Abstract
Background:
Opioid use disorder (OUD) remains a public health crisis in the USA. Although stress and craving are common precipitants of substance use, no research to date has investigated the impact of laboratory-induced stress and craving on subsequent opioid use.
Method:
Participants (N=31) were individuals with prescription OUD who completed a human laboratory study followed by a one-month follow-up visit. Participants were randomly assigned to either a stress task (i.e., Trier Social Tress Task; TSST) or a no-stress condition, and then all participants completed an opioid cue paradigm. Measures of subjective (e.g., stress, craving), and neuroendocrine (e.g., cortisol, dehydroepiandrosterone) reactivity were assessed before and after each task. Survival and regression models tested the association between reactivity to the laboratory tasks and a) time to first opioid use and b) amount of opioid use during follow-up.
Results:
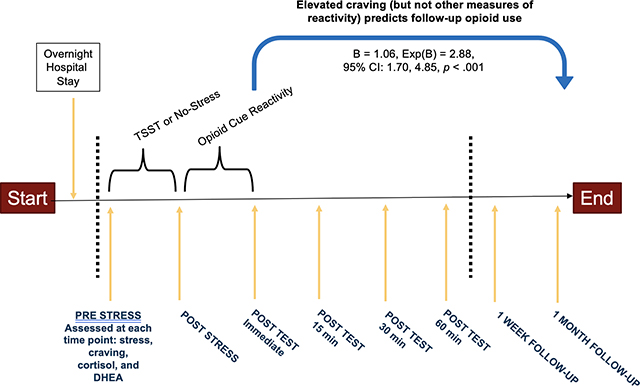
On average, participants first used opioids 3.65 (SD=2.08) days following the study. Craving after the opioid cue paradigm (B=0.44, Exp(B)=1.55, 95% CI [1.06, 2.28], p=.02) and after the TSST/no-stress condition plus opioid cue paradigm (B=1.06, Exp(B)=2.88, 95% CI [1.70, 4.85], p < .001) predicted time to first use. Additionally, there was a significant interaction between randomization to the TSST, stress reactivity, and amount of opioids used.
Conclusions:
Findings demonstrate that elevated cue-induced craving, either in the context of a stressor or not, is associated with shortened time to opioid use, whereas stress reactivity impacts the amount of opioids consumed. Preliminary findings add to the literature on stress, craving and opioid use and implicate treatment.
Keywords: prescription opioids, stress, Trier, cues, opiates
Graphical Abstract
1. Introduction
Prescription opioid use disorder (OUD) is an ongoing public health crisis in the United States. In 2017, the National Survey on Drug Use and Health showed that ~2 million individuals started misusing prescription pain relievers for the first time that year and approximately 18 million misused prescription opioids at least once in the past year (SAMHSA, 2018). Increased misuse parallels increasing rates of prescription opioid overdose. From 1999—2018, there was a four-fold increase in the number of prescription opioid overdose deaths (CDC, 2020).
Previous literature demonstrates that stress is a risk factor for substance use. Stress can be defined as an individual’s emotional, perceptual, and cognitive reaction to difficult acute events (e.g., interpersonal arguments) and chronic conditions (e.g., racism, poverty) (Sinha, 2008). Impaired stress regulation, though, can lead an individual to seek out substances to reduce negative affect and attain physiological homeostasis (Cleck and Blendy, 2008; Koob and Volkow, 2016; Sinha, 2008). Pre-clinical studies (Bossert et al., 2013; Cleck and Blendy, 2008), human laboratory experiments (Back et al., 2015; Back et al., 2010; Daughters et al., 2009; Moran-Santa Maria et al., 2014; Sinha et al., 2006), and ecological momentary assessment (EMA) studies (Preston et al., 2017, 2018b; Preston et al., 2018c) demonstrate that psychological and physiological reactions to stressors impact substance use. However, to our knowledge, no study to date has examined the relationship between laboratory-induced stress response and subsequent prescription opioid use among individuals with prescription OUD.
Stress is often investigated by measuring “reactivity” to a laboratory stress task or drug-related cue. For example, participants are exposed to an acute stress task in the laboratory and the change from baseline in emotions and physiology is measured to assess the impact of the task. The utility of these designs is their high control over extraneous factors, and thus efficiency in detecting the role of stress in substance use (Foley and Kirschbaum, 2010). Subjective responses to a stress task may include changes in self-reported stress or anxiety, whereas neuroendocrine responses may involve changes in cortisol and dehydroepiandrosterone (DHEA). Some studies have shown cortisol and DHEA levels/changes to be associated with craving or substance use (Lovallo, 2006; McRae et al., 2006; Sinha et al., 2006; Wemm and Sinha, 2019) while others have not (Chao et al., 2018).
Subjective craving is also associated with substance use. Cue reactivity studies demonstrate that formerly neutral stimuli (e.g., pill bottle) can be paired with drug cues (e.g., opioid pills) through classical conditioning and increase craving, use, or relapse (Brady et al., 2006; Carter and Tiffany, 1999; McRae-Clark et al., 2011). Given their dual influence on substance use, a handful of research studies have examined the synergistic effects of stress and craving on substance use. Among individuals with OUD, two studies using EMA show that craving is associated with relapse (Marhe et al., 2013) and may have a stronger influence as compared to stress alone (Preston et al., 2018a).
The current study adds to the literature by examining whether reactivity (subjective and neuroendocrine) to a laboratory stress task, the Trier Social Stress Task (TSST; Allen et al., 2016; Kirschbaum et al., 1993), and opioid cue paradigm is associated with subsequent opioid use during follow-up. We hypothesized that higher subjective (i.e., craving and stress) and neuroendocrine reactivity would be associated with a shorter time to use and a greater amount of opioid use at follow-up (Back et al., 2010; Sinha et al., 2006).
2. Methods
2.1. Participants
The parent study enrolled 39 individuals with prescription OUD and 36 healthy controls (Back et al., 2015). In order to examine the associations between reactivity and opioid use, this secondary analysis only examined participants with OUD who completed the laboratory study and the one-week and/or the one-month follow-up visits (N = 31). Information on healthy controls can be found in the parent study (Back et al., 2015). For this study, participants included those who completed all study time points (n = 27), the 1-week follow-up but not the 1-month follow-up (n = 2), and the 1-month follow-up but not the 1-week follow-up (n = 2). Participants met DSM-IV criteria (American Psychiatric Association, 2000) for current opioid dependence (past 6 months) using the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002). The Mini International Neuropsychiatric Interview for DSM-IV was used to assess other Axis I disorders (MINI; Sheehan et al., 1998). Exclusion criteria included: pregnancy or nursing; BMI ≥ 39; methadone use in the prior 3 months; use of medications (e.g., beta-blockers) in the past month or psychiatric conditions (e.g., PTSD) that could influence HPA axis functioning; and major medical problems (e.g., HIV). Participants were compensated $150 after completing the study.
