Abstract
Heroin intake decreases significantly during proestrus in normally cycling female rats, and this effect is mediated by endogenous estradiol but not endogenous progesterone. The purpose of this study was to determine whether chronic administration of exogenous estradiol decreases intake of the semi-synthetic opioid, heroin, and the fully synthetic opioid, remifentanil, in intact female rats. Normally cycling female rats were implanted with intravenous catheters and trained to self-administer heroin on a fixed ratio (FR1) schedule of reinforcement. Rats were treated chronically with daily administration of either a low dose of estradiol (0.5 mcg, sc), a high dose of estradiol (5.0 mcg, sc), or vehicle (peanut oil, sc). After two weeks of heroin self-administration training, dose-effect curves were determined for both heroin and remifentanil. Chronic administration of estradiol non-significantly decreased heroin intake and significantly decreased remifentanil intake. Estradiol-induced decreases in remifentanil intake were dose-dependent, characterized by large effect sizes, and greatest in rats treated with the high dose of estradiol. These data indicate that chronic estradiol administration decreases opioid intake in intact female rats with medium to large effect sizes across opioids. These findings suggest that estrogen-based pharmacotherapies may represent a novel treatment approach for women with opioid use disorder.
Keywords: Estrous Cycle, Opioid, Self-administration, Estrogen, estradiol
1. Introduction
In 2018, opioids were involved in nearly 70% of drug-related overdoses (Wilson et al., 2020), but the risk of overdose over the years has increased more in women than in men (Lopresti et al., 2020). Although significantly more men suffer from opioid use disorder, women using opioids are at greater risk of developing an opioid use disorder than men using opioids (NIDA, 2020). For example, women escalate from casual use to dependence quicker, experience greater craving, are less likely to seek treatment for heroin use, are less likely to adhere to medical treatment, and experience more withdrawal symptoms than men (Greenfield et al., 2007; Kokane and Perrotti, 2020; Lopresti et al., 2020; Sofuoglu et al., 2019). Current pharmacological treatments primarily use long-acting mu-opioid agonists, such as buprenorphine or methadone. Although effective, these agonists have abuse liability of their own and are not always fully accessible because of regulatory burdens related to their schedule-controlled status. Consequently, new pharmacotherapies that lack abuse liability, have low regulatory burden, and possess a proven track record of safety are needed.
Previously, Lacy and colleagues demonstrated heroin intake decreases significantly (~70%) during the proestrus phase of the estrus cycle in normally cycling female rats (Lacy et al., 2016). These proestrus-associated decreases in heroin intake were replicated across multiple cycles in both socially housed and isolated females. Notably, this decrease persisted across a 100-fold dose range of heroin, indicating a decrease in the effectiveness of heroin to serve as a positive reinforcer. During proestrus, both progesterone and estradiol levels reach their peaks, with estradiol peaking first followed by progesterone 8–24 hours later (Scharfman and MacLusky, 2014). Because both estradiol and progesterone reach peak concentrations during proestrus, one or a combination of both could be responsible for the decrease in opioid intake during proestrus.
Early studies examining the effects of estradiol and progesterone on opioid self-administration reported that neither estradiol nor progesterone influences heroin self-administration (Stewart et al., 1996) or that estradiol enhances the acquisition of heroin self-administration (Roth et al., 2002). More recent research, however, has reported that estradiol consistently decreases heroin seeking during extinction and during food restriction (Sedki et al., 2015; Vazquez et al., 2020). Consistent with these latter findings, Smith and colleagues reported that both acute (Smith et al., 2020a) and chronic (Smith et al., 2020b) estradiol administration significantly reduce heroin intake in ovariectomized females, and these effects are not altered by progesterone administration. Similarly, acute administration of an estradiol receptor antagonist, but not a progesterone receptor antagonist, blocks proestrus-associated decreases in heroin intake in normally cycling rats (Smith et al., 2020b). Thus, a converging body of evidence indicates that estradiol, but not progesterone, reduces heroin intake under a wide range of experimental conditions.
Given that estradiol decreases heroin intake in ovariectomized rats and given that blockade of estrogen receptors prevents proestrus-associated decreases in heroin intake in normally cycling rats, the purpose of this study was to determine whether chronic estradiol treatment reduces opioid intake in intact female rats. To this end, female rats were treated chronically with either low (0.5 micrograms or mcg) or high (5.0 mcg) doses of estradiol prior to daily self-administration sessions. Following two weeks of heroin self-administration training, heroin and remifentanil intake were measured separately across a 100-fold dose range. The translational goal of this study was to determine if agonist activity at estrogen receptors reduces the intake of two structurally distinct opioids, both of which have high abuse liability in humans, using intact female rats.
2. Methods
2.1. Animals
The subjects were experimentally naïve, adult, female Long Evans rats (rattus novegicus). Rats were obtained from the vendor (Charles Rivers Laboratories, Raleigh, NC) at postnatal day 49 and individually housed in polycarbonate “shoebox” cages (40 cm wide × 85 cm long × 40 cm high) with environmental enrichment. Ad libitum access to water and food (LabDiet5P00 - ProLabRMH3000) was given to all rats, except during the brief period of lever press training (see below). The animal colony was kept on a 12:12 hour light-dark cycle with testing occurring during the light portion of the cycle. All rats were maintained in accordance with the guidelines of the Animal Care and Use Committee of Davidson College.
