Chimeric antigen receptor T-cell (CAR-T) therapy has significantly improved survival for adult patients with relapsed B-cell acute lymphoblastic leukemia (B-ALL). However, there are novel toxicities after CAR-T infusion which can be associated with significant morbidity and mortality. The two primary toxicities are the cytokine release syndrome (CRS) and the immune effector cell-associated neurotoxicity syndrome (ICANS), which are managed with anti-cytokine therapy (e.g. tocilizumab) and corticosteroids per well-established guidelines [1,2]. In rare cases, CRS can evolve into fulminant hemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS), which has been documented in patients with diffuse large B-cell lymphoma (DLBCL) [3] and B-ALL [4] and is associated with increased mortality [5]. At this time, the optimal treatment for patients with CAR-T-associated HLH/MAS is unknown.
We present the following case of a patient with relapsed B-ALL who developed HLH/MAS after treatment with a commercial CAR-T product and was successfully managed with anakinra, interleukin-6 (IL-6) inhibitors, and corticosteroids. Our case highlights several key challenges surrounding the presentation, diagnosis, and management of CAR-T-associated HLH/MAS, and we discuss the biological rationale and a review of the literature on CAR-T-associated HLH/MAS and the role of anti-cytokine therapy.
A 23-year-old woman with relapsed/refractory Philadelphia chromosome-negative B-ALL was admitted to our hospital for planned administration of CAR-T therapy. The patient was diagnosed with B-ALL with normal cytogenetics at age 21 and was initially treated with the CALGB 10403 regimen, but developed peripheral blasts during maintenance therapy. She was subsequently treated with blinatumomab, 6-mercaptopurine, vincristine, methotrexate, and prednisone (POMP) maintenance therapy, augmented hyper-CVAD, and FLAG-IDA, with multiple disease relapses.
On admission, the patient was pancytopenic with 92% peripheral blasts and >90% bone marrow involvement by B-ALL. She was started on dexamethasone and inotuzumab as cytoreductive therapy for 2 doses, and a follow-up bone marrow biopsy demonstrated persistent 50–60% marrow involvement by B-ALL. The decision was made to proceed with the commercial CAR-T therapy product tisagenlecleucel (Kymriah®), preceded by fludarabine and cyclophosphamide lymphodepletion.
At the time of tisagenlecleucel infusion, the patient was afebrile, hemodynamically within normal limits, and had a baseline ferritin of 3200 ng/mL. Our institution uses the American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading for CRS and ICANS [1]. The patient developed Grade 1 CRS on D+1, 14 h after CAR-T infusion, with a fever of 39.7 °C, without any hypotension or hypoxia. She had no evidence of ICANS at that time. Per the CRS management guidelines by Neelapu et al. we initiated empiric antipyretics and antibiotics for neutropenic fever [2].
As shown in Figure 1, she continued to have daily maximum temperatures >39 °C during D+2 and D+3 after CAR-T. Due to >48 h of persistent fevers refractory to acetaminophen, she was administered tocilizumab 8 mg/kg IV once and scheduled dexamethasone 10 mg IV daily on D+3. She continued to have daily fevers up to 40.1 °C on D+4 through D+6, and received tocilizumab 8 mg/kg on D+5 and again on D+6. She did not defervesce despite these 3 doses of tocilizumab and daily IV dexamethasone, but continued to meet criteria only for Grade 1 CRS without any end-organ damage or evidence of ICANS. Of note, her ferritin had increased to 37,437 mg/mL by D+6. Due to her unrelenting fevers and rising ferritin, we administered siltuximab 11 mg/kg IV once and anakinra 100 mg subcutaneously once on D+6, in addition to increasing scheduled dexamethasone to 10 mg IV every 6h. On D+7, the patient continued to be febrile with ferritin increasing to 55,909 mg/mL, so dexamethasone was increased to 20 mg IV every 6 h.
Figure 1.