2.2. Laboratory Procedures
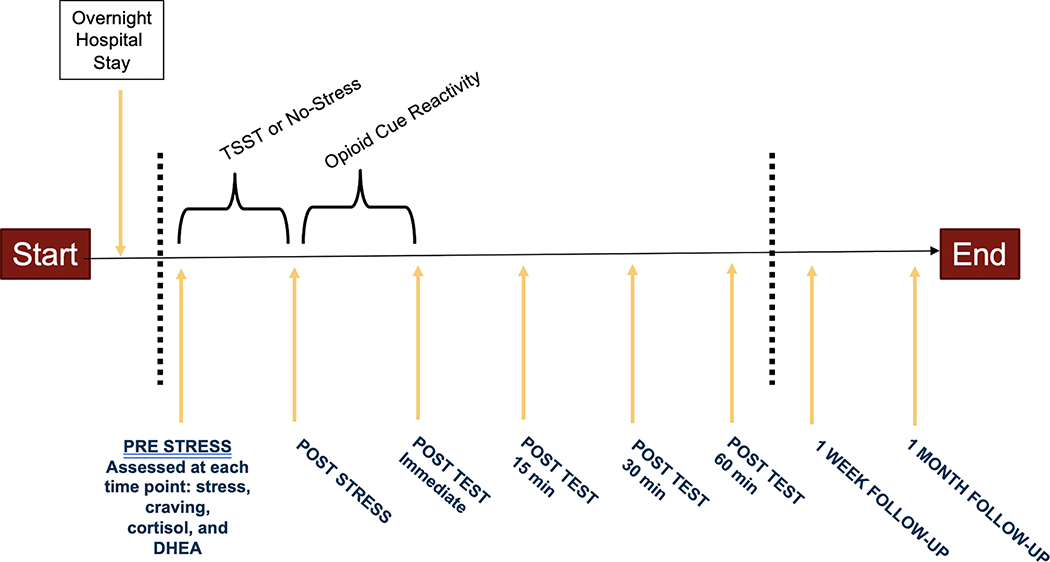
A detailed description of the parent study procedures has been previously reported (Back et al., 2015). Eligible participants were scheduled for a one-night hospital stay followed by testing the next morning. Procedures were conducted at the same time of day to control for diurnal variation in cortisol. Participants were randomly assigned to the TSST or a no-stress condition stratified by sex and using urn randomization (Wei and Lachin, 1988) to control for OUD severity. In the TSST condition, participants completed a standardized 15-min stress provocation where they were asked to prepare (5 min) and deliver (5 min) a speech and then verbally complete serial subtractions (5 min) in front of an audience of three peers (Allen et al., 2016; Kirschbaum et al., 1993). The audience would only comment on the participant’s performance when they miscalculated a subtraction, informing the participant that their subtraction was incorrect and then ask them to start again. In the no-stress condition, participants were allowed to sit and relax for 15 minutes. Participants in both the TSST and no-stress groups then completed a 15-min opioid cue paradigm (Back et al., 2014). During the opioid cue paradigm, participants listened to an audio induction script (5 min), handled and viewed drug paraphernalia (5 min), and watched a video depicting people using prescription opioids (5 min). Participants were assessed at 15-, 30-, and 60-min following the task (Figure 1). They were then debriefed, compensated, and discharged. All participants were given referrals for treatment at the baseline visit and after the laboratory visit. Participants returned to the laboratory 1-week and 1-month following the laboratory visit to complete assessments. All procedures were approved by the Medical University of South Carolina's Institutional Review Board.
Figure 1. Laboratory Study Design.
Note: Participants in this study were randomized to the Trier Social Stress Task (TSST) or a No-Stress condition followed by all participants completing an opioid cue paradigm. Stress, craving, cortisol, heart rate, and dehydroepiandrosterone (DHEA) reactivity were measured at baseline, immediately following each task, and 15-, 30-, and 60- minutes after the opioid cue paradigm. Participants were also asked to come back into the laboratory to complete 1-week and 1-month follow-up visits. For analyses, three change scores in reactivity were calculated: (a) reactivity to the stress condition was calculated by subtracting pre-stress scores from immediate post-stress scores after the TSST/No-Stress condition (i.e., post-stress—pre-stress); (b) reactivity to the TSST/No-Stress condition and the opioid cue paradigm was calculated by subtracting pre-stress scores from peak post-test scores following the opioid cue paradigm (i.e., peak post-test—pre-stress); (c) reactivity to the opioid cue paradigm was calculated by subtracting post-stress scores from peak post-test scores following the opioid cue paradigm (i.e., peak post-test—post-stress).
2.2. Measures
2.2.1. Substance Use.
The Timeline Follow Back (TLFB; Sobell and Sobell, 2000) was used to assess opioid use (frequency and amount) for the past 30 days at baseline, past 7 days at 1-week follow-up, and past 30 days at 1-month follow-up.
2.2.2. Subjective Reactivity.
Subjective stress and craving were assessed using a visual analogue scale (VAS; 0 = not at all to 10 = extremely) adapted from the Within Session Rating Scale (Childress et al., 1994). Stress and craving reactivity were selected as the two predictors of interest based on prior research and to increase model convergence and power (Back et al., 2015; Back et al., 2010; Sinha et al., 2006). Subjective reactivity was assessed prior to and immediately after the stress condition (TSST or No-stress), and at 15-, 30-, and 60-minutes after the opioid cue paradigm.
2.2.3. Neuroendocrine Biomarker Reactivity.
Salivary samples of cortisol and DHEA were collected at the same time points as the subjective reactivity measures. Cortisol and DHEA samples were each assayed in duplicate using enzyme immunoassay systems (precision of 5.6% with a sensitivity of 5 pg/mL for DHEA; precision of 3.35% to 3.65% with a sensitivity of <0.003 μg/dL for cortisol). A PowerWave HT Microplate Spectrophotometer in combination with a Precision Series Automate Liquid Handling System was used to analyze levels of cortisol and DHEA.
