Abstract
The sympathetic nervous system represents a critical mechanism for homeostatic blood pressure regulation in humans. This review focuses on age-related alterations in neurocirculatory regulation in men and women by highlighting human studies that examined the relationship between muscle sympathetic nerve activity (MSNA) acquired by microneurography and circulatory variables (e.g., blood pressure, vascular resistance). We frame this review with epidemiological evidence highlighting sex-specific patterns in age-related blood pressure increases in developed nations. Indeed, young women exhibit lower blood pressure than men, but women demonstrate larger blood pressure increases with age, such that by about age 60 years, blood pressure is greater in women. Sympathetic neurocirculatory mechanisms contribute to sex differences in blood pressure rises with age. Muscle sympathetic nerve activity increases with age in both sexes, but women demonstrate greater age-related increases. The circulatory adjustments imposed by MSNA — referred to as neurovascular transduction or autonomic (sympathetic) support of blood pressure — differ in men and women. For example, whereas young men demonstrate a positive relationship between resting MSNA and vascular resistance, this relationship is absent in young women due to beta-2 adrenergic vasodilation, which offsets alpha-adrenergic vasoconstriction. However, post-menopausal women demonstrate a positive relationship between MSNA and vascular resistance due to a decline in beta-2 adrenergic vasodilatory mechanisms. Emerging data suggest that greater aerobic fitness appears to modulate neurocirculatory regulation, at least in young, healthy men and women. This review also highlights recent advances in microneurographic recordings of sympathetic action potential discharge, which may nuance our understanding of age-related alterations in sympathetic neurocirculatory regulation in humans.
Keywords: Ageing, Blood pressure, Microneurography, Muscle sympathetic nerve activity, Neurovascular transduction, Neural control of the circulation, sex differences
1 |. Introduction
The sympathetic nervous system imposes circulatory adjustments to regulate both short- and long-term arterial blood pressure — a key clinical variable, predictive of cardiovascular disease risk [1,2]. Epidemiological data from developed nations demonstrating that blood pressure rises steadily with age in healthy individuals [3,4], though differently among women and men, provide strong evidence of: i) age-related modifications to sympathetic neurocirculatory regulation in humans, and ii) sex differences in sympathetic vascular control. Indeed, young women have lower resting blood pressures than men [3,4]. However, as highlighted by Fig. 1, women demonstrate larger blood pressure increases across the life course, such that from about 60 years onwards, blood pressure is greater in women than men [3,4]. The incidence of diagnosed high blood pressure follows a similar pattern: young women are less likely to be diagnosed with hypertension than young men, but after about 60 years, hypertension is more common in older women [1,5]. Age-related increases in blood pressure observed in developed nations are not obligatory, however. Data from indigenous Kuna who inhabit islands in the Panamanian Caribbean demonstrate no age-related increase in blood pressure [6]. Elevations in blood pressure observed in developed societies are also linked to age-related arterial stiffening, which is, in part, attributed to aberrant sympathetic discharge, collagen deposition, and vascular smooth muscle hypertrophy, among other factors [7]. Some evidence suggests that similar interactive effects of age and sex exist in arterial stiffening [8]. Although many important caveats exist regarding cardiovascular and anthropometrical sex differences (e.g., heart size and body size) [9], these observations raise important questions regarding neurocirculatory regulation in ageing women and men.
Figure 1. Trends in blood pressure with ageing in men and women.
