Abstract
Cell migration is essential for the development and maintenance of multicellular organisms, contributing to embryogenesis, wound healing, immune response, and other critical processes. It is also involved in the pathogenesis of many diseases, including immune deficiency disorders and cancer metastasis. Recently, extracellular vesicles (EVs) have been shown to play important roles in cell migration. Here, we review recent studies describing the functions of EVs in multiple aspects of cell motility, including directional sensing, cell adhesion, extracellular matrix (ECM) degradation, and leader-follower behavior. We also discuss the role of EVs in migration during development and disease and the utility of imaging tools for studying the role of EVs in cell migration.
Weaver ETOC:
Cell migration is essential for the development and maintenance of multicellular organisms, and also contributes to the pathogenesis of disease. Sung et al. review recent studies describing the functions of extracellular vesicles in various aspects of cell motility, including directional sensing, cell adhesion, extracellular matrix (ECM) degradation, and leader-follower behavior.
INTRODUCTION
General Mechanisms of Cell Migration
The migration of cells from one location to another is critical for the development and maintenance of multicellular organisms. Cells can migrate as individual single cells or in groups (Rørth, 2009; Yamada and Sixt, 2019). Single cell migration is characterized by four steps: polarization, protrusion of the leading edge driven by actin polymerization, adhesion formation and disassembly, and cell body retraction (Horwitz and Webb, 2003; Raftopoulou and Hall, 2004; Ridley et al., 2003). These events are coordinated in a complex manner at the interface between cells and their environment as well as between intracellular cytoskeletal and adhesion machineries, leading to effective forward translocation (Figure 1). On the other hand, collective cell migration is a coordinated movement of a group of cells (Friedl and Gilmour, 2009; Mayor and Etienne-Manneville, 2016; Norden and Lecaudey, 2019). It is utilized for a number of processes, including embryogenesis, wound-healing, cancer metastasis, and angiogenesis. Collective migration is driven by leader cells located at the front of the cluster. Physical and signaling connections, as well as coordinated organization of the actin cytoskeleton by cells in the cluster, are among the mechanisms used to induce migration of follower cells located behind leader cells in the cluster (Friedl and Mayor, 2017).
Figure 1. EV secretion promotes the directional migration of cells.
Cells migrating toward a gradient of soluble chemoattractants secrete extracellular vesicles (EVs) promoting their directional migration. Microvesicles (MVs) bud directly from the plasma membrane. Released MVs carry matrix metalloproteases (MMPs) degrading extracellular matrix to promote cancer cell invasion. Also, tissue transglutaminse 2 (TG2)-carrying MVs activate focal adhesion kinase (FAK) to promote cell contractility. Another type of large EV called a migrasome is formed at the tips or intersections of retraction fibers and enriched with tetraspanins (TSPANs) 4 and 7. Chemokines associated with migrasomes, such as CXCL12, released from leader cells (cyan cell) can promote chemotaxis of follower cells (orange cell) in a paracrine manner. Exosome functions in cell migration are depicted as: A. Multivesicular bodies (MVBs) are formed by inward budding of late endosomal membranes. Endosomal sorting complex required for transport (ESCRT) machinery, neutral sphingomyelinase 2 (nSMASE2), tetraspanins (TSPANs), and Alix-Syndecan-Syntenin complex are all molecules known to drive MVB biogenesis by inducing formation of intraluminal vesicles (ILV). These ILVs are secreted as exosomes after fusion of MVBs with the plasma membrane. B. Exosomes secreted at the leading edge of a migrating cell promote adhesion formation by binding integrin receptors at the cell membrane through fibronectin presented on the surface of the exosomes. C. Cortical branched actin filaments stabilized by cortactin enhance MVB docking at invadopodial protrusions. The secreted exosomes promote invadopodia formation and carry proteinases such as MT1-MMP that degrade ECM and promote cancer cell invasion. D. In Dictyostelium, exosomes carry the adenylyl cyclase ACA, which synthesizes the chemoattractant cAMP in the exosomal lumen and is secreted through specific transporters. The secreted exosomal attractants promote both autocrine and paracrine migration. Created with BioRender.com
EV Biogenesis and Release
Intercellular communication is a key component of a wide array of physiological functions including cell migration. In this context, extracellular vesicles (EVs) have recently emerged as important mediators of cell migration (Maas et al., 2017). EVs are lipid-enclosed moieties that are actively released from cells and mediate cell to cell communication via their bioactive protein, nucleic acid, and lipid cargoes (Kalluri and LeBleu, 2020; Maas et al., 2017). Exosomes are a type of small EV that are distinguished from other types of EVs by their size and biogenesis mechanisms. While exosomes originate from endosomal multivesicular body (MVB) compartments, other EVs - including both small and large EVs - are formed through the outward budding of the plasma membrane and are referred to by a number of names including ectosomes, microvesicles, and large oncosomes (Maas et al., 2017; Teng and Fussenegger, 2020). In addition, a third type of EV called migrasomes was recently described, with features of both microvesicles and MVBs, in that they are formed by the outward budding of the plasma membrane but contain small intralumenal vesicles similar to MVBs (Huang et al., 2019; Ma et al., 2015) (Figure 1). In this review, we will discuss the functions of exosomes and other EVs in multiple aspects of cell motility. Where the endosomal origin of small EVs has been identified, we will use the term exosomes. In cases where the biogenesis mechanism is independent of endocytosis or has not been identified, we will use the terms small and large EVs, noting that small EVs isolated from cells are typically highly enriched in exosomes.
Exosomes are canonically formed by the inward budding of the early endosomal membrane to form intraluminal vesicles (ILVs) within endosomes. A well-understood mechanism for the formation of ILVs is through the recruitment of the endosomal sorting complex required for transport (ESCRT) machinery to ubiquitinated endocytosed cargoes (Hurley, 2010). Alternative cargo recruitment and ILV budding mechanisms have been described involving lipid organization, including the generation of ceramide to induce membrane curvature (Trajkovic et al., 2008), and the formation of tetraspanin-enriched lipid microdomains (Hurwitz et al., 2018; van Niel et al., 2011), and may play roles in both cargo sorting and ILV formation (Zhang et al., 2019). Although more work needs to be done in this area, different biogenesis mechanisms are likely to generate distinct classes of exosomes by recruiting diverse cargoes to specific kinds of ILV formation sites (Kalluri and LeBleu, 2020; Maas et al., 2017; Stahl and Raposo, 2019).
Once formed, MVBs can be transported along microtubules to the plasma membrane to secrete exosomes (Kriebel et al., 2008), or fused with lysosomes for degradation. Once MVBs arrive at the plasma membrane they are docked to the membrane in a process that is dependent on proteins such as the small GTPases Rab27 or Rab35, and the actin regulator cortactin (Hsu et al., 2010; Ostrowski et al., 2010; Sinha et al., 2016). At docking sites, the fusion of MVBs with the plasma membrane to allow the release of ILVs as exosomes is regulated by synaptosomal-associated protein (SNAP) receptor (SNARE) and SNARE-binding proteins, including YKT6, Syntaxin-1a, Syntaxin-5, SNAP23, Munc 13–14, and synaptotagmin-7 (Gross et al., 2012; Hoshino et al., 2013; Hyenne et al., 2015; Koles et al., 2012; Messenger et al., 2018; Wei et al., 2017).
Compared to exosomes, much less is known about the biogenesis mechanisms of ectosomes. Nonetheless, the overall biophysical principles and some molecular mechanisms are conserved. For example, alteration in lipid content and membrane curvature contributes to the formation of ectosomes, including altered lipid flippase activity for small EVs (Beer et al., 2018; Tuck, 2011; Wehman et al., 2011) and acid ceramidase activity for large EVs (Awojoodu et al., 2014; Bianco et al., 2009). For smaller ectosomes, some but not all ESCRT proteins have been shown to promote the formation of at least one type of small EV (Nabhan et al., 2012). Thus, the adapter protein arrestin domain-containing protein 1 (ARRDC1) directly recruits the ESCRT-1 protein TSG101 to the plasma membrane (Nabhan et al., 2012), bypassing ESCRT-0 recruitment by monoubiquitinated proteins that occurs during exosome formation (Lu et al., 2003). For larger ectosomes, the ESCRT machinery does not appear to be involved. Instead, contractility by the actin cytoskeleton, may facilitate pinching off of large ectosomes (also called blebs in the migration literature) (Bergert et al., 2012; Di Vizio et al., 2009; Sheehan and Souza-Schorey, 2019), in an Arf1-, Arf6-, and RhoA-dependent fashion (Clancy et al., 2019; Muralidharan-Chari et al., 2009; Schlienger et al., 2014).