2.3. Apparatus and Chemicals
Rats were trained to lever press in operant conditioning chambers from Med Associates (St. Albans, VT) enclosed in sound attenuating cabinets. Each chamber contained a house light, two response levers with corresponding lights, a food tray between the levers, a food hopper, and an infusion pump located outside the cabinet. Infusions were delivered via a tygon tube connecting the infusion pump to a counterbalanced swivel and connecting the swivel to the implanted catheter port. To prevent damage, the tubing from the swivel to the port was encased in a stainless-steel tether. A white noise machine was also used throughout training and testing. Heroin (diacetylmorphine HCl) was supplied by the National Institute of Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA) and dissolved in sterile saline for intravenous infusion. Remifentanil HCl was purchased from MilliporeSigma (St. Louis, MO, USA) and dissolved in sterile saline. Two doses of estradiol (Low: 0.5 mcg, sc; High: 5.0 mcg, sc) were dissolved in peanut oil as a vehicle. All hormone or vehicle injections were administered at a volume of 0.1 ml. Injections began a week following surgery and occurred daily for the duration of the experiment.
2.4. Lever-Press Training
Rats were food restricted to 90% free feeding weight approximately a week after arrival. Each rat was trained to lever press using food reinforcement on a fixed ratio (FR1) schedule of reinforcement. Training sessions were limited to a maximum of 40 reinforcers or 2 hr, whichever occurred first. Rats completed at least four training sessions.
2.5. Surgery
Rats were implanted with intravenous catheters in the same manner described previously (Smith et al., 2008). Briefly, rats were anesthetized via injection of ketamine (100 mg/kg, ip) and xylazine (8 mg/kg, ip). Next, the catheter was implanted in the right jugular vein and the port was externalized between the scapulae. Postoperatively, rats were given two injections of the analgesic, ketoprofen (3 mg/kg, sc), 24-hours apart. Additionally, catheters were flushed daily with the antibiotic, ticarcillin (20 mg/kg, iv), along with heparinized saline to prevent infection and maintain patency. After one week, ticarcillin was discontinued, but catheters continued to be flushed daily with heparinized saline throughout the experiment to maintain patency.
2.5. Estrous Cycle Monitoring
The estrous cycle was monitored via vaginal lavage using sterile saline to collect samples. Samples were then examined using light microscopy (10x magnification) to analyze cellular composition. Each sample was classified as being metestrus, diestrus, proestrus, or estrus following methods previously described (Lacy et al., 2016).
2.7. Heroin Self-Administration Training and Testing
Heroin self-administration began one week following surgery. Rats were subcutaneously injected with estradiol (0.5 mcg or 5.0 mcg) or peanut oil 30 minutes prior to testing. During testing, rats were placed into the operant conditioning chambers and secured via the stainless-steel tether. Rats were trained to self-administer heroin (0.0075 mg/kg/infusion) on a FR1 schedule. At the beginning of the session, the house light turned on and a noncontingent infusion of heroin was delivered. Both levers, active and inactive, in the chamber were then extended and the light above the active lever was illuminated. Each active lever press yielded an infusion of heroin followed by a 20-s timeout during which the lever was retracted, and the stimulus light was turned off. Each testing session continued in this fashion for 2 hr. Rats were then returned to their homecages and the colony room. Training continued for 10 training sessions across two weeks (5 days a week).
After two weeks of training, heroin intake was measured across five consecutive days, with a single dose of heroin tested each day. Heroin doses (0.0003, 0.001, 0.003, 0.01, and 0.03 mg/kg/infusion) were tested in a pseudorandom order so that no more than two ascending or descending doses were tested in a row. Upon completion of the dose effect curve, a saline substitution test was conducted as a control during which saline was substituted for heroin. After testing with heroin, remifentanil intake was measured across five consecutive days, with a single dose of remifentanil tested each day. Remifentanil doses (0.0001, 0.0003, 0.001, and 0.01 mg/kg/infusion) were tested in a pseudorandom order as described above, followed by an additional saline substitution day. All other methodology during heroin and remifentanil testing matched training sessions.
2.8. Data Analysis
A total of 69 rats completed testing with heroin (vehicle: n = 25; 0.5 mcg estradiol: n = 19 rats; 5.0 mcg estradiol: n = 25 rats). Two rats died during testing with remifentanil for undetermined reasons (vehicle: n = 1; 5.0 mcg estradiol: n = 1). These two rats were excluded from the remifentanil analysis but included in the heroin analysis.
Responding during drug self-administration sessions was expressed as number of infusions (i.e., number of active lever responses; number of reinforcers) per session. Data obtained during training were analyzed via mixed-factor ANOVA, with group serving as a between-subjects factor and day (i.e., training session) serving as the repeated-measure. Data obtained during testing were analyzed via mixed-factor ANOVA, with group serving as a between-subjects factor and dose serving as the repeated-measure. Responding maintained by saline was compared to responding maintained by each dose of heroin and remifentanil by repeated-measures ANOVA, followed by Dunnett post hoc tests in which saline was defined as the control. Area under the curve (AUC) estimates were determined from the dose-response data using the trapezoidal rule and analyzed via one-way ANOVA with group serving as the factor. Data obtained from responding on the inactive lever were analyzed in an identical manner. Responding during saline substitution tests was analyzed via one-way ANOVA using group as the factor. Tukey’s honestly significant difference (HSD) post hoc tests were used to compare differences between groups under conditions in which the omnibus test was significant. All statistical tests were two-tailed, and the alpha level was set to .05. Effect sizes were calculated as either Cohen’s d or partial eta squared (η2).
3. Results
All rats exhibited normal estrous cycles prior to treatment, with vaginal cytology revealing clear transitions across phases (metestrus, diestrus, proestrus, and estrus) over the course of 4- to 5-day cycles. Vehicle treatment did not produce any observable changes to the estrous cycle over the course of the study. Chronic treatment with 0.5 mcg estradiol disrupted cycling in all rats, characterized by a prolongation of the estrous cycle that was attributed to additional days in the high-estradiol phases of proestrus and estrus (~60% of days in proestrus/estrus). Chronic treatment with 5.0 mcg estradiol produced further disruptions in estrous cycling, with an even greater proportion of days in proestrus and estrus (~90% of days in proestus/estrus).
