC, targeted anti-IDH therapy is being tested in clinical trials40.
Conversely, in our series, loss of function in the CDKN2A/B genes in the context of N1 disease conveyed an extremely poor prognosis despite resection or HAIC, with a median survival of 7 months. The INK4b-ARF-INK4α locus spans 35kb on region 9 of chromosome p21 and contains the genes CDKN2A and CDKN2B, which encode two cyclin dependent kinase inhibitors p16INK4a and p15INK4b, which are thought to serve as tumor suppressors41,42. Loss of p16INK4a and p15INK4b through CDKN2A/B mutation, specifically promoter hypermethylation or deletion, is postulated to lead to carcinogenesis in multiple tumor types32. Thus, routine use of genomic sequencing in IHC may help guide decision-making prior to treatment initiation, while also unveiling new areas for targeted therapy.
Routine vs. selective lymphadenectomy at the time of resection of intrahepatic cholangiocarcinoma (IHC) remains controversial43. A multi-institutional study by Bagante et al., demonstrated intermediate survival for patients who did not have formal sampling of the regional nodal basin, with short term outcomes similar to N1 patients, and long-term outcomes comparable to N0 patients44. This variability likely reflects the heterogeneity in this cohort of patients, as routine lymphadenectomy has not been increasingly adopted over time45. Moreover, an analysis of the NCDB by Brauer et al. found no association between a discrete number of lymph nodes retrieved and survival46. In our study, there was no difference in OS between N0 and NX patients (39.2 vs. 37.1 months, respectively), suggesting that contemporary imaging combined with careful intraoperative assessment by experienced hepatopancreatobiliary surgeons can reliably predict lymph node status. This approach likely results in few, if any, misclassified patients and supports the use of selective lymphadenectomy.
This study has several limitations, including the retrospective nature of the analysis and heterogeneity in the data in regards to the proportion of patients receiving adjuvant therapy and the rate of routine lymph node sampling6,45,47. While analysis of the SYS cohort required using imaging findings as a proxy for N1 disease, our supplemental analysis stratified by radiographic lymphadenopathy suggests that imaging alone (without intraoperative assessment) likely over-estimates pathologic nodal metastasis. Survival in the HAIC cohort was identical, and while speculative, may reflect the impact of multifocal disease and tumor-size, or the use of more sensitivite imaging (hepatic angiography) in these patients. It is also likely that a small number of N0 patients were included in the SYS cohort which may over-estimate their survival. Given this, the conclusions in this report are not altered. The heterogeneity in adjuvant therapy (30.8% of all resected patients, 76.3% of N1 patients) is likely reflective of temporal trends due to the retrospective nature of the data. Both the PRODIGE 12-ACCORD 18 and BILCAP trials were published in 2019, and many patients did not receive adjuvant therapy prior the publication of these results6,47. Moreover, these trials were negative for their primary endpoints; while most patients nationally are now receiving adjuvant therapy, the role of adjuvant therapy is still not well understood.
Additionally, these data were not controlled or randomized and there were inherent differences in disease burden between patients who underwent resection or received HAIC patients receiving HAIC had a comparatively greater disease burden. Additionally, patients who were treated with either resection or HAIC were combined into one cohort for genomic analyses which limits the ability to parse the effect of mutational status by individual treatment choice, but would have been limited by small numbers in our series. In terms of the SYS cohort, a high proportion of these patients did not receive gemcitabine plus a platinum-based chemotherapy as their first-line regimen, which is associated with improved survival in the metastatic setting and may have contributed to their poor survival compared to resected and HAIC-treated patients12. Lastly, patients in this cohort were determined to have suspicious lymphadenopathy based on imaging, which has known limitations in predicting the presence of pathologic LNM, and likely provides an over-estimate48,49.
In this retrospective analysis, patients with node-positive IHC treated with HAIC had a survival outcome that was not different from that of patients who underwent resection. HAIC is an attractive method of locoregional disease control for patients with N1 disease and merits further prospective study. Our data also suggest profiling for high-risk genetic alterations, such as CDKN2A/B deletions, and TP53 or KRAS mutations, is a valuable adjunct in guiding decision-making for the treatment of IHC and may allow for better prognostication in the context of LNM. Further studies are warranted to evaluate the optimal treatment strategy in this specific cohort of patients.