Migrasomes are large EVs formed at the tips or intersections of retraction fibers at the back of migrating cells. While the mechanisms by which migrasomes are formed are still being elucidated, like MVBs and exosomes, they have been shown to be enriched for tetraspanins, which are molecules that organize lipid and signaling domains, and cholesterol. It is proposed that this unique protein/lipid composition, together with adhesion and contractility generated by the retraction fibers, increases bending within migrasome structures on the retracting fibers (Huang et al., 2019; Ma et al., 2015) (Figure 1). However, distinct tetraspanins are enriched in and/or control biogenesis of distinct particles. Thus, the tetraspanin CD63 is uniquely enriched in exosomes whereas other tetraspanins control the biogenesis of migrasomes (Huang et al., 2019; Ma et al., 2015; Sung et al., 2020).
Functions of EVs in Cellular Communication
Secreted EVs participate in both autocrine and paracrine communication, which can occur by multiple mechanisms, including: (1) fusion with the plasma or endosomal membranes of recipient cells, delivering membrane proteins into recipient cell membranes and luminal cargoes (including RNAs) into the cytoplasm of recipient cells; (2) direct signaling by ligand-receptor interactions of the EV proteins with receptor proteins on recipient cells; and (3) generation and release of diffusible mediators from EVs (Esser et al., 2010; Kriebel et al., 2018; McKelvey et al., 2015; Pieters et al., 2019).
EV FUNCTIONS DURING CELL MIGRATION
EVs IN CHEMOTAXIS
Cells can, either singly or collectively, bias their migration direction in response to external cues (Figure 1). These cues can be classified as chemical, mechanical and electrical, leading to an expanding list of “taxis” modes (Shellard and Mayor, 2020). For example, while chemotaxis is a type of cell migration toward soluble cues called chemoattractants, haptotaxis refers to the sensing of immobilized chemical cues (typically ECM), durotaxis to sensing of substrate stiffness, and topotaxis to the ability of cells to sense topographical features of the ECM.
EVs in Cancer Cell Chemotaxis and Directional Migration
Studies in cancer cells have shown that genetic inhibition of exosome secretion greatly decreases both directional motility in vivo and chemotaxis in vitro (Sung et al., 2015; Sung and Weaver, 2017). Knockdown (KD) of Rab27a or the ESCRT-0 protein Hrs, which respectively control MVB docking and exosome biogenesis (Ostrowski et al., 2010; Tamai et al., 2010), leads to defects in persistent in vivo migration of fibrosarcoma and squamous carcinoma cells within chick embryo chorioallantoic membranes (Sung et al., 2015). A subsequent in vitro analysis revealed that, while control fibrosarcoma cells migrate directionally toward a chemoattractant gradient of EV-depleted serum, Rab27a-KD fibrosarcoma cells migrate randomly, disregarding the chemoattractant (Sung and Weaver, 2017). As in the chick embryo system, the migration speed of Rab27a-KD cells in vitro was also greatly reduced. In reconstitution rescue experiments, uniformly coating the chemotaxis chambers with small EVs rescued the speed but not directional sensing of Rab27a-KD cells. Interestingly, even control fibrosarcoma cells migrated randomly on small EV-coated surfaces in the presence of the soluble chemoattractant gradient, suggesting that exosomes themselves contain directional cues and must be secreted in an asymmetric manner to promote cell polarization and directional migration (Sung and Weaver, 2017). Furthermore, live confocal imaging of fibrosarcoma cells migrating on polymeric nanopatterned dishes showed that exosomes are secreted at the leading edge of migrating cells (Sung et al., 2020), suggesting that they are secreted in the right place at the right time to respond to external cues and promote autocrine migration
A number of studies have shown that small EVs from one cell type within the tumor microenvironment can induce the directional migration of another cell type. Small EVs secreted from hypoxic breast cancer cells were shown to act in a paracrine manner, by activating mitochondrial dynamics in normal mammary epithelial cells, inducing integrin-linked kinase (ILK)-Akt kinase-dependent migration of the recipient cells (Bertolini et al., 2020). Cancer-associated fibroblast (CAF)-derived small EVs promote migration or metastasis of several types of cancer cells by delivering diverse cargoes, including miRNA, and protein cargoes, including Sonic Hedgehog, TGFβ1, Wnt11, and Snail1 (Bhome et al., 2017; Li et al., 2017; Luga et al., 2012; Sun et al., 2019; Wu et al., 2020; You et al., 2019; Zhao et al., 2020). Exosomes isolated from heat-stressed tumor cells have been reported to contain chemokines that have the ability to attract and activate both dendritic cells and T cells (Chen et al., 2011). In another recent example, small EVs released from M2-polarized macrophages were found to promote the migration and invasion of colorectal cancer cells in wound-healing and Transwell assays, and metastasis in in vivo mouse experiments (Lan et al., 2019).
EVs in Immune Cell Chemotaxis
Exosome-like small EVs secreted from inflamed lymphatic endothelial cells have recently been shown to help guide dendritic cell migration (Brown et al., 2018). Directional migration of dendritic cells toward lymphatic vessels in tissue explants and through primary human lymphatic endothelial cell monolayers was increased in the presence of TNFα-induced small EVs, which were shown to contain many pro-migratory proteins compared to control small EVs. The EVs enhanced the migration response to soluble chemokines, such as CCL19 and CCL21. In particular, it was determined that membrane bound C-X3-C motif chemokine ligand 1 (CX3CL1) on endothelial small EVs induced G-protein-coupled receptor (GPCR) signaling through dendritic cell CX3C receptor 1 (CX3CR1), inducing dynamic protrusion formation and directional motility towards CCL19 chemotactic cues.
Neutrophils have been shown to relay chemotactic signals to neighboring cells at inflammation/injury sites. Upon encountering a primary chemoattractant, e.g., N-formyl-Met-Leu-Phe (fMLF), neutrophils rapidly reorganize their cytoskeleton and adopt a polarized morphology that allows them to migrate up the fMLF gradient (Liew and Kubes, 2019). They also begin secreting the secondary chemoattractant leukotriene B4 (LTB4) within minutes, which has been shown to amplify neutrophil chemotaxis towards fMLF in vitro (Afonso et al., 2012) and toward tissue injury sites in vivo (Lämmermann et al., 2013). Interestingly, the packaging of LTB4 in small EVs has been observed in macrophages and dendritic cells, where the vesicles were shown to promote migration (Esser et al., 2010). EV secretion and LTB4 signaling are also required for neutrophil arrest and extravasation in a sterile mouse footpad model of inflammation (Subramanian et al., 2020). In this context, it is envisioned that LTB4-containing exosomes act to propagate chemotactic signals between cells by protecting LTB4 from degradation in harsh extracellular environments. Attachment of exosomes to the ECM in tissues may also allow maintenance of secondary gradients. Thus, exosomes from cancer cells have been observed attached to collagen fibers in vivo (Sung et al., 2020) and integrin-containing EVs are released from neutrophils in vivo during extravasation (Subramanian et al., 2020). Furthermore, EVs from stimulated neutrophils use integrin signaling to anchor to the ECM (Genschmer et al., 2019).
EVs in Chemotaxis in Genetic Model Systems
Studies in Dictyostelium show that the packaging of chemoattractants in EVs is an evolutionarily conserved process. Dictyostelium cells align in a head-to-tail fashion, or stream, as they migrate in a gradient of cAMP, the main chemoattractant used by these cells (Nichols et al., 2015). In 2008, Kriebel and colleagues showed that the enzyme that synthesizes cAMP, adenylyl cyclase (ACA), is present in MVBs that accumulate at the back of chemotaxing Dictyostelium cells and release ACA-containing small EVs - a process that is necessary for the formation of head-to-tail arrays of migrating cells (Kriebel et al., 2008). Most remarkably, the released small EVs not only contain and synthesize cAMP, but also secrete it via the ATP-binding cassette transporter C8 (Kriebel et al., 2018). Mechanistically, ACA-containing MVBs specifically align on microtubules, which radiate to the back of these cells (Kriebel et al., 2008). Inhibition of microtubule polymerization with nocodazole inhibits the accumulation of ACA vesicles at the back of cells and the formation of streams. The trafficking of these ACA-positive MVBs also requires an intact actin network, and de novo protein synthesis. These findings, together with the data for immune cells, suggest a paradigm in which chemotactic small EVs contain the enzymatic machinery to generate bioactive products critical for sustained directed migration (Sung and Weaver, 2018). Furthermore, vesicular trails containing these machineries may direct migrating cells to specific locations to orchestrate complex biological functions, including immune responses or, in the case of Dictyostelium, organization into a fruiting body. Similar to the vesicular trails deposited from Dictyostelium cells, secreted CD63-positive small EVs are left behind migrating cancer cells (Sung et al., 2015) and neutrophils (Figure 2), and are used by neighboring cells for migration (Sung et al., 2020) (Figure 3).