Responding maintained by heroin was stable by the second week of training (Figure 1) as defined by no significant main effect of day or significant day × group interaction over the last 5 days of training. Rats treated with 5.0 mcg estradiol responded approximately 30% less during this period of training than rats treated with either vehicle or 0.5 mcg estradiol, but this effect was not statistically significant [F(2, 66) = 2.519, p = .088; η2 = .071].
Figure 1.
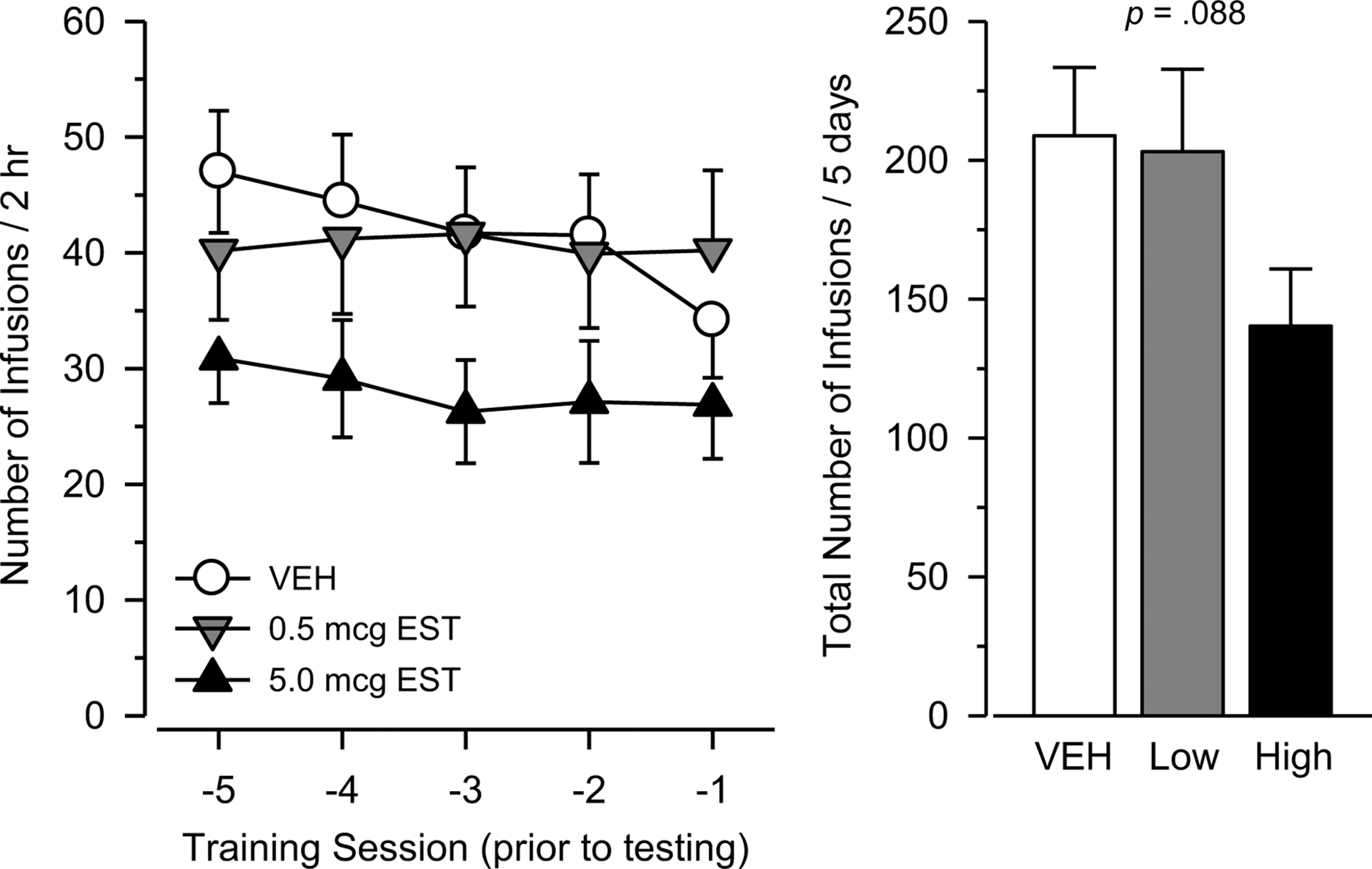
Heroin intake during the last five days of training in female rats treated chronically with vehicle (n = 25), 0.5 mcg estradiol (n = 19), or 5.0 mcg estradiol (n = 25). Left panel depicts number of infusions over 2 hr as a function of training session prior to testing. Right panel depicts total number of infusions over the five sessions. Vertical bars represent the SEM.
During testing, responding maintained by heroin was characterized by an inverted U-shaped dose-effect curve in all groups [main effect of dose: F(4, 264) = 23.080, p < .001; η2 = .259;], with ascending and descending limbs that converged at 0.003 mg/kg (Figure 2). Responding maintained by heroin was greater than responding maintained by saline in all groups. Vehicle-treated rats self-administered more heroin than saline [F(5, 120) = 9.544, p < .001] at doses of .001, .003, .01, and .03 mg/kg/inf (p = .018, p < .001, p = .024, and p = .015, respectively). Similarly, 0.5 mcg-treated rats self-administered more heroin than saline [F(5, 90) = 6.535, p < .001] at a dose of .003 mg/kg/inf (p = .011). Finally, 5.0 mcg-treated rats self-administered more heroin than saline [F(5, 120) = 6.719, p < .001] at doses of .001, .003, and .03 mg/kg/inf (p = .009, p = .008, p = .043, respectively). Dose-effect curves were generally parallel, and no significant dose × group interaction was observed [F(10, 330) = .671, p = .751, η2 = .02]. Rats treated with 5.0 mcg estradiol responded approximately 35% less than rats treated with either vehicle or 0.5 mcg estradiol; however, this effect was not statistically significant in either the dose-response [effect of group: F(2, 66) = 2.662, p = .077; η2 = .075] or the AUC [F(2, 66) = 2.407, p = .098; η2 = .068 analysis].
