Abstract
Objectives:
To describe a cohort of patients presenting with long-term sudden sensorineural hearing loss (SSNHL) treated with prophylactic migraine and intratympanic steroid therapy.
Methods:
Patients presenting to a neurotology clinic at least 6 weeks from SSNHL onset were included. All patients received migraine prophylactic medication (nortriptyline, topiramate, and/or verapamil) and lifestyle changes for at least 6 weeks, as well as intratympanic steroid injections, if appropriate.
Results:
Twenty-one patients (43% female) with a mean age of 64 ± 11 years who presented 9±8 months (median=5) from symptom onset were included. Post-treatment hearing thresholds were significantly improved compared to pre-treatment thresholds at 500 Hz (49±19 dB vs. 55±20 dB, p=0.01), 1000 Hz (52±19 dB vs. 57±21 dB, p=0.03), low-frequency pure tone average (PTA) (53±15 dB vs. 57±17 dB, p=0.01), and speech-frequency PTA (57±13 dB vs. 60±15 dB, p=0.02). Post-treatment word-recognition-score (WRS) and speech-recognition-threshold (SRT) were also significantly improved (45±28% vs. 70±28% and 57±18 dB vs. 50±16 dB, respectively, both p<0.01). Notably, ≥15% improvement in WRS and ≥10 dB improvement in SRT was observed in 13 (68%) and 8 (40%) patients, respectively. Of the 11 patients who presented with initial <50% WRS, 8 (73%) had improved post-treatment >50% WRS with an average improvement of 39±9%.
Conclusions:
Migraine medications in addition to intratympanic steroid injections significantly improved SRT and hearing frequencies in 40% and 29% of SSNHL patients, respectively, while significant WRS recovery was observed in most (68%) patients. This suggests SSNHL may be an otologic migraine phenomenon, which may be at least partially reversible even after the traditional 30-day post-onset window.
Keywords: Sudden sensorineural hearing loss, Migraine, Long-term hearing loss, Chronic hearing loss, Word recognition score, Otologic migraine
Introduction
With an annual incidence of 27 per 100,000 U.S. adults,1 sudden sensorineural hearing loss (SSNHL) can result in functional decline and poor quality of life.2 The U.S. National Institute for Deafness and Communication Disorders (NIDCD) defines SSNHL as a ≥30 dB reduction in ≥3 contiguous audiometric frequencies occurring within 3 days.3 It has been previously shown that up to one- to two-thirds of SSNHL patients can experience spontaneous recovery of hearing loss.4,5 Factors predictive of a worse prognosis may include a higher degree of hearing loss at initial presentation, the absence of steroid therapy, abnormal caloric testing, contralateral hearing impairment, and previous hearing loss or association with disorders of the vestibular system.6–8 Additionally, prompt treatment following the initial onset of SSNHL symptoms is another important prognostic factor.8–10 Although there exists a variety of treatment approaches for SSNHL such as oral and intratympanic (IT) steroids, there is currently no consensus on a standard-of-care or efficacious management.5,11 There also exist a paucity of research on treating patients without spontaneous recovery who neither receive nor respond to early treatment. It has been suggested that treatment needs to occur within 2–4 weeks for efficacy.11,12 As such, most patients who present after this time period are not offered any additional treatment options and continue to suffer from hearing loss. This warrants the investigation of novel treatments for long-term SSNHL for patients who did not seek immediate medical attention or were nonresponsive to initial treatment.
The etiology of SSNHL is yet to be fully understood, but several studies have suggested its association with vascular impairments of the cochlea.13,14 Another complex disorder which is also suggested to be at least partially vascular in nature15 is migraine, and its higher prevalence among SSNHL patients has been demonstrated.16,17 Our group recently showed that SSNHL recovery was improved when oral and IT steroid therapy was supplemented with adjuvant migraine treatment.18 Herein, this retrospective and uncontrolled study reports our institution’s experience with long-term SSNHL patients presenting at least 6 weeks after initial symptomatic onset, managed as an otologic migraine phenomenon with comprehensive migraine treatment.
Methods
With Institutional Review Board approval, a retrospective chart review of patients presenting to our tertiary care neurotology clinic from February 2017 to March 2020 was performed. SSNHL was defined as ≥30 dB reduction in ≥3 contiguous audiometric frequencies occurring within 3 days and diagnosed by the senior author. Initial audiograms for all but three patients were obtained at the authors’ home institution. Long-term SSNHL was defined as presenting with a chief complaint of SSNHL at least 6 weeks after the onset of the hearing loss. This included those who did not seek prompt (<6 weeks) treatment, and those who sought previous treatment but did not experience improvements. Patients who received previous treatments (i.e., steroid therapy) with hearing improvement were excluded. Patients who reported recent direct or sound trauma, or those with a history of Meniere’s disease or fluctuating hearing loss were also excluded. Those already receiving medications for migraine management were excluded.