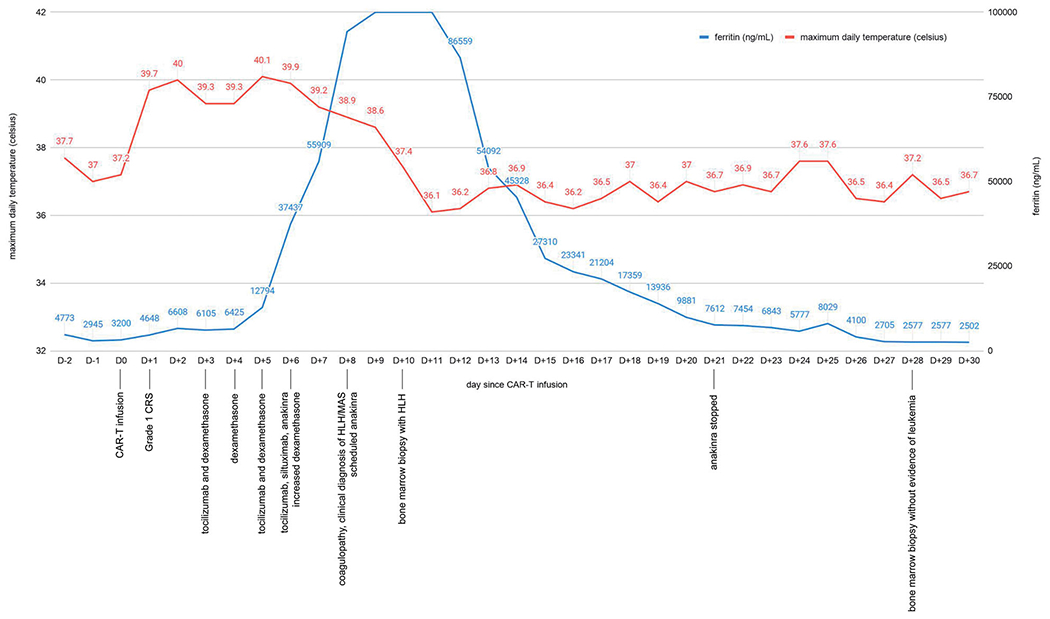
Graph of maximum daily temperature and ferritin before and after CAR-T infusion
Despite escalating doses of dexamethasone and administration of multiple anti-cytokine agents, the patient continued to have fevers on D+8, with a rapidly increasing ferritin to 94,286 ng/mL, and a new coagulopathy with fibrinogen of 48 mg/dL, INR 2.0, and D-dimer >20. Her triglycerides also newly increased to 226 mg/dL. She was diagnosed clinically with fulminant HLH/MAS, switched to methylprednisolone 500 mg IV twice daily, and initiated on scheduled anakinra 100mg daily. Neelapu et al. have published diagnostic criteria for CART-associated HLH/MAS, defined as peak ferritin >10,000 mg/mL with at least two of the following additional toxicities: hepatic transaminases or bilirubin ≥ grade 3, creatinine ≥ grade 3, pulmonary edema ≥ grade 3, and/or evidence of HLH on bone marrow biopsy [2]. Our patient did not develop ≥ grade 3 changes in her hepatic transaminases, bilirubin, or creatinine at any point during her hospital course. Although our patient only fulfilled the first diagnostic criterion of hyperferritinemia, tocilizumab can mask signs and symptoms of HLH/MAS [6], so we initiated high-dose steroids in addition to siltuximab and anakinra.
On D+9, the ferritin rose to >100,000 mg/mL, and on D+10, the patient finally defervesced. Due to persistent hyperferritinemia and coagulopathy, we increased anakinra 100 mg to three times daily on D+10 and performed a bone marrow biopsy to further assess for HLH. On D+12, the patient’s ferritin finally decreased to 86,559 mg/mL, and she remained afebrile and her coagulopathy resolved. Steroids were deescalated from methylprednisolone 500 mg twice daily to dexamethasone 20 mg every 6 h. At that time, results from the bone marrow biopsy from D+10 confirmed the presence of hemophagocytosis (Figure 2(A,B)) with no morphologic evidence for involvement by residual B-ALL. During her immediate post-CAR-T course, she did not have any evidence of infection on cultures or imaging, and was maintained on broad-spectrum antibiotics. Of note, serum cytokine levels are not readily available at our institution for targeted therapy.
Figure 2.

(A) D+10 bone marrow biopsy demonstrating hemophagocytosis. Hematoxylin and eosin staining demonstrating ingested nuclei and some platelet and red cell debris at 100× for a total magnification of 1000. (B) D+10 bone marrow biopsy demonstrating hemophagocytosis. CD68 immunostain highlighting macrophages with ingested debris at 50× with a total magnification of 500.