2.3. Statistical Analyses.
Three sets of predictor variables were calculated for this study (Figure 1). Changes in subjective and neuroendocrine reactivity were calculated following (a) the TSST/no-stress condition, (b) the TSST/no-stress condition plus the opioid cue paradigm, and (c) the opioid cue paradigm. Change scores were computed, respectively, by (a) subtracting the baseline value from the reactivity value immediately after the TSST/no-stress condition; (b) subtracting the baseline value from the peak reactivity value measured 15-, 30-, and 60-min following the opioid cue paradigm; and (c) subtracting the post-TSST/no-stress condition value from the peak post cue-reactivity levels. The computation of change scores aligns with procedures in prior literature on reactivity (Back et al., 2015; Daughters et al., 2009; McRae-Clark et al., 2011). Randomization to the TSST/no-stress condition was included as a factor in all analyses.
Opioid use during follow-up was measured in two ways: 1) time to first opioid use in days following the laboratory visit, and 2) average amount of opioid pills used per using day during the 1-month follow-up. Cox proportional hazards regression analysis was completed on models predicting time to use in days. Subjective and neuroendocrine reactivity variables did not show statistical multicollinearity with squared multiple correlations greater than 0.90 (Tabachnick and Fidell, 2007). Craving reactivity violated the proportionality assumption in models assessing changes in reactivity following the opioid cue paradigm and the TSST/No-Stress condition plus the opioid cue paradigm. Thus, a time variable (craving reactivity*log(days to use)) was included to adjust for the interaction between the covariate and time (Tabachnick and Fidell, 2007). Participants who did not endorse using within the 1-month follow-up were considered right-censored cases meaning opioid use did not occur during follow-up and it is unknown if it will occur in the future. To include these cases in the analysis a value of 30 was inputted to demonstrate lack of known use within the 30-day measurement period while use status was defined as 0 which equates to no use during follow-up (Tabachnick and Fidell, 2007).
Multiple linear regressions were completed on the average amount of opioids used at 1-month follow-up. For all predictors, tolerance and variance inflation factor values were within guidelines (Field, 2009). Residuals violated a Gaussian distribution and multiple linear regression was used because this approach is robust to non-normality. Models with significant predictors were re-run as hierarchical multiple linear regressions where all predictors were entered in the first step, followed by interaction terms in the second step. Interaction terms were centered for interpretability. Gender was included in initial models due to prior significant findings (Gilmore et al., 2019), but removed due to nonsignificant effects and to increase model parsimony and power. For participants who were lost to 1-month follow-up (n = 2), average amount of opioid use on using days at 1-month follow-up was replaced with the amount participants reported using at 1-week follow-up. Four missing values for DHEA were also mean-replaced. Data are presented as mean ± SD, and all analyses were completed in IBM SPSS v25.
2. Results
3.1. Demographic Sample
Characteristics of the sample by stress condition are reported in Table 1. Across all OUD participants, the most commonly used opioids included: Lortab (87.1%), Hydrocodone (58.1%), Percocet (58.1%), oxycodone/OxyContin (53.25%), and Vicodin (54.8%). Participants had last used opioids M = 5.70 (SD = 3.23) days prior to the laboratory visit. At 1-month follow up, 64.5% participants self-reported using opioid pills since the laboratory visit. Of the ten participants who did not use during the 1-month follow-up period, three had been randomized to the TSST and seven had been randomized to the No-Stress condition. There was no significant difference on follow-up use and randomization to the TSST or No-Stress condition (p = .22). Mean time to first use of opioids was M = 3.65 (SD = 2.08) days with an average of 2.91 opioid pills consumed per using day. All participants who reported using during the follow-up period, used within the 7 days following the laboratory visit. Mean change scores in response to the stress condition, the opioid cue paradigm, and the stress condition plus opioid cue paradigm by group assignment (TSST/No-Stress) are reported in Table 2.
Table 1.
Demographics and Clinical Characteristics of Individuals with Opioid Use Disorder (N = 31)
| Variable | TSST (n = 16) | No Stress (n = 15) |
|---|---|---|
|
Mean (SD) or n (%) | ||
| Age | 36.19 (12.69) | 32.60 (12.31) |
| Gender (% Male) | 9 (56.3%) | 9 (53.3%) |
| Education (% some college or more) | 8 (50%) | 12 (80%) |
| Employment | ||
| Employed (part/full time) | 5 (31.3%) | 2 (13.3%) |
| Student | 2 (12.5%) | 3 (20%) |
| Unemployed | 9 (56.3%) | 10 (66.7%) |
| Race & Ethnicity | ||
| Caucasian | 13 (81.3%) | 11 (73.3%) |
| Black/African American | 2 (12.5%) | 0 (0%) |
| Hispanic/Latino | 2 (6.3%) | 1 (6.7%) |
| Native American | 0 (0%) | 3 (20%) |
| Relationship Status (% single, never married) | 10 (62.5%) | 8 (53.3%) |
| Smokes Nicotine (% yes) | 13 (81.3%) | 13 (86.7%) |
|
Prescription Opioid Use | ||
| Age first used prescription opioids | 22.13 (10.04) | 24.13 (10.97) |
| Age of onset of opioid dependence | 27.48 (10.31) | 27.87 (11.42) |
| Total days using opiates at baseline a | 18.69 (8.70) | 19.40 (8.27) |
| Average number of opioid pills used per using day at baseline a | 4.09 (2.37) | 3.93 (2.44) |
| Days since last opioid use before laboratory visit | 5.42 (4.08) | 6.00 (2.41) |
| Days to first use of opioids during follow-up b | 4.08 (2.19) | 3.00 (1.85) |
| Average number of opioid pills used per using day during follow-up | 3.80 (2.77) | 1.96 (1.87) |
|
Comorbid Psychiatric Conditions | ||
| Alcohol use disorder | 3 (18.8%) | 1 (6.7%) |
| Cannabis use disorder | 0 (0%) | 3 (20%) |
| Sedative use disorder | 0 (0%) | 2 (13.3%) |
| Cocaine use disorder | 0 (0%) | 0 (0%) |
| Major Depression | 0 (0%) | 1 (6.7%) |
| Panic disorder* | 3 (18.8%) | 6 (40.0%) |
| Posttraumatic Stress disorder (PTSD) | 0 (0%) | 0 (0%) |
| Social Anxiety disorder | 3 (18.8%) | 1 (6.7%) |
| Generalized anxiety disorder | 3 (18.8%) | 2 (13.3%) |
| Bipolar I and II disorder | 0 (0%) | 0 (0%) |
| Pain disorder | 1 (6.3%) | 1 (6.7%) |
| Obsessive compulsive disorder | 0 (0%) | 2 (13.3%) |
| Attention-deficit/hyperactivity disorder | 1 (6.3%) | 1 (6.7%) |
|
History of Treatment (% yes) | ||
| History of chronic pain treatment | 6 (37.5%) | 4 (26.7%) |
| History of addiction treatment | 8 (50%) | 5 (33.3%) |
| History of mental health treatment | 5 (31.3%) | 5 (33.3%) |
Baseline measures assessed use for past 30 days. All participants reported using in the past 30 days.
