Abstract
Background:
The brain–gut–microbiota axis plays a crucial role in the bidirectional interactions between the brain and the gut. Soluble epoxide hydrolase (coded by the Ephx2 gene) plays an important role in inflammation, which has been implicated in stress-related depression. Ephx2 knock-out (KO) mice exposed to chronic social defeat stress (CSDS) did not show depression-like behaviors, indicating stress resilience. Here we examined whether the brain–gut–microbiota axis influences the resilience in Ephx2 KO mice.
Methods:
Effects of fecal microbiota transplantation (FMT) from CSDS-susceptible (or control) mice in wild-type (WT) mice and Ephx2 KO mice treated with an antibiotic cocktail (ABX) were investigated. Behavioral, biochemical tests and 16S ribosome RNA analysis were performed.
Results:
FMT from CSDS-susceptible mice produced anhedonia-like behavior in ABX-treated WT and Ephx2 KO mice. The 16S ribosome RNA analysis showed that Faecalibaculum rodentium (F. rodentium) may be responsible for the observed anhedonia-like behavior following FMT from CSDS-susceptible mice. Ingestion of F. rodentium for 14 days produced depression- and anhedonia-like behaviors, higher blood levels of interleukin-6, and reduced expression of synaptic proteins in the prefrontal cortex of ABX-treated Ephx2 KO mice. Furthermore, subdiaphragmatic vagotomy blocked the development of these behavioral abnormalities after ingestion of F. rodentium.
Limitations:
Detailed mechanisms are unclear.
Conclusions:
These findings suggest that F. rodentium might contribute to the conversion of resilient Ephx2 KO mice into KO mice with depression-like phenotypes. The brain–gut–microbiota axis via the subdiaphragmatic vagus nerve plays a crucial role in susceptibility and resilience to stress.
Keywords: Depression, Microbiome, Resilience, Susceptibility, Vagus nerve
1. Introduction
Humans vary widely in their response to stress. Multiple lines of evidence have suggested that resilience is the ability of several neural circuits that are involved in the interface between the brain and the periphery to adapt to changing stress levels (Anacker et al., 2018; Cathomas et al., 2019; Dantzer et al., 2018; Feder et al., 2009; Franklin et al., 2012; Russo et al., 2012; Southwick et al., 2005). However, the precise mechanisms underlying resilience and vulnerability to stress remain poorly understood.
The brain–gut–microbiota axis is a complex multi-organ bidirectional signaling system between the brain and the gastrointestinal microbiota and plays an important role in host homeostasis (Cryan et al., 2019; Cussotto et al., 2018; Dinan and Cryan, 2017; Fung et al., 2017; Long-Smith et al., 2020). Numerous preclinical studies have demonstrated that an abnormal composition of gut microbiota may contribute to the resilience or susceptibility in rodents exposed to stress (Bear et al., 2021; Jianguo et al., 2019; Szyszkowicz et al., 2017; Wang et al., 2020a; Wang et al., 2020b; Yang et al., 2019; Yang et al., 2017; Zhang et al., 2019; Zhang et al., 2020).
Epoxy fatty acids, which are produced from polyunsaturated fatty acids through the action of cytochrome P450s, have shown to possess potent anti-inflammatory actions. These epoxy fatty acids are metabolized into the corresponding diols by soluble epoxide hydrolase (sEH; coded by the Ephx2 gene), and inhibition of sEH can enhance the beneficial effects of epoxy fatty acids (Atone et al., 2020; Hashimoto, 2019; Imig et al., 2009; Morisseau and Hammock, 2005, 2013; Swardfager et al., 2018; Wagner et al., 2017). We previously reported that sEH inhibitors have potent beneficial effects in animal models of depression, Parkinson’s disease, schizophrenia, and autism spectrum disorder (Hashimoto, 2016; Ma et al., 2019; Pu et al., 2020; Ren et al., 2016; Ren et al., 2018). Interesting, Ephx2 KO did not show anhedonia- and depression-like phenotypes after exposure to chronic social defeat stress (CSDS), which suggested that Ephx2 KO mice are resilient to stress (Hashimoto, 2016; Ren et al., 2016).Furthermore, we demonstrated that microbiome depletion by treatment with an antibiotic cocktail (ABX) was associated with resilience in mice after CSDS exposure (Wang et al., 2020b), and that fecal microbiome transplantation (FMT) from CSDS-susceptible mice caused anhedonia-like phenotypes in ABX-treated wild-type (WT) mice (Wang et al., 2020a), which suggested a crucial role of the brain–gut– microbiota axis in resilience and susceptibility. However, it remains unclear whether FMT from CSDS-susceptible mice causes depression-related behavioral abnormalities in resilient Ephx2 KO mice.
The purpose of this study was to examine the role of the brain–gut–microbiota axis in resilience of Ephx2 KO mice. First, we examined whether FMT from CSDS-susceptible mice causes behavioral and biochemical abnormalities in ABX-treated WT and Ephx2 KO mice. Furthermore, we determined the blood levels of pro-inflammatory cytokine interleukin-6 (IL-6) and synaptic proteins (i.e., GluA1 [α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor A1] and PSD-95 [postsynaptic density 95]) in the prefrontal cortex (PFC) because the expression of these synaptic proteins in the PFC from rodents with depression-like phenotypes was shown to be lower than that of rodents without depression-like phenotypes (Duman and Aghajanian, 2012; Ohgi et al., 2015; Zhang et al., 2017). Second, using 16S ribosome RNA gene sequencing, we examined the diversity and composition of the gut microbiota in fecal samples. In this study, we identified the microbe (Faecalibaculum rodentium [F. rodentium]) to be potentially responsible for the behavioral and biochemical changes in recipient mice following FMT from CSDS-susceptible mice. Third, we investigated whether ingestion of F. rodentium for 14 days causes depression-related behaviors in ABX-treated Ephx2 KO mice. Finally, because the vagus nerve is known to play a role in the communication between the brain and microbiota (Bonaz et al., 2018; Bravo et al., 2011; Cawthon and de La Serre, 2018; Cryan et al., 2019; Long-Smith et al., 2020; Pu et al., 2021; Wang et al., 2020a; Wang et al., 2020c; Zhang et al., 2020), we examined whether subdiaphragmatic vagotomy (SDV) blocks depression-related behaviors in ABX-treated Ephx2 KO mice after ingestion of F. rodentium.
2. Materials and Methods
2.1. Animals
Male adult C57BL/6 mice (n = 30, 8 weeks old, body weight = 20–25 g, Japan SLC, Inc., Hamamatsu, Japan) and male adult CD1 (ICR) mice (n = 20, 13–15 weeks old, body weight > 40 g, Japan SLC, Inc.) were used. A colony of Ephx2 KO mice (provided from UC Davis) with targeted deletion of sEH gene (Ephx2) which was backcrossed to C57BL/6 background were used (Sinal et al., 2000). Adult male Ephx2 KO mice (n = 83) were used in this study. Animals were housed under controlled temperatures and 12 h/12 h light/dark cycles (lights on between 07:00–19:00 h) with ad libitum access to food (CE-2; CLEA Japan, Inc., Tokyo, Japan) and water. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee (Permission number: 31–149, and 2–409). Animals were deeply anesthetized with isoflurane before being sacrificed via cervical dislocation. All efforts were made to minimize suffering.