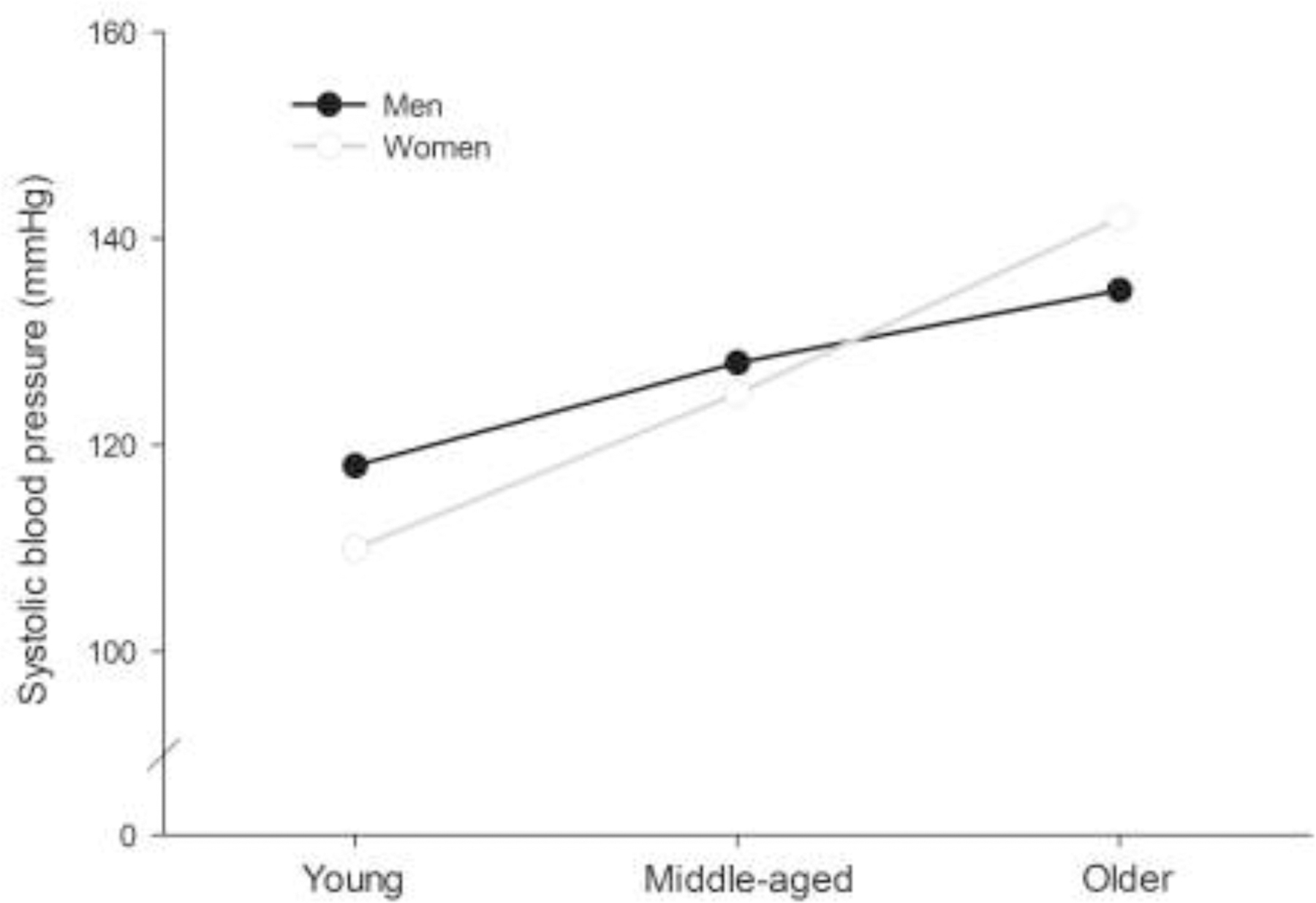
Blood pressure rises steadily with age in healthy humans in developed nations. However, sex-specific blood pressure trajectories exist across the life course. Young women exhibit lower blood pressure than men, but the magnitude of blood pressure increase is greater in women than men. At about 60 years, men and women’s blood pressure intersects and from this point onwards, women demonstrate greater systolic blood pressure than men. Figure based on data from [3,4].
Accordingly, this work aims to review the evidence supporting age-related alterations in sympathetic neurocirculatory regulation in humans, highlighting important sex differences that exist across the lifespan. We also review the emerging literature targeting the impact of physical exercise and aerobic fitness on sympathetic neurocirculatory control in humans. Core to this discussion are two key fundamental physiological processes which will be reviewed: i) muscle sympathetic nerve activity (MSNA), which represents the action potential (AP) discharge patterns emanating from sympathetic nerves innervating the peripheral vasculature, and ii) neurovascular transduction or autonomic support of blood pressure, which describes the circulatory and hemodynamic effects of sympathetic discharge in humans.
2 |. Sympathetic neurocirculatory regulation
The sympathetic nervous system controls vasomotor adjustments to maintain blood pressure homeostasis at rest and during physiological perturbations such as maintaining an upright posture and physical exercise. The connection between sympathetic neurocirculatory regulation and blood pressure can be explained by Ohm’s law applied to the circulation, which highlights key roles for cardiac output and total peripheral resistance in the determination of mean arterial pressure: mean arterial pressure = cardiac output X total peripheral resistance (TPR). Importantly, sympathetic regulation of vascular diameter represents the core element of vascular resistance. Although cardiac output is also governed by sympathetic (and parasympathetic) neural mechanisms, this review focuses on sympathetic regulation of the peripheral vasculature in the context of human ageing [10]. Indeed, the inability to perform activities of daily living such as physical exercise or maintaining an upright posture in patients with sympathetic disorders or clinical sympathectomy reinforces the importance of sympathetic neurocirculatory control in humans [11,12].
