Abstract
Background.
Opioid overdose remains a leading cause of death. Office-based buprenorphine could expand access to treatment to the many opioid users who are not in treatment and who are at risk for opioid overdose. However, many people in need of buprenorphine treatment do not enroll in treatment. This randomized pilot trial evaluated efficacy of a remotely delivered incentive intervention in promoting engagement in buprenorphine treatment in out-of-treatment adults with opioid use disorder.
Methods.
Participants (N=41) were offered referrals to buprenorphine treatment and randomly assigned to Control or Incentive groups for 6 months. Incentive participants were offered incentives for enrolling in buprenorphine treatment, verified by providing documentation showing that they received a buprenorphine prescription, and providing videos taking daily buprenorphine doses. Participants used a smartphone application to record and submit a video of their buprenorphine prescription and daily buprenorphine administration. Incentive earnings were added remotely to reloadable credit cards.
Results.
Incentive participants were significantly more likely to enroll in treatment compared to control participants (71.4% versus 30.0% of participants; OR [95% CI]: 6.24 [1.46–26,72], p=.014). Few participants in either group adhered to buprenorphine treatment, and the two groups continued to use opioids, including fentanyl at high and comparable rates. The two groups did not differ in the percentage of urine samples that were positive for buprenorphine, opiates, fentanyl, or methadone at monthly assessments conducted during the 6-month intervention.
Conclusions.
Remotely delivered incentives can connect out-of-treatment adults with opioid use disorder to treatment, but additional supports are needed to promote buprenorphine adherence.
Keywords: buprenorphine, incentives, mobile health, opioids, overdose, treatment
1. Introduction
Opioid overdose remains a leading cause of injury and death in the United States (Ahmad et al., 2020; Scholl et al., 2019; Slavova et al., 2020; Wilson, 2020). In 2018 alone, nearly 47,000 people died from an overdose involving opioids. This equates to approximately 128 opioid-related overdose deaths per day (Wilson et al., 2020). Recent provisional data suggest that the opioid overdose epidemic is worsening (Ahmad et al., 2020).
Among the three medications approved by the United States Food and Drug Administration (FDA) for treatment of opioid use disorder (i.e., methadone, buprenorphine, and naltrexone), buprenorphine offers the greatest opportunity for expanding treatment access and combating the opioid overdose epidemic. Although methadone can reduce opioid overdose (Larochelle et al., 2018; Pearce et al., 2020; Sordo et al., 2017), it can only be dispensed in federally approved opioid treatment programs that typically require daily observed dosing (Connery, 2015). Although naltrexone can be prescribed in office-based treatment settings, there is insufficient evidence to suggest that it reduces opioid overdose (Jarvis et al., 2018; Larochelle et al., 2018; Wakeman et al., 2020). Buprenorphine can reduce opioid overdose (Larochelle et al., 2018; Pearce et al., 2020; Sordo et al., 2017) and can be prescribed in office-based treatment settings by physicians, nurse practitioners, and physician assistants in up to 30-day supplies. Office-based buprenorphine treatment permits patients to receive medication by prescription to be taken at home, thereby avoiding the requirement for daily dosing at a federally-approved opioid treatment program (Mattick et al., 2014). Despite these benefits, many individuals in need of opioid use disorder treatment do not enter buprenorphine treatment (Macmadu et al., 2020; Wakeman et al., 2020).
Research over the past 50 years on the use of incentives to treat substance use disorders suggests that incentive interventions (also called “contingency management” interventions) could be effective in promoting enrollment in buprenorphine treatment in out-of-treatment adults with opioid use disorder (Benishek et al., 2014; Davis et al., 2016; Higgins et al., 2011; Lussier et al., 2006; Silverman et al., 2019b; Silverman, 2004). Incentive interventions provide immediate incentives for health behaviors and thereby increase the frequency of those health behaviors. Furthermore, the remote delivery of incentive interventions via mobile-health technology has been shown to be a feasible and efficacious approach that could improve treatment reach and accessibility (Dallery et al., 2019; Getty et al., 2019). This randomized controlled pilot trial sought to evaluate the efficacy of a remotely delivered incentive intervention in promoting enrollment and engagement in buprenorphine treatment in out-of-treatment adults with opioid use disorder.
2. Materials and Methods
2.1. Setting and Study Participants
The study was conducted by the Center for Learning and Health on the Johns Hopkins Bayview Campus in Baltimore, Maryland, USA. All procedures were approved by the Johns Hopkins University School of Medicine Institutional Review Board, and all participants provided written informed consent. This study was registered at ClinicalTrials.gov (NCT03677986).
2.1.1. Inclusion and Exclusion Criteria
Applicants were eligible for the study if they were 18 years or older, met DSM-5 criteria for opioid use disorder, provided an opioid-positive urine sample (other than methadone), reported not receiving any type of drug abuse treatment in the past 30 days, and were interested in receiving buprenorphine treatment. Applicants were excluded from the study if they had current suicidal or homicidal ideation, were pregnant or nursing, or were unwilling or unable to use their own smartphone.
2.1.2. Recruitment Procedures
Participants were recruited from May 2019 through January 2020 via community agencies that served the target population (the Baltimore City Needle Exchange Program, Project Connections at Re-Entry, and Johns Hopkins Emergency Departments) and a referral system in which study participants were paid for successfully referring others to the study. Interested individuals completed a brief phone interview. Potentially eligible participants were invited for a full in-person interview. Eligible participants completed a computerized course about opioid overdose prevention and treatment strategies and FDA-approved medications for opioid use disorder (Toegel et al., Under Review).