Figure 2.
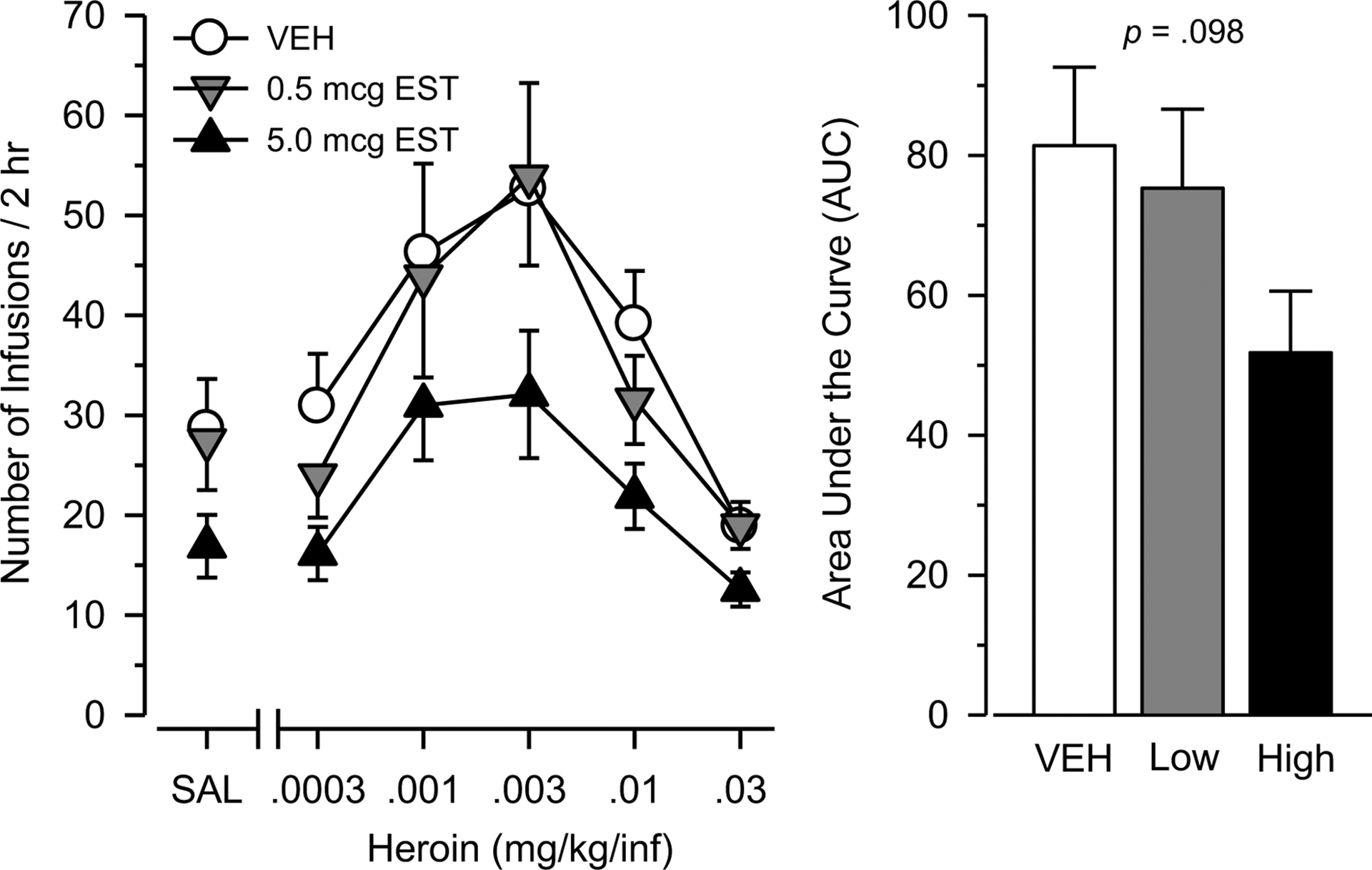
Heroin intake during testing in female rats treated chronically with vehicle (n = 25), 0.5 mcg estradiol (n = 19), or 5.0 mcg estradiol (n = 25). Left panel depicts number of infusions over 2 hr as a function of dose of heroin (mg/kg/infusion) or saline (SAL). Right panel depicts AUC estimates as determined from the dose-response data. Vertical bars represent the SEM.
Similar to that observed with heroin, responding maintained by remifentanil was characterized by an inverted U-shaped dose-effect curve in all groups [main effect of dose: F(4, 256) = 23.102, p < .001; η2 = .265], with steep ascending and descending limbs centered at .001 mg/kg (Figure 3). Responding maintained by remifentanil was greater than responding maintained by saline in all groups. Vehicle-treated rats self-administered more remifentanil than saline [F(5, 115) = 15.869, p < .001] at doses at doses of .0003, .001, and .003 mg/kg/inf (p = .010, p = .001, and p < .001, respectively). Similarly, rats treated with 0.5 mcg estradiol self-administered more remifentanil than saline [F(5, 90) = 7.762, p < .001] at doses of .0003, .001, .003, and .01 mg/kg/inf (p = .003, p < .001, p = .003, and p = .008, respectively). Finally, rats treated with 5.0 mcg estradiol self-administered more remifentanil than saline [F(5, 110) = 4.83, p < .001] at doses of .0003, .001, and .003 mg/kg/inf (p = .020, p = .007, and p = .048, respectively).
Figure 3.