Supplementary Material
Statement of Translational Relevance.
In this manuscript, we demonstrate that hepatic arterial infusion chemotherapy (HAIC) may provide comparable survival to resection for patients with liver-limited intrahepatic cholangiocarcinoma (IHC) and lymph node metastases (LNM). HAIC is an attractive form of liver-directed therapy and can be offered with lower morbidity and mortality risk than liver resection, while also providing adequate disease control for traditionally unresectable patients. Moreover, this manuscript bridges the translational connection between genomics and clinical care by analyzing the next generation sequencing (NGS) data for this cohort of patients, treated with both modalities. Patients with N1 disease and high-risk alterations, which included mutations in KRAS and TP53, and deletions in CDKN2A and CDKN2B, had significantly worse prognosis, regardless of treatment. Conversely, mutations in IDH1 and IDH2 as well as FGFR2 fusion events, had no association with survival. Our results support the routine use of NGS in all patients with IHC, which may provide valuable insights into which patients are unlikely to derive benefit from any form of liver-directed therapy
Acknowledgements:
We gratefully acknowledge Erin Patterson (Memorial Sloan Kettering Cancer Center [MSKCC]) for editorial assistance, as well as the members of the Molecular Diagnostics Service in the Department of Pathology at MSKCC. The authors would also like to thank the following Cycle for Survival Teams for fundraising support: Here Comes the Sun, Notorious HPB, Team Dany Testa, and The Jersey Girls.
Financial Support:
This work was supported in part by the Marie-Josée and Henry R. Kravis Center for Molecular Oncology, the National Cancer Institute (NCI) Cancer Center Core Grant P30-CA008748, and NCI U01-CA238444 to W. Jarnagin. J. Jolissaint receives support from the Weill Cornell Medical College (WCMC) Clinical and Translational Science Center (CTSC) funded by NIH/NCATS UL1TR002384.
Conflict of Interest Statement:
ACW has received travel funding from Intuitive Surgical and honorarium from AstraZeneca. JJH has received research support from Bristol Myers Squibb and consulting fees from Bristol Myers Squibb, Merck, Eli Lilly, Eisai, Exelexis, Imvax, QED and CytomX. NEK has received research funding from Amgen. VPB has received research funding from Bristol Myers Squibb and Genentech. TPK received one-time compensation from Olympus.
Footnotes
Presentations: Data from this manuscript were presented virtually at the American College of Surgeons (ACS) Clinical Congress, October 4, 2020
Data Availability: Sequencing data from the cohorts analyzed in this manuscript are publicly available at cBioPortal (https://www.cbioportal.org/study/summary?id=ihch_mskcc_2020).
References:
- 1.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. doi: 10.1097/SLA.0b013e318176c4d3 [DOI] [PubMed] [Google Scholar]
- 2.Buettner S, ten Cate DWG, Bagante F, et al. Survival after Resection of Multiple Tumor Foci of Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2019;23(11):2239–2246. doi: 10.1007/s11605-019-04184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jutric Z, Johnston WC, Hoen HM, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford). 2016;18(1):79–87. doi: 10.1016/j.hpb.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X-F, Xue F, Dong D-H, et al. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann Surg. 2020;XX(Xx):1. doi: 10.1097/sla.0000000000003788 [DOI] [PubMed] [Google Scholar]
- 5.Zhang X-F, Beal EW, Bagante F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. BJS (British J Surgery). 2018;105(7):848–856. doi: 10.1002/bjs.10676 [DOI] [PubMed] [Google Scholar]
- 6.Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37(8):658–667. doi: 10.1200/JCO.18.00050 [DOI] [PubMed] [Google Scholar]
- 7.De Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: An international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–3145. doi: 10.1200/JCO.2011.35.6519 [DOI] [PubMed] [Google Scholar]