2.2. Study Design
This was a two-group randomized controlled pilot study. Participants (N=41) were offered referrals to buprenorphine treatment and randomly assigned (1:1) to a Control group or an Incentive group using a computerized urn randomization procedure to balance groups on two characteristics that could influence outcome: (1) cocaine-positive urine sample (yes/no) and (2) sex (male/female). Various research staff members operated the randomization program. The participants and research staff were not blind to the conditions. Participants were taught the details of their group with written instructions and quizzes with incentives for correct responses. All participants received instructions about the study assessment procedures (e.g., assessment frequency and payments for assessments). Participants in the Incentive group received additional instructions about the incentive system (e.g., possible incentive earnings, requirements for submitting buprenorphine videos, when to expect research staff review of the videos).
2.3. Referrals to Buprenorphine Treatment
Participants in both groups were offered referrals to buprenorphine treatment. A research staff member met individually with participants to help them identify a preferred buprenorphine treatment provider or program. The research staff member and participant then called the provider or program to schedule a buprenorphine intake appointment. Participants also were provided with a list of 10–15 community buprenorphine treatment providers and programs that included location and contact information.
2.4. Incentive Group
Participants assigned to the Incentive group were offered a financial incentive for enrolling in buprenorphine treatment. Specifically, participants could earn $70 for documenting that they received a prescription for buprenorphine. Participants used a smartphone application offered by emocha Mobile Health, Inc. to record and submit a video of their buprenorphine prescription. These videos were encrypted, transmitted to a secure web portal, and subsequently deleted from the participant’s smartphone. Research staff then reviewed the participant videos on the web portal to verify and deliver incentives for receipt of a buprenorphine prescription. Incentive earnings were added remotely to a reloadable credit card that was given to the participant at study intake. After receiving a buprenorphine prescription, participants could then earn additional incentives ($10 per day) for submitting videos of themselves taking their daily buprenorphine dose via the emocha smartphone application. The incentives were available to participants for 6 months. In total, participants in the Incentive group could earn a maximum of $1,890 over the 6-month study period. The incentive magnitudes were selected based on results from a prior incentive-based medication adherence study in adults living with HIV (Silverman et al., 2019a). Research staff assisted participants in installing the emocha Mobile Health, Inc. application on their smartphones and showed them how to use the application. Participants were asked to independently submit a practice video via the smartphone application as a part of this training. Participants were instructed to call research staff if they encountered issues with the smartphone application (e.g., difficulty logging-into the application).
2.5. Control Group
Participants assigned to the Control group did not receive incentives for enrolling in buprenorphine treatment or taking buprenorphine. This group was included to determine the percentage of the study population that would enroll in buprenorphine treatment under routine, off-site referral conditions over a 6-month period.
2.6. Study Assessments
Participants completed assessments at study intake and every month after random assignment for 6 months. At intake we administered the DSM-5 checklist to screen for opioid use disorder (DSM-5, 2013) and an 8-item questionnaire that we developed to assess self-reported barriers and facilitators to accessing opioid use disorder treatment. At all assessments, we administered a brief questionnaire that we developed to assess smartphone access and ownership, a modified time-line follow-back procedure to assess self-reported use of opioid use disorder medications (i.e., buprenorphine, methadone, and naltrexone) (Sobell and Sobell, 1992), a modified opioid overdose risk questionnaire to assess rates at which participants self-reported engaging in behaviors that may put them at risk for an opioid overdose (Bohnert et al., 2016), the Addiction Severity Index-Lite to assess functioning in areas commonly affected by drug use (McLellan et al., 1985), the Beck Depression Inventory to screen for depression (Beck et al., 1996), and urine toxicology testing (unless an in-person visit could not be conducted). Participants were paid $50 for completing each of the intake and monthly assessments.
2.7. Outcome Measures
The outcome measures were based on data collected at the monthly assessments. The primary outcome measures were the percentage of participants who ever self-reported enrollment in buprenorphine treatment during the 6-month intervention (based on responses on the time-line follow-back) and the percentage of participants with buprenorphine-positive urine samples (urinary buprenorphine glucuronide concentrations greater than 20 ng/mL). Secondary outcome measures included the percentage of participants with urine samples positive for opiates (urinary morphine concentrations greater than 300 ng/mL), fentanyl (urinary fentanyl concentrations greater than 2 ng/mL), and methadone (urinary methadone concentrations greater than 300 ng/mL). Additional outcome measures included the percentage of participants with cocaine-positive (urinary benzoylecgonine concentrations greater than 300 ng/mL) urine samples and assessment collection rates.
2.8. Statistical Analyses
Measures assessed once were analyzed using logistic regression. Measures assessed repeatedly over time were analyzed with longitudinal logistic regression models. Within-person correlated outcomes were handled using the method of generalized estimating equations (Zeger et al., 1988). The magnitude of effect was expressed using odds ratios with 95% confidence intervals. Intention-to-treat analyses were adjusted for covariates used for stratification (Pocock et al., 2002). All missing values were considered missing (imputation of missing values was not conducted). Stata Statistical Software: Release 15 (College Station, TX; StataCorp LLC) was used to perform these analyses.
We followed Liu and Liang to determine the total number of participants required to detect a difference between groups with 80% power (Liu and Liang, 1997). A sample size of 64 was expected to be sufficient to detect a difference of 25% between the groups in the percentage of participants with buprenorphine-positive urine samples at the six monthly assessments. We stopped recruitment after randomly assigning 41 participants to the study groups due to a university-wide mandate to pause recruitment in response to the COVID-19 pandemic.