2.2. Chronic social defeat stress (CSDS) model and behavioral tests
Detailed methods of CSDS and behavioral tests were given in Supplemental Information.
2.3. Collection of fecal samples, treatment with an antibiotic cocktail and FMT
Fresh fecal samples were collected from CSDS-susceptible mice and control (no CSDS) mice on days 12–14 in sterilized screw cap microtubes immediately after defecation and stored at −80°C until FMT, as previously reported (Wang et al., 2020b). The fecal samples were collected from each mouse around 9:00 – 10:00 on each day to avoid circadian effects on the microbiome. Totally about 30 collected tubes containing approximately 0.5 g of feces per each tube were used for FMT.
Based on previous reports (Pu et al., 2019; Pu et al., 2021; Wang et al., 2020a; 2020b; Yang et al., 2019; Zhan et al., 2018), broad-spectrum antibiotics (ABX: ampicillin 1 g/L, FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan: neomycin sulfate 1 g/L, Sigma-Aldrich Co. Ltd, St. Louis, MO, USA: metronidazole 1 g/L, FUJIFUILM Wako Pure Chemical Corporation, Tokyo, Japan) dissolved in drinking water were given ad libitum to male C57BL/6 mice for 14 consecutive days.
The drinking solution was renewed every 2 days.
Before FMT, fecal samples were removed from the freezer in every morning, and they were allowed to thaw for 10 – 15 min at room temperature. Then drinking water (10 mL/g feces) was added to the tube including the fecal samples. The drinking water including fecal samples (0.2 mL/mouse) was given to the ABX-treated wild-type (WT) and Ephx2 KO mice using gastric gavage for consecutive 14 days.
2.4. Transplantation of F. rodentium
Ephx2 KO mice were given drinking water alone or drinking water containing ABX on days 1–14. Subsequently, mice were divided into three groups: water + water group, ABX + water group, and ABX + microbe (Faecalibaculum rodentium) group.
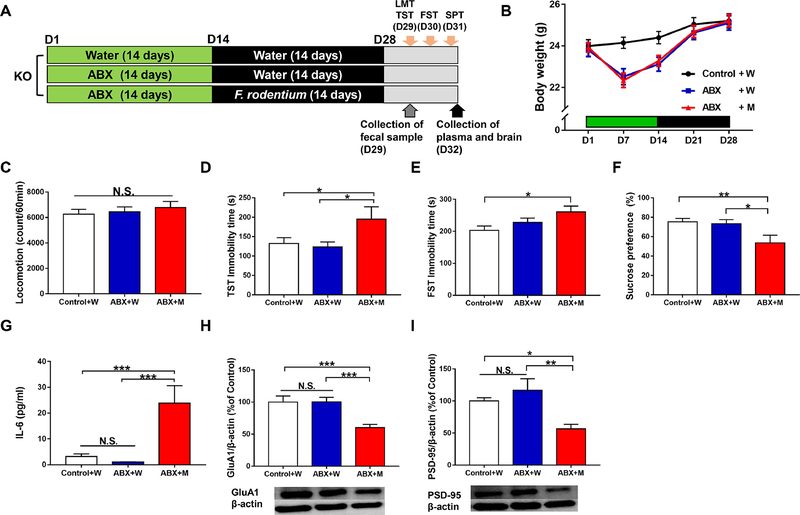
Faecalibaculum rodentium (F. rodentium, catalog number: JCM30274) (Chang et al., 2015; Lim et al., 2016) were purchased from RIKEN BioResource Research Center (Tsukuba, Ibaraki, Japan). Mice were orally administered water or water containing the microbes (approximately 1 × 108 CFU/day) for 14 days (days 15–28) using gastric gavage (Figure 4A). The locomotion test and tail suspension test (TST) were performed on day 29. The forced swimming test (FST) and 1% SPT were performed on days 30 and 31, respectively. On day 32, plasma samples and PFC samples were collected as described above, and stored at −80°C until use.
Figure 4. Effects of ingestion of F. rodentium in the antibiotic-treated Ephx2 KO mice.
(A): The schedule of treatment of antibiotic cocktail (ABX), microbiota transplantation, collection of fecal samples, behavioral tests and collection of plasma and brain. Antibiotic cocktail (ABX) or water in drinking water was given to adult male Ephx2 KO mice for 14 days (day 1 – day 14). Subsequently, F. rodentium were administered orally for 14 days (day 15 – day 28) using gastric gavage. On day 29, fresh fecal samples were collected before behavioral tests. On day 29, locomotion test (LMT) and tail suspension test (TST) were performed. Forced swimming test (FST) and 1% SPT were performed on day 30 and 31, respectively. On day 32, plasma and brain were collected. (B): Body weight (repeated measures one-way ANOVA, F2,29 = 2.265, P = 0.122). (C): Locomotion (LMT) (one-way ANOVA, F2,29= 0.380, P = 0.687). (D): TST (one-way ANOVA, F2,29 = 3.364, P = 0.049). (E): FST (one-way ANOVA, F2,29 = 3.545, P = 0.042). (F): SPT (one-way ANOVA, F2,29 = 4.767, P = 0.016). (G): Plasma IL-6 (one-way ANOVA, F2,29 = 11.308, P < 0.001). (H): GluA1 (one-way ANOVA, F2,29 = 9.126, P = 0.001). (I): PSD-95 (one-way ANOVA, F2,29 =6.630, P = 0.004). Data are shown as mean ± S.E.M. (n = 10 or 11). *P < 0.05, **P < 0.01, ***P < 0.001. NS: not significant. Control + W: water + water treated KO mice. ABX + W: antibiotic + water treated KO mice. ABX + M: antibiotic + microbe treated KO mice.