Subsample of participants who reported using opioids at follow-up, n = 20
p < .05 between-group differences
Table 2.
Average Reactivity Change Scores by Stress Condition (N = 31)
| TSST (n = 16) | No-Stress (n = 15) | |
|---|---|---|
| Laboratory Task | Mean (SD) | |
| Stress condition (TSST/No-Stress) | ||
| Stress* | 4.31 (3.14) | −0.60 (1.68) |
| Craving* | 2.13 (3.01) | −0.27 (1.03) |
| DHEA* | 109.30 (102.94) | 23.81 (67.84) |
| Cortisol* | 0.15 (0.25) | −0.03 (0.08) |
| TSST/No-stress condition plus Opioid Cue Paradigm | ||
| Stress | 2.44 (3.90) | 1.93 (1.94) |
| Craving | 3.38 (2.28) | 3.33 (1.88) |
| DHEA | 119.88 (150.21) | 96.45 (99.28) |
| Cortisol | 0.07 (0.13) | 0.06 (0.15) |
| Opioid Cue Paradigm | ||
| Stress* | −1.88 (2.78) | 2.53 (2.20) |
| Craving* | 1.25 (1.98) | 3.60 (1.76) |
| DHEA | 10.39 (133.80) | 72.64 (100.43) |
| Cortisol* | −0.08 (0.22) | 0.09 (0.14) |
Note: Mean changes scores included in the survival analysis by group assignment to the Trier Social Stress Task (TSST) or the No-Stress condition. For example, the first row shows the average change score for individuals following the stress condition (either randomization to the TSST or No-Stress task). Participants who were randomized to the TSST showed an average change score of M=4.31 (SD = 3.14), suggesting increases in stress following the TSST. Oneway ANOVAs tested for group differences.
p < .05; DHEA = dehydroepiandrosterone
3.2. Reactivity to the TSST/No-Stress Condition
The Cox proportional hazards regression model that assessed whether changes in response to the TSST or no-stress condition were associated with time to first use was not significant (X2 (5) = 3.97, p = .55) and none of the predictors were significantly associated with time to use. When the average amount of opioid pills used in the past month was regressed on reactivity to the TSST/no-stress condition, the model approached significance (R2 = 0.31, p = .08). Randomization to the TSST was the only significant predictor (B = 3.83, SE = 1.31, β= 0.77, p = .007). Participants in the TSST showed a 3.83 increase in the average amount of opioid pills used in comparison to participants in the no-stress condition.
3.3. Reactivity to the TSST/No-stress Condition and the Opioid Cue Paradigm
The Cox proportional hazards regression model assessing changes in reactivity to the TSST/No-stress condition plus the opioid cue paradigm was significant (X2 (6) = 43.9, p < .001). Change in subjective craving was significantly associated with a greater likelihood of follow-up use (B = 1.06, Exp(B) = 2.88, 95% Confidence Interval [CI]: 1.70, 4.85, p < .001). While holding all other predictor variables in the model constant (randomization to TSST/no-stress condition, subjective stress reactivity, cortisol, and DHEA), there was a 1.06 increase in the expected log of the relative hazard for follow-up opioid use for each one-point increase in subjective craving reactivity; that is, there is an expected hazard of follow-up opioid use that is 188 times higher in a person who is one point higher in craving reactivity following the TSST/No-stress condition plus opioid cue paradigm. The survival rate to follow-up use decreased one week after the laboratory study, suggesting greater risk for follow-up use in the week after the study.
A hierarchical multiple regression model examining the relationship between changes in reactivity following the TSST/no-stress condition plus the opioid cue paradigm with the average amount of opioids use at follow-up was completed (Table 3). Step 1 included all main effects and showed a significant model (R2 = .35, p = .04), where predictors accounted for 35.4% of the variance in average amount of opioid pills used on using days at follow-up. In Step 2, inclusion of an interaction variable of subjective stress reactivity multiplied by group (TSST/no-stress) significantly improved the model (ΔR2 = .13, p = .02) and was significant (B = −0.78, SE = 0.32, β = −.85, p = .02). Inspection of the simple slopes (Figure 2) by splitting stress reactivity into three groups based on the mean and standard deviation showed that average amount of opioids used at follow-up varied by subjective stress response to the TSST. Among participants randomized to the TSST, those with one standard deviation of greater subjective stress following the TSST plus opioid cue paradigm showed lower average opioid use at follow-up. In comparison, those with one standard deviation of lesser subjective stress reactivity after the TSST plus opioid cue paradigm showed higher average opioid use at follow-up.
Table 3.
Average Amount of Opioid Use during Follow-up Regressed on Change in Reactivity to the TSST/No-stress condition and Opioid Cue Paradigm
| B | Standard Error | ß | ||
|---|---|---|---|---|
| Step 1 | ||||
| Constant | 2.64* | 1.00 | ||
| TSST/No-Stress Group | 2.09* | 0.81 | −.42 | |
| Stress | −0.39* | 0.14 | −.47 | |
| Craving | 0.09 | 0.21 | .07 | |
| Cortisol | 0.36 | 3.27 | .02 | |
| DHEA | −0.002 | 0.003 | −.12 | |
| Step 2 | ||||
| Constant | 1.52 | 1.03 | ||
| TSST/No-Stress Group | 2.00* | 0.74 | .40 | |
| Stress | 0.22 | 0.28 | .27 | |
| Craving | 0.12 | 0.20 | .10 | |
| Cortisol | −0.66 | 3.02 | −.04 | |
| DHEA | −0.003 | 0.003 | −0.18 | |
| Stress x TSST/No-Stress Group | −0.78* | 0.32 | −.85 | |
Note: Results from multiple regression examining how change in reactivity to the stress condition (TSST/No-Stress Task) and the opioid cue paradigm predicted follow-up opioid use. In Step 1, predictors include changes in reactivity for stress, craving, cortisol, and DHEA in addition to randomization to either the TSST or No-Stress task. In Step 2, the interaction of the randomization to the TSST or No-Stress task multiplied by stress reactivity is included in the model.