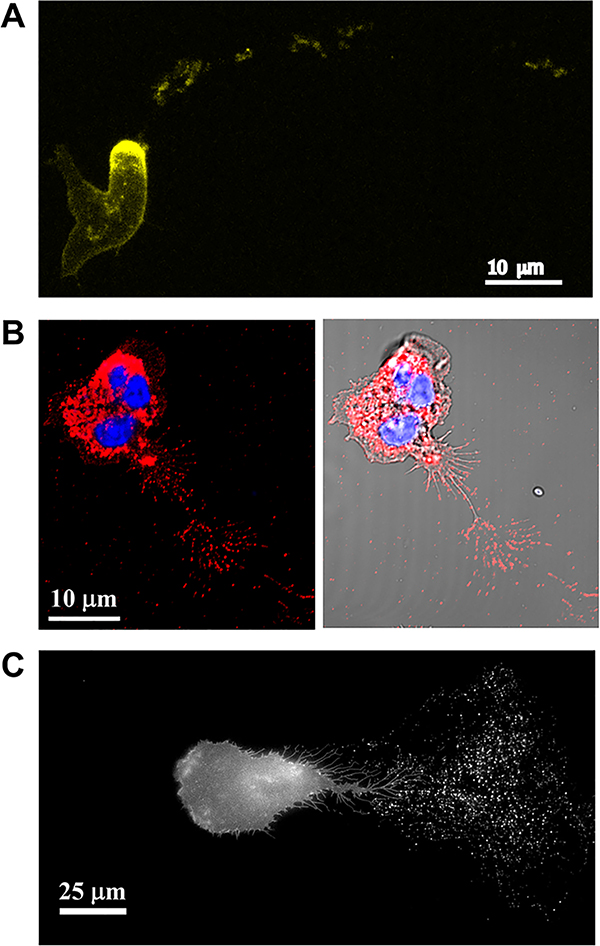
Figure 2. Cells leave trails of EVs behind during migration.
A. Fluorescence maximum intensity projection of Dictyostelium discoideum aca− cells expressing ACA-YFP and chemotaxing towards cAMP. Note the trails of ACA-YFP-positive vesicles left from the rear of the migrating cell. Reproduced under the term of the Creative Commons CC BY-NC-SA 3.0 license (Kriebel et al., 2008). Copyright © 2008, Rockefeller University Press. B. Confocal images of primary human neutrophils migrating towards fMLF, fixed and stained using an antibody against CD63 (red) and the nuclear dye Hoechst (blue). The image shows CD63-positive vesicles left behind the migrating neutrophil. C. Fluorescence image of HT1080 fibrosarcoma cells stably expressing pHluo_M153R-CD63. Note the exosome trails left behind the cell as it migrates toward the left side of the image. Reproduced under the term of the Creative Commons CC BY 4.0 license (Sung et al., 2020). Copyright © 2020, Springer Nature.
Figure 3. Cells exhibit pathfinding behavior on EV trails.
A. Left: Fluorescence maximum intensity images taken from a time lapse recording of Dictyostelium discoideum aca− cells expressing ACA-YFP moving towards an aggregate of cells. Cell outline traces depicting the relative position of 4 cells is presented as a time evolution of outlines from lighter to darker colors for each cell. The secreted ACA-YFP-containing vesicles are depicted in gray. Right: Plot showing the correlation between the distance of a cell to its nearest vesicle and the angle θ between the cell direction of motion and the direction vector to the nearest vesicle. In this context, the closer a cell is to a vesicle, the higher the chemotactic response of the cells, which is measured by smaller angles of deviation. Reproduced under the term of the Creative Commons CC BY-NC-SA 4.0 license (Kriebel et al., 2018). Copyright © 2018, Rockefeller University Press. B. Left: Fluorescence time series images of HT1080 fibrosarcoma cells co-expressing pHluo_M153R-CD63 (exosome label) and mCherry-CAAX (plasma membrane label), showing leader-follower cell migration behavior in 3D collagen gels. Exosome trails (arrowheads) deposited behind migrating leader cells promote directional migration of follower cells (arrows). Right: Scatter plot with median and quartile range showing pathfinding index quantitated by the cosine value of the angle θ between the cell direction of motion and the nearest exosome trail. Note that migration towards exosome trails yields positive cosine θ values, with perfect migration along an exosome trail yielding a cosine θ = 1, whereas migration away from exosome trails yields negative cosine θ values, with a maximum possible value of −1. Reproduced under the term of the Creative Commons CC BY 4.0 license (Sung et al., 2020). Copyright © 2020, Springer Nature.
In another developmental model, gastrulating zebrafish embryos, migrasome formation regulated by TSPAN4a and TSPAN7 was shown to be important for the correct migration of dorsal forerunner cells and subsequent organ morphogenesis (Jiang et al., 2019). Migrasomes carrying the chemokines CXCL12a/b accumulate in the embryonic shield area and serve as a chemoattractant for the dorsal forerunner cells. The essential role of migrasomes in this process was shown with rescue experiments, in which purified migrasomes were injected into embryos and rescued both laterality defects of MZtspan4a and MZtspan7 embryos and chemotaxis of dorsal forerunner cells. Furthermore, injection of migrasomes purified from cxcl12a/b morphants were unable to induce chemotaxis of dorsal forerunner cells.
In addition to carrying attractive cues, exosomes can transmit repulsive signals in the parasite Trypanosoma brucie. Thus, heat shock induces exosome secretion in an ESCRT-dependent manner, and exosomes taken up by neighboring trypanosome cells transmit repulsive signals that regulate social motility (Eliaz et al., 2017). While these exosomes carry a specialized form of RNA (spliced leader RNA) that is involved in trans-splicing, it is unknown whether the RNA transmits the repulsive signal. Interestingly, a distinct type of EV originating from membrane nanotubules of trypanosomes is responsible for the transfer of virulence factors to other trypanosomes, thereby providing resistance to human innate immunity. These same nanotubule-derived EVs were shown to fuse with human erythrocytes and induce clearance and anemia (Szempruch et al., 2016). Exosomes and EVs therefore represent an important mechanism by which parasites communicate with each other and with their hosts, similar to the observations in mammalian systems.
EVs IN CELL ADHESION
For cells using a mesenchymal mode of migration, such as fibroblasts and many types of cancer cells, cell migration speed is closely tied to the ability of cells to form and disassemble cell adhesions, and a number of studies have reported that exosomes and other EVs promote adhesion to a variety of substrates (Altei et al., 2020; Koumangoye et al., 2011; Singh et al., 2016; Yue et al., 2015). As cell adhesion molecules are highly enriched in small EVs (Jimenez et al., 2019; Mears et al., 2004; Rai et al., 2020; Wubbolts et al., 2003), these data suggest that exosomes/small EVs have the ability to promote cell adhesion during migration. Indeed, live imaging of adhesion dynamics in control and Rab27a-KD fibrosarcoma cells revealed a specific role for exosome secretion in adhesion assembly. Notably, adhesion disassembly was not affected (Sung et al., 2015).
Integrins are a major type of adhesion receptor that is present in EVs secreted from diverse cell types (Demory Beckler et al., 2013; Hosseini-Beheshti et al., 2012; Jimenez et al., 2019; Liang et al., 2013; Peinado et al., 2012; Skogberg et al., 2013; Sung et al., 2015). Over a decade ago, Clayton and colleagues demonstrated that integrins in B-cell-derived exosomes mediate anchorage to ECM and cytokine-activated fibroblasts (Clayton et al., 2004; Wubbolts et al., 2003). More recently, integrins present in small EVs derived from cancer cells were found to be important for organotropism of cancer metastatic site selection (Hoshino et al., 2015). In particular, α6β4 and αvβ5 present in small EVs derived from breast cancer cells were proposed to respectively mediate lung- and liver-specific metastasis of breast cancer cells by facilitating EV binding to distinct ECM repertoires of the target organs (Hoshino et al., 2015). In prostate cancer, small EV-associated integrins have been shown to be delivered to neighboring nontumorigenic and tumorigenic cells and to promote their adhesion and migration (Fedele et al., 2015; Singh et al., 2016). In these cases, integrins appear to be directly incorporated into the membranes of recipient cells – promoting direct adhesion of the cells to the ECM and other integrin ligands (Fedele et al., 2015; Singh et al., 2016). This scenario is probably most effective when the recipient cells endogenously express low levels of the transferred receptor.