All included long-term SSNHL patients were offered adjuvant migraine prophylactic medications and lifestyle changes, as well as IT steroid injections (dexamethasone 10 mg/ml placed in the anterior superior quadrant with oral suctioning for 30 minutes) if the patient did not improve to their baseline after reaching the maximum tolerable dosage of the migraine medications. A minimum of two injections were given on a twice-a-week regimen unless the patient refused the second injection. If the patient had improvement in thresholds (≥10 dB in two frequencies) or word recognition score (WRS) after two injections, then more IT injections were given until there was no improvement. Migraine prophylactic medications included nortriptyline, topiramate, and/or verapamil. Dosage was escalated gradually every 1–2 weeks, with the maximum allowed dosage for nortriptyline, topiramate, and verapamil being 75 mg, 150 mg, and 240 mg, respectively. Dosages were titrated based on side effects and vital signs until symptomatic improvement was achieved. Patients were moved up on medication doses to the maximum dosages (nortriptyline 75mg and topiramate 150mg) and seen at the 6-week time point. At six weeks, the patients had been on topiramate 150 mg for one week and on nortriptyline 75 mg for two weeks. Medication adherence was evaluated on each follow-up visit, and migraine medications were tapered off 4–8 weeks after reaching maximum dosage. If there was no improvement and the patient did not want to receive IT steroid injections, the medications were tapered off. If the patient opted to receive IT steroid injections, the medications were tapered after the last IT steroid injection. In addition to medication, as part of a comprehensive migraine prophylactic regimen, patients were also strongly advised on migraine lifestyle modifications. This included dietary recommendations (e.g., avoiding food preservatives, fermented products, alcohol, chocolate, processed meat, etc.), supplementations (magnesium 400 mg orally twice a day and riboflavin 200 mg orally twice a day), and regular sleep and meal schedules.
All included patients had normal magnetic resonance imaging, tympanometry at 226 Hz, and microscopic examination. Chart reviews of all patient visits were performed to evaluate for clinical history, assessments, treatments, and patient-reported hearing quality and medication compliance. Comprehensive audiologic testing for each patient was extracted for analysis, including pure tone average (PTA), speech recognition threshold (SRT), and WRS measured in accordance with the American Academy of Otolaryngology-Head and neck Surgery guidelines.19 Low-frequency PTA included hearing thresholds at 500, 1000, and 2000 Hz, while speech-frequency PTA included hearing thresholds at 500, 1000, 2000, and 4000 Hz. Post-treatment audiometry evaluation was defined as the final visit’s audiometric results and varied among patients. Pre- and post-treatment WRS was not analyzed for 2 (10%) patients due to their language barriers. Pre- and post-treatment SRT was not analyzed for 1 (5%) patient due to lack of pre-treatment SRT availability for that patient. As such, the calculated percentage of patients with WRS and SRT improvements use 19 and 20, respectively, as the denominator values. In addition to analyzing average improvement in the various hearing parameters, we also identified patients who had ≥10 dB improvements in PTA, SRT, and audiometric frequencies, and ≥15% improvement in WRS, to further designate patients with clinically significant hearing improvements according to set thresholds. In the case of bilateral hearing loss, audiometric data for the affected ear was followed. Migraine diagnosis in accordance with the International Classification of Headache Disorders 3rd edition (ICHD-3) beta criteria was assessed for each patient via a comprehensive questionnaire completed at the initial visit. Paired samples t-test was used to compare pre- and post-treatment audiometric data, while chi-square tests were performed to analyze categorical variables. Statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL) and a p value <0.05 was considered significant.