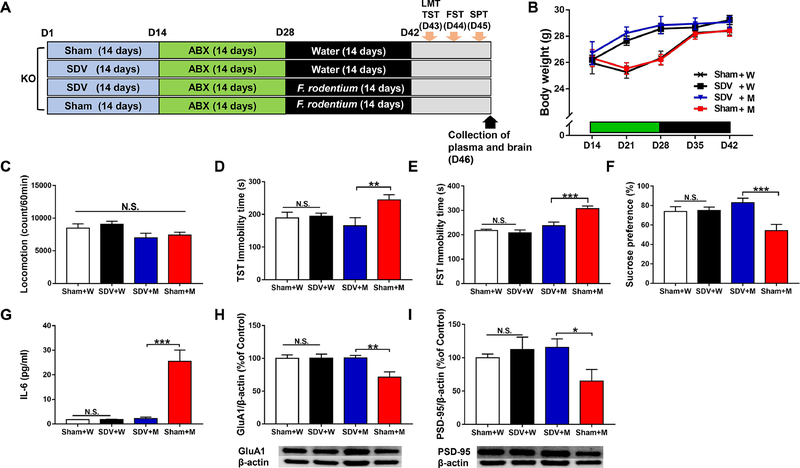
To examine the role of vagus nerve in the behavioral abnormalities in Ephx2 KO mice after ingestion of F. rodentium, we performed subdiaphragmatic vagotomy (SDV), as previously reported (Pu et al., 2021; Wang et al., 2020b; Zhang et al., 2020). Sham surgery or SDV was performed under anesthesia with 5% isoflurane. Mice were put under a microscope (Leica LEICA S9E, Germany), and hair was removed from the abdomen. The esophagus of each mouse was exposed to the full view. The ventral and dorsal vagus nerves of the esophagus were severed. After muscle and skin were sutured, mice were kept in clean cages until complete recovery from anesthesia. Then, mice were housed in cages for 14 days (days 1–14) (Figure 5A). ABX was given to all KO mice in drinking water for 14 days (from day 15 to day 28) (Figure 5A). Subsequently, mice were divided into four groups: sham + water group, SDV + water group, SDV + microbe (F. rodentium) group, and sham + microbe (F. rodentium) group. Water alone or water containing the microbes (approximately 1 × 108 CFU/day) was administered orally for 14 days (day 29 to day 42) using gastric gavage (Figure 5A). The locomotion test and TST were performed on day 43. The FST and 1% SPT were performed on days 44 and 45, respectively. On day 46, plasma samples and PFC tissues were collected and stored at −80°C until use.
Figure 5. Effects of SDV on behavioral abnormalities after transplantation of F. rodentium in the antibiotic-treated Ephx2 KO mice.
(A): The schedule of subdiaphragmatic vagotomy (SDV), treatment of antibiotic cocktail (ABX), F. rodentium transplantation, behavioral tests, and collection of plasma and brain. SDV or sham was performed in adult male Ephx2 KO mice, and mice were recovered 14 days after surgery (day 1 – day 14). Subsequently, antibiotic cocktail (ABX) in drinking water was given to all mice for 14 days (day 15 – day 28). Subsequently, transplantation of F. rodentium was performed for 14 days (day 29 – day 42). LMT and TST were performed on day 43. FST and 1% SPT were performed on day 44 and day 45, respectively. On day 46, plasma and brain were collected. (B): Body weight (repeated measures two-way ANOVA, SDV: F1,27 = 0.323, P = 0.575, microbe transplantation: F1,27 = 2.012, P = 0.168, interaction: F1,27 = 0.163, P = 0.689). (C): Locomotion (two-way ANOVA, SDV: F1,27 = 0.024, P = 0.878, microbe transplantation: F1,27 = 7.632, P = 0.010, interaction: F1,27 = 0.763, P = 0.390). (D): TST (two-way ANOVA, SDV: F1,27 = 4.645, P = 0.040, microbe transplantation: F1,27 = 0.594, P =0.448, interaction: F1,27= 6.088, P = 0.020). (E): FST (two-way ANOVA, SDV: F1,27 = 13.367, P = 0.001, microbe transplantation: F1,27 = 30.209, P <0.001, interaction: F1,27= 7.604, P = 0.010). (F): SPT (two-way ANOVA, SDV: F1,27 = 8.593, P = 0.007, microbe transplantation: F1,27 =1.349, P = 0.256, interaction: F1,27 = 7.482, P = 0.011). (G): Plasma IL-6 (two-way ANOVA, SDV: F1,27 = 23.632, P < 0.001, microbe transplantation: F1,27 = 25.599, P < 0.001, interaction: F1,27 = 24.023, P < 0.001). (H): GluA1 (two-way ANOVA, SDV: F1,27 = 5.622, P = 0.025, microbe transplantation: F1,27 =5.073, P = 0.033, interaction: F1,27 = 5.447, P = 0.027). (I): PSD-95 (two-way ANOVA, SDV: F1,27 = 4.564, P = 0.042, microbe transplantation: F1,27 = 1.172, P = 0.288, interaction: F1,27 = 1.684, P = 0.205). Data are shown as mean ± S.E.M. (n = 7 or 8). *P < 0.05, **P < 0.01, ***P < 0.001. NS: not significant. Sham + W: sham + water treated KO mice. SDV + W: SDV + water treated KO mice. SDV + M: SDV + microbe transplantation treated KO mice. Sham + M: sham + microbe transplantation treated KO mice.
2.5. Measurement of IL-6 and Western blot analysis
Detailed methods of IL-6 ELISA and Western blot were given in Supplemental Information.
2.6. 16S ribosome RNA analysis and measurement of short-chain fatty acids
Detailed methods of 16S ribosomal RNA analysis and measurement of SCFAs in fecal samples were given in Supplemental Information.
2.7. Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). The body weight data were analyzed using repeated measures one-way or two-way analysis of variance (ANOVA), followed by Fisher’s least significant difference (LSD) test. Comparisons among four groups were performed using two-way ANOVA followed by Fisher’s LSD test or the Kruskal–Wallis test, followed by the Mann-Whitney U-test. Data for alpha-diversity of the gut microbiota were analyzed using the Kruskal–Wallis test, followed by the Mann-Whitney U-test. For beta-diversity of the gut microbiota, principal component analysis (PCA) based on the OTU level was performed using analysis of similarities (ANOSIM) by R package vegan (2.5.4) (Xia and Sun, 2017). Correlations between SCFAs and the relative abundance of bacteria were analyzed by Spearman’s correlation. The P-values of less than 0.05 were considered statistically significant.
3. Results
3.1. FMT from CSDS-susceptible mice induced an anhedonia-phenotype in antibiotic-treated mice
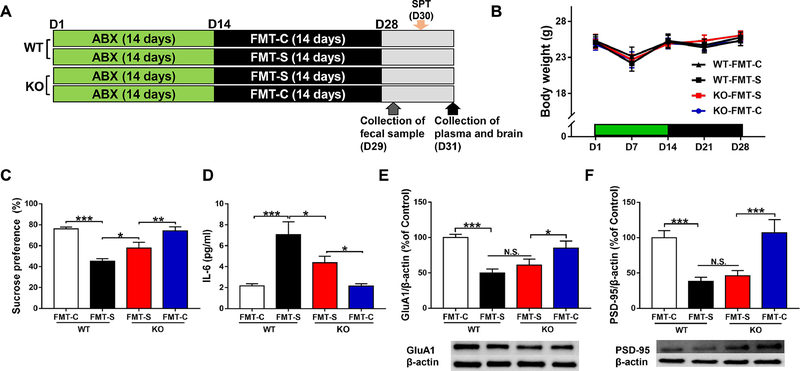
We used an antibiotic cocktail (ABX: ampicillin 1 g/L, neomycin sulfate 1 g/L, and metronidazole 1 g/L) for microbiome depletion as reported previously (Wang et al., 2020a; Wang et al., 2020b). First, we investigated the effects of FMT from CSDS-susceptible mice in WT mice and Ephx2 KO mice treated with ABX (Figure 1A). There were no changes in body weight among the four groups (Figure 1B).