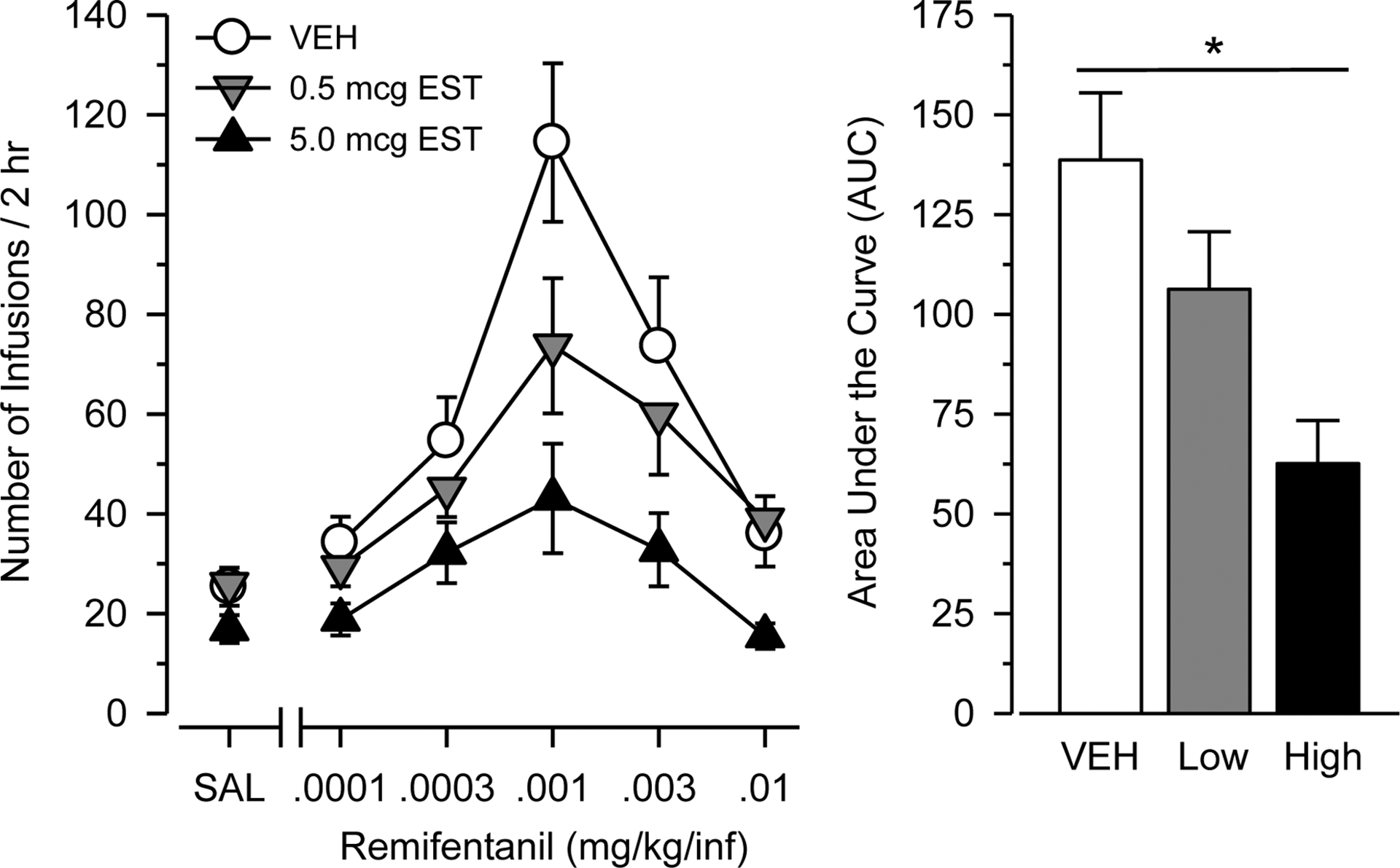
Remifentanil intake during testing in female rats treated chronically with vehicle (n = 24), 0.5 mcg estradiol (n = 19), or 5.0 mcg estradiol (n = 24). Left panel depicts number of infusions over 2 hr as a function of dose of remifentanil (mg/kg/infusion) or saline (SAL). Right panel depicts AUC estimates as determined from the dose-response data. Vertical bars represent the SEM. Asterisk indicates significant difference between groups.
Responding maintained by remifentanil differed significantly across groups in the dose-response analysis [main effect of group: F(2, 64) = 7.894, p = .001; η2 = .198], with a rank order of vehicle > 0.5 mcg estradiol > 5.0 mcg estradiol. Similar effects were obtained in the AUC analysis [F(2, 66) = 7.713, p = .001; η2 = .194], with rats treated with 5.0 mcg estradiol responding significantly less than rats treated with vehicle (p = .001; d = 1.102). Differences across groups varied across dose [significant group × dose interaction: F(10,315) = 3.144, p = .009, η2 = .091]. Rats treated with 5.0 mcg estradiol self-administered less remifentanil than vehicle-treated rats at doses of .0001, .0003, .001, .003, and .01 mg/kg/infusion (p = .017, p = .043, p = .001, p = .013, and p = .005, respectively) and less remifentanil than rats treated with 0.5 mcg estradiol at doses of .0001 and .01 mg/kg/infusion (p = 0.041 and p = .001, respectively).
Responding during the saline subjection test was ~37% lower in rats treated with the 5.0 mcg estradiol than in rats treated with vehicle, but this effect did not approach statistical significance during testing with heroin [F(2, 68)= 2.292, p = .109) or remifentanil F(2, 66) = 1.180, p = .214). Responding on the inactive lever was low throughout the study, and no significant differences in inactive-lever responding were observed across groups during training (Supplemental Figure 1), testing with heroin (Supplemental Figure 2), or testing with remifentanil (Supplemental Figure 3).