Results
A total of 21 patients (43% female) with a mean age of 64 ± 11 years (range: 36–81, median: 67) were included. On average, patients presented 9 ± 8 months (range: 2–36, median: 5) from the onset of SSNHL. Common past medical histories included migraine headache (n=17, 81%) and previous lifetime history of vertigo (n=15, 71%). Of note, the cohort had not experienced vertigo in the preceding six months, and the only signs of active migraine in the cohort were neck stiffness or aural pressure. In the cohort, 13 (62%) had tinnitus and 12 (57%) had aural fullness. The distribution of baseline audiograms is demonstrated in Figure 1. All patients received migraine medications for at least 6 weeks, while 15 subjects (71%) also received IT steroid injections (usually given towards the end of the treatment period if they did not experience hearing restoration back to near baseline). Individual patient presentations and treatment approaches are outlined in Table 1.
Figure 1.
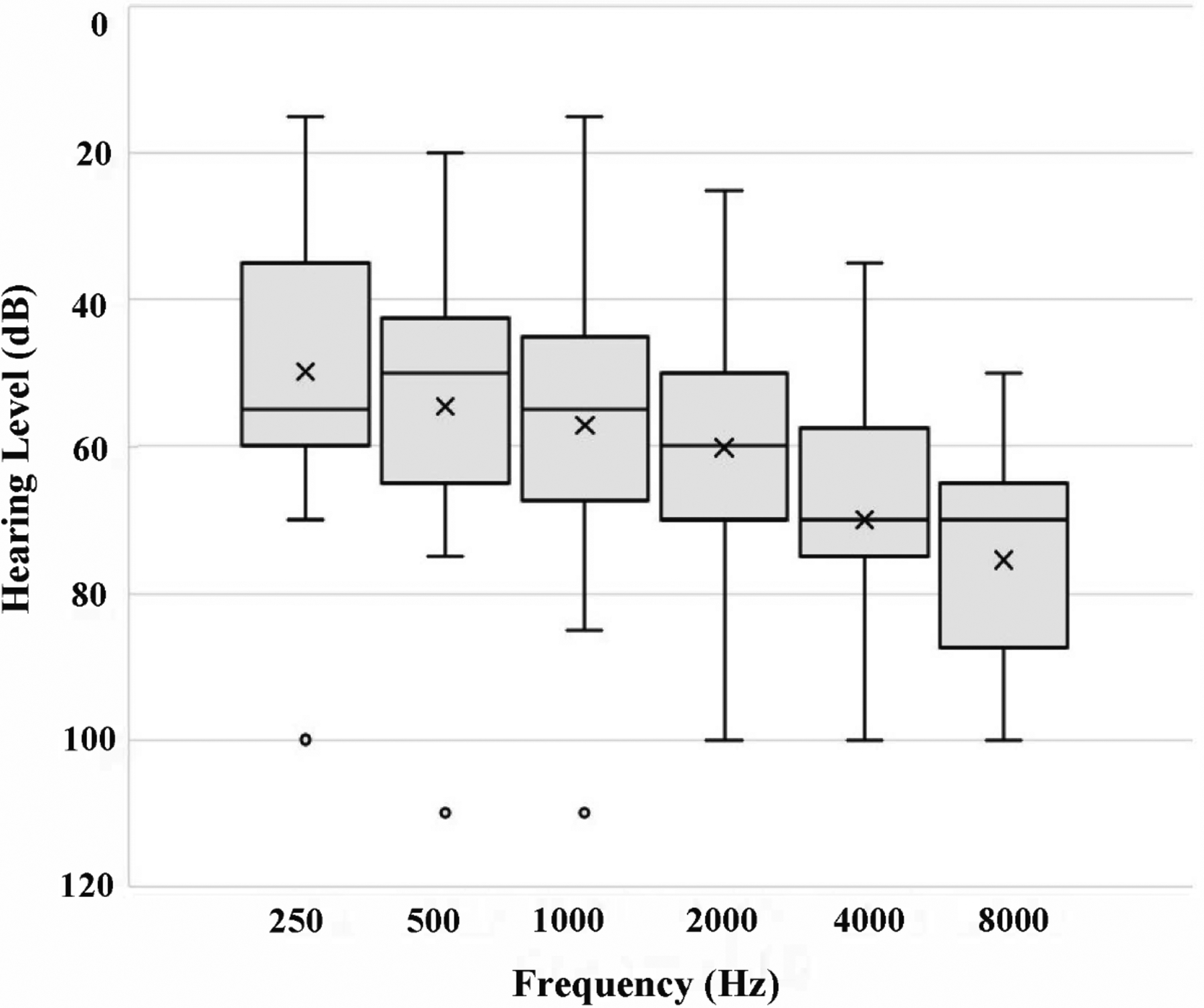
Distribution of baseline audiograms in the cohort. Within each box, the horizontal line and × mark represent the median and mean, respectively. The box sizes correspond to the 25th–75th percentile (1st–3rd quartile), and the whiskers represent the full range, excluding the outliers. Represented by a circle, outliers were defined as data exceeding 1.5 times the interquartile range below the 1st quartile or above the 3rd quartile.
Table 1.
Cohort patients’ presentations and treatment approaches.
| # | Age, Sex | Met migraine criteria | Other symptoms | HL laterality, time from SSNHL to visit | Previous treatment | Treatment (dosage at approx. 6 week) | Improvement in WRS (Δ%) |
|---|---|---|---|---|---|---|---|
| 1 | 74, F | Yes | AF, Tinnitus | R, 11 m | None | Med: N (10), V (120) IT injections: 3 |
44 |
| 2 | 60, M | No | None | L, 2 m | Prednisone + IT | Med: N (60), T (150) IT injections: 4 |
0 |
| 3 | 62, M | Yes | Tinnitus | L, 4 m | None | Med: N (50), T (125) IT injections: 2 |
0 |
| 4 | 56, M | Yes | AF, Tinnitus | R, 6 m | Prednisone | Med: V (180), T (25) IT injections: 0 |
48 |
| 5 | 58, F | Yes | MS, AF, Tinnitus | L, 24 m | None | Med: N (50), T (25) IT injections: 0 |
32 |
| 6 | 72, F | Yes | MS | L, 3 m | Prednisone | Med: N (10), T (100) IT injections: 5 |
28 |
| 7 | 70, M | No | AF | R, 9 m | Prednisone | Med: N (40), V (120), T (150) IT injections: 3 |
32 |
| 8 | 73, F | Yes | Tinnitus | L, 15 m | Prednisone | Med: N (10), T (25) IT injections: 0 |
4 |
| 9 | 67, M | No | AF | R, 5 m | Prednisone | Med: N (20), T (150) IT injections: 3 |
48 |
| 10 | 49, F | Yes | AF, Tinnitus | L, 11 m | Prednisone + IT | Med: N (30), T (150) IT injections: 3 |
52 |
| 11 | 79, M | Yes | AF, Tinnitus | L, 4 m | Prednisone | Med: N (50), V (40) IT injections: 3 |
23 |
| 12 | 50, M | Yes | Tinnitus | R, 4 m | None | Med: N (25), V (180), T (180) IT injections: 3 |
36 |
| 13 | 63, M | Yes | AF, Tinnitus | L, 36 m | None | Med: N (25), V (180), T (150) IT injections: 2 |
- |
| 14 | 36, F | Yes | MS | R, 3 m | Prednisone + IT | Med: N (25), T (25) IT injections: 1 |
32 |
| 15 | 81, F | Yes | None | L, 10 m | None | Med: N (40), V (40) IT injections: 0 |
28 |
| 16 | 71, F | Yes | AF, Tinnitus | R, 2 m | None | Med: N (40), V (120), T (150) IT injections: 2 |
20 |
| 17 | 70, M | Yes | None | R, 5 m | None | Med: N (50), T (75) IT injections: 0 |
0 |
| 18 | 51, M | No | Tinnitus | R, 4 m | None | Med: N (25), T (25) IT injections: 4 |
11 |
| 19 | 72, F | Yes | AF, Tinnitus | R, 3 m | None | Med: N (25), T (50) IT injections: 2 |
- |
| 20 | 58, M | Yes | AF, Tinnitus | R, 7 m | Prednisone + Myringotomy | Med: N (75), V (120), T (150) IT injections: 6 |
40 |
| 21 | 71, M | Yes | AF | L, 18 m | None | Med: N (25), T (25) IT injections: 0 |
8 |
HL: hearing loss; SSNHL: sudden sensorineural hearing loss; M: male; F: female; AF: aural fullness; MS: motion sickness; R: right; L: left; IT: intratympanic steroid; N: nortriptyline; V: verapamil; T: topiramate; WRS: word recognition score; m: months.