= p < .05; R2 = .35 for Step 1 (p = .04), ΔR2 = .13 for Step 2 (p = .02).
Figure 2. Predicted Values from the Interaction between Stress Reactivity to the Trier Social Stress Task (TSST) or No-Stress Task and Average Amount of Prescription Opioids Used during Follow-Up.
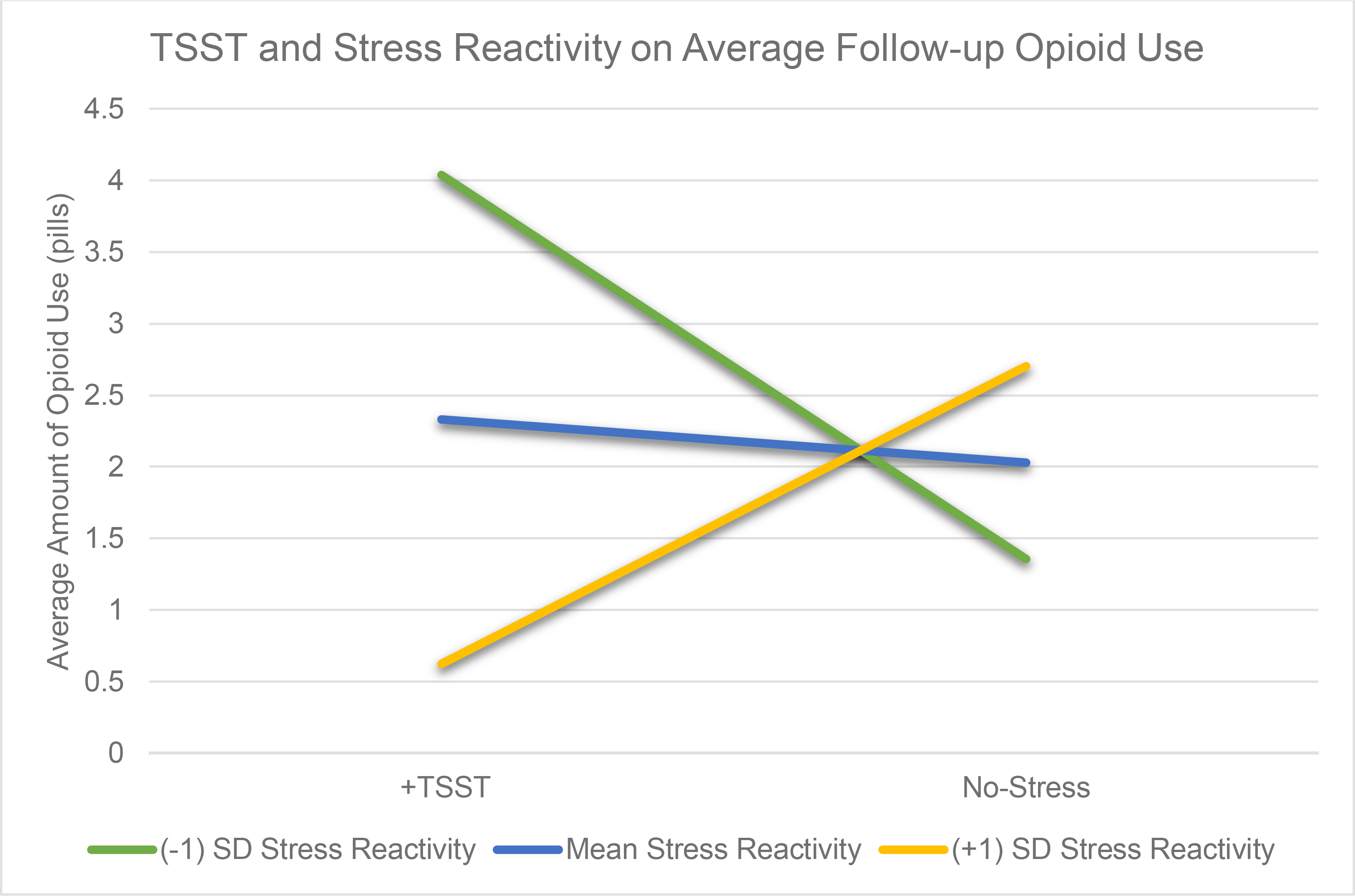
Note: This figure demonstrates the predicted values from the multiple regression model for the interaction between stress reactivity and randomization to the Trier Social Stress Task (TSST) or to the No-Stress condition with follow-up opioid use. The model outcomes show that individuals randomized to the TSST showed variation in the number of opioid pills they used at 1-month follow-up depending on their changes in stress reactivity. Following the TSST, those with a greater change in stress reactivity (+1 SD Stress Reactivity; yellow line) consumed fewer opioid pills at one month follow up whereas those with less change in stress reactivity (−1 SD Stress Reactivity, green line) used more pills at 1-month follow-up.
3.4. Reactivity to the Opioid Cue Paradigm
When examining changes in response to the opioid cue paradigm, the Cox proportional hazards regression model was significant (X2 (6) = 28.37, p < .01). Increases in subjective craving following the opioid cue paradigm were associated with fewer days to first use after the laboratory visit (B = 0.44, Exp(B) = 1.55, 95% CI [1.06, 2.28], p = .02). Specifically, there was a 0.44 increase in the expected log of the relative hazard for each one-point increase in subjective craving while holding all other predictor variables in the model constant; thus, there was an expected hazard 55 times higher in a person who was one-point higher in subjective craving reactivity following the opioid cue paradigm when holding randomization to the TSST/No-stress condition, subjective stress reactivity, cortisol, and DHEA constant. The final multiple regression model assessing changes in reactivity following the opioid cue paradigm approached significance (R2 = .31, p = .08). None of the predictors in this model were significant.