4. Discussion
Preclinical studies report that both acute and chronic estradiol administration decreases opioid intake in ovariectomized female rats. The present study assessed the potential of chronic estradiol to reduce opioid intake of structurally dissimilar opioids with high abuse liability in fully intact female rats. Here, we demonstrate that chronic treatment with a high daily dose of estradiol non-significantly reduces heroin intake and significantly reduces remifentanil intake across a 100-fold dose range. These reductions reflected 35% decreases in drug intake relative to vehicle for both drugs, with medium and large effect sizes for heroin and remifentanil, respectively. Collectively, these data suggest that estrogenic therapies may reduce opioid intake in women with opioid use disorder.
Both heroin and remifentanil produced biphasic doses-effect curves with clear ascending and descending limbs. Chronic treatment with low and high doses of estradiol led to dose-dependent downward shifts in the curves for both drugs. The observation that the curves were shifted vertically, as opposed to horizontally, suggests that estradiol reduced intake by decreasing the effectiveness, as opposed to the potency, of these drugs to serve as positive reinforcers. Indeed, the peak of the dose-effect curves were unchanged by estradiol treatment, but the area under the curve was significantly lower in estradiol- than vehicle-treated rats for remifentanil and non-significantly lower for heroin. Notably, there were no significant differences in responding on an inactive lever, indicating the observed differences were not due to differences in non-specific motor activity.
We emphasize that estradiol’s effects on heroin self-administration failed to reach statistical significance. The 35% reduction in heroin intake at the high dose of estradiol was characterized by an effect size smaller than remifentanil but still defined as “medium” by traditional standards (Cohen, 2013). Rats used in this study were obtained in multiple cohorts over a 12-month period, with all three treatment groups represented in each cohort. All cohorts showed between-group differences in heroin intake similar in magnitude to that depicted in the final analysis. It is possible that statistical significance may have been achieved with a larger number of subjects; however, a power analysis revealed that approximately 300 rats would be needed to reach statistical significance with this effect size. We believe the 35% reduction in heroin intake and medium effect size is accurate and potentially meaningful when applied to human populations, but nonetheless modest when compared to remifentanil. We do not believe the differences obtained between heroin and remifentanil reflect differences in the receptor-mediated mechanisms of these drugs. Remifentanil is a selective, high-efficacy mu opioid agonist (Scott & Perry, 2012; Michelsen & Hug, 2013); heroin is a prodrug whose primary metabolites (i.e., morphine, 6-MAM, and M6G; Gottas et al., 2013) are all mu agonists with greater selectivity for mu than non-mu receptors. It is possible that the smaller effect size observed with heroin was due to the lower levels of responding maintained by heroin, thus creating less separation from a hypothetical “floor”. We do not find this explanation convincing because estradiol did not decrease responding to saline-control levels for either drug, suggesting that the floor had not been reached. Finally, it is possible that estradiol was less effective at decreasing heroin than remifentanil intake because heroin was tested before remifentanil and estradiol-treated rats had one less week of estradiol exposure. We do not find this explanation convincing because we reported previously that a single dose of estradiol significantly decreases heroin intake when given as a bolus 22 hours before a test session (Smith et al., 2020a).
We previously demonstrated that daily heroin self-administration under limited-access conditions can be maintained for long periods of time in female rats with no disruption to the estrous cycle (Lacy et al., 2016). All rats were cycling normally prior to heroin self-administration, and vehicle-treated rats continued to cycle normally for the duration of the study. As expected, chronic treatment with estradiol disrupted cycling by increasing the frequency and duration of high-estrogenic phases of the cycle, with the majority of rats assigned to the high-dose consistently generating vaginal cytology characteristic of late proestrus. We previously demonstrated that heroin intake decreases significantly during proestrus, and these effects are due to increases in estradiol rather than progesterone (Smith et al., 2020a; Smith et al., 2020b). The present study adds further evidence that high estrogenic states inhibit opioid self-administration and extend those findings to a fully synthetic opioid.
The present study used scheduling conditions characterized by low and consistent levels of use and did not necessarily model states of compulsive drug use and drug seeking characteristic of substance use disorders. Potential therapies are typically evaluated for both their ability to reduce drug intake but also to reduce drug seeking following a history of use and subsequence abstinence, which may be akin to “drug craving” in human populations. Recent studies report that estradiol reliably decreases drug seeking following a history of heroin self-administration and forced abstinence. For instance, estradiol decreases heroin seeking during extinction training (Vazquez et al., 2020) and during augmentation by food restriction (Sedki et al., 2015), suggesting its potential to reduce both drug intake in individuals using heroin and drug craving in individuals abstinent from heroin. In the present study, estradiol non-significantly decreased responding during a saline substitutions test, which may be viewed as a measure of heroin seeking. Future studies should expand the conditions under which estradiol may reduce opioid intake, including under conditions of high opioid intake (e.g., extended-access conditions) and high unit price (e.g., progressive ratio schedules). Finally, the current experiment examined the effects of estradiol in female rats given the increased risk of developing an opioid use disorder in women using opioids relative to men using opioids (NIDA, 2020), but future experiments should assess these effects in male rats to further identify the mechanisms underlying estradiol’s effect on opioid intake.
The role of estradiol in opioid intake differs from its role in stimulant intake. For instance, rats self-administer more cocaine during estrus when estradiol is high relative to progesterone (Lynch, 2008; Lynch et al., 2000), and ovariectomized rats treated with estradiol acquire cocaine self-administration faster and self-administer more cocaine than vehicle-treated rats (Hu and Becker, 2008; Jackson et al., 2006; Lynch and Carroll, 2001; Zhao and Becker, 2010). Cocaine sensitization and cocaine seeking are also enhanced with estradiol treatment (Anker et al., 2007; Feltenstein and See, 2007; Sell et al., 2002), and blockade of estrogen receptors with tamoxifen significantly reduces cocaine intake (Lynch and Carroll, 2001). The mechanisms by which estradiol increases stimulant intake and seeking has been explored previously (see Kokane and Perrotti, 2020, Meitzen et al., 2018 for review; Satta et al., 2017; Calipari et al., 2017); the mechanisms by which estradiol reduces opioid intake are not known, but some possibilities have been proposed (Smith et al., 2020b).