Pre- and post-treatment audiometric thresholds are compared in Table 2. Notably, post-treatment hearing thresholds significantly improved compared to pre-treatment values at 500 Hz (49 ± 19 dB vs. 55 ± 20 dB, p=0.01), 1000 Hz (52 ± 19 dB vs. 57 ± 21 dB, p=0.03), low-frequency PTA (53 ± 15 dB vs. 57 ± 17 dB, p=0.01), and speech-frequency PTA (57 ± 13 dB vs. 60 ± 15 dB, p=0.02). Mean WRS was significantly improved from pre-treatment to post-treatment (45 ± 28% vs. 70 ± 28%, p<0.01). Likewise, mean SRT was significantly improved from pre-treatment to post-treatment (57 ± 18 dB vs. 50 ± 16 dB, p=0.01). Schematic diagrams of speech-frequency PTA and WRS hearing results, in accordance with standardized audiologic reporting formats,19 are represented in Figure 2. Pre- to post-treatment audiogram changes among all patients and the sub-cohort with ≥10 dB improvement in at least two audiometric frequencies (n=6, 29%) are demonstrated in Figure 3. The figure shows that, especially in the latter group, post-treatment improvement was more predominant in lower-frequency hearing than high-frequency hearing.
Table 2.
Comparison of pre- and post-treatment audiometry of long-term sudden sensorineural hearing loss patients (n=21). All values are in mean ± standard deviation.
| Tested frequency | Pre-treatment hearing threshold | Post-treatment hearing threshold | p value |
|---|---|---|---|
| 250 Hz (dB) | 50 ± 20 | 46 ± 17 | 0.08 |
| 500 Hz (dB) | 55 ± 20 | 49 ± 19 | 0.01 |
| 1000 Hz (dB) | 57 ± 21 | 52 ± 19 | 0.03 |
| 2000 Hz (dB) | 60 ± 17 | 59 ± 16 | 0.46 |
| 4000 Hz (dB) | 70 ± 17 | 68 ± 16 | 0.34 |
| 8000 Hz (dB) | 76 ± 13 | 72 ± 17 | 0.17 |
| Low-frequency PTA (dB) | 57 ± 17 | 53 ± 15 | 0.01 |
| Speech-frequency PTA (dB) | 60 ± 15 | 57 ± 13 | 0.02 |
| High-frequency PTA (dB) | 73 ± 14 | 70 ± 15 | 0.18 |
| WRS (%) | 45 ± 28 | 70 ± 28 | <0.01 |
| SRT (dB) | 57 ± 18 | 50 ± 16 | 0.01 |
PTA: pure tone average; WRS: word recognition score; SRT: speech recognition threshold.
Low-frequency PTA: averages 500, 1000, and 2000. Speech-frequency PTA: averages 500, 1000, 2000, and 4000. High-frequency PTA: averages 4000 and 8000. WRS and SRT were not available for analysis in 2 and 1 patients, respectively.
Figure 2.
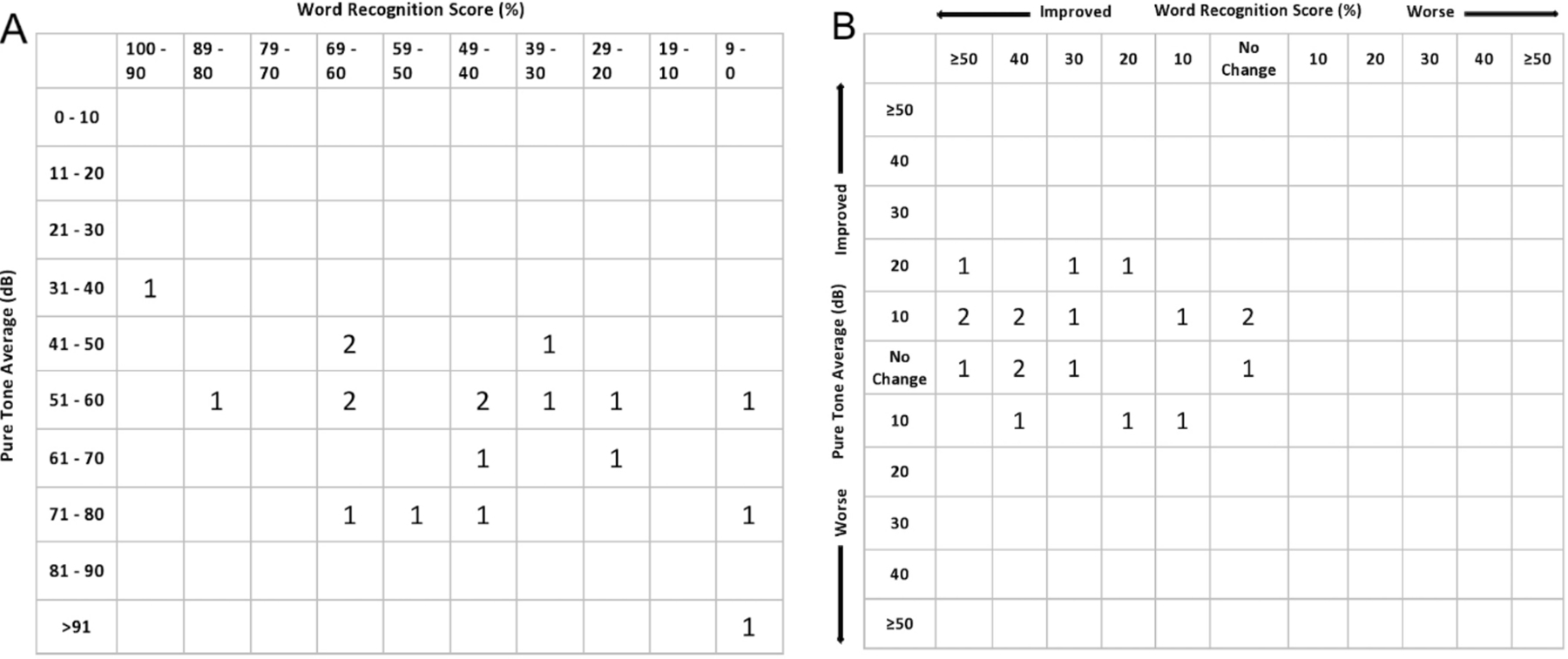
Scattergram of (A) pre-treatment and (B) post-treatment hearing results in 19 patients. Pure tone averages (dB) are labeled on the Y-axis and word recognition scores (%) are labeled on the X-axis. Each number represents the number of patients with audiometric data that can be categorized in each square. The post-operative scattergram shows 11 out of 19 patients with minor PTA improvements of ≤20 dB, and 16 out of 19 patients with varied WRS score improvements.