Figure 1. Effects of FMT in WT and Ephx2 KO mice treated with antibiotic cocktail.
(A): The schedule of treatment with antibiotics (ABX), fecal microbiota transplantation (FMT), feces collection, sucrose preference test (SPT), and collection of plasma and brain. Antibiotic cocktail in drinking water was given to adult male WT and KO mice for 14 days (day 1 – day 14). Subsequently, FMT of control (no CSDS) mice or CSDS-susceptible mice was performed for 14 days (day 15 – day 28). On day 29, fecal samples were collected. On day 30, 1% SPT was performed. On day 31, plasma and brain (i.e., PFC) were collected. (B): Body weight (repeated measures two-way ANOVA, genotype: F1,36 = 0.001, P =0.981, FMT: F1,36 = 0.078, P = 0.782, interaction: F1,36= 0.038, P = 0.846). (C): SPT (two-way ANOVA, genotype: F1,36 = 2.102, P =0.156, FMT: F1,36=41.424, P<0.001, interaction: F1,36 = 3.650, P = 0.064). (D): Plasma IL-6 (two-way ANOVA, genotype: F1,36 = 3.532, P = 0.068, FMT: F1,36 = 24.926, P < 0.001, interaction: F1,36 =3.497, P = 0.070). (E): GluA1 (two-way ANOVA, genotype: F1,36 = 0.062, P = 0.804, FMT: F1,36 = 23.814, P < 0.001, interaction: F1,36 = 2.924, P = 0.096). (F): PSD-95 (two-way ANOVA, genotype: F1,36 = 0.381, P = 0.541, FMT: F1,36 = 27.647, P < 0.001, interaction: F1,36 =0.001, P = 0.973). Data are shown as mean ± S.E.M. (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001. FMT: fecal microbiota transplantation. NS: not significant. WT + FMT-C: Wild-type mice + FMT from control (no CSDS) mice. WT + FMT-S: Wild-type mice + FMT from CSDS susceptible mice. KO + FMT-S: Ephx2 KO mice + FMT from CSDS-susceptible mice. KO + FMT-C: Ephx2 KO mice + FMT from control (no CSDS) mice.
In the 1% SPT, FMT from CSDS-susceptible mice produced significant reductions in sucrose preference in ABX-treated WT mice (Figure 1C), which is consistent with a previous report (Wang et al., 2020b). Furthermore, FMT from CSDS-susceptible mice produced the reduced sucrose preference of SPT in ABX-treated Ephx2 KO mice, which was indicative of an anhedonia-like phenotype in the KO mice. Interestingly, we observed significantly higher sucrose preference in Ephx2 KO mice compared with WT mice following FMT from CSDS-susceptible mice (Figure 1C), which suggested that KO mice were more resistant than WT mice. In contrast, sucrose preference of WT mice and Ephx2 KO mice did not differ after FMT from control (no CSDS) mice (Figure 1C).
FMT from CSDS-susceptible mice caused significant increases in blood IL-6 levels in both the Ephx2 KO and WT mice, although blood IL-6 levels were significantly lower in Ephx2 KO mice compared with WT mice (Figure 1D). Blood IL-6 levels did not differ significantly in either the WT or Ephx2 KO mice after FMT from control (no CSDS) mice (Figure 1D).
FMT from CSDS-susceptible mice significantly decreased protein expression of GluA1 and PSD-95 in the PFC of both ABX-treated WT and Ephx2 KO mice (Figure 1E and 1F). Furthermore, there were no changes between two groups after FMT from CSDS-susceptible mice. There were no significant changes in GluA1 or PSD-95 expression in the PFC of either group after FMT from control mice (Figure 1E and 1F).
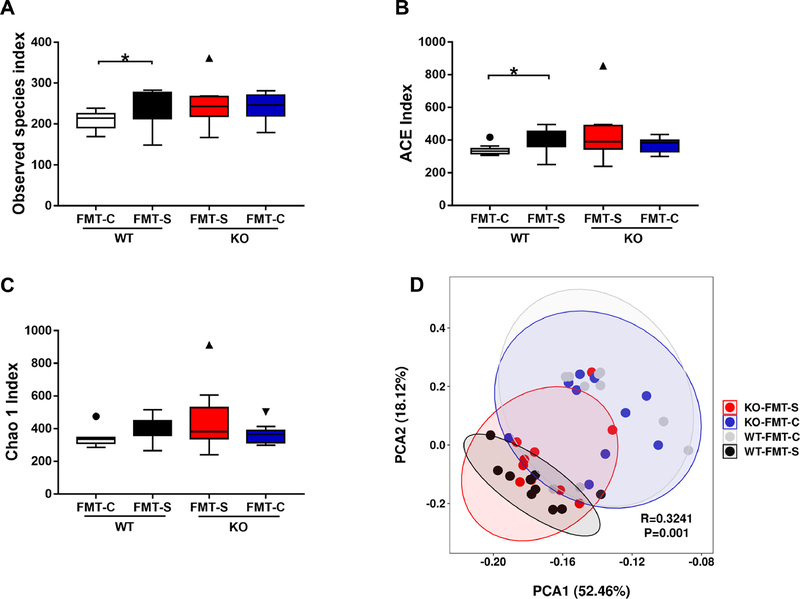
3.2. Abnormal diversity of gut microbiota after FMT from CSDS-susceptible mice
The diversity and composition of gut microbiota in the four groups were analyzed using alpha- and beta-diversity. The Kruskal–Wallis test revealed significant differences in the observed species index and ACE index between the four groups (Figure 2A and 2B). Post-hoc analyses showed that the alpha-diversity in the WT mice after FMT from CSDS-susceptible mice was significantly higher than that in WT mice after FMT from control mice, whereas alpha-diversity was not altered in the Ephx2 KO mice after FMT from either CSDS-susceptible or control mice (Figure 2A and 2B). In contrast, there was no difference in Chao 1 index between the four groups (Figure 2C). Regarding beta-diversity, PCA was applied to analyze the bacterial community composition of gut microbiota in the four groups (Figure 2D). PCA revealed significant separation in the community composition, which was evaluated using an analysis of similarities (R = 0.3241, P = 0.001) (Figure 2D).
Figure 2. Effects of antibiotic cocktail and different genotype of mice in the diversity of gut microbiota after FMT.