We emphasize that the effects of estradiol in this study cannot be attributed to a general suppression of motor behavior. As noted above, these doses of estradiol increase responding maintained (or previously maintained) by stimulants. Furthermore, estradiol did not alter responding on an inactive lever, and high estrogen-states enhance morphine-induced locomotion more than low estrogen-states (Craft et al., 2006).
A growing body of preclinical evidence suggests that estrogenic therapies may be an effective intervention to reduce drug use and craving in women with opioid use disorder. Estrogen-based hormone replacement and birth control therapies already exist and have been approved by the FDA. Currently, over 30 hormonal drugs containing either estradiol alone or estradiol in combination with progesterone are clinically available (Stefanick, 2005). Unfortunately, hormone therapies can be accompanied by potentially harmful side effects and increased risks of health complications (Depypere et al., 2020; Manyonda et al., 2020). These risks, however, can be mitigaged by using estradiol-only treatments or by more careful prescription and monitoring (Gambacciani et al., 2019; Manyonda et al., 2020; Stengler, 2019). Data obtained from vaginal cyclotomy suggested blood concentrations of estradiol were comparable to those observed during late proestrus in rats receiving 5.0 mcg estradiol. Estradiol concentrations during proestrus are similar to estradiol concentrations during mid-to-late pregnancy in rats and similar to estradiol concentrations following ingestion of an oral contraceptive in women (c.f., Smith, 1975; Anadol et al., 2014; Brenner et al., 1980). These data suggest the dosing regimen used in the present study was sufficient to mimic the physiological and pharmacological effects of estradiol contained in oral contraceptives for women. We are not aware of any clinical studies published on the effects of estradiol on opioid intake; however, ovarian hormones have already shown their utility for treating substance use disorders. For example, exogenous treatment with progesterone decreases cocaine use, cannabis craving, and short-term tobacco abstinence in women (Allen et al., 2016; Sherman et al., 2019; Yonkers et al., 2014). Given evidence that women are more susceptible to opioid addiction and overdose (Kokane and Perrotti, 2020), and that estradiol decreases opioid intake and opioid seeking in preclinical models, a justification can be made that clinical trials of opioid use disorder should be expanded to include exogenous estradiol administration.
Supplementary Material
Highlights.
Estradiol non-significantly reduced heroin intake with a moderate effect size
Estradiol significantly reduced remifentanil intake with a large effect size
Estrogen-based pharmacotherapy may represent novel treatment for women with OUD
Acknowledgements:
The authors thank the National Institute on Drug Abuse for supplying the study drug.
Role of Funding Source:
This work was supported by NIH Grants DA045364, DA031725, and DA045714. The NIH had no role in the writing of the manuscript or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflict declared.
References
- Allen SS, Allen AM, Lunos S, Tosun N, 2016. Progesterone and Postpartum Smoking Relapse: A Pilot Double-Blind Placebo-Controlled Randomized Trial. Nicotine Tob Res 18, 2145–2153. 10.1093/ntr/ntw156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anadol E, Kanca H, Yar AS, Helvacioğlu F, Menevşe S, Çalgüner E, & Erdoğan D (2014). Prostaglandin F receptor expression in intrauterine tissues of pregnant rats. Journal of veterinary science, 15(1), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME, 2007. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Experimental and Clinical Psychopharmacology 15, 472–480. 10.1037/1064-1297.15.5.472 [DOI] [PubMed] [Google Scholar]
- Brenner PF, Goebelsmann U, Stanczyk FZ, & Mishell DR Jr (1980). Serum levels of ethinylestradiol following its ingestion alone or in oral contraceptive formulations. Contraception, 22(1), 85–95. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, … & Nestler EJ (2017). Dopaminergic dynamics underlying sex-specific cocaine reward. Nature communications, 8(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 2013. Statistical Power Analysis for the Behavioral Sciences. Elsevier Science, Burlington. [Google Scholar]
- Craft RM, Clark JL, Hart SP, & Pinckney MK (2006). Sex differences in locomotor effects of morphine in the rat. Pharmacology Biochemistry and Behavior, 85(4), 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depypere H, Dierickx A, Vandevelde F, Stanczyk F, Ottoy L, Delanghe J, Lapauw B, 2020. A randomized trial on the effect of oral combined estradiol and drospirenone on glucose and insulin metabolism in healthy menopausal women with a normal oral glucose tolerance test. Maturitas 138, 36–41. 10.1016/j.maturitas.2020.04.009 [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE, 2007. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug and Alcohol Dependence 89, 183–189. 10.1016/j.drugalcdep.2006.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambacciani M, Cagnacci A, Lello S, 2019. Hormone replacement therapy and prevention of chronic conditions. Climacteric 22, 303–306. 10.1080/13697137.2018.1551347 [DOI] [PubMed] [Google Scholar]
- Gottås A, Øiestad EL, Boix F, Vindenes V, Ripel Å, Thaulow CH, & Mørland J (2013). Levels of heroin and its metabolites in blood and brain extracellular fluid after iv heroin administration to freely moving rats. British journal of pharmacology, 170(3), 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, Miele GM, 2007. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend 86, 1–21. 10.1016/j.drugalcdep.2006.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB, 2008. Acquisition of cocaine self-administration in ovariectomized female rats: Effect of estradiol dose or chronic estradiol administration. Drug and Alcohol Dependence 94, 56–62. 10.1016/j.drugalcdep.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB, 2006. Sex Differences and Hormonal Influences on Acquisition of Cocaine Self-Administration in Rats. Neuropsychopharmacol 31, 129–138. 10.1038/sj.npp.1300778 [DOI] [PubMed] [Google Scholar]