Figure 3.
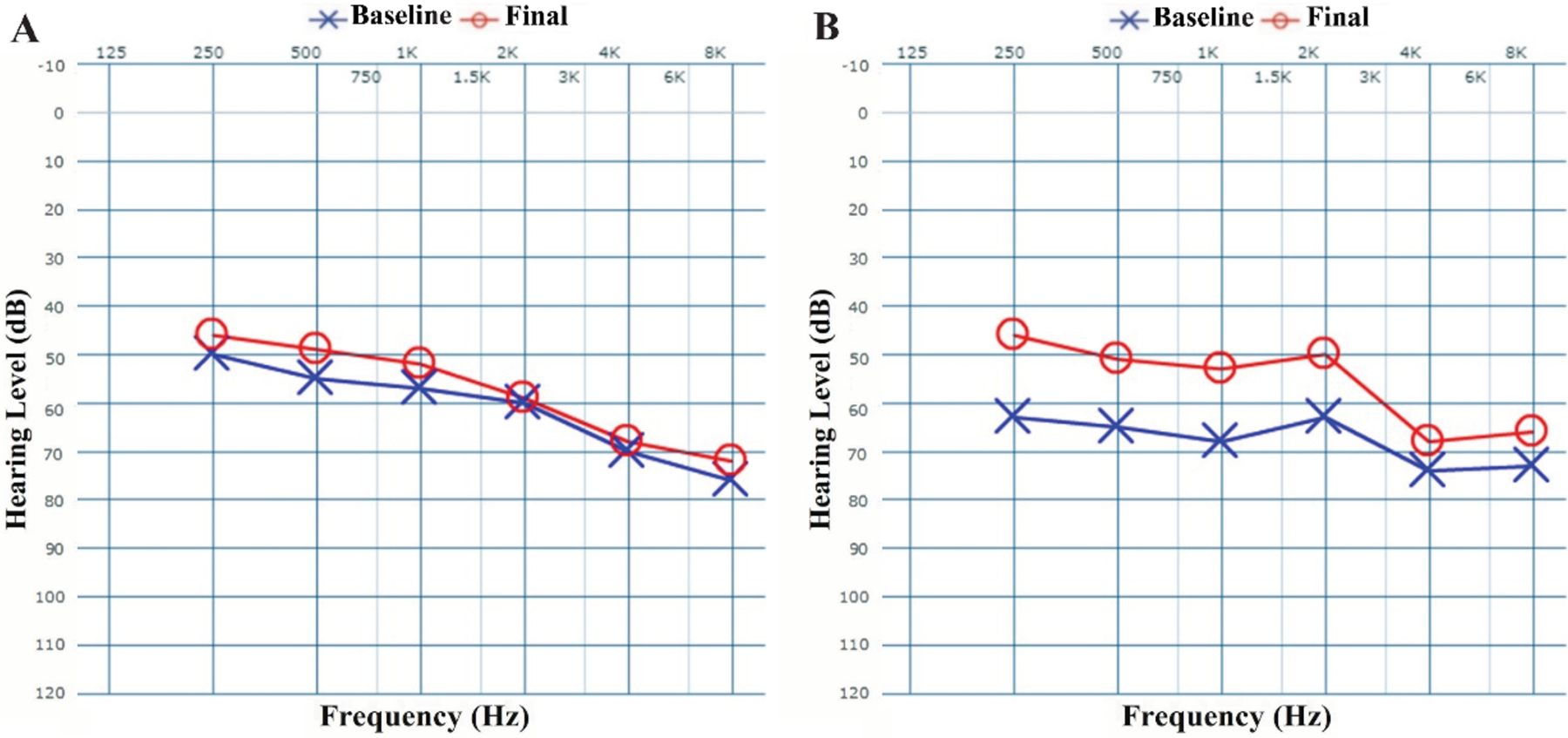
Baseline and final audiograms for (A) all long-term SSNHL patients (n=21) and (B) patients who experienced ≥10 dB improvement in at least two frequencies (n=6).