Another mechanism by which EVs may induce cell adhesion during migration is by carrying ECM (Figure 1). In fibrosarcoma cells, fibronectin bound to α5β1 integrin on exosomes was shown to directly promote adhesion formation and cell speed (Sung et al., 2015). Fibronectin and laminins can also be carried by large EVs where, in association with the ECM crosslinking molecule tissue transglutaminase 2 (TG2), they have been shown to enhance FAK activation and trophoblast cell migration (Antonyak et al., 2011; Desrochers et al., 2016; Hur et al., 2020). As a variety of ECM components have been identified to be associated with EVs (Jimenez et al., 2019; Rilla et al., 2019; Yeung et al., 2020), an interesting future direction will be to determine whether the presentation of ECM molecules by EVs is a general mechanism that promotes adhesion formation by diverse types of migrating cells.
EVs IN ECM DEGRADATION
Migration through tissues often requires breakdown of ECM barriers to create pores large enough to allow cell passage (Sabeh et al., 2009). Matrix metalloproteinases (MMPs) are key proteinases that digest both stromal and epithelial ECM assembly to drive cell invasion (Kessenbrock et al., 2010). EVs can facilitate this process in several ways (Figure 1). First, both small and large EVs released from cancer cells carry MMPs and promote degradation of ECM substrates (Clancy et al., 2015; Hakulinen et al., 2008; Hoshino et al., 2013; Laghezza Masci et al., 2016; Mu et al., 2013; Muralidharan-Chari et al., 2009). Second, exosome secretion drives both formation and activity of invadopodia, which are actin-rich invasive protrusions that serve as hotspots for proteinase secretion (Hoshino et al., 2013). Invadopodia serve as docking sites for MVBs, thereby facilitating release of invasion-promoting exosomes (Hoshino et al., 2013). While MT1-MMP is known to be a key invasion-promoting MMP carried by exosomes, it is not the only cargo driving invasion. Thus, small EVs purified from MT1-MMP-KD cells are still able to induce invasion of head and neck cancer cells through Matrigel-coated Transwell filters (Sinha et al., 2016). Thus, it is likely that multiple proteinase, growth factor and ECM molecules carried by EVs are involved in driving invasive motility.
EVs IN CELL MIGRATION IN DISEASE
Throughout development, cell growth and patterning are essential for the formation of tissues and organs. For this to occur, coordinated signaling between cells is necessary. EVs have been shown to play an important role in mediating cell-cell signaling, including for molecules that are important for pattern formation such as Notch ligands, and Hedgehog and Wnt families of secreted proteins (reviewed in (McGough and Vincent, 2016)). Similarly, cell-cell communication and cell migration are integral parts of many disease processes. One notable example is cancer, where exosomes and other EVs have been shown to drive multiple aspects of cancer metastasis, including promotion of cancer cell motility and invasiveness and seeding of premetastatic niches (Sato and Weaver, 2018; Tao and Guo, 2020; Wortzel et al., 2019).
The response to infections requires the recruitment of immune cells from distant locations. Humans with defects in Rab27A (Griscelli Syndrome Type 2) have immune defects as well as albinism (Klein et al., 1994; Ménasché et al., 2000) and Rab27 knockout mice have been shown to exhibit defects in neutrophil chemotaxis and recruitment, as well as defects in antibody and melanosome transport (Singh et al., 2014). Similarly, neutrophils derived from synaptotagmin 7-deficient mice exhibit chemotaxis defects in vitro and in vivo (Colvin et al., 2010).
EV secretion may also affect the recruitment and migration of cells during chronic inflammatory processes. In an in vitro model of kidney stone formation, macrophages treated with crystals of calcium oxalate monohydrate (COM) were shown to secrete small EVs containing altered proteins involved in immune processes and cell migration (Singhto et al., 2018). These altered EVs promoted migration of monocytes and T cells and also induced monocyte activation and phagocytic activity of macrophages. In addition, small EVs purified from neutrophils of patients with generalized pustular psoriasis have been shown to stimulate keratinocytes to produce chemokines, including CXCL1, CXCL2, and CXCL8 (Shao et al., 2019). These chemokines then induced neutrophil migration, which may explain the increased neutrophil infiltration in patient skin lesions.
Inflammation frequently contributes to atherosclerosis. During atherogenesis, EVs secreted from atherogenic macrophages are enriched with microRNAs that target cell adhesion and migration pathways, resulting in decreased motility of naïve macrophages in the vessel wall. Specifically, miR-146a carried by small EVs was shown to downregulate insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) and human antigen R, both of which affected macrophage motility (Nguyen et al., 2018). Such entrapment of macrophages in the vessel wall may lead to macrophage accumulation and acceleration of the development of atherosclerosis. EVs secreted from macrophage foam cells enhance the adhesion and motility of vascular smooth muscle cells by promoting the phosphorylation of extracellular signal-regulated kinase (ERK) and Akt (Niu et al., 2016). In a separate study, small EVs isolated from the plasma of patients with peripheral artery disease were found to increase the migration of vascular smooth muscle cells and decrease the migration of endothelial cells, although the mechanisms by which this occurs remain to be determined (Sorrentino et al., 2020).
Cell migration is a critical component of wound healing, in which multiple waves of cells are recruited to fight infection, lay down matrix, form scars, and re-epithelialize the wound. While the intrinsic role of exosomes or EVs in wound repair has not been investigated in in vivo systems, in vitro scratch wounding of renal tubular cells induces exosome secretion and these scratch-wounding-derived exosomes inhibit in vitro wound healing (Zhou et al., 2017). The induced exosome secretion from scratch-wounding is inhibited by epidermal growth factor (EGF) and promoted by the EGF receptor inhibitor gefitinib, suggesting a suppressive role of EGFR signaling in exosome production in these cells. In contrast, small EVs isolated from fibroblast growth factor 2 (FGF2)-stimulated human fibrocytes promote in vitro wound-healing of human diabetic keratinocytes (Geiger et al., 2015). In this study, the use of therapeutic EVs for the treatment of wounds was also studied in an in vivo model, where small EVs isolated from stimulated human fibrocytes accelerated excised wound closure by increasing the content of MECA32, collagen 1, and α-smooth muscle actin (α-SMA) in the wound sites (Geiger et al., 2015). In a similar in vivo model, small EVs purified from adipose-derived mesenchymal stem cells enhanced chronic diabetic wound-healing, angiogenesis, re-epithelialization, and collagen deposition (Wang et al., 2019). While more studies are needed to identify key cargoes and secreting cells, it appears that some types of EVs promote wound healing.
IMAGING TOOLS FOR STUDYING THE ROLE OF EVs IN CELL MIGRATION
Live imaging is an important tool for studying cell migration, as it allows the visualization and quantification of key events involved in migration, including cell polarization, cytoskeletal reorganization, adhesion dynamics, as well as cell directionality and speed. In order to assess how exosomes and EVs regulate these events, it is important to visualize the spatial and temporal dynamics of their secretion and interactions with cells.
While many tools have been constructed to visualize EVs (Table 1), not all of these have the spatiotemporal resolution required for migration studies. For example, although conjugation of luciferase to lipid anchoring signals or to EV marker proteins is useful for tracking the organ accumulation of EVs and does not require an excitation source (Danzer et al., 2012; Hikita et al., 2018; Lai et al., 2014; Takahashi et al., 2013), the resulting signal lacks resolution and cannot give the type of spatiotemporal granularity required to gain insight into migratory mechanisms, either in vitro or in vivo. Lipophilic membrane dyes such as DiI, DiD-DS, PKH26, and PKH67 all label purified EVs efficiently (Dabrowska et al., 2018; Macklin et al., 2016; Nicola et al., 2009); however they cannot be used to study spatiotemporal dynamics of EV secretion coupled to cell migration. An alternative approach is to label cell membranes, using cyanine-based lipophilic MemBright membrane dyes (Collot et al., 2019), which are compatible with long-term live imaging as well as two-photon microscopy. By injecting MemBright-labeled melanoma cells and EVs into zebrafish embryos, Hyenne et al. studied the fate of tumor EVs in vivo and found that EV-targeted patrolling macrophages exhibited significantly decreased motility (Hyenne et al., 2019). The colocalization of MemBright with CD63-GFP in late endosomal compartments showed that MemBright can label exosomes as well as plasma membrane-derived EVs. However, not all puncta labeled with MemBright were positive for CD63-GFP and vice versa, suggesting that MemBright does not label all exosomes.
Table 1. List of imaging tools for EV studies.