- Kokane SS, Perrotti LI, 2020. Sex Differences and the Role of Estradiol in Mesolimbic Reward Circuits and Vulnerability to Cocaine and Opiate Addiction. Front. Behav. Neurosci 14, 74. 10.3389/fnbeh.2020.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA, 2016. The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology (Berl.) 233, 3201–3210. 10.1007/s00213-016-4368-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti NM, Esguerra M, Mermelstein PG, 2020. Sex Differences in Animal Models of Opioid Reward. Curr Sex Health Rep 12, 186–194. 10.1007/s11930-020-00266-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, 2008. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 197, 237–246. 10.1007/s00213-007-1028-0 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME, 2000. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 152, 132–139. 10.1007/s002130000488 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME, 2001. Regulation of drug intake. Exp Clin Psychopharmacol 9, 131–143. [DOI] [PubMed] [Google Scholar]
- Manyonda I, S Talaulikar V, Pirhadi R, Onwude J, 2020. Progestogens are the problem in hormone replacement therapy: Time to reappraise their use. Post Reprod Health 26, 26–31. 10.1177/2053369119876490 [DOI] [PubMed] [Google Scholar]
- Meitzen J, Meisel RL, & Mermelstein PG (2018). Sex differences and the effects of estradiol on striatal function. Current opinion in behavioral sciences, 23, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen LG, & Hug CC Jr (1996). The pharmacokinetics of remifentanil. Journal of clinical anesthesia, 8(8), 679–682. [DOI] [PubMed] [Google Scholar]
- NIDA. 2020, May 28. Sex and Gender Differences in Substance Use. Retrieved from https://www.drugabuse.gov/publications/research-reports/substance-use-in-women/sex-gender-differences-in-substance-use
- Roth ME, Casimir AG, Carroll ME, 2002. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol. Biochem. Behav 72, 313–318. 10.1016/s0091-3057(01)00777-8 [DOI] [PubMed] [Google Scholar]
- Satta R, Certa B, He D, & Lasek AW (2018). Estrogen receptor β in the nucleus accumbens regulates the rewarding properties of cocaine in female mice. International Journal of Neuropsychopharmacology, 21(4), 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ, 2014. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis 72 Pt B, 180–192. 10.1016/j.nbd.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, & Perry M (2005). Remifentanil: A review of its use during the induction and maintenance of general anaesthesia (vol 65, pg 1793, 2005). DRUGS, 65(16), 2286–2286. [DOI] [PubMed] [Google Scholar]
- Sedki F, Gardner Gregory J, Luminare A, D’Cunha TM, Shalev U, 2015. Food restriction-induced augmentation of heroin seeking in female rats: manipulations of ovarian hormones. Psychopharmacology 232, 3773–3782. 10.1007/s00213-015-4037-4 [DOI] [PubMed] [Google Scholar]
- Sell SL, Thomas ML, Cunningham KA, 2002. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend 67, 281–290. 10.1016/s0376-8716(02)00085-6 [DOI] [PubMed] [Google Scholar]
- Sherman BJ, Caruso MA, McRae-Clark AL, 2019. Exogenous progesterone for cannabis withdrawal in women: Feasibility trial of a novel multimodal methodology. Pharmacology Biochemistry and Behavior 179, 22–26. 10.1016/j.pbb.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ethridge SB, Gibson AN, Schmidt KT, Sharp JL, 2020a. The effects of artificially induced proestrus on heroin intake: A critical role for estradiol. Experimental and Clinical Psychopharmacology. 10.1037/pha0000428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ethridge SB, Pearson T, Zhang H, Marcus MM, Ballard SL, … & Robinson AM (2020b). Modulation of heroin intake by ovarian hormones in gonadectomized and intact female rats. Psychopharmacology, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, & Neill JD (1975). The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology, 96(1), 219–226. [DOI] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML, 2008. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend 98, 129–135. 10.1016/j.drugalcdep.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Carroll KM, 2019. Pharmacological and Behavioral Treatment of Opioid Use Disorder. Psychiatr. res. clin. pract 1, 4–15. 10.1176/appi.prcp.20180006 [DOI] [Google Scholar]
- Stefanick ML, 2005. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. The American Journal of Medicine 118, 64–73. 10.1016/j.amjmed.2005.09.059 [DOI] [PubMed] [Google Scholar]
- Stengler MD, 2019. Examining the safe and effective use of estrogen replacement for menopausal women. EMIJ 7. 10.15406/emij.2019.07.00266 [DOI] [Google Scholar]
- Stewart J, Woodside B, Shaham Y, 1996. Ovarian hormones do not affect the initiation and maintenance of intravenous self-administration of heroin in the female rat. Psychobiology 24, 154–159. 10.3758/BF03331967 [DOI] [Google Scholar]
- Vazquez M, Frazier JH, Reichel CM, Peters J, 2020. Acute ovarian hormone treatment in freely cycling female rats regulates distinct aspects of heroin seeking. Learn. Mem 27, 6–11. 10.1101/lm.050187.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H, Davis NL, 2020. Drug and Opioid-Involved Overdose Deaths — United States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep 69, 290–297. 10.15585/mmwr.mm6911a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Forray A, Nich C, Carroll KM, Hine C, Merry BC, Shaw H, Shaw J, Sofuoglu M, 2014. Progesterone for the reduction of cocaine use in post-partum women with a cocaine use disorder: a randomised, double-blind, placebo-controlled, pilot study. The Lancet Psychiatry 1, 360–367. 10.1016/S2215-0366(14)70333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Becker JB, 2010. Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm Behav 58, 8–12. 10.1016/j.yhbeh.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