(A): Observed species index (Kruskal-Wallis test, X2 = 7.861 P = 0.049) (B): ACE index (Kruskal-Wallis test, X2 = 8.537 P = 0.036). (C): Chao 1 index (Kruskal-Wallis test, antibiotics: X2 = 6.631 P = 0.085). (D): PCA analysis of gut bacteria data R = 0.3241 P = 0.001) Data are shown as mean ± S.E.M. (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001. NS: not significant. FMT: fecal microbiota transplantation. NS: not significant. WT + FMT-C: Wild-type mice+ FMT from control (no CSDS) mice. WT + FMT-S: Wild-type mice + FMT from CSDS susceptible mice. KO + FMT-S: Ephx2 KO mice + FMT from CSDS-susceptible mice. KO + FMT-C: Ephx2 KO mice + FMT from control (no CSDS) mice.
3.3. Abnormal composition of gut microbiota at the taxonomic level
At the genus level, five bacteria (Akkermansia, Butyricimonas, Faecalibaculum Parabacteroides, and Ruminococcus) were detected in the four groups (Figure S1A–F). Interestingly, FMT from CSDS-susceptible mice significantly increased the relative abundance of Faecalibaculum in both WT and KO groups compared with the two groups that underwent FMT from control mice (Figure S1D).
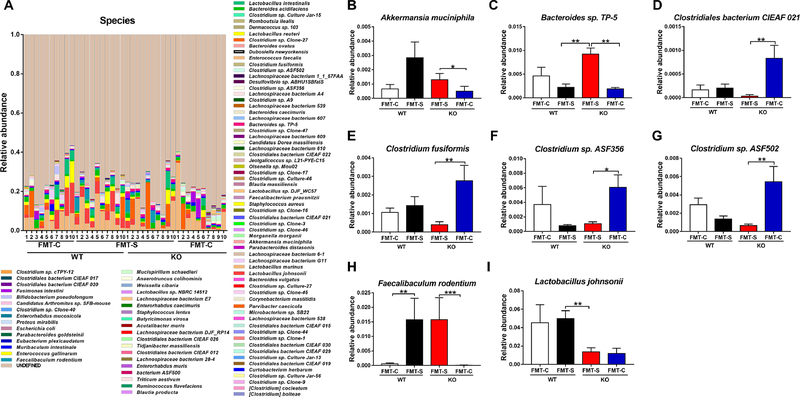
At the species level, eight bacteria [Akkermansia muciniphila, Bacteroides sp. TP-5, Clostridiales bacterium CIEAF 021, Clostridium fusiformis, Clostridum sp. ASF356, Clostridium sp. ASF502, F. rodentium, and Lactobacillus johnsonii (L. johnsonii)] were detected in the four groups (Figure 3A–I). Interestingly, FMT from CSDS-susceptible mice significantly increased the relative abundance of F. rodentium in both WT and KO groups (Figure 3H), which suggested that F. rodentium may be responsible for the anhedonia-like behavior observed in both groups after FMT from CSDS-susceptible mice.
Figure 3. Altered composition in the gut microbiota at the species level.
(A): The relative abundances of species in fecal samples of the four groups. (B): Akkermansia muciniphila (Kruskal-Wallis test, X2 = 8.607 P = 0.035). (C): Bacteroides sp. TP-5 (Kruskal-Wallis test, X2 = 14.821 P = 0.002). (D): Clostridiales bacterium CIEAF 021 (Kruskal-Wallis test, X2 = 13.240 P = 0.004). (E): Clostridium fusiformis (Kruskal-Wallis test, X2 = 10.053 P = 0.018). (F): Clostridium sp. ASF356 (Kruskal-Wallis test, X2 = 10.858 P = 0.013). (G): Clostridium sp. ASF502 (Kruskal-Wallis test, X2 = 11.198 P = 0.011). (H): Faecalibaculum rodentium (Kruskal-Wallis test, X2 = 21.971 P < 0.001). (I): Lactobacillus johnsonii (Kruskal-Wallis test, X2 = 11.701 P = 0.008). Data are shown as mean ± S.E.M. (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001. FMT: fecal microbiota transplantation. NS: not significant. WT + FMT-C: Wild-type mice+ FMT from control (no CSDS) mice. WT + FMT-S: Wild-type mice + FMT from CSDS-susceptible mice. KO + FMT-S: Ephx2 KO mice + FMT from CSDS-susceptible mice. KO + FMT-C: Ephx2 KO mice + FMT from control (no CSDS) mice.
3.4. Levels of short chain fatty acids in fecal samples
Short-chain fatty acids (SCFAs) produced by microbiome play a role in brain-gut communication (Dalile et al., 2019; Silva et al., 2020; Wu et al., 2020). There were significant genetic effects in acetic acid, lactic acid, and propionic acid (Figure S2A–C). However, there were no changes in butyric acid and succinic acid in any of the four groups (Figure S2D and S2E). Levels of lactic acid and propionic acid after FMT from CSDS-susceptible mice were significantly higher in WT mice than in Ephx2 KO mice (Figure S2B and S2C). There was a positive correlation (r = 0.621, P <0.001) between the relative abundance of Lactobacillus johnsonii and lactic acid levels in all groups (Figure S2F).
3.5. Ingestion of F. rodentium caused depression-like behaviors in antibiotic-treated Ephx2 KO mice
Next, we investigated whether F. rodentium causes depression-related behavior phenotypes in ABX-treated Ephx2 KO mice (Figure 4A). There were no changes in either body weight (Figure 4B) or locomotion (Figure 4C) in any of the three groups. Immobility time in the tail suspension test (TST) in the ABX + microbe group was significantly higher than that in both the water + water and antibiotic + water groups (Figure 4D). Immobility time in the forced swimming test (FST) in the ABX + microbe group was significantly higher than that in the water + water group, but not in the ABX + water group (Figure 4E). Furthermore, sucrose preference of SPT in the ABX + microbe group was significantly lower than that in both the water + water and ABX + water groups (Figure 4F).
Blood IL-6 levels in the ABX + microbe group were significantly higher than in the other two groups (Figure 4G). Moreover, the expression of synaptic proteins (i.e., GluA1 and PSD-95) in the PFC of the ABX + microbe group was significantly lower than in the other two groups (Figure 4H and 4I). Unexpectedly, we did not detect increases in F. rodentium abundance in host intestine after repeated ingestion of F. rodentium (Figure S3G). The data suggest that showed that repeated ingestion of F. rodentium causes depression-related behaviors, systemic inflammation, and reduction in the expression of synaptic proteins in the PFC in antibiotic-treated Ephx2 KO mice.
3.6. Blocking of anhedonia- and depression-like behaviors by SDV in antibiotic-treated Ephx2 KO mice after ingestion of F. rodentium
To examine the role of the vagus nerve in depression-related behaviors in antibiotic-treated Ephx2 KO mice after ingestion of F. rodentium, we performed SDV (Figure 5A). There were no changes in body weight (Figure 5B) or locomotion (Figure 5C) in any of the four groups. The immobility times of the TST and FST in the SDV + microbe group were significantly lower than those in the sham + microbe group (Figure 5D and 5E). Furthermore, sucrose preference of SPT in the SDV + microbe group was significantly higher than that in the sham + microbe group (Figure 5F). Moreover, blood IL-6 levels in the SDV + microbe group were significantly lower than in the sham + microbe group (Figure 5G). The expression of GluA1 and PSD-95 in the PFC of the SDV + microbe group was significantly higher than that in the sham + microbe group (Figure 5H and I).