EV, extracellular vesicles; MVB, multivesicular bodies; GFP, green fluorescent protein, RFP, red fluorescent protein; FP, fluorescent protein. Cartoons in Table were created with BioRender.com.
| Labeling Methods | Dyes | Labeling targets | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Bioluminescence | Luciferase |  |
• In vivo imaging • Excitation source not required |
• Low resolution • Not suitable to spatiotemporal imaging • Limited to the marker-specific EVs if EV marker proteins are labeled |
• (Danzer et al., 2012; Hikita et al., 2018; Lai et al., 2014; Takahashi et al., 2013) |
| Lipophilic fluorescent dyes | DiO, DiI, DiD, PKH26, PKH67 |
 |
• Directly label purified EV membrane • Tracking added-up EVs |
• Purified EV required • Off-target labeling, e.g. lipoproteins • EV-sized dye aggregates generated • Not suitable to study EV secretion |
• (Dabrowska et al., 2018; Lai et al., 2015; Macklin et al., 2016; Nicola et al., 2009; Pužar Dominkuš et al., 2018; Simonsen, 2019) |
| MemBright |  |
• Six different colors • Long-term live imaging • Two-photon microscopy • Label cell membrane to get labeled • EVs |
• Endosomal compartments not distinguished to each other (e.g. early endosomes vs. late endosomes) • Not suitable to track MVB fusion events |
• (Collot et al., 2019; Hyenne et al., 2019) | |
| Immunofluorescent reporters | Fluorescencetagged antibodies |  |
• Optimizable to low background noise • Based on a simple immunofluorescence technique |
• Purified EV required • Limited to target antibody-specific EVs Special antibodies required (e.g. extracellular domain-specific) • Not suitable to study EV secretion |
• (Mondal et al., 2019) |
| Genetically encoded fluorescent probes | Conventional fluorescent proteins (e.g. GFP, RFP, mCherry, etc.) |  |
• Bright • Live imaging for MVB tracking and exosome secretion and uptake • Tracking EV exchange between cells in vitro and in vivo |
• Limited to the marker-specific EVs if EV marker proteins are labeled • Not suitable to track MVB fusion events |
• (Corrigan et al., 2014; Koumangoye et al., 2011; Lai et al., 2015; Liu et al., 2016; Men et al., 2019; Neckles et al., 2019; Shen et al., 2011; Sung et al., 2015; Yoshimura et al., 2016) |
| pH-sensitive fluorescent proteins (e.g. pHluorin) |  |
• Only fluorescent at the MVB fusion and exosome secretion sites • Tracking exosome secretion due to a good signal-to-noise ratio • Capable to be labeled with non-pH sensitive FPs enabling to monitor MVB trafficking and fusion and a sequence of exosome endocytosis, internalization, and endosomal acidification events. • Available in vivo |
• Limited to the marker-specific exosomes • Likely exosome-specific labeling |
• (Messenger et al., 2018; Sung et al., 2015; Sung et al., 2020; Verweij et al., 2018; Verweij et al., 2019) |
To study the dynamic trafficking and secretion of EVs, EVs are typically labeled with genetically encoded fluorescent proteins. One approach has been to link fluorescent proteins such as enhanced green fluorescent protein (EGFP) or tandem dimer Tomato (tdTomato) to a lipid anchoring signal, such as a palmitoylation signal (Lai et al., 2015). The resulting palmitoylated EGFP/tdTomato is both anchored in the plasma membrane and endocytosed, allowing labeling of both shed microvesicles and exosomes (Lai et al., 2015). Plasma membrane-derived EVs can also be preferentially labeled by adding a myristoylation tag or a phosphatidylinositol-(4,5)-bisphosphate (PIP2)-binding domain to GFP (Shen et al., 2011). Another approach has been to label specific EV proteins, such as tetraspanins, with fluorescent proteins. Conventional fluorescent proteins, including GFP, RFP, and mCherry, have all been used to label the tetraspanin CD63 and study MVB trafficking, exosome secretion, and uptake (Koumangoye et al., 2011; Liu et al., 2016; Sung et al., 2015). This approach has been particularly useful for tracking EV exchange between cells in vivo, including in transgenic models and in xenograft tumor models (Corrigan et al., 2014; Men et al., 2019; Neckles et al., 2019; Yoshimura et al., 2016). However, there are some limitations to using fluorescently protein-tagged CD63. Due to the concentration of CD63 in internal endosomes and consequent bright fluorescence, it is difficult to distinguish MVB trafficking events in the cytoplasm from MVB fusion events with the plasma membrane. This difficulty in detecting fusion events is due to a poor signal-to-noise ratio, since the internal fluorescence is so high. An approach that we and others have used to solve this problem is to use the pH-sensitive GFP derivative pHluorin. pHluorin is virtually nonfluorescent under acidic conditions but fluoresces at neutral pH (Miesenböck et al., 1998), and is widely used to visualize the fusion of synaptic vesicles in neurons (Kyung et al., 2016; Royle et al., 2008; Sankaranarayanan et al., 2000). As MVBs are also acidic, we adapted this approach by inserting superecliptic pHluorin (pKa ~ 7.11) (Sankaranarayanan et al., 2000) into a small extracellular loop of CD63 (Sung et al., 2015). Indeed, this allowed us to observe MVB fusion with the plasma membrane, revealing bursts of pHluorin-CD63 fluorescence at the plasma membrane appearing just before nascent adhesion formation in spreading fibrosarcoma cells. We also observed that secreted exosomes are left behind migrating cells in adhesive trails. A similar construct was also used to demonstrate that histamine-mediated GPCR activation triggers MVB fusion with the plasma membrane (Verweij et al., 2018) as well as to image the fate of CD63-positive EVs released from yolk cells in zebrafish (Verweij et al., 2019).
The pHluorin-CD63 construct was improved by mutating methionine 153 to arginine (M153R) in pHluorin (Sung et al., 2020), a mutation known to stabilize the protein in bacteria (Morimoto et al., 2011). Indeed, this led to greatly increased stability and brightness of the fusion protein in cells, allowing improved visualization of exosome trails left behind migrating cells (Fig. 2), and the use of those trails for migration by follower cells (Fig. 3). Using intravital imaging, bright pHluorin_M153R-CD63-positive puncta secreted from breast cancer cells in mouse mammary fat pads and from fibrosarcoma cells in the chick chorioallantoic membrane were observed to be associated with filopodia-like protrusions and ECM fibers (Sung et al., 2020). In order to monitor MVB trafficking inside the cell before fusion with the plasma membrane, a dual tagged reporter in which pHluorin_M153R-CD63 was tagged at the C-terminus with a pH-insensitive red fluorescent protein, mScarlet was created. This reporter, pHluorin_M153R-CD63-mScarlet, allowed the observation of MVB trafficking and fusion events at protruding cell edges as well as the uptake of the released exosomes by cellular filopodia. Further use of this construct will allow more in-depth dissection of how exosomes contribute to autocrine and paracrine migration.
Besides CD63, other tetraspanins, such as CD9 and CD81, have been labeled and used to study EV exchange between cells (Neckles et al., 2019; Oleksiuk et al., 2015). Unlike CD63, these are primarily localized to the plasma membrane instead of endosomes (Mathieu et al., 2020; Pols and Klumperman, 2009). Thus, they may be more useful for labeling non-exosomal EV populations or EVs released from cells that secrete little CD63 in EVs (Saunderson et al., 2008). Thus far, there has been little to no use of other tetraspanins such as CD82 or CD151 as imaging tools, probably because they are less widely expressed.
CONCLUSIONS
Cell migration is a complex process, involving rapid and dynamic responses of the internal cellular machinery to external cues. In tissues, these cues include soluble chemical mediators, molecular and topological aspects of the extracellular matrix, and EVs (Fig. 1). While this field is in this infancy, already some themes are emerging about the role of EVs in guiding migrating cells. One unique feature of EVs is that they can bind to ECM substrates, leaving a stationary and long-lasting remnant behind the cell from which they were secreted. Indeed, breadcrumb-like trails have been observed behind cells across the evolutionary scale (Fig. 2) and are used as paths for cells to follow (Fig. 3). By contrast, soluble gradients would dissipate much more quickly. In addition, while themselves stationary, EVs have been shown to contain the enzymatic machinery to generate soluble chemoattractants (Kriebel et al., 2018)), thus serving as a source of soluble molecules even after the secreting cell has moved far away. The enrichment of EVs in adhesion molecules, not only serves to bind surrounding ECM in the tissue but also to carry associated ECM from the secreting cell (Antonyak et al., 2011; Desrochers et al., 2016; Jimenez et al., 2019; Sung et al., 2015; Wu et al., 2017) which may serve as a further guidance cue during leaderfollower migratory behavior. Finally, EVs carry additional molecules such as membrane-linked MMPs and crosslinking enzymes which act to remodel the surrounding ECM and facilitate cell invasion (Fig. 1, (Antonyak et al., 2011; Clancy et al., 2015; Hakulinen et al., 2008; Hoshino et al., 2013; Laghezza Masci et al., 2016; Mu et al., 2013; Muralidharan-Chari et al., 2009)).