With no further evidence of CRS or ICANS, and with decreasing ferritin as shown in Figure 1, the patient was gradually tapered off anakinra by D+21 and steroids by D+38. The patient had a prolonged hospital course after CAR-T infusion due recurrent fevers later in her hospital course from candidemia and candida meningitis, which resolved after a prolonged course of antifungal therapy. Her D+28 and D+84 bone marrow biopsies demonstrated a remission with no morphologic or flow cytometric evidence of B-ALL.
HLH/MAS shares many overlapping features with CRS, and the classical criteria for HLH/MAS – fever, splenomegaly, cytopenias in 2 cell lineages, hypertriglyceridemia, hypofibrinogenemia, hyperferritinemia, high levels of soluble CD25, low or absent NK cell activity, or hemophagocytosis – are clearly not specific in the setting of CRS [1,2]. Distinguishing HLH/MAS from severe CRS can be challenging, and the two likely exist on a spectrum of systemic hyperinflammatory disorders induced by T-cell-mediated reticuloendothelial dysfunction. Given the lack of specific biomarkers, we advocate for early bone marrow biopsy in patients with suspected CAR-T-associated HLH/MAS to confirm the diagnosis, as we performed in our patient.
With respect to diagnostic criteria for CAR-T-associated HLH/MAS, some authors have argued that because most patients with at least moderate CRS will meet many criteria for classic HLH/MAS, and that these criteria often resolve with CRS resolution, specific grading for HLH/MAS need not be included in the ASTCT consensus guidelines [1]. Others argue that while the incidence of fulminant HLH/MAS is rare, occurring in approximately 1% of patients receiving CAR-T, it is associated with a high mortality rate if not treated urgently, and therefore specific diagnostic criteria are warranted [2]. We argue that the adoption of diagnostic criteria, as done by Neelapu et al. [2] is critical in identifying patients who may require early HLH-directed therapy to mitigate its high risk of mortality.
No formal guidelines for the management of CAR-T-associated HLH/MAS currently exist. Neelapu et al. recommend anti-IL-6 therapy and steroids, with initiation of etoposide if no improvement within 48 h [2]. However, because etoposide is associated with T-cell killing in HLH [7] and there is no evidence on the use of etoposide in CAR-T-associated HLH/MAS, we elected to defer etoposide administration due to concern for ablating the patient’s CAR-T cells. Although we elected to not pursue cytotoxic chemotherapies, T-cell ablation may be required in patients with hemodynamic instability or organ failure.
Anakinra, a recombinant interleukin-1 (IL-1) receptor antagonist, is an emerging therapeutic agent for CRS and CAR-T-associated HLH/MAS. The biologic rationale for anakinra has been demonstrated in studies implicating macrophage-produced IL-1 in CRS, with IL-1 blockade demonstrating efficacy in mouse models of CAR-T CRS [8,9]. Anakinra has therapeutic benefit in HLH/MAS and has predominantly been used in pediatric patients, with emerging evidence of its efficacy in adult patients [10,11]. One single-center retrospective review of 8 critically-ill adult patients with HLH revealed a 50% survival rate with anakinra in combination with steroids and IVIG, with anakinra dosed at 100–200 mg thrice daily at a range of 4–8 mg/kg/day [12]. Another single-center retrospective review of 19 adult patients with HLH/MAS proposed a diagnostic and therapeutic algorithm that involves empiric anakinra (100 mg IV twice daily), IVIG and steroids with the possible addition of cyclosporine or tocilizumab if patients are not responding; however, none of the patients with leukemia/lymphoma-related HLH survived in the study [13].