- 8.Tsilimigras DI, Hyer JM, Paredes AZ, et al. Tumor Burden Dictates Prognosis Among Patients Undergoing Resection of Intrahepatic Cholangiocarcinoma: A Tool to Guide Post-Resection Adjuvant Chemotherapy? Ann Surg Oncol. December 2020. doi: 10.1245/s10434-020-09393-7 [DOI] [PubMed] [Google Scholar]
- 9.Fruscione M, Pickens RC, Baker EH, et al. Conversion therapy for intrahepatic cholangiocarcinoma and tumor downsizing to increase resection rates: A systematic review. Curr Probl Cancer. June 2020:100614. doi: 10.1016/j.currproblcancer.2020.100614 [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Shimizu H, Ohtsuka M, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20(1):318–324. doi: 10.1245/s10434-012-2312-8 [DOI] [PubMed] [Google Scholar]
- 11.Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105(7):839–847. doi: 10.1002/bjs.10641 [DOI] [PubMed] [Google Scholar]
- 12.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 13.Lamarca A, Ross P, Wasan HS, et al. Advanced Intrahepatic Cholangiocarcinoma: Post Hoc Analysis of the ABC-01, −02, and −03 Clinical Trials. JNCI J Natl Cancer Inst. 2020;112(2):200–210. doi: 10.1093/jnci/djz071 [DOI] [PubMed] [Google Scholar]
- 14.Bekaii-Saab TS, Valle JW, Van Cutsem E, et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020;16(30):2385–2399. doi: 10.2217/fon-2020-0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;2045(20):1–14. doi: 10.1016/S1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cercek A, Boerner T, Tan BR, et al. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination with Systemic Gemcitabine and Oxaliplatin in Patients with Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2019;10065:1–8. doi: 10.1001/jamaoncol.2019.3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193(4):384–391. doi: 10.1016/s1072-7515(01)01016-x [DOI] [PubMed] [Google Scholar]
- 19.Ammori JB, D’Angelica MI, Fong Y, et al. Hepatic artery infusional chemotherapy in patients with unresectable colorectal liver metastases and extrahepatic disease. J Surg Oncol. 2012;106(8):953–958. doi: 10.1002/jso.23204 [DOI] [PubMed] [Google Scholar]
- 20.Cardona K, Donataccio D, Peter Kingham T, et al. Treatment of extensive metastatic colorectal cancer to the liver with systemic and hepatic arterial infusion chemotherapy and two-stage hepatic resection: The role of salvage therapy for recurrent disease. Ann Surg Oncol. 2014;21(3):815–821. doi: 10.1245/s10434-013-3351-5 [DOI] [PubMed] [Google Scholar]
- 21.Konstantinidis IT, Koerkamp BG, Do RKG, et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122(5):758–765. doi: 10.1002/cncr.29824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki M, Ohtsuka M, Miyakawa S, et al. Classification of biliary tract cancers established by Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3rd english edition. J Hepatobiliary Pancreat Sci. 2015;22(3):181–196. doi: 10.1002/jhbp.211 [DOI] [PubMed] [Google Scholar]
- 23.Seo N, Kim DY, Choi J-Y. Cross-Sectional Imaging of Intrahepatic Cholangiocarcinoma: Development, Growth, Spread, and Prognosis. Am J Roentgenol. 2017;209(2):W64–W75. doi: 10.2214/AJR.16.16923 [DOI] [PubMed] [Google Scholar]
- 24.Cerami E, Gao J, Dogrusoz U, et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012;2(5):401 LP–404. doi: 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017;2017. doi: 10.1200/PO.17.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–4161. doi: 10.1158/1078-0432.CCR-18-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma B, Meng H, Tian Y, et al. Distinct clinical and prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer. 2020;20(1):1–12. doi: 10.1186/s12885-020-06804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan H, Parsons DW, Jin G, et al. Mutations in Gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller PJ, Duraisamy S, Newell JA, et al. Classifying variants of CDKN2A using computational and laboratory studies. Hum Mutat. 2011;32(8):900–911. doi: 10.1002/humu.21504 [DOI] [PubMed] [Google Scholar]