EVs were long thought to be a mechanism for cells to rid themselves of unwanted material. However, EVs are now established to be irreplaceable messengers for cell-cell communication, regulating the physiology of recipient cells. Over the past few years, it has become clear that one of the major functions of EVs is to regulate cell migration in a variety of contexts. This essential function appears to be due to their diverse cargoes regulating multiple subcellular processes critical for cell migration, including cell signaling, cell polarization, and cell adhesion. An important future direction will be to further identify cargoes controlling specific aspects of cell migration, such as directional sensing, adhesion formation, and cytoskeletal reorganization. In addition, the question of how EV functions in cell migration impact development and disease remains relatively understudied. Thus, another future research direction is to understand how EV secretion from diverse cell types controls migration in complex environments. The use of live imaging tools and genetically engineered cells with altered EV biogenesis and/or secretion will greatly facilitate this work, to allow sophisticated assessment of cell migration in 3D and in vivo systems. Such studies will be important to better understand how EVs control autocrine and paracrine cellular migration in different microenvironments, physiologic conditions, and diseases.
Acknowledgements:
We apologize for references that we were not able to include due to space constraints. We would like to thank Dr. Subhash Arya for providing the confocal images of primary human neutrophils in Fig. 2B and acknowledge support from the NIH (R01AI152517 to CAP and R01CA206458, R01CA249424, and R01 CA249684 to AMW).
Footnotes
Declaration of Interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, and Parent CA (2012). LTB4 is a signal-relay molecule during neutrophil chemotaxis. Developmental cell 22, 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altei WF, Pachane BC, Dos Santos PK, Ribeiro LNM, Sung BH, Weaver AM, and Selistre-de-Araújo HS (2020). Inhibition of αvβ3 integrin impairs adhesion and uptake of tumor-derived small extracellular vesicles. Cell Commun Signal 18, 158–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, and Cerione RA (2011). Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proceedings of the National Academy of Sciences 108, 4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awojoodu AO, Keegan PM, Lane AR, Zhang Y, Lynch KR, Platt MO, and Botchwey EA (2014). Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood 124, 1941–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer KB, Rivas-Castillo J, Kuhn K, Fazeli G, Karmann B, Nance JF, Stigloher C, and Wehman AM (2018). Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry. Proceedings of the National Academy of Sciences 115, E1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergert M, Chandradoss SD, Desai RA, and Paluch E (2012). Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proceedings of the National Academy of Sciences 109, 14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini I, Ghosh JC, Kossenkov AV, Mulugu S, Krishn SR, Vaira V, Qin J, Plow EF, Languino LR, and Altieri DC (2020). Small Extracellular Vesicle Regulation of Mitochondrial Dynamics Reprograms a Hypoxic Tumor Microenvironment. Developmental Cell 55, 163–177.e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhome R, Goh RW, Bullock MD, Pillar N, Thirdborough SM, Mellone M, Mirnezami R, Galea D, Veselkov K, Gu Q, et al. (2017). Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging (Albany NY) 9, 2666–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, and Clementi E (2009). Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO journal 28, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Johnson LA, Leone DA, Majek P, Vaahtomeri K, Senfter D, Bukosza N, Schachner H, Asfour G, and Langer B (2018). Lymphatic exosomes promote dendritic cell migration along guidance cues. Journal of Cell Biology 217, 2205–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Guo J, Yang M, Zhu X, and Cao X (2011). Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol 186, 2219–2228. [DOI] [PubMed] [Google Scholar]
- Clancy JW, Sedgwick A, Rosse C, Muralidharan-Chari V, Raposo G, Method M, Chavrier P, and D’Souza-Schorey C (2015). Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat Commun 6, 6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JW, Zhang Y, Sheehan C, and D’Souza-Schorey C (2019). An ARF6-Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nature cell biology 21, 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, and Hallett MB (2004). Adhesion and signaling by B cell-derived exosomes: the role of integrins. Faseb j 18, 977–979. [DOI] [PubMed] [Google Scholar]
- Collot M, Ashokkumar P, Anton H, Boutant E, Faklaris O, Galli T, Mély Y, Danglot L, and Klymchenko AS (2019). MemBright: a family of fluorescent membrane probes for advanced cellular imaging and neuroscience. Cell chemical biology 26, 600–614. e607. [DOI] [PubMed] [Google Scholar]
- Colvin RA, Means TK, Diefenbach TJ, Moita LF, Friday RP, Sever S, Campanella GS, Abrazinski T, Manice LA, Moita C, et al. (2010). Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol 11, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan L, Redhai S, Leiblich A, Fan SJ, Perera SM, Patel R, Gandy C, Wainwright SM, Morris JF, Hamdy F, et al. (2014). BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior. J Cell Biol 206, 671–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska S, Del Fattore A, Karnas E, Frontczak-Baniewicz M, Kozlowska H, Muraca M, Janowski M, and Lukomska B (2018). Imaging of extracellular vesicles derived from human bone marrow mesenchymal stem cells using fluorescent and magnetic labels. Int J Nanomedicine 13, 1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, and McLean PJ (2012). Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, and Coffey RJ (2013). Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 12, 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers LM, Bordeleau F, Reinhart-King CA, Cerione RA, and Antonyak MA (2016). Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nature Communications 7, 11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, True LD, Rubin MA, Adam RM, Beroukhim R, et al. (2009). Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer research 69, 5601–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliaz D, Kannan S, Shaked H, Arvatz G, Tkacz ID, Binder L, Waldman Ben-Asher H, Okalang U, Chikne V, Cohen-Chalamish S, et al. (2017). Exosome secretion affects social motility in Trypanosoma brucei. PLoS Pathog 13, e1006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser J, Gehrmann U, D’Alexandri FL, Hidalgo-Estévez AM, Wheelock CE, Scheynius A, Gabrielsson S, and Rådmark O (2010). Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. Journal of Allergy and Clinical Immunology 126, 1032–1040. [DOI] [PubMed] [Google Scholar]
- Fedele C, Singh A, Zerlanko BJ, Iozzo RV, and Languino LR (2015). The αvβ6 integrin is transferred intercellularly via exosomes. J Biol Chem 290, 4545–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, and Gilmour D (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nature Reviews Molecular Cell Biology 10, 445–457. [DOI] [PubMed] [Google Scholar]
- Friedl P, and Mayor R (2017). Tuning Collective Cell Migration by Cell-Cell Junction Regulation. Cold Spring Harbor perspectives in biology 9, a029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger A, Walker A, and Nissen E (2015). Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochemical and Biophysical Research Communications 467, 303–309. [DOI] [PubMed] [Google Scholar]
- Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, Abdul Roda M, Xu X, Rezonzew G, Viera L, et al. (2019). Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 176, 113–126.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JC, Chaudhary V, Bartscherer K, and Boutros M (2012). Active Wnt proteins are secreted on exosomes. Nature Cell Biology 14, 1036–1045. [DOI] [PubMed] [Google Scholar]
- Hakulinen J, Sankkila L, Sugiyama N, Lehti K, and Keski-Oja J (2008). Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. Journal of cellular biochemistry 105, 1211–1218. [DOI] [PubMed] [Google Scholar]
- Hikita T, Miyata M, Watanabe R, and Oneyama C (2018). Sensitive and rapid quantification of exosomes by fusing luciferase to exosome marker proteins. Sci Rep 8, 14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz R, and Webb D (2003). Cell migration. Current Biology 13, R756–R759. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, and Weaver AM (2013). Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 5, 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Beheshti E, Pham S, Adomat H, Li N, and Tomlinson Guns ES (2012). Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol Cell Proteomics 11, 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, et al. (2010). Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 189, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zucker B, Zhang S, Elias S, Zhu Y, Chen H, Ding T, Li Y, Sun Y, Lou J, et al. (2019). Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nature Cell Biology 21, 991–1002. [DOI] [PubMed] [Google Scholar]
- Hur YH, Feng S, Wilson KF, Cerione RA, and Antonyak MA (2020). Embryonic Stem Cell-Derived Extracellular Vesicles Maintain ESC Stemness by Activating FAK. Developmental Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH (2010). The ESCRT complexes. Crit Rev Biochem Mol Biol 45, 463–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz SN, Cheerathodi MR, Nkosi D, York SB, and Meckes DG Jr. (2018). Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1. J Virol 92, e01969–01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, Tak S, Lefebvre O, Schwab Y, and Goetz JG (2015). RAL-1 controls multivesicular body biogenesis and exosome secretion. Journal of Cell Biology 211, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyenne V, Ghoroghi S, Collot M, Bons J, Follain G, Harlepp S, Mary B, Bauer J, Mercier L, Busnelli I, et al. (2019). Studying the Fate of Tumor Extracellular Vesicles at High Spatiotemporal Resolution Using the Zebrafish Embryo. Dev Cell 48, 554–572.e557. [DOI] [PubMed] [Google Scholar]