Anatomically, the sympathetic nervous system elicits vasomotor adjustments through postganglionic c-fibres which traverse arteries within the adventitia and interface with the smooth muscle via bulging varicosities containing synaptic vesicles. A single sympathetic postganglionic c-fibre innervates a large area of the vasculature through a process known as synapse en passant which describes the formation of intermittent varicosities along the length of the axon that enable a single c-fibre to interface with many vascular smooth muscle cells [10]. Postganglionic sympathetic c-fibres conduct APs, which upon arrival at sympathetic varicosities initiate the release of neurotransmitters responsible for vascular adjustments — the primary neurotransmitter, norepinephrine (NE), and co-transmitters, neuropeptide Y (NPY) and adenosine triphosphate (ATP) [13]. The primary avenue for NE-mediated vasoconstriction results from binding postsynaptic alpha-1 and alpha-2 adrenergic receptors on the vascular smooth muscle. However, NE can also elicit vasodilation through the binding of vascular smooth muscle beta-2 adrenergic receptors [14]. Neuropeptide Y and ATP initiate vasoconstriction by binding NPY-Y1 and P2X1 receptors on the vascular smooth muscle, respectively [13]. Overall, the degree of sympathetic regulation of the vasculature depends on the distribution, concentration, and sensitivity of vascular smooth muscle receptors [15]. A detailed discussion of the vascular smooth muscle intracellular cascades responsible for vascular adjustments related to individual receptor types is available elsewhere [10,13,14].
2.1 |. Muscle sympathetic nerve activity
Fundamental to the understanding of human sympathetic neurocirculatory regulation is a thorough comprehension of the discharge patterns emanating from the sympathetic postganglionic c-fibres innervating the vasculature and affecting neurotransmitter release. Muscle sympathetic nerve activity (MSNA) describes sympathetic postganglionic c-fibre discharge directed towards the vasculature supplying skeletal muscle [16,17]. Developed by Hagbarth and Vallbo in 1968, microneurography involves the insertion of a thin tungsten microelectrode into a common nerve carrying postganglionic c-fibres to provide direct measurement of MSNA in humans [16,18]. Although the sympathetic nervous system broadly innervates the human vasculature and heart, microneurographic measurements in humans are commonly performed on superficial peripheral nerves such as the peroneal (fibular) nerve in the lower leg or the median nerve in the arm [19,20]. However, an intriguing new study by Macefield and colleagues highlights the possibility of performing ultrasound-guided microneurographic recordings from deeper (sympathetic) nerves under supervised clinical conditions [21]. In general, sympathetic nerve activity recorded from the peroneal nerve reflects sympathetic traffic towards other circulatory beds and the heart, as assessed from NE spillover [22–24].
Microneurographic recordings of MSNA require delicate positioning of the microelectrode tip near spontaneously discharging bundles of unmyelinated postganglionic c-fibres supplying the skeletal muscle vasculature [25]. Postganglionic sympathetic nerves vary in diameter from 0.5 to 2 μm and form small bundles most often comprised of approximately five c-fibres, with a broad range of 1 to about 40 c-fibres per bundle [26]. Because sympathetic c-fibres fire synchronously, or at about the same time, MSNA is fundamentally characterized by bursts of activity that demonstrate time-varying amplitude [27]. Synchronous MSNA discharge is governed by the arterial baroreflex, a critical neural reflex which imposes rhythmic sympathoinhibition related to pressure pulsatility accompanying the cardiac cycle, such that MSNA discharge is suppressed by the arterial baroreflex during systole [28,29]. As reviewed elsewhere [15,30], several reflexive mechanisms communicate physiological information to the brain (e.g., skeletal muscle fatigue) to regulate MSNA discharge and maintain blood pressure homeostasis during various physiological challenges (e.g., physical exercise). Sympathetic control of cutaneous circulation can also be assessed with microneurography [18]. Skin sympathetic nerve activity is not under strong baroreflex regulation, resulting in bursts that span multiple cardiac cycles and are morphologically distinct from MSNA [18]. Skin sympathetic nerve activity has been discussed in detail elsewhere [31]; however, this text focuses on MSNA which represents the fundamental neural signal for blood pressure regulation [32].
Due to the low signal-to-noise ratio of microneurographic recordings, MSNA is often measured at the multi-unit level (e.g., simultaneous recording from multiple active c-fibres) by integrating an amplified and filtered neurogram (Fig. 2). Inspection of the integrated signal enables quantification of sympathetic discharge through burst frequency (i.e., total bursts per min) or burst incidence metrics (i.e., total bursts per 100 heartbeats) [19]. Burst amplitude (size) represents an additional measure of sympathetic outflow [33]. Though repositioning of the microelectrode tip in relation to a c-fibre bundle during a microneurographic recording also produces variations in burst size, highlighting the importance of stable recordings and the microneurographers ability to detect such changes based on the morphological features of the neurogram [34]. Integrated MSNA bursts of variable size and frequency can be visualized in Fig. 2. Nonetheless, focusing entirely on the integrated MSNA signal conceals AP discharge emanating from individual sympathetic c-fibres.