3. Results
3.1. Study Population
Figure 1 shows the flow of participants through the study. We assessed 100 participants for eligibility. We excluded 57 participants who did not meet the study eligibility criteria and 2 participants who did not return for randomization. We randomized 41 participants (20 to the Control group and 21 to the Incentive group). All randomized participants were included in the analyses.
Figure 1.
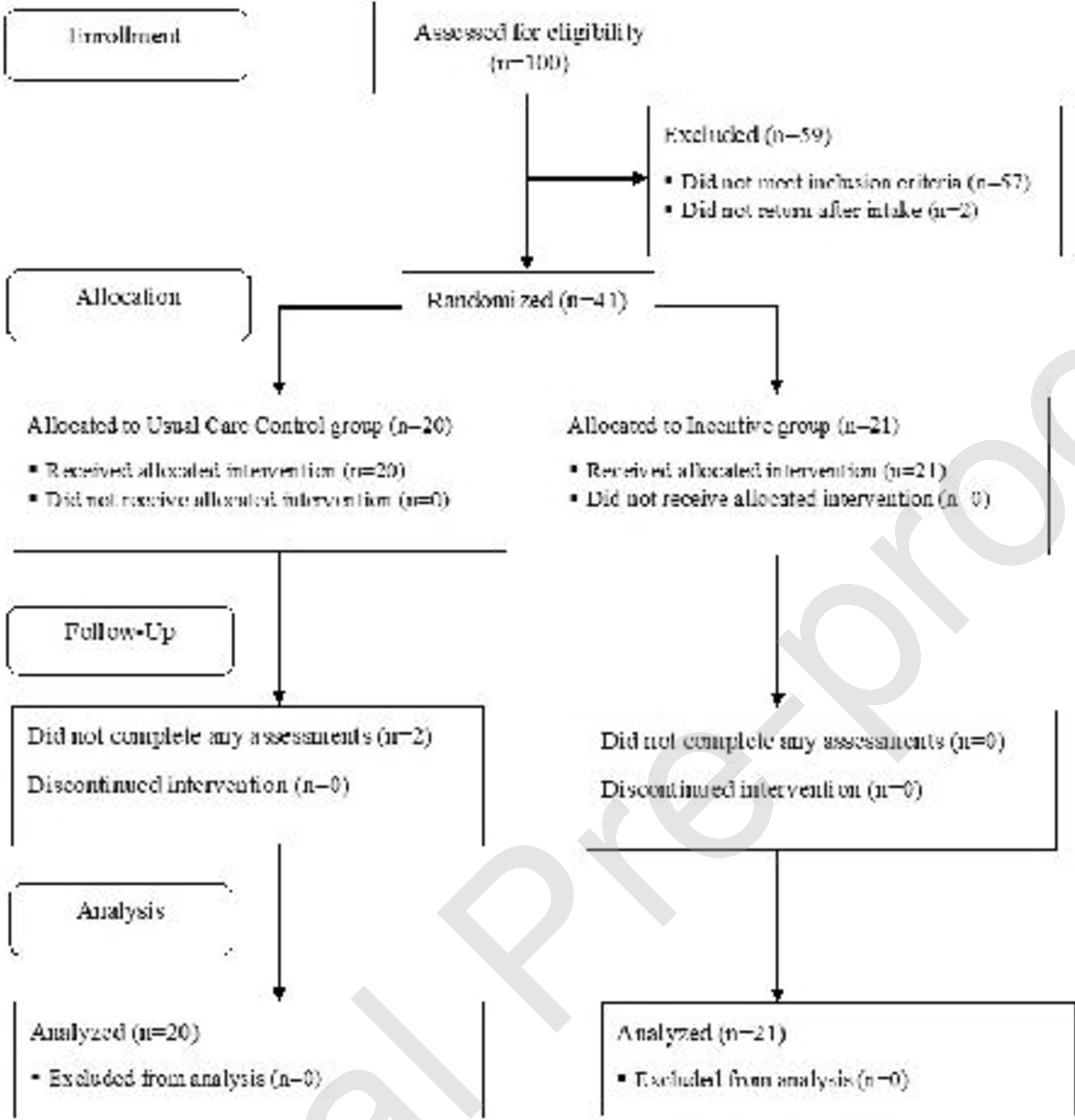
Flow of participants through the study.
Participants in the Control and Incentive groups were an average (SD) of 49.4 (8.9) and 44.7 (11.1) years old, respectively. Table 1 shows participant characteristics at study intake. Most participants identified as male (73%) and black (63%). Most participants were living in poverty (85%) and most were usually unemployed during the past 3 years (61%). All participants met DSM-5 criteria for severe opioid use disorder (100%). Few participants (7%) reported that they had never received opioid use disorder treatment in their lifetime. In most cases, participant urine samples were positive for opiates (88%), fentanyl (90%), or cocaine (66%). Some participants provided urine samples that were positive for buprenorphine (12%) or methadone (12%).
Table 1.
Participant characteristics at study intake.
| Percentage | ||
|---|---|---|
| Characteristic |
Control (n=20) |
Incentive (n=21) |
| Male | 70 | 76 |
| Race | ||
| Black | 80 | 48 |
| White | 15 | 5 |
| Other | 5 | 48 |
| Married | 15 | 14 |
| No stable living arrangement, past 3 years | 0 | 14 |
| High school diploma or GED | 70 | 71 |
| Living in povertya | 85 | 86 |
| Ever incarcerated | 80 | 71 |
| Usually unemployed, past 3 years | 65 | 57 |
| DSM-5 severe opioid use disorder | 100 | 10b |
| Injection drug use, past 30 days | 30 | 38 |
| Prior treatment: Buprenorphine | 60 | 67 |
| Prior treatment: Methadone | 60 | 38 |
| Prior treatment: Detox | 40 | 67 |
| Prior treatment: None | 10 | 5 |
| Self-reported street buprenorphine use | 25 | 29 |
| Drug positive urine sample | ||
| Opiates | 85 | 90 |
| Fentanyl | 95 | 86 |
| Cocaine | 70 | 62 |
| Buprenorphine | 15 | 10 |
| Methadone | 15 | 10 |
Poverty status was calculated using income and age data from the Addiction Severity Index-Lite and 2020 Poverty Thresholds from the US Census Bureau for one person with no related children.