- 32.Zhao R, Choi BY, Lee MH, Bode AM, Dong Z. Implications of Genetic and Epigenetic Alterations of CDKN2A (p16INK4a) in Cancer. EBioMedicine. 2016;8(127):30–39. doi: 10.1016/j.ebiom.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu Q, Hao J, Zhou X, et al. CDKN2B deletion is essential for pancreatic cancer development instead of unmeaningful co-deletion due to juxtaposition to CDKN2A. Oncogene. 2018;37(1):128–138. doi: 10.1038/onc.2017.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin SP, Drake J, Wach MM, et al. Resection and chemotherapy is the optimal treatment approach for patients with clinically node positive intrahepatic cholangiocarcinoma. HPB (Oxford). 2020;22(1):129–135. doi: 10.1016/j.hpb.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin S, Fu Q, Wuyun G, Wuyun T. Management of post-hepatectomy complications. World J Gastroenterol. 2013;19(44):7983–7991. doi: 10.3748/wjg.v19.i44.7983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimick JB, Cowan JA, Knol JA, Upchurch GR. Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg. 2003;138(2):185–191. http://www.ncbi.nlm.nih.gov/pubmed/12578418. Accessed August 8, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann K, Hinz U, Stravodimos C, et al. Risk assessment for liver resection. Surgery. 2018;164(5):998–1005. doi: 10.1016/j.surg.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 38.Qadan M, D’Angelica MI, Kemeny NE, Cercek A, Kingham TP. Robotic hepatic arterial infusion pump placement. HPB (Oxford). 2017;19(5):429–435. doi: 10.1016/j.hpb.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito K, Ito H, Kemeny NE, et al. Biliary Sclerosis after Hepatic Arterial Infusion Pump Chemotherapy for Patients with Colorectal Cancer Liver Metastasis: Incidence, Clinical Features, and Risk Factors. Ann Surg Oncol. 2012;19(5):1609–1617. doi: 10.1245/s10434-011-2102-8 [DOI] [PubMed] [Google Scholar]
- 40.Lowery MA, Burris HA 3rd, Janku F, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. lancet Gastroenterol Hepatol. 2019;4(9):711–720. doi: 10.1016/S2468-1253(19)30189-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popov N, Gil J. Epigenetic regulation of the INK4B-ARF-INK4a locus: In sickness and in health. Epigenetics. 2010;5(8):685–690. doi: 10.4161/epi.5.8.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7(9):667–677. doi: 10.1038/nrm1987 [DOI] [PubMed] [Google Scholar]
- 43.Li D-Y, Zhang H-B, Yang N, Quan Y, Yang G-S. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol. 2013;19(47):9084–9091. doi: 10.3748/wjg.v19.i47.9084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagante F, Gani F, Spolverato G, et al. Intrahepatic Cholangiocarcinoma: Prognosis of Patients Who Did Not Undergo Lymphadenectomy. J Am Coll Surg. 2015;221(6):1031–1040.e4. doi: 10.1016/j.jamcollsurg.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 45.Zhang XF, Chen Q, Kimbrough CW, et al. Lymphadenectomy for Intrahepatic Cholangiocarcinoma: Has Nodal Evaluation Been Increasingly Adopted by Surgeons over Time?A National Database Analysis. J Gastrointest Surg. 2018;22(4):668–675. doi: 10.1007/s11605-017-3652-2 [DOI] [PubMed] [Google Scholar]
- 46.Brauer DG, Fields RC, Tan BRJ, et al. Optimal extent of surgical and pathologic lymph node evaluation for resected intrahepatic cholangiocarcinoma. HPB (Oxford). 2018;20(5):470–476. doi: 10.1016/j.hpb.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi: 10.1016/S1470-2045(18)30915-X [DOI] [PubMed] [Google Scholar]
- 48.Tsilimigras DI, Sahara K, Paredes AZ, et al. Predicting Lymph Node Metastasis in Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. July 2020. doi: 10.1007/s11605-020-04720-5 [DOI] [PubMed] [Google Scholar]
- 49.Bartsch F, Hahn F, Müller L, Baumgart J, Hoppe-lotichius M, Kloeckner R. Relevance of suspicious lymph nodes in preoperative imaging for resectability, recurrence and survival of intrahepatic cholangiocarcinoma. 2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