Figure 2. Representative integrated muscle sympathetic nerve activity and sympathetic action potential discharge in a healthy individual.
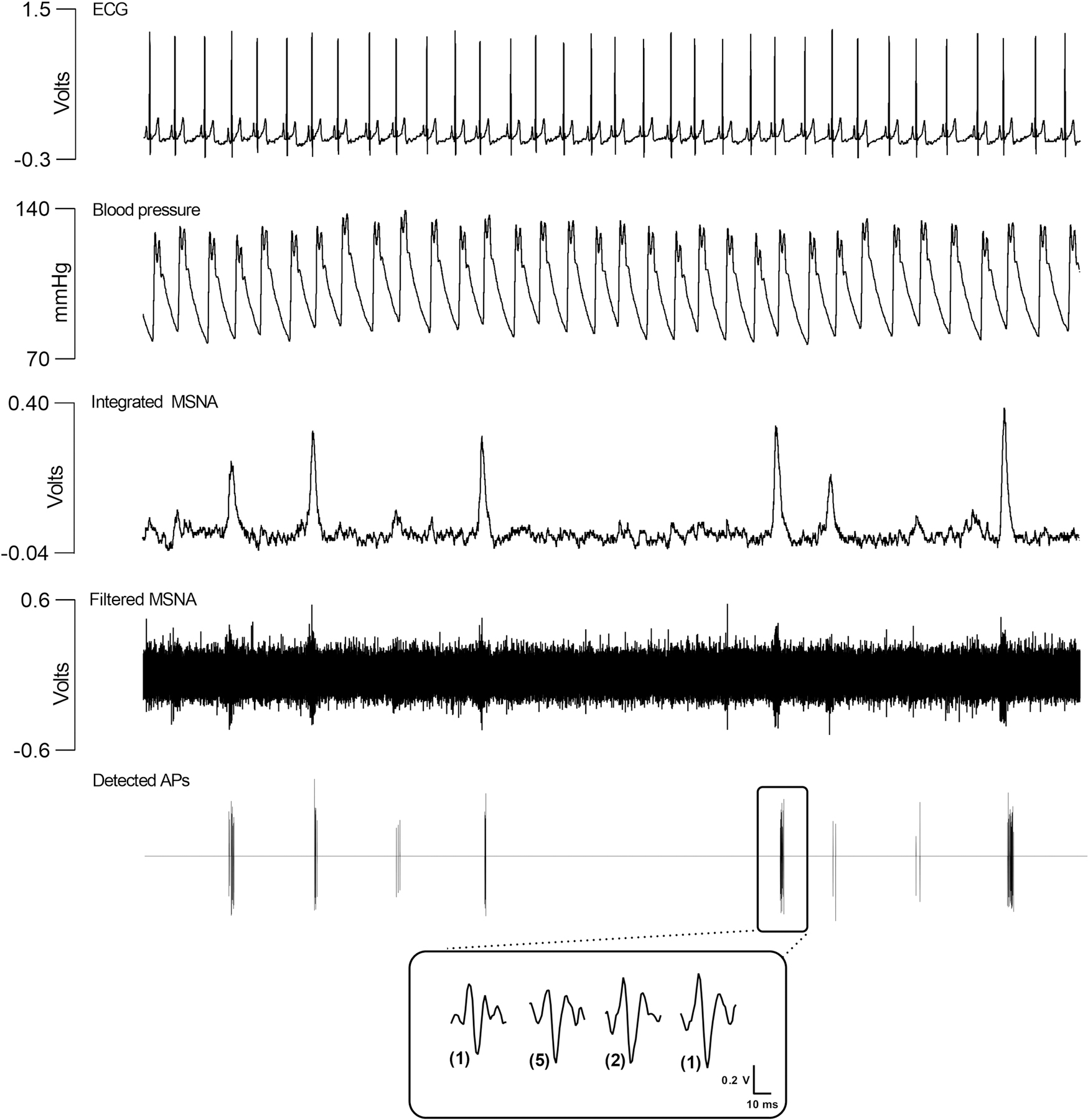
The integrated neurogram highlights that muscle sympathetic nerve activity (MSNA) is fundamentally characterized by bursts of activity with time-varying frequency and size. In this figure, APs were detected and extracted from the filtered neurogram using a continuous wavelet transform [39]. The filtered neurogram and the detected action potentials (AP) show that MSNA bursting discharge is attributed to synchronous firing of varying-sized sympathetic APs. Fundamentally, larger integrated bursts are comprised by greater AP content and larger AP firing. Featured in the magnified inset at the bottom of the figure are the sympathetic AP clusters, representing APs of similar morphology, that comprise one selected MSNA burst. The size of each AP cluster is related to the sympathetic c-fibre that generated the AP. In the magnified inset, the AP clusters firing in the selected burst are organized by peak-to-peak amplitude, from smallest to largest. The number of times each AP cluster fired in this selected burst is displayed in parentheses: two AP clusters each fired once, one AP cluster fired two times, and one AP cluster fired five times, for a total of nine APs firing in the selected burst. For simplicity, not shown in this figure are asynchronously discharging sympathetic APs which fire between synchronous bursts of MSNA. The interested reader is directed to Salmanpour et al. [39] and Shoemaker et al. [25] for more information regarding the continuous wavelet transform for sympathetic AP detection and discharge sympathetic AP discharge patterns in humans.