In the overall cohort, the average change in WRS and SRT was 26 ± 17% and 7 ± 12 dB, respectively. Moreover, the cohort’s mean change in low- and speech-frequency PTA was 4 ± 6 dB and 3 ± 6 dB, respectively. Clinically significant changes in WRS, SRT, PTA, and hearing thresholds are summarized in Table 3. Notably, ≥15% improvement in WRS and ≥10 dB improvement in SRT was observed in 13 (68%) and 8 (40%) patients, respectively. Within the sub-cohort with clinically significant (≥15%) WRS improvement, mean WRS change was 36 ± 10%. Furthermore, within the sub-cohort with clinically significant SRT improvement (≥10 dB), mean SRT change was 20 ± 8 dB. Six (29%) and 3 (14%) subjects experienced ≥10 dB improvement in at least two or three audiometric frequencies, respectively. The 29% of subjects with ≥10 dB improvement in at least two audiometric frequencies also had higher mean improvements in SRT (18 ± 12 dB vs. 3 ± 9 dB, p=0.03) and speech-frequency PTA (9 ± 8 dB vs. 1 ± 4 dB, p=0.049) compared to the rest of the cohort, but improvement in WRS remained the same (25 ± 19% vs. 26 ± 18%, p=0.89). However, of the 11 patients who presented with initial WRS of <50%, 8 (73%) had post-treatment WRS improved to >50%. The average percent improvement in WRS in these 9 patients was 39 ± 9%.
Table 3.
Summary of patients with clinically significant improvements in WRS, SRT, PTA, and hearing thresholds among long-term sudden sensorineural hearing loss patients.
| Patients with ≥15% WRS improvement* | 13 (68%) |
| Patients with ≥10 SRT improvement* | 8 (40%) |
| Patients with ≥10 PTA improvement | 3 (14%) |
| Patients with ≥10 dB improvement in at least one audiometric frequency | 8 (38%) |
| Patients with ≥10 dB improvement in at least two audiometric frequencies | 6 (29%) |
| Patients with ≥10 dB improvement in at least three audiometric frequencies | 3 (14%) |
| Patients with self-perceived hearing improvement† | 12 (57%) |
WRS: word recognition score; SRT: speech recognition threshold; PTA: pure tone average.
WRS and SRT were not available for analysis in 2 and 1 patients, respectively.
Self-perceived hearing improvement as reported by patient during post-treatment visit.
Age was not associated with ≥15% improvement in WRS (p=0.82), ≥10 dB improvement in at least two audiometric frequencies (p=0.88), or ≥10 dB improvement in SRT (p=0.13). Neither meeting migraine criteria nor receiving earlier treatment were associated with better improvement in any of the audiometric parameters. Patients with aural fullness were more likely to have ≥15% improvement in WRS (9/10, 90%) compared to those without aural fullness (4/9, 44%; p=0.03). Patients who received IT steroid injections in addition to their migraine treatment (n=15) had similar rates of significant improvement in SRT, WRS, and PTA compared to patients who did not receive IT steroid injections (all p>0.05). Independent samples t-test analysis also demonstrated that the numeric improvement in these parameters was similar between patients who received IT steroids and those who did not receive IT steroid injections (all p>0.05).
The reported medication adverse events, which were not mutually exclusive and usually occurred at higher dosage, included fatigue (n=5, 24%), nausea/lightheadedness (n=2, 10%), dry mouth (n=2, 10%), and heart palpitations (n=1, 5%), which resulted in de-escalating the dosage and subsequently improved patient tolerance. To our knowledge and the extent of follow-up data, the responders did not experience recurrence of hearing loss after discontinuation of the medications.
Discussion
This retrospective study of 21 long-term SSNHL patients demonstrated that adjuvant migraine treatment and IT steroid injection led to clinically significant improvement in WRS, SRT, and at least two audiometric frequencies in 68%, 40%, and 29% of the subjects, respectively. Moreover, 73% of subjects presenting with non-useful hearing (<50% initial WRS) improved to >50% WRS (usable hearing) post-treatment, which can have major implications for the consideration of hearing aid use. Patients who presented with continued aural fullness on the same side as the SSNHL had a higher chance of improvement in WRS. The observed improvement in at least two audiometric frequencies was more predominant among low frequency thresholds (Figure 3B), which is similar to our recent report of this treatment regimen in addressing acute (<14 days) SSNHL.18 These findings are significant because the literature on long-term and refractory SSNHL is scarce and these patients are often limited to only steroid therapy recommendations by the clinicians. Our findings suggest a novel treatment for this debilitating condition and may suggest further evidence of an underlying vascular etiology relating SSNHL and migraine.
A recent longitudinal study by Xie and colleagues reported that 27% of SSNHL patients had persistent hearing loss after one month, despite presenting ≤10 days from symptom onset and receiving steroid therapy.20 Another study which followed SSNHL patients for 2–18 months without treatment showed that of the 113 patients with standard-of-care treatment failure (as measured by PTA), only 13 (12%) had significant improvement in WRS.21 As such, this study’s significantly higher percentage of patients with WRS improvement (68%), and the relatively higher WRS percentage increase within the sub-cohort with clinically significant WRS improvement (35.6% vs. 23.8%21) may suggest a novel and efficacious treatment for long-term SSNHL. According to our results, adjuvant migraine medications significantly improved WRS in most patients above adequate thresholds fit for general quality of life benefits or consideration for hearing rehabilitation. Although only present in a sub-cohort of patients, we observed that SRT and PTA could also be improved following this treatment regimen. In our experience, this added efficacy of migraine management is independent of concurrent or previous steroid therapy. This notion is in line with our recent study reporting superior hearing recovery in short-term SSNHL when supplementing standard-of-care steroid therapy with adjuvant migraine medications.18 This efficacy is likely attributed to underlying migraine-induced cochlear symptoms, including SSNHL. We hypothesize that the possible relationship between migraine and SSNHL is due to the trigeminal innervation of the cochlear vasculature and stria vascularis.22,23 Further supported by our previous reports of the therapeutic benefits of migraine treatments for prolonged aural fullness,24 hyperacusis,25 persistent post-stapedotomy vertigo,26 and Meniere’s disease,27 this “otologic migraine” (otologic manifestations of migraine) phenomenon warrants further in-depth research.