The data show that SDV blocked the development of behavioral abnormalities, increased blood IL-6 levels, and decreased expression of synaptic proteins in the PFC in antibiotic-treated Ephx2 KO mice after oral ingestion of F. rodentium.
4. Discussion
There were several major findings in the current study. First, FMT from CSDS-susceptible mice produced anhedonia-like behavior, systemic inflammation, and downregulation of synaptic proteins in the PFC of WT mice and Ephx2 KO mice treated with ABX. Sucrose preference of Ephx2 KO mice after FMT from CSDS-susceptible mice was higher than that of WT mice after FMT from CSDS-susceptible mice, which suggested that KO mice are more resilient than WT mice. In contrast, sucrose preference of both WT and Ephx2 KO mice after FMT from control (no CSDS) mice was not different. Second, 16S ribosome RNA analysis showed that alpha-diversity in WT mice was increased after FMT from CSDS-susceptible mice, but not in Ephx2 KO mice. The PCA showed different distributions of bacteria among the four groups. At the species level, the relative abundance of F. rodentium was higher in both WT and KO mice after FMT from CSDS-susceptible mice than those mice that underwent FMT from control (no CSDS) mice, which suggested that F. rodentium may be responsible for these changes. Third, oral ingestion of F. rodentium for 14 days caused depression-related abnormal behaviors, higher blood IL-6 levels, and downregulation of synaptic proteins in the PFC in ABX-treated Ephx2 KO mice. Finally, SDV blocked these behavioral and biochemical changes in the ABX-treated Ephx2 KO mice after oral injection of F. rodentium. Collectively, it is likely that F. rodentium induces depression-related abnormal behavior in ABX-treated Ephx2 KO mice through the brain–gut–microbiota axis via the vagus nerve. It is noteworthy that ingestion of F. rodentium can convert resilient Ephx2 KO mice into KO mice with depression-like phenotypes via the brain– gut–microbiota axis.
It is known that treatment with ABX can induce dramatic alterations in the diversity and composition of gut microbiota in the host intestine (Becattini et al., 2016). We have previously reported that FMT from CSDS-susceptible mice do not produce anhedonia-like phenotype, higher blood IL-6 levels, or downregulation of synaptic proteins in the PFC in water-treated WT mice but do so in ABX-treated WT mice after FMT from CSDS-susceptible mice (Wang et al., 2020a). Thus, microbiome depletion by ABX is necessary for these changes to occur in recipient WT mice after administration of “anhedonia-related microbes” obtained from CSDS-susceptible mice (Wang et al., 2020a). In this study, we found that FMT from CSDS-susceptible mice produced anhedonia-like behavior, systemic inflammation, and reduced expression of synaptic proteins in the PFC of WT mice and Ephx2 KO mice treated with ABX. In contrast, we previously reported that Ephx2 KO mice did not show anhedonia- and depression-like phenotypes after CSDS, which indicated that KO mice are resilient to stress (Ren et al., 2016). Taken together, we demonstrated that FMT in antibiotic-treated Ephx2 KO mice using “anhedonia-related microbes” obtained from CSDS-susceptible mice causes anhedonia-like behavior through systemic inflammation. The precise mechanisms underlying the anhedonia-like phenotype in ABX-treated Ephx2 KO mice caused by FMT of “anhedonia-related microbes” from CSDS-susceptible mice remain unknown. It seems that FMT using “anhedonia-related microbes” from CSDS-susceptible mice may produce systemic inflammation in recipient mice, resulting in an anhedonia-like phenotype.
We found that the relative abundance of F. rodentium in mice with FMT from CSDS-susceptible mice was higher than that in mice with FMT from control (no CSDS) mice, which suggested that F. rodentium is responsible for the anhedonia-like behavior. Oral ingestion of F. rodentium for 14 days produced depression-related behaviors and reduced the expression of synaptic proteins in the PFC in ABX-treated Ephx2 KO mice through systemic inflammation, even though these KO mice were resilient to CSDS (Ren et al., 2016). However, we could not detect a higher abundance of F. rodentium in the ABX + microbiome group although the microbiome were given orally for 14 days. The data suggest that F. rodentium did not colonize in the intestine after repeated ingestion. Although the precise mechanisms by which ingestion of F. rodentium causes depressive-like behaviors in Ephx2 KO mice are currently unknown, it seems that metabolites or bacterial products from F. rodentium may contribute to systemic inflammation, resulting in depression-like phenotypes in mice. Nonetheless, further study is needed to investigate the mechanisms for F. rodentium-induced depression-like phenotypes in ABX-treated Ephx2 KO mice.
Yang et al. (2019) showed that FMT from “anhedonia susceptible rats” exaggerated depression-like phenotypes, such as anhedonia, in ABX-treated mice. In contrast, FMT from “anhedonia resilient rats” could improve depression-like phenotypes, including anhedonia, in ABX-treated mice (Yang et al., 2019). Furthermore, we previously reported that microbiome depletion by ABX might contribute to stress resilience in mice after exposure to CSDS via the brain–gut– microbiome axis (Wang et al., 2020b). In this study, we found that repeated ingestion of F. rodentium produced behavioral and biochemical abnormalities in ABX-treated Ephx2 KO mice through systemic inflammation. The data suggested that F. rodentium facilitates susceptibility in ABX-treated resilient Ephx2 KO mice, although it remains unclear how Ephx2 regulates gut microorganisms in the host. A recent study showed that sEH is an endogenous regulator of obesity-induced intestinal barrier dysfunction, which leads to systemic inflammation (Wang et al., 2020c). Although detailed mechanisms underlying depression-like phenotypes by ingestion of F. rodentium are unknown, it seems that systemic inflammation may play a role in the conversion from resilience to susceptibility. Increasing evidence suggests that promoting the growth of commensal bacteria is likely to confer some increase in resilience under stress (Bear et al., 2021). Future research is needed to study the relationship between F. rodentiusm in the host gastrointestinal tract, susceptibility and resilience.
The fastest and most direct pathway for gut microbiota to influence the brain may be by hijacking vagus nerve signaling (Fulling et al., 2019). The subdiaphragmatic vagus nerve plays a crucial role in the crosstalk between the brain and gut microbiota, and is thought to contribute to depression (Pu et al., 2021; Wan et al., 2021; Wang et al., 2020a; Zhang et al., 2020). We recently reported that SDV blocks depression-like behaviors and altered composition of gut microbiota after a single injection of lipopolysaccharide (Zhang et al., 2020). It has also been reported that the vagus nerve mediates anti-inflammatory effects of antidepressants (Ondicova et al., 2019). In addition, we have previously reported that SDV significantly blocks depression-related behaviors in ABX-treated mice following repeated ingestion of Lactobacillus intestinalis and Lactobacillus reuteri (Wang et al., 2020a). In this study, we found that SDV significantly blocked blood IL-6 levels and depression-related behaviors in ABX-treated Ephx2 KO mice after repeated ingestion of F. rodentium. A recent study showed that systemic IL-6 is originated from adipocytes during acute psychological stress (Qing et al., 2020). Collectively, it seems that the subdiaphragmatic vagus nerve plays an important role in systemic inflammation and depression-like behaviors in Ephx2 KO mice after ingestion of F. rodentium.