Major advances in the microneurographic technique have provided insight to AP discharge emanating from postganglionic sympathetic c-fibres — the actual neural signal imposing neurocirculatory adjustments. First, Macefield and colleagues developed the single-unit microneurographic approach which relies on highly selective electrodes with higher impedance to study the discharge characteristics of one (or a few) sympathetic c-fibres [35–37]. While this method enables the tracking of the discharge of a few c-fibres over time, it does not provide insight to the varying-sized sympathetic c-fibres that lie beyond the focused recording field provided by high impedance electrodes. This concept prompted the development of additional complementary microneurographic signal processing methods.
Second, and more recently, Shoemaker and colleagues developed a continuous wavelet transform approach for detecting and extracting individual sympathetic APs from the multi-unit filtered neurogram [38]. This technique enables morphological sorting of sympathetic APs into clusters which represent the discharge of size-based subpopulations of sympathetic postganglionic c-fibres [25,39]. This technique has revealed important information regarding sympathetic AP firing patterns. As visualized by Fig. 2, bursts of sympathetic activity are formed by the synchronous discharge of varying-sized sympathetic AP subpopulations and bursts demonstrate time-varying amplitude related to the number and size of synchronized APs [40]. While ‘bursty’ discharge describes the hallmark feature of MSNA, a recent multi-unit AP study revealed that sympathetic bursts are not separated by complete neural silence but rather about 30% of total sympathetic APs fire asynchronously, between synchronized bursts [41]. As this finding was reproduced in two separate cohorts, it appears that asynchronous discharge represents a fundamental component of MSNA [41,42]. For simplicity, asynchronous discharge is not presented in Fig. 2, but the interested reader is directed to a recent review of the topic [43]. Under baseline conditions, AP clusters can be grouped into three general subpopulations (small, medium, and large APs) that vary in discharge characteristics and reflect the diameter of the sympathetic c-fibre that generated the AP [43–45]. While the subpopulation of medium APs fires most often, the subpopulations of small and large APs fire less frequently, suggesting differential regulation within the central sympathetic neurocircuitry and/or variable recruitment thresholds [38,42].
By imposing physiological perturbations to modify multi-unit AP firing patterns, investigators using the wavelet transform approach have revealed the fundamental sympathetic discharge strategies supporting blood pressure homeostasis. Consistent with single-unit microneurographic data [35], Steinback et al. [40] found that the firing frequency of sympathetic c-fibres active during baseline conditions increased during maximal voluntary inspiratory apnea. Importantly, during the breath-hold period, Steinback and colleagues also observed the firing of previously-silent larger and faster-conducting APs, representing the recruitment of a latent subpopulation of larger sympathetic c-fibres that are reserved for periods of severe physiological stress [40,46]. This recruitment feature is analogous to the size-principle for motor-unit recruitment [47]. The increase in the previously-active sympathetic AP subpopulations (rate-coding mechanism) and the recruitment of a previously-silent AP subpopulations (population-coding mechanism) during physiological stress underlie the increase in sympathetic burst occurrence and burst amplitude during physiological stress to defend blood pressure homeostasis. These strategies augment total sympathetic outflow to maintain blood pressure homeostasis during various physiological challenges including maintaining an upright posture [44], physical activity [48], and hypoxia [49].
Although the technical demands of microneurography preclude its role in routine clinical assessment, resting MSNA is an important factor in circulatory health. From cross sectional evidence it is well established that individuals with cardiovascular diseases and vascular risk factors show elevated supine resting MSNA [50,51]. Importantly, sympathetic dysregulation plays a deleterious role in the progression of cardiovascular diseases. For example, elevated MSNA (> 49 bursts/min) independently predicted one-year mortality in a large sample of heart failure patients [52]. Thus, pathophysiological alterations in sympathetic discharge contribute to the development and progression of cardiovascular diseases.