Although a history of migraine was largely prevalent in our cohort, we observed that hearing improvement was similar between those who did and did not fulfill migraine criteria. Likewise in a study by Arslan et al., while there was a higher prevalence of migraine among SSNHL patients, hearing recovery was shown to be similar among those with or without migraine.28 These suggest that the therapeutic benefit of adjuvant migraine management for long-term SSNHL is not just limited to patients with a history of headache, and that migraine and SSNHL may be independently related. This relationship is further shown by recent Korean and Taiwanese nationwide studies in which migraineurs had an increased risk of developing SSNHL.16,17 Specifically, Chu et al. demonstrated that migraine patients (n=10,280) had a significantly increased incidence rate ratio (1.8, 95% CI 1.22–2.61) of developing SSNHL compared to matched cohorts (n=41,120).16 Similarly, in another study of 45,114 migraine patients and 180,456 controls, Kim and colleagues showed that the adjusted hazard ratio of migraine for SSNHL was 1.34 (95% CI 1.19–1.50).17 In patients with pre-existing migraine, it is possible that SSNHL is caused by migraine-induced vasospasm of cochlear vasculature29 or increased vascular permeability within the cochlea.15 It is hypothesized that cortical spreading depression, which is a wave of slowly propagating neuronal changes resulting in the release of various neuropeptides particularly from the trigeminal nerve, may play an important role in migraine development.30–32 Given that the spiral modiolar artery, cochleovestibular artery, and stria vascularis are innervated by trigeminal nerve fibers15,23 and trigeminal nerve stimulation results in fluid extravasation within the cochlea,15 we hypothesize that the possible link between SSNHL and migraine is rooted in the trigeminal innervation of the stria vascularis and cochlear vasculature.
Despite our efforts to cautiously collect and interpret data, this study contains several limitations. First, the retrospective nature of the study and lack of adequate follow-up for several excluded patients precludes us from concluding the efficacy of this treatment for all long-term SSNHL patients. As such, the results should be interpreted with caution, since they are based on a small and non-controlled retrospective case series. Future large, randomized controlled studies with appropriate effect size considerations will better determine the true and independent efficacy of migraine medications while controlling for spontaneous recovery, steroid therapy, and other confounders. This future randomized controlled trial can be possibly achieved by enrolling long-term SSNHL patients who do not respond to initial steroid treatment and randomizing them into migraine treatment and placebo groups for comparison. Another limitation of this study was that although migraine management consisted of comprehensive counseling on lifestyle and diet changes, compliance with these parameters was not objectively evaluated. Similarly, this study assumes full compliance with medication regimens and recommended dosage changes, but future prospective studies can further control this by incorporating self-reporting medication adherence measures.33 Furthermore, the regimen of migraine medications was not standardized among patients due to the retrospective nature of the study and personalized treatment of each subject based on side effects and other medications and comorbidities by the senior author. Lastly, while our identification of patients who met migraine criteria was in accordance with the ICHD-3 beta guidelines due to the timeline of data collection, future investigations will need to adopt the newly published and finalized ICHD-3 guidelines.34 Despite these limitations, this study shows a promising and novel treatment for long-term SSNHL patients, many of whom exhaust the limited standard-of-care options without benefits. This study encourages future investigations to further elucidate the efficacy of migraine medications for patients with refractory and long-term SSNHL.
Conclusion
In this case series of 21 patients, migraine medications and lifestyle changes resulted in significant hearing improvements in a subset of patients with long-term SSNHL presenting at least six weeks after symptom onset. Clinically significant improvements in SRT and audiometric frequencies were seen in 40% and 29% of patients, respectively, while WRS was improved in 68% of the cohort, including significant WRS improvements in most patients presenting with initial <50% WRS. For long-term SSNHL patients who are often left without additional treatment options, migraine therapy may be a novel therapeutic approach resulting in improved hearing levels, regardless of whether classic migraine symptoms are present. Future large-scale, controlled investigations are warranted to further explore this treatment for patients with long-term and refractory SSNHL.
Financial Disclosure:
Mehdi Abouzari is supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant TL1TR001415.