Interestingly, vagus nerve stimulation has been used in the treatment of treatment-resistant epilepsy and other brain disorders such as depression, anxiety, autism spectrum disorder, and Alzheimer’s disease (Mridha et al., 2021). It is suggested that the benefits of vagus nerve stimulation may be at least partially mediated by the recruitment of the acetylcholine system (Mridha et al., 2021). Therefore, it may be interesting to investigate the role of acetylcholine system in the effects of SDV in depression-like behaviors after ingestion of F. rodentium.
It has been reported that F. rodentium is enriched in mice fed with a diet free comprising aryl hydrocarbon receptor, which leads to inflammatory diseases such as hepatic steatosis (Brawner et al., 2019; Wada et al., 2016) and obesity (Sharma et al., 2018). A recent study showed that F. rodentium strongly correlated with phospholipoids such as phosphatidylethanolamine, phosphatidylcholine, and phosphoglyceride (Lee et al., 2020), indicating the important role of F. rodentium in the phospholipid synthesis. Furthermore, a recent study using a mouse model of colorectal cancer showed that F. rodentium (F. PB1), an endogenous strain of the mouse microbiota, is underrepresented in early stage of tumorigenesis (Zagato et al., 2020). Although the detailed functions of F. rodentium are currently unknown, it seems that F. rodentium may play a role in the phospholipid synthesis which is involved in progression of cancer (Cheng et al., 2016). Here, we found that oral ingestion of F. rodentium in antibiotic-treated resilient Ephx2 KO mice caused depression-like behaviors via the vagus nerve, although the detrimental effects in the gastrointestinal tract of hosts due to F. rodentium remains unclear. Future research is needed to examine the effects of F. rodentium in the host gastrointestinal tract.
In conclusion, the present data showed that FMT of “depression-related microbes” from CSDS-susceptible mice into antibiotic-treated Ephx2 KO mice results in anhedonia-like phenotypes. Moreover, repeated oral ingestion of F. rodentium causes depression-like behaviors in antibiotic-treated Ephx2 KO mice via the subdiaphragmatic vagus nerve. Thus, oral ingestion of F. rodentium could convert resilient Ephx2 KO mice into KO mice with depression-like phenotypes. This study provides new avenues for investigating the brain–gut–microbiota axis via the vagus nerve in susceptibility and resilience to stress.
Supplementary Material
Highlight.
Ephx2 KO mice do not show depression-like phenotypes after chronic social defeat stress (CSDS).
Fecal microbiota transplantation (FMT) from CSDS-susceptible mice caused depression-like phenotype in antibiotic-treated Ephx2 KO mice.
Ingestion of F. rodentium caused depression-like phenotypes in antibiotic-treated Ephx2 KO mice.
Subdiaphragmatic vagotomy did not show depression-like phenotypes in the antibiotic-treated Ephx2 mice after ingestion of F. rodentium.
The brain–gut–microbiota axis via the subdiaphragmatic vagus nerve plays an important role in susceptibility and resilience.
Acknowledgements
This study was supported by AMED (to K.H., JP20dm0107119), KAKENHI (to K.H., 19H05203 and 21H00184), Smoking Research Foundation, Japan (to K.H.), the National Institute of Environmental Health Sciences (NIEHS) River Award R35 ES030443-01 (to B.D.H.), NIEHS Superfund Program P42 ES004699 (to B.D.H.). Dr. Siming Wang was supported by TAKASE Scholarship Foundation (Tokyo, Japan). Dr. Lijia Chang was supported by the Japan China Sasakawa Medical Fellowship (Tokyo, Japan). Dr. Yan Wei was supported by the China Scholarship Council (China).
Role of the Funding Source
This study was supported by AMED (to K.H., JP20dm0107119), KAKENHI (to K.H., 19H05203), Smoking Research Foundation, Japan (to K.H.), the National Institute of Environmental Health Sciences (NIEHS) River Award R35 ES030443-01 (to B.D.H.), NIEHS Superfund Program P42 ES004699 (to B.D.H.).
Footnotes
Statement of Interest
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R, 2018. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atone J, Wagner K, Hashimoto K, Hammock BD, 2020. Cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases. Prostaglandins Other Lipid Mediat 147, 106385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear T, Dalziel J, Coad J, Roy N, Butts C, Gopal P, 2021. The microbiome-gut-brain axis and resilience to developing anxiety or depression under stress. Microorganisms 9, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becattini S, Taur Y, Pamer EG, 2016. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med 22, 458–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Bazin T, Pellissier S, 2018. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 12, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner KM, Yeramilli VA, Duck LW, Van Der Pol W, Smythies LE, Morrow CD, Elson CO, Martin CA, 2019. Depletion of dietary aryl hydrocarbon receptor ligands alters microbiota composition and function. Sci Rep 9, 14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF, 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108, 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomas F, Murrough JW, Nestler EJ, Han MH, Russo SJ, 2019. Neurobiology of resilience: Interface between mind and body. Biol Psychiatry 86, 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon CR, de La Serre CB, 2018. Gut bacteria interaction with vagal afferents. Brain Res 1693, 134–139. [DOI] [PubMed] [Google Scholar]
- Chang DH, Rhee MS, Ahn S, Bang BH, Oh JE, Lee HK, Kim BC, 2015. Faecalibaculum rodentium gen. nov., sp. nov., isolated from the faeces of a laboratory mouse. Antonie Van Leeuwenhoek 108:1309–1318. [DOI] [PubMed] [Google Scholar]
- Cheng M, Bhujwalla ZM, Glunde K, 2016. Targeting phospholipid metabolism in cancer. Front Oncol 6, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG, 2019. The microbiota-gut-brain axis. Physiol Rev 99, 1877–2013. [DOI] [PubMed] [Google Scholar]
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF, 2018. The neuroendocrinology of the microbiota-gut-brain axis: A behavioural perspective. Front Neuroendocrinol 51, 80–101. [DOI] [PubMed] [Google Scholar]
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K, 2019. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 16, 461–478. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Cohen S, Russo SJ, Dinan TG, 2018. Resilience and immunity. Brain Behav Immun 74, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF, 2017. Brain-gut-microbiota axis and mental health. Psychosom Med 79, 920–926. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, 2012. Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS, 2009. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci 10, 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM, 2012. Neural mechanisms of stress resilience and vulnerability. Neuron 75, 747–761. [DOI] [PubMed] [Google Scholar]