2.2 |. The impact of ageing on muscle sympathetic nerve activity in men and women
Similar to the blood pressure trends discussed at the outset of this report, MSNA increases with age but the magnitude of sympathoexcitation varies among men and women. A recent large cross-sectional report of healthy normotensive men and women demonstrated the differential impact of ageing on sympathetic activity in men and women [53]. As highlighted in Fig. 3, MSNA burst frequency was lower in young women than men. Both sexes demonstrated increased MSNA with age, but the magnitude of change was greater in women than men. Age-related modifications also exist in sympathetic AP discharge patterns at rest and during periods of physiological stress. For example, compared to young healthy individuals, Badrov and colleagues [54] observed greater resting sympathetic AP discharge in older healthy individuals. Also, during maximal voluntary breath-holds, older individuals exhibited attenuated rises in the firing probability of previously-active APs (rate-coding) and impaired recruitment of previously-silent larger and faster-conducting sympathetic APs (population-coding), compared to young healthy participants [54]. To date, sex differences in the age-related increases in sympathetic AP discharge and recruitment have not been investigated and this remains an interesting area for research. Although robust age-related MSNA increases occur, broad inter-individual variability exists in MSNA across the lifespan in both men and women. Indeed, even in individuals with similar blood pressures, MSNA may vary as much as 5–10 fold [32,55]. Complex and multi-factorial mechanisms likely produce both the wide inter-individual variations and age-related changes in sympathetic outflow including the central sympathetic neurocircuitry, arterial baroreflex regulation of sympathetic discharge, and/or vascular nitric oxide production [30,56].
Figure 3. Trends in muscle sympathetic nerve activity with ageing in men and women.
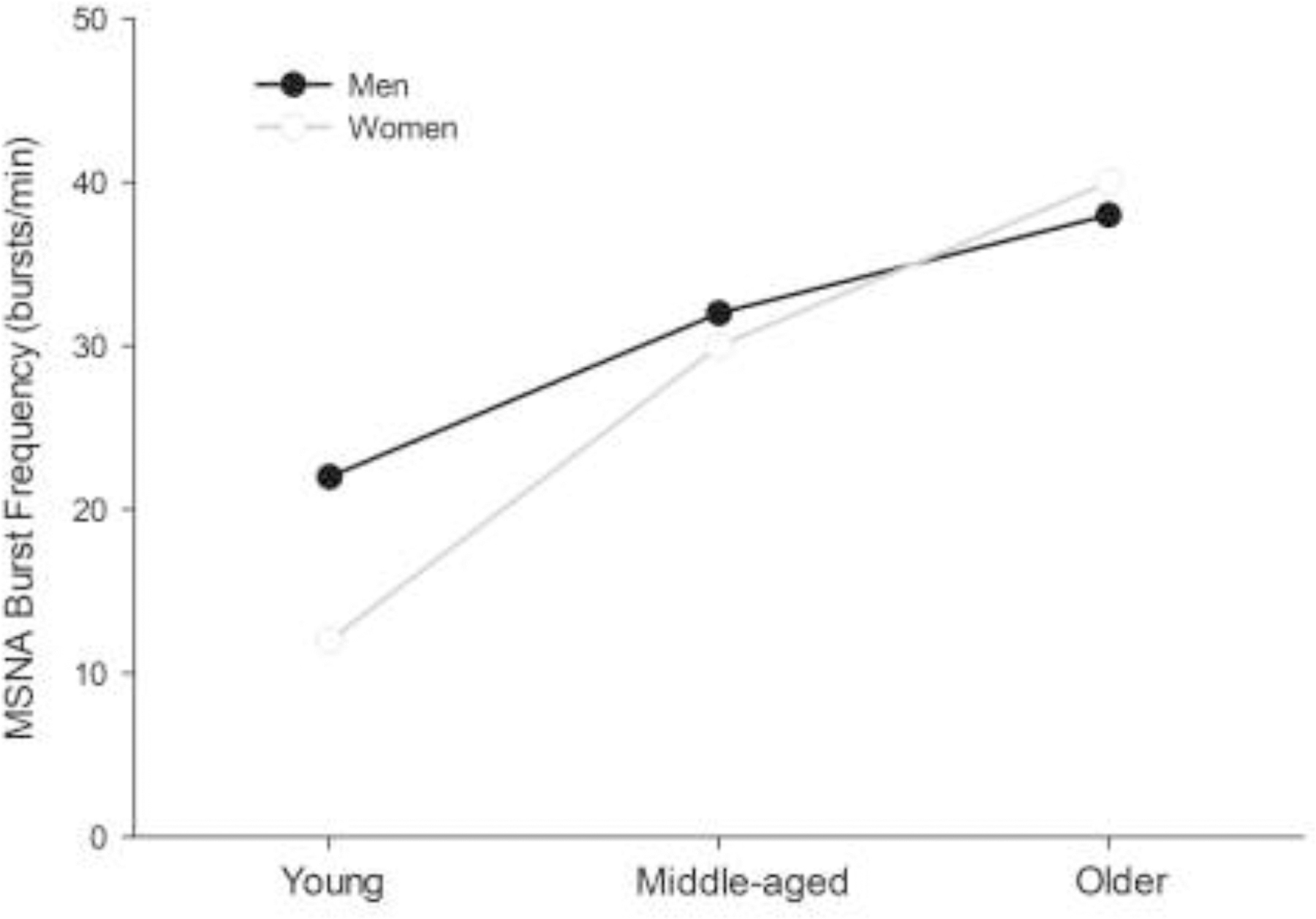
In healthy normotensive individuals, muscle sympathetic nerve activity (MSNA) burst frequency approximately doubles from the age of 20 to 70 years. However, the magnitude of MSNA increase with age differs in men and women. Young women have lesser MSNA burst frequency than men. Both sexes exhibit increases in MSNA with age but the magnitude of change is greater in women than men. Figure based on data from [53,73].