- Jiang D, Jiang Z, Lu D, Wang X, Liang H, Zhang J, Meng Y, Li Y, Wu D, Huang Y, et al. (2019). Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation. Nature Cell Biology 21, 966–977. [DOI] [PubMed] [Google Scholar]
- Jimenez L, Yu H, McKenzie AJ, Franklin JL, Patton JG, Liu Q, and Weaver AM (2019). Quantitative Proteomic Analysis of Small and Large Extracellular Vesicles (EVs) Reveals Enrichment of Adhesion Proteins in Small EVs. J Proteome Res 18, 947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, and LeBleu VS (2020). The biology, function, and biomedical applications of exosomes. Science 367, eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, and Werb Z (2010). Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Philippe N, Le Deist F, Fraitag S, Prost C, Durandy A, Fischer A, and Griscelli C (1994). Partial albinism with immunodeficiency (Griscelli syndrome). J Pediatr 125, 886–895. [DOI] [PubMed] [Google Scholar]
- Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, and Budnik V (2012). Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. The Journal of biological chemistry 287, 16820–16834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, and Ochieng J (2011). Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS One 6, e24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel PW, Barr VA, Rericha EC, Zhang G, and Parent CA (2008). Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. Journal of Cell Biology 183, 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel PW, Majumdar R, Jenkins LM, Senoo H, Wang W, Ammu S, Chen S, Narayan K, Iijima M, and Parent CA (2018). Extracellular vesicles direct migration by synthesizing and releasing chemotactic signals. J Cell Biol 217, 2891–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyung JW, Bae JR, Kim D-H, Song WK, and Kim SH (2016). Epsin1 modulates synaptic vesicle retrieval capacity at CNS synapses. Scientific Reports 6, 31997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghezza Masci V, Taddei AR, Gambellini G, Giorgi F, and Fausto AM (2016). Microvesicles shed from fibroblasts act as metalloproteinase carriers in a 3-D collagen matrix. Journal of circulating biomarkers 5, 1849454416663660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, and Breakefield XO (2015). Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nature Communications 6, 7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, and Breakefield XO (2014). Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, and Germain RN (2013). Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, et al. (2019). M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res 79, 146–158. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang X, Wang J, Li M, Cao C, Tan J, Ma D, and Gao Q (2017). TGFβ1 in fibroblasts-derived exosomes promotes epithelial-mesenchymal transition of ovarian cancer cells. Oncotarget 8, 96035–96047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Peng P, Chen S, Li L, Zhang M, Cao D, Yang J, Li H, Gui T, Li X, et al. (2013). Characterization and proteomic analysis of ovarian cancer-derived exosomes. J Proteomics 80, 171–182. [DOI] [PubMed] [Google Scholar]
- Liew PX, and Kubes P (2019). The Neutrophil’s Role During Health and Disease. Physiological Reviews 99, 1223–1248. [DOI] [PubMed] [Google Scholar]
- Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan MLG, Gibson GA, Watkins SC, Larregina AT, et al. (2016). Donor dendritic cell–derived exosomes promote allograft-targeting immune response. The Journal of Clinical Investigation 126, 2805–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Hope LW, Brasch M, Reinhard C, and Cohen SN (2003). TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proceedings of the National Academy of Sciences 100, 7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, and Wrana JL (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151, 1542–1556. [DOI] [PubMed] [Google Scholar]
- Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, Chen L, Yan X, Du Y, and Yu L (2015). Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Research 25, 24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas SLN, Breakefield XO, and Weaver AM (2017). Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol 27, 172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin R, Wang H, Loo D, Martin S, Cumming A, Cai N, Lane R, Ponce NS, Topkas E, Inder K, et al. (2016). Extracellular vesicles secreted by highly metastatic clonal variants of osteosarcoma preferentially localize to the lungs and induce metastatic behaviour in poorly metastatic clones. Oncotarget 7, 43570–43587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M, Névo N, Jouve M, Valenzuela JI, Maurin M, Verweij F, Palmulli R, Lankar D, Dingli F, Loew D, et al. (2020). Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking and synchronized extracellular vesicle release of CD9 and CD63. bioRxiv, 2020.2010.2027.323766. [Google Scholar]
- Mayor R, and Etienne-Manneville S (2016). The front and rear of collective cell migration. Nature Reviews Molecular Cell Biology 17, 97–109. [DOI] [PubMed] [Google Scholar]
- McGough IJ, and Vincent J-P (2016). Exosomes in developmental signalling. Development 143, 2482. [DOI] [PubMed] [Google Scholar]
- McKelvey KJ, Powell KL, Ashton AW, Morris JM, and McCracken SA (2015). Exosomes: Mechanisms of Uptake. J Circ Biomark 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, and Banks RE (2004). Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 4, 4019–4031. [DOI] [PubMed] [Google Scholar]
- Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, Brown E, Jarvis R, and Yang Y (2019). Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nature Communications 10, 4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, et al. (2000). Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25, 173–176. [DOI] [PubMed] [Google Scholar]
- Messenger SW, Woo SS, Sun Z, and Martin TFJ (2018). A Ca(2+)-stimulated exosome release pathway in cancer cells is regulated by Munc13–4. The Journal of cell biology 217, 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, and Rothman JE (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195. [DOI] [PubMed] [Google Scholar]
- Mondal A, Ashiq KA, Phulpagar P, Singh DK, and Shiras A (2019). Effective Visualization and Easy Tracking of Extracellular Vesicles in Glioma Cells. Biol Proced Online 21, 4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto YV, Kojima S, Namba K, and Minamino T (2011). M153R mutation in a pH-sensitive green fluorescent protein stabilizes its fusion proteins. PLoS One 6, e19598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu W, Rana S, and Zöller M (2013). Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia 15, 875–IN874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, and D’Souza-Schorey C (2009). ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Current Biology 19, 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan JF, Hu R, Oh RS, Cohen SN, and Lu Q (2012). Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proceedings of the National Academy of Sciences 109, 4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckles VN, Morton MC, Holmberg JC, Sokolov AM, Nottoli T, Liu D, and Feliciano DM (2019). A transgenic inducible GFP extracellular-vesicle reporter (TIGER) mouse illuminates neonatal cortical astrocytes as a source of immunomodulatory extracellular vesicles. Scientific Reports 9, 3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M-A, Karunakaran D, Geoffrion M, Cheng Henry S, Tandoc K, Perisic Matic L, Hedin U, Maegdefessel L, Fish Jason E, and Rayner Katey J (2018). Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arteriosclerosis, Thrombosis, and Vascular Biology 38, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JME, Veltman D, and Kay RR (2015). Chemotaxis of a model organism: progress with Dictyostelium. Current Opinion in Cell Biology 36, 7–12. [DOI] [PubMed] [Google Scholar]
- Nicola AM, Frases S, and Casadevall A (2009). Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryot Cell 8, 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C, Wang X, Zhao M, Cai T, Liu P, Li J, Willard B, Zu L, Zhou E, Li Y, et al. (2016). Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J Am Heart Assoc 5, e004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden C, and Lecaudey V (2019). Collective cell migration: general themes and new paradigms. Current Opinion in Genetics & Development 57, 54–60. [DOI] [PubMed] [Google Scholar]
- Oleksiuk O, Abba M, Tezcan KC, Schaufler W, Bestvater F, Patil N, Birk U, Hafner M, Altevogt P, Cremer C, et al. (2015). Single-Molecule Localization Microscopy allows for the analysis of cancer metastasis-specific miRNA distribution on the nanoscale. Oncotarget 6, 44745–44757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature Cell Biology 12, 19–30. [DOI] [PubMed] [Google Scholar]
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters BCH, Cappariello A, van den Bosch MHJ, van Lent PLEM, Teti A, and van de Loo FAJ (2019). Macrophage-Derived Extracellular Vesicles as Carriers of Alarmins and Their Potential Involvement in Bone Homeostasis. Frontiers in Immunology 10, 1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pols MS, and Klumperman J (2009). Trafficking and function of the tetraspanin CD63. Exp Cell Res 315, 1584–1592. [DOI] [PubMed] [Google Scholar]
- Pužar Dominkuš P, Stenovec M, Sitar S, Lasič E, Zorec R, Plemenitaš A, Žagar E, Kreft M, and Lenassi M (2018). PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochimica et Biophysica Acta (BBA) - Biomembranes 1860, 1350–1361. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, and Hall A (2004). Cell migration: Rho GTPases lead the way. Developmental Biology 265, 23–32. [DOI] [PubMed] [Google Scholar]