3.2. Study Outcomes
Figure 2 shows the cumulative percentage of participants in each of the two groups who enrolled in buprenorphine treatment during the study. By the end of the 6-month intervention period, 71.4% of participants in the Incentive group enrolled in buprenorphine treatment compared with 30.0% of participants in the Control group. Incentive participants were significantly more likely to have enrolled in buprenorphine treatment by the end of the 6-month intervention than Control participants (Figure 2 and Table 2).
Figure 2.
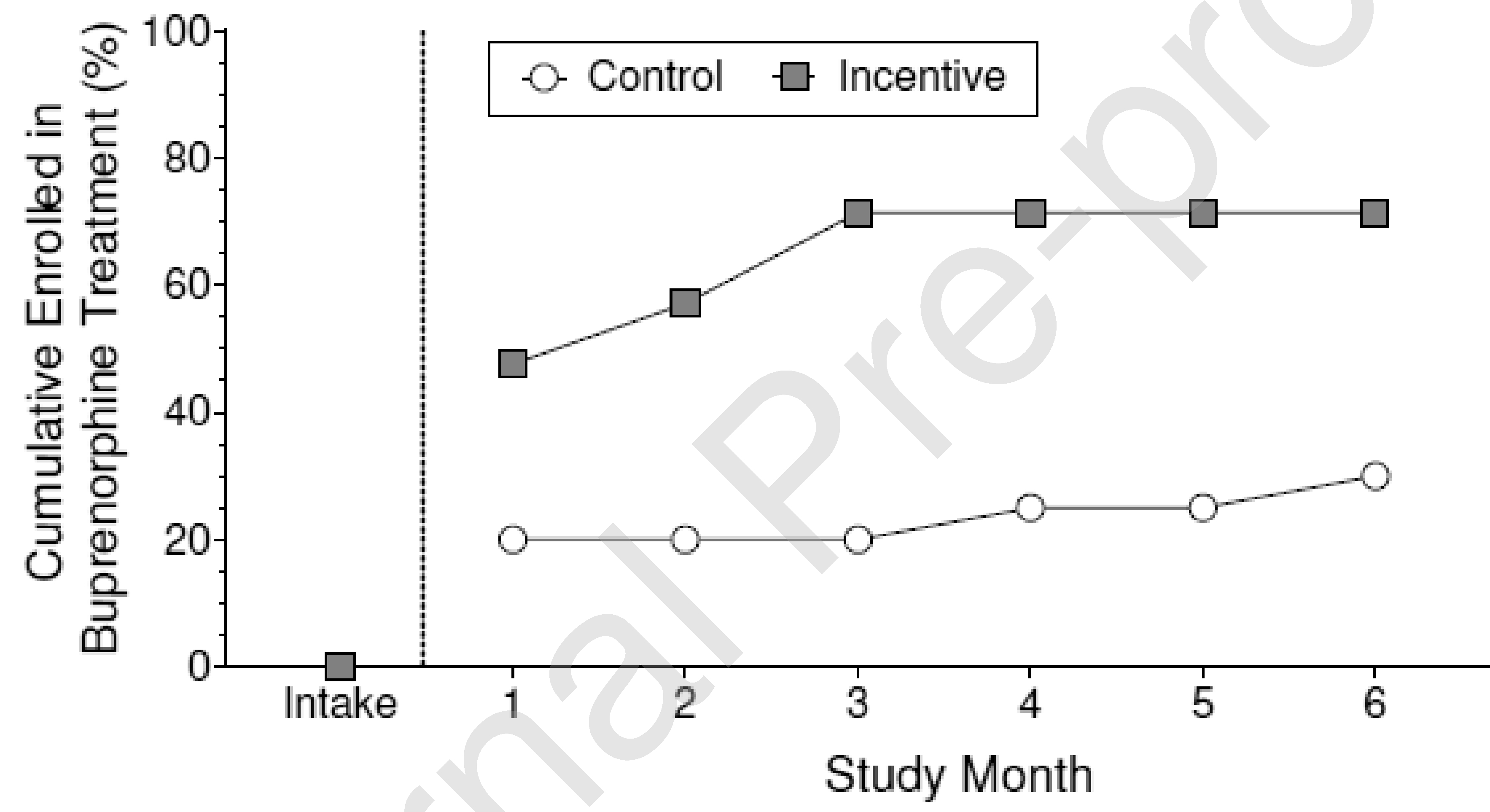
Cumulative percentage of participants who enrolled in buprenorphine treatment in the control group (circles) and the incentive group (squares) at intake and across consecutive months during the 6-month intervention. The difference between groups at the end of the intervention period was statistically significant (Odds Ratio=6.24, 95% Confidence Interval= 1.46–26.72, p=.014).
Table 2.