4.1 |. Quantifying sympathetic neurocirculatory regulation: Neurovascular transduction
In the seminal microneurographic studies, consistent observations of pressor responses following bursts of MSNA formed the first evidence for the vasoconstrictor nature of sympathetic discharge in humans [16,32,57]. Thus, several methodological strategies examining the relationship between MSNA and resultant circulatory adjustments – known commonly as neurovascular transduction – have been developed to understand the fundamental mechanisms of sympathetic neurocirculatory regulation and the factors modifying this process [15,58]. These techniques range in complexity from simply computing the quotient of mean TPR and mean MSNA over a given recording period, to quantifying the beat-by-beat circulatory (e.g., blood pressure, blood flow, vascular conductance) responses ensuing from a single burst of MSNA [59,60]. Nonetheless, questions remain unanswered regarding the assessment of neurovascular transduction in humans: Which aspect of the sympathetic neurogram best represents the message directed towards the vascular and what circulatory variable does the sympathetic system regulate? This section will draw attention to these questions while providing an overview of the key techniques currently used to assess sympathetic neurovascular control in humans.
Quantifying the relationship between beat-by-beat MSNA and resultant cardiovascular adjustments offers the opportunity to study neurovascular transduction in the context of the time-varying nature of sympathetic discharge. The first investigation of beat-by beat sympathetic circulatory regulation was performed in the early 1980’s by Wallin and Nerhed who performed simultaneous recordings of integrated MSNA and beat-by-beat blood pressure which enabled tracking of the magnitude of pressure changes following an integrated burst of MSNA [61]. This group consistently observed a transient increase in diastolic pressure that began 1–2 beats and peaked 5–7 beats after a MSNA burst in all individuals (peak change: ~ 2–3 mmHg), with the magnitude of the pressor responses demonstrating inter-individual variability [61]. Using a similar approach about 30 years later, Vianna and colleagues discovered that the pressor responses stemming from MSNA bursts were primarily mediated by reductions in total vascular conductance, calculated from the quotient of cardiac output and mean pressure [62]. While these studies gained insight to neurocirculatory impact of sympathetic discharge, their indices of vasomotor adjustments were based on systemic hemodynamic variables [61,62]. Importantly, studies exploring beat-by-beat neurovascular transduction have revealed rapid cardiac responses to MSNA bursts, which underscore the complexity of interpreting systemic blood pressure as the regulated variable in the context of neurovascular transduction in humans [61,63]. Blood pressure represents an integrated variable regulated by several physiological factors including not only vascular conductance (or resistance) but also cardiac output, MSNA, myogenic mechanisms, and vascular compliance [64,65].
To provide greater specificity in understanding the impact of sympathetic discharge on circulatory control, Fairfax and colleagues implemented Doppler ultrasound to facilitate simultaneous measures of femoral artery blood flow (to calculate vascular conductance) during microneurographic recordings of peroneal MSNA [60,62]. This enabled assessment of the MSNA discharge patterns that produced the most robust vascular adjustments. Upon grouping MSNA bursts into quartiles based on size, the reduction in vascular conductance scaled to burst size, that is, larger bursts produced greater reductions in vascular conductance [60]. Considering that a critical role for burst size has emerged consistently across neurocirculatory studies [60,62,66,67], this feature likely represents an important aspect of the sympathetic signal for neurocirculatory control.