Footnotes
Conflicts of Interest: None
References
- 1.Alexander TH, Harris JP. Incidence of sudden sensorineural hearing loss. Otol Neurotol. 2013; 34:1586–1589. [DOI] [PubMed] [Google Scholar]
- 2.Arlinger S Negative consequences of uncorrected hearing loss-a review. Int J Audiol. 2003; 42:2S17–12S20. [PubMed] [Google Scholar]
- 3.National Institute of Health Sudden Deafness. Bethesda, MD: National Institutes of Health; 2000. NIH publication; 00–4757. [Google Scholar]
- 4.Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1977; 86:463–480. [DOI] [PubMed] [Google Scholar]
- 5.Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: I. A systematic review. Arch Otolaryngol Hea Neck Surg. 2007; 133:573–581. [DOI] [PubMed] [Google Scholar]
- 6.Wen Y-H, Chen P-R, Wu H-P. Prognostic factors of profound idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2014; 271:1423–1429. [DOI] [PubMed] [Google Scholar]
- 7.Weiss D, Böcker AJ, Koopmann M, Savvas E, Borowski M, Rudack C. Predictors of hearing recovery in patients with severe sudden sensorineural hearing loss. J Otolaryngol Head Neck Surg. 2017; 46:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cvorovic L, Ðeric D, Probst R, Hegemann S. Prognostic model for predicting hearing recovery in idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2008; 29:464–469. [DOI] [PubMed] [Google Scholar]
- 9.Narozny W, Kuczkowski J, Kot J, Stankiewicz C, Sicko Z, Mikaszewski B. Prognostic factors in sudden sensorineural hearing loss: our experience and a review of the literature. Ann Otol Rhinol Laryngol. 2006; 115:553–558. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald DC, McGuire JF. Intratympanic steroids for idiopathic sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2007; 116:253–256. [DOI] [PubMed] [Google Scholar]
- 11.Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012; 146:S1–S35. [DOI] [PubMed] [Google Scholar]
- 12.Slattery WH, Fisher LM, Iqbal Z, Friedman RA, Liu N. Intratympanic steroid injection for treatment of idiopathic sudden hearing loss. Otolaryngol Head Neck Surg. 2005; 133:251–259. [DOI] [PubMed] [Google Scholar]
- 13.Gündoğan F, Bayram A, Kalkan M, Özcan İ. Plasma levels of endothelial cell-specific molecule-1 and pentraxin-3 in idiopathic sudden sensorineural hearing loss. J Laryngol Otol. 2018; 132:995–999. [DOI] [PubMed] [Google Scholar]
- 14.Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. 2010; 120:1011–1021. [DOI] [PubMed] [Google Scholar]
- 15.Vass Z, Steyger P, Hordichok A, Trune D, Jancso G, Nuttall A. Capsaicin stimulation of the cochlea and electric stimulation of the trigeminal ganglion mediate vascular permeability in cochlear and vertebro-basilar arteries: a potential cause of inner ear dysfunction in headache. Neuroscience. 2001; 103:189–201. [DOI] [PubMed] [Google Scholar]
- 16.Chu C-H, Liu C-J, Fuh J-L, Shiao A-S, Chen T-J, Wang S-J. Migraine is a risk factor for sudden sensorineural hearing loss: a nationwide population-based study. Cephalalgia. 2013; 33:80–86. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Kim MK, Lim J-S, Kong IG, Choi HG. Migraine increases the proportion of sudden sensorineural hearing loss: A longitudinal follow-up study. Auris Nasus Larynx. 2019; 46:353–359. [DOI] [PubMed] [Google Scholar]
- 18.Abouzari M, Goshtasbi K, Chua JT, et al. Adjuvant Migraine Medications in the Treatment of Sudden Sensorineural Hearing Loss. Laryngoscope. 2021; 131:E283–E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurgel RK, Jackler RK, Dobie RA, Popelka GR. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg. 2012; 147:803–807. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y, Orabi NA, Zwolan TA, Basura GJ. Outcomes of unilateral idiopathic sudden sensorineural hearing loss: Two decades of experience. Laryngoscope Investig Otolaryngol. 2019; 4:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jan TA, Kozin ED, Kanumuri VV, Sethi RK, Jung DH. Improvement in word recognition following treatment failure for sudden sensorineural hearing loss. World J Otorhinolaryngol Head Neck Surg. 2016; 2:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vass Z, Shore S, Nuttall A, Jancsó G, Brechtelsbauer P, Miller J. Trigeminal ganglion innervation of the cochlea—a retrograde transport study. Neuroscience. 1997; 79:605–615. [DOI] [PubMed] [Google Scholar]
- 23.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004; 84:903–934. [DOI] [PubMed] [Google Scholar]
- 24.Moshtaghi O, Ghavami Y, Mahboubi H, et al. Migraine-related aural fullness: a potential clinical entity. Otolaryngol Head Neck Surg. 2018; 158:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abouzari M, Tan D, Sarna B, et al. Efficacy of multi-modal migraine prophylaxis therapy on Hyperacusis patients. Ann Otol Rhinol Laryngol. 2020; 129:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moshtaghi O, Mahboubi H, Haidar YM, Sahyouni R, Lin HW, Djalilian HR. Resolution of persistent post-stapedotomy vertigo with migraine prophylactic medication. Otol Neurotol. 2017; 38:1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghavami Y, Haidar YM, Moshtaghi O, Lin HW, Djalilian HR. Evaluating quality of life in patients with Meniere’s disease treated as migraine. Ann Otol Rhinol Laryngol. 2018; 127:877–887. [DOI] [PubMed] [Google Scholar]
- 28.Arslan Y, Arslan IB, Aydin H, Yagiz Ö, Tokuçoglu F, Çukurova I. The etiological relationship between migraine and sudden hearing loss. Otol Neurotol. 2017; 38:1411–1414. [DOI] [PubMed] [Google Scholar]
- 29.Viirre ES, Baloh RW. Migraine as a cause of sudden hearing loss. Headache. 1996; 36:24–28. [DOI] [PubMed] [Google Scholar]
- 30.Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurosci. 2013; 9:637. [DOI] [PubMed] [Google Scholar]
- 31.Malhotra R Understanding migraine: Potential role of neurogenic inflammation. Ann Indian Acad Neurol. 2016; 19:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harriott AM, Takizawa T, Chung DY, Chen S-P. Spreading depression as a preclinical model of migraine. J Headache Pain 2019; 20:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care. 2004; 42:649–652. [DOI] [PubMed] [Google Scholar]
- 34.Arnold M Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia. 2018; 38:1–211. [DOI] [PubMed] [Google Scholar]