Primary, secondary, and other outcome measures from monthly assessments conducted during the 6-month intervention period.
| Percentage | ||||
|---|---|---|---|---|
| Control | Incentive | OR (95% CI) | P value | |
| Primary Outcome Measures | ||||
| Enrolled in buprenorphine treatment | 30.0 | 71.4 | 6.24 (1.4626.72) | .014 |
| Buprenorphine positive urine | 19.7 | 16.1 | 0.92 (0.21–4.14) | .914 |
| Secondary Outcome Measures | ||||
| Methadone positive urine | 27.6 | 18.4 | 0.68 (0.16–2.8.; | .594 |
| Opiate positive urine | 60.5 | 62.1 | 1.13 (0.40 –3.K) | .816 |
| Fentanyl positive urine | 77.6 | 82.8 | 1.54 (0.36–6.53) | .558 |
| Other Outcome Measures | ||||
| Cocaine positive urine | 61.8 | 54.0 | 0.79 (0.20–3.10) | .730 |
| Assessment collection rate | ||||
| Collected self-reports | 80.8 | 80.2 | 0.96 (0.33–2.84) | .946 |
| Collected urine samples | 63.3 | 69.0 | 1.30 (0.49–3.45) | .594 |
Figure 3 shows the percentage of opioid-positive urine samples provided by participants in the two groups during the study. The percentage of buprenorphine-positive urine samples and methadone-positive urine samples was low and similar among participants in both groups at intake and throughout the intervention (Figure 3, top panels; Table 2). The percentage of opiate-positive urine samples and fentanyl-positive urine samples was high and similar among participants in both groups at intake and throughout the intervention (Figure 3, bottom panels; Table 2).
Figure 3.
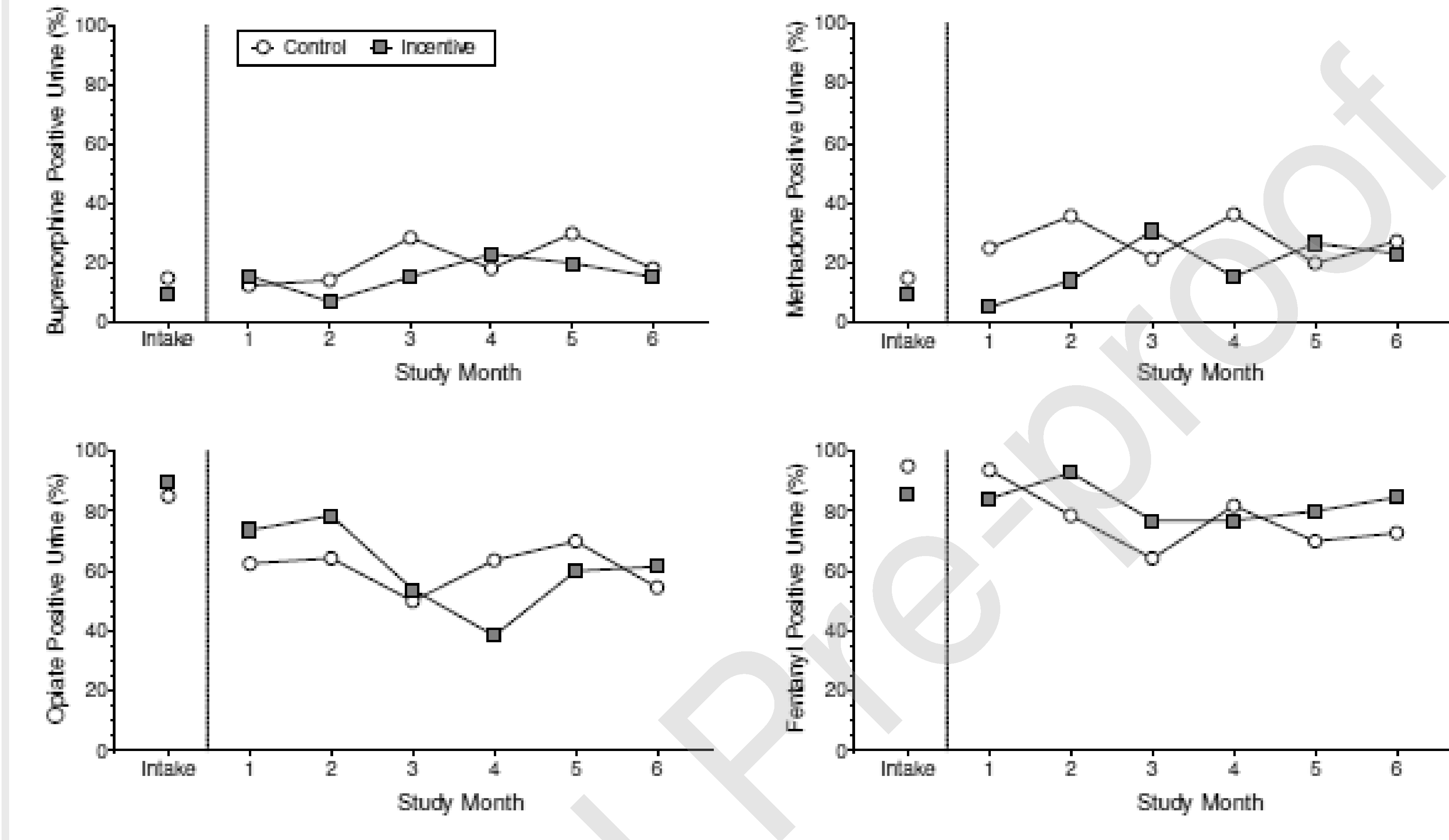
The percentage of opioid-positive urine samples provided by participants in the control group (circles) and the incentive group (squares) at intake and at each of the monthly assessments during the 6-month intervention. Results are shown for buprenorphine (top left panel), methadone (top right panel), opiates (bottom left panel), and fentanyl (bottom right panel). The difference between groups during the intervention was not statistically significant for any of the opioids (all ps >.05).