Studies on the use of anakinra specifically for CAR-T-associated HLH/MAS have recently been published, with the aim of guiding practice as CAR-T therapy is more widely adopted. A phase I trial of patients with relapsed/refractory CD22+ malignancies, which were predominantly B-ALL, evaluated an anti-CD22 CAR-T product [14]. In the trial of 58 patients, who were predominantly pediatric with a median age of 17.5 years, 19 patients developed HLH/MAS at an average of 14 days after CAR-T infusion. Although HLH/MAS self-resolved in 5 of the patients, the remainder required treatment with anakinra 5–8 mg/kg/day subcutaneously, steroids alone, or both anakinra and steroids due to worsening laboratory parameters or symptoms. All of these patients had resolution of HLH/MAS without any reported impact on CAR-T efficacy. It is important to note that, in this study, CRS was mostly resolved prior to the onset of HLH/MAS, suggesting that HLH/MAS was not temporally associated with severe CRS and may have a unique CAR-T-related pathophysiology. In another study of 100 patients with DLBCL treated with axicabtagene ciloleucel, 8 patients were treated with anakinra, 6 for high-grade ICANS and 2 for HLH, starting at a median of 12 days after CAR-T infusion and at a median dose of 100 mg daily [15]. The two patients with CAR-T-associated HLH did not respond to anakinra and both died, one due to progressive lymphoma and the other due to HLH. The authors concluded that anakinra may be effective for ICANS, with a higher dose or earlier administration required for treatment of HLH/MAS.
Preclinical studies [9] our experience (Figure 1), and a report from another clinical series [16] all suggest that there may be an additive effect of combining anakinra with other anti-inflammatory agents. Because these studies all utilized different CAR-T products in different disease settings, the ideal dosing and dose schedule for anakinra, as well as the sequence of anakinra with other agents to limit overall immunosuppression and total steroid dose, merit further prospective study.
Our illustrative case demonstrates the utility of anakinra in CAR-T-associated HLH/MAS without impacting the efficacy of CAR-T cells, and also illustrates the role of early bone marrow biopsy in patients suspected to have HLH/MAS. Understanding risk factors for progression to fulminant HLH, and developing specific diagnostic criteria for CAR-T-associated HLH/MAS, are critical steps in developing future HLH-targeted treatment algorithms.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
Informed consent
Granted by the patient and her mother.
References
- [1].Lee D, Santomasso B, Locke F, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PubMed] [Google Scholar]
- [2].Neelapu S, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Neelapu S, Locke F, Bartlett N, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ombrello A, Yates B, Shalabi H, et al. Experience with and management of HLH-like toxicities following chimeric antigen receptor T-cell therapy for treatment of relapsed/refractory pre-B ALL [abstract]. Arthritis Rheumatol. 2020. 72 (suppl 4). [cited 2020 Aug 12]. https://acrabstracts.org/abstract/experience-with-and-management-of-hlh-like-toxicities-following-chimeric-antigen-receptor-t-cell-therapy-for-treatment-of-relapsed-refractory-pre-b-all/. [Google Scholar]
- [5].Ahmed S, Furqan F, Strati P, et al. Haemophagocytic lymphohistiocytosis (HLH) in patients with large B-cell lymphoma treated with standard of care (SOC) axicabtagene ciloleucel (Axi-cel). J Clin Oncol. 2020;38(15_suppl):8057–8057. [Google Scholar]
- [6].Shimizu M, Nakagishi Y, Kasai K, et al. Tocilizumab masks the clinical symptoms of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome: the diagnostic significance of interleukin-18 and interleukin-6. Cytokine. 2012;58(2):287–294. [DOI] [PubMed] [Google Scholar]
- [7].Johnson T, Terrell C, Millen S, et al. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192(1):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018; 24(6):739–748. [DOI] [PubMed] [Google Scholar]
- [9].Giavridis T, van der Stegen S, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6): 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Murthy H, Iqbal M, Chavez J, et al. Cytokine release syndrome: current perspectives. Immunotargets Ther. 2019;8: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477. [DOI] [PubMed] [Google Scholar]
- [12].Wohlfarth P, Agis H, Gualdoni G, et al. Interleukin 1 receptor antagonist Anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med. 2019;34(9):723–731. [DOI] [PubMed] [Google Scholar]
- [13].Kumar B, Aleem S, Saleh H, et al. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J Clin Immunol. 2017;37(7):638–643. [DOI] [PubMed] [Google Scholar]
- [14].Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol. 2020;38(17):1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jatiani SS, Aleman A, Madduri D, et al. Myeloma CAR-T CRS management with IL-1R antagonist anakinra. Clin Lymphoma Myeloma Leuk. 2020;20(9):632–636.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]