- Rai A, Greening DW, Xu R, Suwakulsiri W, and Simpson RJ (2020). Exosomes Derived from the Human Primary Colorectal Cancer Cell Line SW480 Orchestrate Fibroblast-Led Cancer Invasion. Proteomics 20, e2000016. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, and Horwitz AR (2003). Cell Migration: Integrating Signals from Front to Back. Science 302, 1704. [DOI] [PubMed] [Google Scholar]
- Rilla K, Mustonen A-M, Arasu UT, Härkönen K, Matilainen J, and Nieminen P (2019). Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biology 75–76, 201–219. [DOI] [PubMed] [Google Scholar]
- Rørth P (2009). Collective cell migration. Annu Rev Cell Dev Biol 25, 407–429. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Granseth B, Odermatt B, Derevier A, and Lagnado L (2008). Imaging phluorin-based probes at hippocampal synapses. Methods Mol Biol 457, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, and Weiss SJ (2009). Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol 185, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, De Angelis D, Rothman JE, and Ryan TA (2000). The use of pHluorins for optical measurements of presynaptic activity. Biophys J 79, 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, and Weaver AM (2018). Extracellular vesicles: important collaborators in cancer progression. Essays Biochem 62, 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunderson SC, Schuberth PC, Dunn AC, Miller L, Hock BD, MacKay PA, Koch N, Jack RW, and McLellan AD (2008). Induction of Exosome Release in Primary B Cells Stimulated via CD40 and the IL-4 Receptor. The Journal of Immunology 180, 8146. [DOI] [PubMed] [Google Scholar]
- Schlienger S, Campbell S, and Claing A (2014). ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Molecular Biology of the Cell 25, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Fang H, Zhang J, Jiang M, Xue K, Ma J, Zhang J, Lei J, Zhang Y, Li B, et al. (2019). Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis via activating keratinocytes. FASEB J 33, 6813–6828. [DOI] [PubMed] [Google Scholar]
- Sheehan C, and Souza-Schorey C (2019). Tumor-derived extracellular vesicles: molecular parcels that enable regulation of the immune response in cancer. Journal of Cell Science 132, jcs235085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellard A, and Mayor R (2020). All Roads Lead to Directional Cell Migration. Trends in Cell Biology 30, 852–868. [DOI] [PubMed] [Google Scholar]
- Shen B, Wu N, Yang JM, and Gould SJ (2011). Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem 286, 14383–14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen JB (2019). Pitfalls associated with lipophilic fluorophore staining of extracellular vesicles for uptake studies. J Extracell Vesicles 8, 1582237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Fedele C, Lu H, Nevalainen MT, Keen JH, and Languino LR (2016). Exosome-mediated Transfer of αvβ3 Integrin from Tumorigenic to Nontumorigenic Cells Promotes a Migratory Phenotype. Mol Cancer Res 14, 1136–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Furze RC, Birrell MA, Rankin SM, Hume AN, and Seabra MC (2014). A role for Rab27 in neutrophil chemotaxis and lung recruitment. BMC Cell Biology 15, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhto N, Kanlaya R, Nilnumkhum A, and Thongboonkerd V (2018). Roles of Macrophage Exosomes in Immune Response to Calcium Oxalate Monohydrate Crystals. Front Immunol 9, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M, Tyska MJ, and Weaver AM (2016). Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol 214, 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogberg G, Gudmundsdottir J, van der Post S, Sandström K, Bruhn S, Benson M, Mincheva-Nilsson L, Baranov V, Telemo E, and Ekwall O (2013). Characterization of human thymic exosomes. PLoS One 8, e67554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino TA, Duong P, Bouchareychas L, Chen M, Chung A, Schaller MS, Oskowitz A, Raffai RL, and Conte MS (2020). Circulating exosomes from patients with peripheral artery disease influence vascular cell migration and contain distinct microRNA cargo. JVS Vasc Sci 1, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl PD, and Raposo G (2019). Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 34, 169–177. [DOI] [PubMed] [Google Scholar]
- Subramanian BC, Melis N, Chen D, Wang W, Gallardo D, Weigert R, and Parent CA (2020). The LTB4-BLT1 axis regulates actomyosin and β2-integrin dynamics during neutrophil extravasation. J Cell Biol 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LP, Xu K, Cui J, Yuan DY, Zou B, Li J, Liu JL, Li KY, Meng Z, and Zhang B (2019). Cancer‑associated fibroblast‑derived exosomal miR‑382‑5p promotes the migration and invasion of oral squamous cell carcinoma. Oncol Rep 42, 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, Ketova T, Hoshino D, Zijlstra A, and Weaver AM (2015). Directional cell movement through tissues is controlled by exosome secretion. Nat Commun 6, 7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, von Lersner A, Guerrero J, Krystofiak ES, Inman D, Pelletier R, Zijlstra A, Ponik SM, and Weaver AM (2020). A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat Commun 11, 2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, and Weaver AM (2017). Exosome secretion promotes chemotaxis of cancer cells. Cell Adh Migr 11, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, and Weaver AM (2018). Directed migration: Cells navigate by extracellular vesicles. Journal of Cell Biology 217, 2613–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szempruch AJ, Sykes SE, Kieft R, Dennison L, Becker AC, Gartrell A, Martin WJ, Nakayasu ES, Almeida IC, Hajduk SL, et al. (2016). Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell 164, 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, and Takakura Y (2013). Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol 165, 77–84. [DOI] [PubMed] [Google Scholar]
- Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, Shiina M, Fukushima K, Hoshino T, Sano K, et al. (2010). Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochemical and Biophysical Research Communications 399, 384–390. [DOI] [PubMed] [Google Scholar]
- Tao SC, and Guo SC (2020). Role of extracellular vesicles in tumour microenvironment. Cell Commun Signal 18, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F, and Fussenegger M (2020). Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv Sci (Weinh) 8, 2003505–2003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, and Simons M (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247. [DOI] [PubMed] [Google Scholar]
- Tuck S (2011). Extracellular Vesicles: Budding Regulated by a Phosphatidylethanolamine Translocase. Current Biology 21, R988–R990. [DOI] [PubMed] [Google Scholar]
- van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, and Raposo G (2011). The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 21, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij FJ, Bebelman MP, Jimenez CR, Garcia-Vallejo JJ, Janssen H, Neefjes J, Knol JC, de Goeij-de Haas R, Piersma SR, Baglio SR, et al. (2018). Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. Journal of Cell Biology 217, 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM, Follain G, Allio G, Goetz JG, Zimmermann P, et al. (2019). Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo. Dev Cell 48, 573–589.e574. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Xu H, Lei B, and Mao C (2019). Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 9, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehman AM, Poggioli C, Schweinsberg P, Grant BD, and Nance J (2011). The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Current Biology 21, 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H, Li M, Shi L, Pan C, and Zhu D (2017). Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nature communications 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortzel I, Dror S, Kenific CM, and Lyden D (2019). Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell 49, 347–360. [DOI] [PubMed] [Google Scholar]
- Wu D, Xu Y, Ding T, Zu Y, Yang C, and Yu L (2017). Pairing of integrins with ECM proteins determines migrasome formation. Cell Research 27, 1397–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Hao M, Yeo SK, and Guan JL (2020). FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene 39, 2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Möbius W, Hoernschemeyer J, Slot JW, Geuze HJ, and Stoorvogel W (2003). Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 278, 10963–10972. [DOI] [PubMed] [Google Scholar]
- Yamada KM, and Sixt M (2019). Mechanisms of 3D cell migration. Nature Reviews Molecular Cell Biology 20, 738–752. [DOI] [PubMed] [Google Scholar]
- Yeung C-YC, Schoof EM, Tamáš M, Mackey AL, and Kjaer M (2020). Proteomics identifies differences in fibrotic potential of extracellular vesicles from human tendon and muscle fibroblasts. Cell Communication and Signaling 18, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Kawamata M, Yoshioka Y, Katsuda T, Kikuchi H, Nagai Y, Adachi N, Numakawa T, Kunugi H, Ochiya T, et al. (2016). Generation of a novel transgenic rat model for tracing extracellular vesicles in body fluids. Sci Rep 6, 31172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Li M, Cao LM, Gu QH, Deng PB, Tan Y, and Hu CP (2019). Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. Qjm 112, 581–590. [DOI] [PubMed] [Google Scholar]
- Yue S, Mu W, Erb U, and Zöller M (2015). The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget 6, 2366–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Liu H, and Tang WH (2019). Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Li H, Guo Q, Zhou A, Wang X, Li P, and Zhang S (2020). Exosomal Sonic Hedgehog derived from cancer-associated fibroblasts promotes proliferation and migration of esophageal squamous cell carcinoma. Cancer Medicine 9, 2500–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhang W, Yao Q, Zhang H, Dong G, Zhang M, Liu Y, Chen JK, and Dong Z (2017). Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am J Physiol Renal Physiol 312, F963–F970. [DOI] [PMC free article] [PubMed] [Google Scholar]