Table 2 shows monthly assessment collection rates. Due to COVID-19, for 19.1% of the monthly assessments, we could not conduct in-person visits to conduct urine collection and testing. However, we were able to collect participant self-reports via phone for these assessments. Assessment collection rates did not significantly differ between the two groups.
3.3. Incentive Group Video Submissions
Of the 21 participants in the Incentive group, 19 were able to successfully upload a practice video; two participants were unable to upload videos due to software issues with their personal smartphones, which we reported to the developers of the smartphone application. Fourteen participants provided documentation and received the incentive for showing that they received a buprenorphine prescription. Of those 14 participants, 7 submitted at least one video of themselves taking buprenorphine, and thereafter these 7 participants submitted buprenorphine videos on 1%, 5%, 35%, 91%, 98%, 99%, and 100% of the remaining study days.
4. Discussion
Opioid overdose remains a leading cause of injury and death in the United States, and is particularly severe among individuals who are not in treatment (Larochelle et al., 2018; Pearce et al., 2020; Sordo et al., 2017). Buprenorphine can reduce opioid overdose, but many individuals who could benefit from buprenorphine treatment do not enter treatment. This randomized controlled pilot trial sought to evaluate the efficacy of a remotely delivered incentive intervention in promoting engagement in buprenorphine treatment in out-of-treatment adults with opioid use disorder. Participants who were offered an incentive for enrolling in buprenorphine treatment were significantly more likely to enroll in treatment compared to control participants who were not offered an incentive. However, few participants adhered to buprenorphine treatment, and the two groups did not differ in the percentage of urine samples that were positive for buprenorphine. Participants in both groups continued high rates of opiate and fentanyl use. The two groups did not differ in the percentage of urine samples that were positive for opiates or fentanyl at monthly assessments conducted during the 6-month intervention period. Although the intervention was effective at connecting out-of-treatment adults with opioid use disorder to treatment, it did not effectively promote buprenorphine adherence.
Recent reports have noted difficulty with initiating buprenorphine treatment in individuals who have been exposed to or use fentanyl, such as the present study population (Antoine et al., 2020; Bisaga, 2019; Silverstein et al., 2019). Individuals exposed to fentanyl may be at increased risk for precipitated withdrawal when initiating buprenorphine treatment (i.e., during buprenorphine induction), which is likely related to fentanyl’s pharmacokinetic profile relative to other opioids (Antoine et al., 2020; Huhn et al., 2020; Silverstein et al., 2019). In the present study, we expected that individuals who self-reported an interest in receiving buprenorphine treatment and successfully connected to buprenorphine treatment - as evidenced by obtaining a buprenorphine prescription - would in turn initiate buprenorphine treatment. This does not appear to be the case. Additional supports during buprenorphine induction may be particularly needed in populations with high rates of fentanyl use, such as the present sample. Providing remotely delivered coaching during the buprenorphine induction based on clinical judgements and patient response could be applied to improve initiation outcomes. Nevertheless, novel buprenorphine induction protocols may be needed to promote successful buprenorphine induction in the current fentanyl era (Antoine et al., 2020; Moe et al., 2020; Randhaw et al., 2020). For example, “microdosing” - in which a small buprenorphine dose (e.g., 0.5 mg) is administered initially with incremental increases to both dose and frequency over time - is a buprenorphine induction approach that has been proposed to avoid withdrawal and minimize precipitated withdrawal. However, similar to other emerging buprenorphine induction protocols, rigorous studies evaluating their effectiveness and safety are needed (Moe et al., 2020).
Prior research has shown that the remote delivery of incentive interventions is feasible and efficacious. One approach used an internet-based method to deliver an incentive intervention for smoking cessation. The procedure required patients to remotely video record collection of breath samples with a carbon monoxide monitor, and delivered incentives through a web-based platform (Dallery et al., 2013). This intervention has been shown to be effective in promoting smoking cessation (Dallery and Glenn, 2005; Dallery et al., 2013; Reynolds et al., 2008; Stoops et al., 2009), including in a nationwide study of smokers from around the United States (Dallery et al., 2017). To facilitate dissemination, another approach used mobile technology to implement incentive interventions for cigarette smoking (Alessi et al., 2017), alcohol use disorder (Alessi and Petry, 2013; Koffarnus et al., 2018), and cannabis use disorder (Beckham et al., 2018). Results from the present study further support the feasibility of remotely delivered incentive interventions.
A few limitations should be noted. First, treatment enrollment was measured via participant self-report, which can be inaccurate and subject to recall or social desirability biases. Indeed, although participants self-reported that they were not enrolled in drug abuse treatment in the past 30 days at intake to the study, some participants (12%) provided urine samples that tested positive for buprenorphine or methadone. Participants self-reported street use of these substances, however, it is possible that these participants were not out of treatment. Second, for some of the study assessments, we could not conduct in-person visits for urine collection and testing due to COVID-19. In part because of this, we collected a relatively low rate of urine samples. Third, many participants reported a prior history of receiving treatment for opioid use disorder. Results may not generalize to other participant populations, such as individuals with no prior treatment experience. Finally, we did not ask participants about their buprenorphine induction experiences or reasons for low adherence to buprenorphine treatment at the monthly assessments. We do not know for certain the degree to which a poor buprenorphine induction experience (e.g. withdrawal precipitation) caused the low rates of buprenorphine treatment adherence.