- Fulling C, Dinan TG, Cryan JF, 2019. Gut microbe to brain signaling: What happens in vagus. Neuron 101, 998–1002. [DOI] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY, 2017. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, 2016. Soluble epoxide hydrolase: a new therapeutic target for depression. Expert Opin Ther Targets 20, 1149–1151. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, 2019. Role of soluble epoxide hydrolase in metabolism of PUFAs in psychiatric and neurological disorders. Front Pharmacol 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Carpenter MA, Shaw S, 2009. The soluble epoxide hydrolase inhibitor AR9281 decreases blood pressure, ameliorates renal injury and improves vascular function in hypertension. Pharmaceuticals (Basel) 2, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianguo L, Xueyang J, Cui W, Changxin W, Xuemei Q, 2019. Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl Psychiatry 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Lee SM, Jung J, 2020. Integrated omics analysis unraveled the microbiome-mediated effects of Yijin-Tang on hepatosteatosis and insulin resistance in obese mouse. Phytomedicine 79, 153354. [DOI] [PubMed] [Google Scholar]
- Lim S, Chang DH, Ahn S, Kim BC, 2016. Whole genome sequencing of “Faecalibacterium rodentium” AL017, isolated from C57BL/6J laboratory mouse feces. Gut Pathog 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-Smith C, O’Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF, 2020. Microbiota-gut-brain axis: New therapeutic opportunities. Annu Rev Pharmacol Toxicol 60, 477–502. [DOI] [PubMed] [Google Scholar]
- Ma M, Ren Q, Yang J, Zhang K, Xiong Z, Ishima T, Pu Y, Hwang SH, Toyoshima M, Iwayama Y, Hisano Y, Yoshikawa T, Hammock BD, Hashimoto K, 2019. Key role of soluble epoxide hydrolase in the neurodevelopmental disorders of offspring after maternal immune activation. Proc Natl Acad Sci U S A 116, 7083–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD, 2005. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol 45, 311–333. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD, 2013. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 53, 37–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgi Y, Futamura T, Hashimoto K, 2015. Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr Mol Med 15, 206–221. [DOI] [PubMed] [Google Scholar]
- Ondicova K, Tillinger A, Pecenak J, Mravec B, 2019. The vagus nerve role in antidepressants action: Efferent vagal pathways participate in peripheral anti-inflammatory effect of fluoxetine. Neurochem Int 125, 47–56. [DOI] [PubMed] [Google Scholar]
- Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, Wang X, Hashimoto K, 2021. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun 94, 318–326. [DOI] [PubMed] [Google Scholar]
- Pu Y, Yang J, Chang L, Qu Y, Wang S, Zhang K, Xiong Z, Zhang J, Tan Y, Wang X, Fujita Y, Ishima T, Wang D, Hwang SH, Hammock BD, Hashimoto K, 2020. Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc Natl Acad Sci U S A 117, 11753–11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, Rashed S, Palm NW, Sinha R, Picciotto MR, Perry RJ, Wang A, 2020. Origin and function of stress-induced IL-6 in murine models. Cell 182, 372–387 e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Ma M, Ishima T, Morisseau C, Yang J, Wagner KM, Zhang JC, Yang C, Yao W, Dong C, Han M, Hammock BD, Hashimoto K, 2016. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci U S A 113, E1944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Ma M, Yang J, Nonaka R, Yamaguchi A, Ishikawa KI, Kobayashi K, Murayama S, Hwang SH, Saiki S, Akamatsu W, Hattori N, Hammock BD, Hashimoto K, 2018. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc Natl Acad Sci U S A 115, E5815–E5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ, 2012.Neurobiology of resilience. Nat Neurosci 15, 1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Smolin J, Nayak J, Ayala JE, Scott DA, Peterson SN, Freeze HH, 2018. Mannose alters gut microbiome, prevents diet-induced obesity, and improves host metabolism. Cell Rep 24, 3087–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva YP, Bernardi A, Frozza RL, 2020. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ, 2000. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem 275, 40504–40510. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS, 2005. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol 1, 255–291. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Hennebelle M, Yu D, Hammock BD, Levitt AJ, Hashimoto K, Taha AY, 2018. Metabolic/inflammatory/vascular comorbidity in psychiatric disorders; soluble epoxide hydrolase (sEH) as a possible new target. Neurosci Biobehav Rev 87, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszkowicz JK, Wong A, Anisman H, Merali Z, Audet MC, 2017. Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav Immun 66, 45–55. [DOI] [PubMed] [Google Scholar]
- Wada T, Sunaga H, Miyata K, Shirasaki H, Uchiyama Y, Shimba S, 2016. Aryl hydrocarbon receptor plays protective roles against high fat diet (HFD)-induced hepatic steatosis and the subsequent lipotoxicity via direct transcriptional regulation of Socs3 gene xxpression. J Biol Chem 291, 7004–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KM, McReynolds CB, Schmidt WK, Hammock BD, 2017. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther 180, 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ishima T, Zhang J, Qu Y, Chang L, Pu Y, Fujita Y, Tan Y, Wang X, Hashimoto K, 2020a. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J Neuroinflammation 17, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Qu Y, Chang L, Pu Y, Zhang K, Hashimoto K, 2020b. Antibiotic-induced microbiome depletion is associated with resilience in mice after chronic social defeat stress. J Affect Disord 260, 448–457. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang J, Wang W, Sanidad KZ, Cinelli MA, Wan D, Hwang SH, Kim D, Lee KSS, Xiao H, Hammock BD, Zhang G, 2020c. Soluble epoxide hydrolase is an endogenous regulator of obesity-induced intestinal barrier dysfunction and bacterial translocation. Proc Natl Acad Sci U S A 117, 8431–8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Chang L, Hashimoto K, 2021. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry 2021 May 7. doi: 10.1038/s41380-021-01121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Tian T, Mao Q, Zou T, Zhou CJ, Xie J, Chen JJ, 2020. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry 10, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Sun J, 2017. Hypothesis testing and statistical analysis of microbiome. Genes Dis 4, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Fang X, Zhan G, Huang N, Li S, Bi J, Jiang R, Yang L, Miao L, Zhu B, Luo A, Hashimoto K, 2019. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl Psychiatry 9, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K, 2017. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep 7, 45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, Fosso B, Melocchi L, Nizzoli G, Troisi J, Marzano M, Oresta B, Spadoni I, Atarashi K, Carloni S, Arioli S, Fornasa G, Asnicar F, Segata N, Guglielmetti S, Honda K, Pesole G, Vermi W, Penna G, Rescigno M, 2020. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol 5, 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K, 2020. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry 10, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Hashimoto K, 2017. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry 7, e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Fujita Y, Chang L, Qu Y, Pu Y, Wang S, Shirayama Y, Hashimoto K, 2019. Abnormal composition of gut microbiota is associated with resilience versus susceptibility to inescapable electric stress. Transl Psychiatry 9, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.