4. Discussion
This study examined associations between laboratory-induced stress and craving and subsequent prescription opioid use among individuals with current OUD. Two main findings emerged. First, greater increase in craving following the opioid cue paradigm was associated with fewer days to first opioid use during follow-up; this was true regardless of whether the opioid cue was preceded by the TSST/no-stress condition or not. Secondly, changes in subjective stress reactivity following the TSST and opioid cue paradigm were associated with the amount of opioid pills consumed during follow-up. Exposure to the TSST plus opioid cue paradigm increased the amount of use during follow-up but in an unexpected direction: those with higher subjective stress in response to both the TSST and the opioid cue paradigm consumed fewer opioid pills during follow-up, whereas those with lower subjective stress reactivity to the TSST and opioid cue paradigm consumed more opioid pills during follow-up.
In survival models, subjective craving was the only significant predictor of time to first use of opioid pills at 1-month follow-up, and all participants used within a week after the laboratory visit. This corresponds with research demonstrating that craving is a better predictor of opioid use or relapse (Baxley et al., 2019; Tsui et al., 2014) than subjective stress (Furnari et al., 2015; Preston et al., 2018a). It also corresponds with research suggesting that the mechanisms of classical conditioning, cue reactivity, and attentional bias may be the most salient triggers for use among individuals with OUD (Garland and Howard, 2014; Marhe et al., 2013). The current study also found that the hazard ratio of subjective craving reactivity following the opioid cue paradigm was lower (HR = 55) than subjective craving reactivity in response to the TSST/no-stress condition plus the opioid cue (HR = 188). This could suggest that combined exposure to the TSST task and opioid cue paradigm may increase subjective craving, and thus have a greater impact on the time to use. The additive effect of stress and drug cues has been documented by Preston and colleagues, where the authors note that “craving and the likelihood of relapse are greater in the presence of stress” (Preston et al., 2018b, p. 864). However, given that half of the participants did not receive the TSST and there was no significant interaction of randomization to the TSST/no-stress group on time to follow-up use, these interpretations are preliminary and require replication on a larger sample that may be powered to detect effects.
An important ethical implication from these results is the amount of time it took for participants with OUD to use following completion of the laboratory task. Prior research has shown that a laboratory stressor task followed by an opioid cue paradigm was associated with reduced substance use (DeSantis et al., 2009). The current findings show that participants used 3.65 days following the study and 2.91 pills on using days. In comparison, participants used 5.70 days before the laboratory visit and used 4.01 pills on using days at baseline. This suggests that after the study, time to opioid use is shorter, but the amount of use is less than at baseline. This makes it hard to determine whether laboratory stress tasks increase opioid use. Instead, findings may highlight the importance of continuing to provide informed consent regarding potential risks of greater craving, referral to evidence-based treatments, and follow-up monitoring.
Findings also revealed that individuals with OUD who were randomized to the TSST plus the opioid cue paradigm and reported higher levels of subjective stress, consumed less opioid pills during follow-up. In contrast, those with lower levels of subjective stress in response to the TSST plus opioid cue paradigm reported greater opioid pill use during follow-up. The mechanisms underlying this association are somewhat unclear, given that prior pre-clinical research has found stress increases heroin self-administration (Stafford et al., 2019). However, we speculate whether these results could point to unique profiles of subjective stress in individuals with OUD. Findings could be indicative of a “blunted” response in some individuals with OUD where the difficulty to experience acute stress may have yielded delayed experiences of stress and consequently increased follow-up use. Prior neuroendocrine research demonstrates that higher basal levels of cortisol from chronic stress can yield blunted responses to acute stressors (Lee et al., 2015). Other research shows that individuals with OUD who have a greater number of adverse childhood experiences show higher blunted heart rate variability and cue-elicited craving (Garland et al., 2019). However, the current findings could also point to bias in the reporting of subjective stress levels. It may be that some individuals with OUD who were in the TSST struggled to identify or minimized the intensity of their experience (e.g., Marhe et al., 2013). Alexithymia (i.e., difficulty in identifying emotions, including stress) is characteristic of many mental health disorders and may inhibit individuals with OUD from detecting and acknowledging craving or subjective stress within themselves. Another interpretation of these findings could be that some individuals with OUD may be avoidant of experiencing stress. Avoiding stressful experiences has been shown to have long-term consequences in other disorders, such as anxiety disorders (Foa et al., 2006). If following a similar pattern, the avoidance of state stress could have a deleterious consequence in which individuals with OUD use greater amounts of opioids during follow-up.
Contrary to our hypotheses, cortisol and DHEA were not associated with time to first use. This finding is in contrast to prior research showing cortisol is associated with heroin relapse (Fatseas et al., 2011). There are a several ways to interpret the absence of this finding. Chronic opioid use is associated with complex changes in the stress response system, including cortisol and DHEA (Brennan, 2013; Daniell, 2006; de Vries et al., 2019). Exposure to a short-term stressor typically results in activation of the HPA axis and increased cortisol. However, in response to long-term stressors, the body allostatically adapts with a higher basal level of cortisol and cortisol responses to additional acute stressors are blunted (See Figure 2 in Lee et al., 2015). Although participants in this study did not have a higher basal cortisol levels than healthy controls in post-hoc tests, they appear to have shown a blunted response to the laboratory paradigm. Table 2 shows the change scores in cortisol following each part of the laboratory task. For the TSST group, cortisol levels decreased following the TSST and opioid cue paradigm, which may suggest a blunted response. However, another consideration is the difficulty in measuring neuroendocrine variables which are indicators of biological changes and may fluctuate widely within a day and across individuals (Heck and Handa, 2019; Lee et al., 2015).
Although this was not a treatment-seeking sample, results lend support to the use of clinical interventions that reduce craving. Previous research has shown that both laboratory-induced and personally-relevant drug cues increase craving, but personally-relevant cues have a stronger effect on craving (Childress et al., 1994; Childress et al., 1986). This may account for why all participants in the current study used within the first week of follow-up. Medications for OUD (MOUD) are well-known to address opioid withdrawal and target craving (Sofuoglu et al., 2019), but other pharmacological (e.g., clonidine, Kowalczyk et al., 2015) and psychotherapeutic interventions may also enhance MOUD despite some mixed findings (Carroll and Weiss, 2017; Dugosh et al., 2016; Schacht et al., 2017; Schiff et al., 2015; Sofuoglu et al., 2019). Many psychotherapies bring greater awareness to situations that trigger cravings and teach skills that are useful in managing craving. In addition, the results from this study also suggest lower subjective stress in response to a stressor is associated with greater opioid use at follow-up. Although more research is needed to examine the mechanisms underlying this finding, if blunted stress responses or cognitive avoidance helps explain these results, adjunctive psychotherapy treatments focused on decreasing stress, mindfulness, and approaching (versus avoiding) situations may be beneficial for this subpopulation. For example, previous research has shown that an adjunctive mindfulness-based treatment in individuals with OUD and chronic pain is associated with reductions in craving, pain, stress, and self-control (Garland et al., 2019). Likewise, in individuals with OUD and posttraumatic stress disorder, a disorder characterized in part by a blunted stress response, exposure-based treatments have shown efficacy in preventing opioid relapse (Schacht et al., 2017; Schiff et al., 2015).