Out-of-treatment adults with opioid use disorder have been difficult to engage in treatment (Booth et al., 1996; Kidorf et al., 2009; Macmadu et al., 2020). For example, in a multi-site study (N=2,973) that sought to promote treatment entry in out-of-treatment people who used opioids, only 8% of participants initiated treatment (Booth et al., 1996). In a recent cohort study (N=17,449) that examined enrollment in medication-based treatment within six months of an opioid overdose or opioid use disorder diagnosis, only 42% initiated treatment (Macmadu et al., 2020). Psychosocial interventions have shown some promise in engaging out-of-treatment opioid users into treatment, but they did not promote treatment initiation in approximately 60–70% of participants (Langabeer et al., 2020; Scott et al., 2020; Winhusen et al., 2020). Clearly there is a need for continued development of effective ways to help people with opioid use disorder who are not in treatment enter and stay in treatment. Improving the success of starting buprenorphine in individuals with fentanyl use disorder will be essential.
5. Conclusion
This pilot study suggests that remotely delivered incentives can connect out-of-treatment adults with opioid use disorder, a population at high risk for opioid overdose, to buprenorphine treatment. However, the incentive intervention did not promote adherence to buprenorphine treatment. Investigation and modification of the parameters of the intervention that might promote buprenorphine treatment initiation and adherence are needed.
Highlights.
Out-of-treatment adults with opioid use disorder are at risk for opioid overdose
Remotely delivered incentives promoted enrollment in buprenorphine treatment
Incentives did not promote buprenorphine treatment adherence
Fentanyl use may have contributed to the low rates of buprenorphine adherence
Acknowledgements
This journal article was supported by Grants T32DA07209 funded by the National Institute on Drug Abuse of the National Institutes of Health and R01CE003069 funded by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the U.S. Department of Health and Human Services.
We are grateful to our staff who helped to conduct this study: Jacqueline Hampton, Andrew Rodewald, Sarah Pollock, Meghan Arellano, India Harper, and Calvin Jackson.
Footnotes
Conflict of Interest. Under a license agreement with emocha Mobile Health Inc, the Johns Hopkins University is entitled to fees and royalty distributions related to technology used in the study described in this publication. In addition, the University owns equity in emocha Mobile Health Inc. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad F, Rossen L, Spencer M, Warner M, Sutton P, 2020. Provisional drug overdose death counts. National Center for Health Statistics. Centers for Disease Control and Prevention; Atlanta, GA. [Google Scholar]
- Alessi SM, Petry NM, 2013. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction. 108(5), 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Rash CJ, Petry NM, 2017. A randomized trial of adjunct mHealth abstinence reinforcement with transdermal nicotine and counseling for smoking cessation. Nicotine. Tob. Res. 19(3), 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine D, Huhn AS, Strain EC, Turner G, Jardot J, Hammond AS, Dunn KE, 2020. Method for successfully inducting individuals who use illicit fentanyl onto buprenorphine/naloxone. Am. J. Addict. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Beck depression inventory-II. San Antonio 78(2), 490–498. [Google Scholar]
- Beckham JC, Adkisson KA, Hertzberg J, Kimbrel NA, Budney AJ, Stephens RS, Moore SD, Calhoun PS, 2018. Mobile contingency management as an adjunctive treatment for co-morbid cannabis use disorder and cigarette smoking. Addict. Behav. 79, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, Festinger DS, 2014. Prize- based contingency management for the treatment of substance abusers: A meta- analysis. Addiction. 109(9), 1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, 2019. What should clinicians do as fentanyl replaces heroin? Addicton. 114(5), 782–783.. [DOI] [PubMed] [Google Scholar]
- Bohnert AS, Bonar EE, Cunningham R, Greenwald MK, Thomas L, Chermack S, Blow FC, Walton M, 2016. A pilot randomized clinical trial of an intervention to reduce overdose risk behaviors among emergency department patients at risk for prescription opioid overdose. Drug. Ale. Depend. 163, 40–47. [DOI] [PubMed] [Google Scholar]
- Booth RE, Crowley TJ, Zhang Y, 1996. Substance abuse treatment entry, retention and effectiveness: out-of-treatment opiate injection drug users. Drug. Ale. Depend. 42(1), 11–20.. [DOI] [PubMed] [Google Scholar]
- Connery HS, 2015. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harvard. Rev. Psychiatry. 23(2), 63–75. [DOI] [PubMed] [Google Scholar]
- Dallery J, Glenn IM, 2005. Effects of an Internet- based voucher reinforcement program for smoking abstinence: A feasibility study. J. App. Rebav. Anal. 38(3), 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ, 2013. Internet- based contingency management to promote smoking cessation: A randomized controlled study. J. App. Behav. Anal. 46(4), 750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ, Marsch LA, 2019. Technology-Based Contingency Management in the Treatment of Substance-Use Disorders. Perspect. Behav. Sci. 42(3), 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, Grabinski MJ, 2017. Nationwide access to an internet- based contingency management intervention to promote smoking cessation: a randomized controlled trial. Addiction. 112(5), 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST, 2016. A review of the literature on contingency management in the treatment of substance use disorders. 2009–2014. Prev. Med. 92, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-5, 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub. [Google Scholar]
- Getty CA, Morande A, Lynskey M, Weaver T, Metrebian N, 2019. Mobile telephonedelivered contingency management interventions promoting behaviour change in individuals with substance use disorders: a meta- analysis. Addiction. 114(11), 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S, Sigmon S, Heil S, 2011. Contingency management in the treatment of substance use disorders: trends in the literature. Lowinson and Ruiz’s substance abuse: A comprehensive textbook, 603–621. [Google Scholar]
- Huhn AS, Hobelmann JG, Oyler GA, Strain EC, 2020. Protracted renal clearance of fentanyl in persons with opioid use disorder. Drug. Ale. Depend. 214, 108147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, Silverman K, 2018. Extended- release injectable naltrexone for opioid use disorder: a systematic review. Addiction. 113(7), 1188–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidorf M, King VL, Neufeld K, Peirce J, Kolodner K, Brooner RK, 2009. Improving substance abuse treatment enrollment in community syringe exchangers. Addiction. 104(5), 786–795. [DOI] [PubMed] [Google Scholar]
- Koffamus MN, Bickel WK, Kablinger AS, 2018. Remote alcohol monitoring to facilitate incentive- based treatment for alcohol use disorder: a randomized trial. Ale. Clin. Exp. Res. 42(12), 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langabeer J, Champagne-Langabeer T, Luber SD, Prater SJ, Stotts A, Kirages K, Yatsco A, Chambers KA, 2020. Outreach to people who survive opioid overdose: Linkage and retention in treatment. J. Sub. Abuse. Treat. 111, 11–15. [DOI] [PubMed] [Google Scholar]
- Larochelle MR, Bemson D, Land T, Stopka TJ, Wang N, Xuan Z, Bagley SM, Liebschutz JM, Walley AY, 2018. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann. Intern. Med. 169(3), 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Liang KY, 1997. Sample size calculations for studies with correlated observations. Biometrics. 937–947. [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST, 2006. A meta- analysis of voucher- based reinforcement therapy for substance use disorders. Addiction. 101(2), 192–203.. [DOI] [PubMed] [Google Scholar]
- Macmadu A, Pauli K, Youssef R, Batthala S, Wilson KH, Samuels EA, Yedinak JL, Marshall BD, 2020. Predictors of Enrollment in Opioid Agonist Therapy after Opioid Overdose or Diagnosis with Opioid Use Disorder: A cohort study. Drug. Ale. Depend. 108435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane. Database. Syst. Rev. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans L, Barr HL, O’Brien CP, 1985. New data from the Addiction Severity Index: reliability and validity in three centers. J. Nerv. Ment. 173(7), 412–423. [DOI] [PubMed] [Google Scholar]
- Moe J, O’Sullivan F, Hohl CM, Doyle-Waters MM, Ronsley C, Cho R, Liu Q, Azar P, 2020. Systematic review on effectiveness of micro-induction approaches to buprenorphine initiation. Addict. Behav. 114, 106740. [DOI] [PubMed] [Google Scholar]
- Pearce LA, Min JE, Piske M, Zhou H, Homayra F, Slaunwhite A, Irvine M, McGowan G, Nosyk B, 2020. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 368, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, Kasten LE, 2002. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practiceand problems. Stat. Med. 21(19), 2917–2930. [DOI] [PubMed] [Google Scholar]
- Randhawa PA, Brar R, Nolan S, 2020. Buprenorphine-naloxone “microdosing”: an alternative induction approach for the treatment of opioid use disorder in the wake of North America’s increasingly potent illicit drug market. CMAJ. 192(3), E73–E73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Dallery J, Shroff P, Patak M, Leraas K, 2008. A web- based contingency management program with adolescent smokers. J. App. Behav. Anal. 41(4), 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, 2019. Drug and opioid-involved overdose deaths—United States, 2013–2017. Morbid. Mortal. Wkly. Rep. 67(51–52), 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Dennis ML, Grella CE, Kurz R, Sumpter J, Nicholson L, Funk RR, 2020. A community outreach intervention to link individuals with opioid use disorders to medication-assisted treatment. J. Sub. Abuse. Treat. 108, 75–81. [DOI] [PubMed] [Google Scholar]
- Silverman K, 2004. Exploring the limits and utility of operant conditioning in the treatment of drug addiction. Behav. Anal. 27(2), 209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Holtyn AF, Rodewald AM, Siliciano RF, Jarvis BP, Subramaniam S, Leoutsakos J-M, Getty C-A, Ruhs S, Marzinke MA, 2019a. Incentives for viral suppression in people living with HIV: a randomized clinical trial. AIDS. Behav. 23(9), 2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Holtyn AF, Toegel F, 2019b. The Utility of Operant Conditioning to Address Poverty and Drug Addiction. Perspect. Behav. Sci. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Daniulaityte R, Martins SS, Miller SC, Carlson RG, 2019. “Everything is not right anymore”: Buprenorphine experiences in an era of illicit fentanyl. Int. J. Drug. Policy. 74, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavova S, Rock P, Bush HM, Quesinberry D, Walsh SL, 2020. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug. Ale. Depend. 214, 108176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back, Measuring alcohol consumption. Springer, pp. 41–72. [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, PastorBarriuso R, 2017. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, Casey B, Wong CJ, 2009. An internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug. Ale. Depend. 105, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toegel F, Novak MD, Rodewald AM, Leoutsakos JM, Silverman K, Holtyn AF, Under Review. Technology-assisted opioid education for out-of-treatment adutls with opioid use disorder. Psychol. Addict. Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, Azocar F, Sanghavi DM, 2020. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA. Netw. Open. 3(2), e1920622–e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H, Davis N, 2020. Drug and opioid-involved overdose deaths—United States, 2017–2018. Morbid. Mortal. Wkly. Rep. 69, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Wilder C, Kropp F, Theobald J, Lyons M, Lewis D, 2020. Brief telephonedelivered peer intervention to encourage enrollment in medication for opioid use disorder in individuals surviving an opioid overdose: results from a randomized pilot trial. Drug. Ale. Depend. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y, Albert PS, 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics, 1049–1060. [PubMed] [Google Scholar]