Several limitations of the current study warrant consideration. The sample size was small, and thus, the findings need to be replicated in a larger sample. The parent study’s focus on prescription OUD, rather than heroin use disorder, led us to have a less racially and ethnically diverse sample due to health disparities between individuals with prescription OUD who tend to self-identify as white/Caucasian as compared to heroin users, who tend to self-identify as Black/African American or of another non-white race and ethnicity (Alexander et al., 2018). Similarly, prior work on this data has shown gender differences in stress reactivity and OUD when examining participants who completed the laboratory study but were lost follow-up (i.e., a larger sample, Gilmore et al., 2019). No differences were observed in this secondary analysis most likely due to low power. An additional limitation to this study was the parent study’s exclusion criteria (e.g., psychiatric and medical comorbidities, BMI > 39) which limited generalizability of these findings to a more diverse patient population, although prevented possible confounds on study results. Furthermore, research on stress induction within the laboratory does not perfectly translate to stress in real-life experiences (Sinha, 2008). It may be useful for future work to assess ongoing stressors to examine the potential impact with laboratory induced-stress or use EMA to compare the impact of real-life stressors with laboratory stressors on opioid use.
4.1. Conclusions
This study is the first to our knowledge to examine the relationship between laboratory-induced stress and craving, and subsequent prescription opioid use. Results suggest that for individuals with OUD, craving predicts the time to first use, and all users will use within one week following the study. This points to the strong influence of craving, rather than stress, on future opioid use. Subjective stress also seems to play a role in the amount of opioids used during follow-up. However, the mechanisms of this relationship are unclear and require additional work. It will be important for future work to consider unique responses to stress among individuals with OUD, how those responses may be related to craving and use, and means to mitigate craving to assist in long-term recovery.
Highlights.
Participants with opioid use disorder used opioids, on average, 3.65 days after the study.
Subjective craving predicted time to follow-up opioid use.
The stress task and subjective stress levels predicted the amount of opioids used.
Acknowledgments
Role of Funding Source: This work was supported in part by grants from the National Institute on Drug Abuse T32 DA007288 (PI: McGinty), K23 DA021228 (PI: Back), R25 DA020537 (PI: Back), K23 AA027307 (PI: Jarnecke), K12 DA031794 (PI: Jones), and in partnership with the Office of Research on Women’s Health and the Medical University of South Carolina U54 DA016511 (PI: Brown).
Footnotes
Conflict of Interest: No conflicts declared.
Ethics Approval: This study was approved by the Institutional Review Board at the Medical University of South Carolina. Informed consent was obtained from all participants prior to their participation in the study.
Data Availability: Data not available. The data that has been used is confidential.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander MJ, Kiang MV, Barbieri M, 2018. Trends in Black and White Opioid Mortality in the United States, 1979–2015. Epidemiology (Cambridge, Mass.) 29(5), 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Dockray S, Cryan JF, Dinan TG, Clarke G, 2016. The Trier Social Stress Test: Principles and practice. Neurobiology of stress 6, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association; Washington, D.C. [Google Scholar]
- Back SE, Gros DF, McCauley JL, Flanagan JC, Cox E, Barth KS, Brady KT, 2014. Laboratory-induced cue reactivity among individuals with prescription opioid dependence. Addict. Behav 39(8), 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Gros DF, Price M, et al. Laboratory-induced stress and craving among individuals with prescription opioid dependence. Drug Alcohol Depend. 2015;155:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT, 2010. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend 106(1), 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxley C, Weinstock J, Lustman PJ, Garner AA, 2019. The influence of anxiety sensitivity on opioid use disorder treatment outcomes. Exp. Clin. Psychopharmacol 27(1), 64–77. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y, 2013. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology 229(3), 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK, 2006. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol. Clin. Exp. Res 30(6), 938–946. [DOI] [PubMed] [Google Scholar]
- Brennan MJ, 2013. The Effect of Opioid Therapy on Endocrine Function. The American Journal of Medicine 126(3), S12–S18. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Weiss RD, 2017. The role of behavioral interventions in buprenorphine maintenance treatment: A review. Am. J. Psychiatry 174(8), 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST, 1999. Meta-analysis of cue-reactivity in addiction research. Addiction 94(3), 327–340. [PubMed] [Google Scholar]
- CDC, 2020. Wide-ranging online data for epidemiologic research (WONDER). CDC, Atlanta, GA. [Google Scholar]
- Chao T, Radoncic V, Hien D, Bedi G, Haney M, 2018. Stress responding in cannabis smokers as a function of trauma exposure, sex, and relapse in the human laboratory. Drug Alcohol Depend 185, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O’Brien CP, 1994. Can induced moods trigger drug-related responses in opiate abuse patients? J. Subst. Abuse Treat. 11(1), 17–23. [DOI] [PubMed] [Google Scholar]
- Childress AR, Thomas McLellan A, O’Brien CP, 1986. Conditioned responses in a methadone population: A comparison of laboratory, clinic, and natural settings. J. Subst. Abuse Treat. 3(3), 173–179. [DOI] [PubMed] [Google Scholar]
- Cleck JN, Blendy JA, 2008. Making a bad thing worse: adverse effects of stress on drug addiction. The Journal of clinical investigation 118(2), 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell HW, 2006. DHEAS deficiency during consumption of sustained-action prescribed opioids: evidence for opioid-induced inhibition of adrenal androgen production. J. Pain 7(12), 901–907. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Richards JM, Gorka SM, Sinha R, 2009. HPA axis response to psychological stress and treatment retention in residential substance abuse treatment: a prospective study. Drug Alcohol Depend 105(3), 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries F, Bruin M, Lobatto DJ, Dekkers OM, Schoones JW, van Furth WR, Pereira AM, Karavitaki N, Biermasz NR, Zamanipoor Najafabadi AH, 2019. Opioids and Their Endocrine Effects: A Systematic Review and Meta-analysis. The Journal of Clinical Endocrinology & Metabolism 105(4), 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis SM, Bandyopadhyay D, Back SE, Brady KT, 2009. Non-treatment laboratory stress- and cue-reactivity studies are associated with decreased substance use among drug-dependent individuals. Drug Alcohol Depend 105(3), 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D, 2016. A systematic review on the use of psychosocial Interventions in conjunction with medications for the treatment of opioid addiction. J. Addict. Med. 10(2), 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatseas M, Denis C, Massida Z, Verger M, Franques-Rénéric P, Auriacombe M, 2011. Cue-induced reactivity, cortisol response and substance use outcome in treated heroin dependent individuals. Biol. Psychiatry 70(8), 720–727. [DOI] [PubMed] [Google Scholar]
- Field AP, 2009. Discovering statistics using SPSS: (and sex and drugs and rock ‘n’ roll). SAGE Publications, Thousand Oaks, CA. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Institute; New York, NY. [Google Scholar]
- Foa EB, Huppert JD, Cahill SP, 2006. Emotional processing theory: An update., in: Rothbaum BO (Ed.) Pathological Anxiety: Emotional Processing in Etiology and Treatment. Guilford, New York, NY, pp. 3–24. [Google Scholar]
- Foley P, Kirschbaum C, 2010. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci Biobehav Rev 35(1), 91–96. [DOI] [PubMed] [Google Scholar]
- Furnari M, Epstein DH, Phillips KA, Jobes ML, Kowalczyk WJ, Vahabzadeh M, Lin JL, Preston KL, 2015. Some of the people, some of the time: field evidence for associations and dissociations between stress and drug use. Psychopharmacology (Berl.) 232(19), 3529–3537. [DOI] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, Yack BP, Bedford CE, Bryan MA, Atchley R, Nakamura Y, Froeliger B, Howard MO, 2019. Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. J. Consult. Clin. Psychol 87(10), 927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Howard MO, 2014. Opioid attentional bias and cue-elicited craving predict future risk of prescription opioid misuse among chronic pain patients. Drug Alcohol Depend 144, 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AK, Guille C, Baker NL, Brady KT, Hahn CK, Davis CM, McCauley JL, Back SE, 2019. Gender differences in subjective stress and neuroendocrine response to a stress task among individuals with opioid dependence: A pilot study. Addict. Behav. 92, 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck AL, Handa RJ, 2019. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 44(1), 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The ‘Trier Social Stress Test’ – A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3(8), 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk WJ, Phillips KA, Jobes ML, Kennedy AP, Ghitza UE, Agage DA, Schmittner JP, Epstein DH, Preston KL, 2015. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: A randomized controlled trial with ecological momentary assessment. Am. J. Psychiatry 172(8), 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Kim E, Choi MH, 2015. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep 48(4), 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, 2006. Cortisol secretion patterns in addiction and addiction risk. Int. J. Psychophysiol 59(3), 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, Waters AJ, van de Wetering BJ, Franken IH, 2013. Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: an ecological momentary assessment study. J. Consult. Clin. Psychol 81(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA, 2006. Stress reactivity: biological and subjective responses to the cold pressor and Trier Social stressors. Hum Psychopharmacol 21(6), 377–385. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, Giarla K, Nicholas K, Brady KT, 2011. Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology (Berl.) 218(1), 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT, 2014. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl.) 231(21), 4157–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2017. Context and craving during stressful events in the daily lives of drug-dependent patients. Psychopharmacology (Berl.) 234(17), 2631–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2018a. Before and after: craving, mood, and background stress in the hours surrounding drug use and stressful events in patients with opioid-use disorder. Psychopharmacology (Berl.) 235(9), 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2018b. Exacerbated craving in the presence of stress and drug cues in drug-dependent patients. Neuropsychopharmacology 43(4), 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Schroeder JR, Kowalczyk WJ, Phillips KA, Jobes ML, Dwyer M, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2018c. End-of-day reports of daily hassles and stress in men and women with opioid-use disorder: Relationship to momentary reports of opioid and cocaine use and stress. Drug Alcohol Depend 193, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA, 2018. Center for Behavioral Health Statistics and Quality. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD. [Google Scholar]
- Schacht RL, Brooner RK, King VL, Kidorf MS, Peirce JM, 2017. Incentivizing attendance to prolonged exposure for PTSD with opioid use disorder patients: A randomized controlled trial. J. Consult. Clin. Psychol 85(7), 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M, Nacasch N, Levit S, Katz N, Foa EB, 2015. Prolonged Exposure for treating PTSD among female methadone patients who were survivors of sexual abuse in Israel. Soc. Work Health Care 54(8), 687–707. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sinha R, 2008. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 1141, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville J, 2006. Stress-Induced Cocaine Craving and Hypothalamic-Pituitary-Adrenal Responses Are Predictive of Cocaine Relapse Outcomes. Arch. Gen. Psychiatry. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 2000. Alcohol Timeline Followback (TFLB), in: Association, A.P. (Ed.) Handbook of Psychiatric Measures. American Psychiatric Associaiton, Washington, D.C., pp. 477–479. [Google Scholar]
- Sofuoglu M, DeVito EE, Carroll KM, 2019. Pharmacological and behavioral treatment of opioid use disorder. Psychiatric Research and Clinical Practice 1(1), 4–15. [Google Scholar]
- Stafford NP, Kazan TN, Donovan CM, Hart EE, Drugan RC, Charntikov S, 2019. Individual Vulnerability to Stress Is Associated With Increased Demand for Intravenous Heroin Self-administration in Rats. Front. Behav. Neurosci. 13, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, 2007. Using multivariate statistics. Pearson/Ally & Bacon, Boston, MA [Google Scholar]
- Tsui JI, Anderson BJ, Strong DR, Stein MD, 2014. Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: a longitudinal study. Am J Drug Alcohol Abuse 40(2), 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LJ, Lachin JM, 1988. Properties of the urn randomization in clinical trials. Control. Clin. Trials 9(4), 345–364. [DOI] [PubMed] [Google Scholar]
- Wemm SE, Sinha R, 2019. Drug-induced stress responses and addiction risk and relapse. Neurobiology of Stress 10, 100148. [DOI] [PMC free article] [PubMed] [Google Scholar]