Given that MSNA burst size stems from the number and size of synchronously firing sympathetic APs [68], the consistent observation that burst size is linked to vasomotor adjustments suggests that sympathetic AP discharge patterns affect neurocirculatory responses. Although measuring AP discharge carries additional technical demands, sympathetic AP discharge likely represents the optimal signal for assessing neurovascular transduction in humans. As proposed recently, subpopulations of differently sized APs demonstrating heterogeneous patterns of discharge, recruitment, and synchronization enable the sympathetic nervous system to fine-tune circulatory adjustments [25,69]. The observations made by Fairfax and colleagues suggest a robust role for larger sympathetic APs that fire with low probability under baseline conditions. However, the circulatory responses ensuing from the discharge of specific AP subpopulations including APs active under baseline conditions and previously-silent APs that are recruited during physiological stress remain unclear [69]. Also, although the previously described investigations focusing on integrated MSNA bursts point towards a key role for synchronous sympathetic AP discharge, a large proportion (~30%) of sympathetic AP discharge occurs asynchronously under baseline conditions and may also affect vasomotor tone [41].
In addition to studies evaluating spontaneous beat-by-beat changes in MSNA and circulatory adjustments, intravenous infusion of pharmacological agents affecting neural control of the circulation has provided valuable insight to the role of the sympathetic (and parasympathetic) nervous system in human blood pressure regulation. In particular, the intravenous infusion of trimethaphan camsylate – a competitive ganglionic nicotinic receptor antagonist – abolishes sympathetic and parasympathetic activity directed towards cardiovascular end-targets [32]. This experimental model holds that changes in blood pressure attributed to reductions in neural discharge towards the circulation are used to determine “autonomic support” of blood pressure [70]. Commonly, a larger blood pressure reduction during trimethaphan infusion is interpreted to suggest that an individual or cohort has greater autonomic support of blood pressure. Important to the interpretation of the drop in blood pressure with trimethaphan is the concomitant change in cardiac output that stems, in part, from parasympathetic blockade. For example, compared to young individuals, older participants show larger reductions in blood pressure during trimethaphan infusion, in part because they have greater baseline sympathetic vasoconstriction, but also because there is less tonic parasympathetic suppression of heart rate during rest in older participants, so blocking parasympathetic control leads to a smaller increase in heart rate, and therefore cardiac output compared to young participants [71].
4.2 |. Sympathetic neurocirculatory regulation with ageing in men and women
Considerable interactive effects of age and sex exist in sympathetic neurocirculatory control (Table 1). Although sympathetic discharge represents the essential vasoconstrictor stimulus [32,61], investigations of healthy cohorts of both sexes have consistently revealed broad inter-individual differences in MSNA among individuals with similar blood pressures [55,72]. Notably, in young healthy individuals no significant relationship exists between MSNA and blood pressure under supine resting conditions [73,74]: young individuals with high MSNA do not necessarily demonstrate high blood pressures.
Table 1 |.
The interactive effects of age and sex on sympathetic neurocirculatory control
| Young Men | Young Women | Older Men | Older Women | |
|---|---|---|---|---|
| Integrated MSNA | Greater than young women | Lesser than young men | Greater than young men | Greater than young women |
| Sympathetic AP dischargea | Rate-coding and population-coding mechanisms exist to increase total MSNA during physiological stress | Greater resting sympathetic AP discharge plus impaired AP rate-coding and population-coding during physiological stress compared to young cohort | ||
| Relationship with integrated MSNA | ||||
| Blood Pressure | No relationship | No relationshipb | Positive relationship | Positive relationship |
| Total Peripheral Resistance | Positive relationship | No relationshipb | Positive relationship | Positive relationship |
| Cardiac Output | Inverse relationship | No relationship | No relationship | No relationship |
| Autonomic (Sympathetic) Support of Blood Pressure | Greater than young women | Lesser than young men | Greater than young men | Greater than young women and similar to older men |
| Neurovascular Transduction | Greater than young women | Lesser than young men | Lesser than young men | Greater than young women |
| Alpha-adrenergic Vasoconstriction | Inverse relationship with MSNA | Lesser than young men c | Lesser than young men | Greater than young women |
| Beta-adrenergic Vasodilation | Does not buffer alpha-adrenergic vasoconstriction | Buffers alpha-adrenergic vasoconstriction | Does not buffer alpha-adrenergic vasoconstriction | Lesser than young women |
AP, action potential; MSNA, muscle sympathetic nerve activity.
Footnotes:
Sex differences in sympathetic AP discharge have not been investigated. As such, male and female groups are collapsed and only age comparisons are provided.
Beta-adrenergic blockade produces positive relationship
Beta-adrenergic blockade increases alpha-adrenergic constriction
Further study of this paradoxical finding revealed that in young men and women less than 40 years of age, integrative neural and hemodynamic factors buffer sympathetic vasoconstriction to maintain homeostatic blood pressures and render no relationship between MSNA burst frequency and blood pressure [74,75]. However, the mechanisms buffering the relationship between sympathetic outflow and blood pressure in young healthy individuals differ in men and women. In young men, a positive relationship exists between MSNA and total peripheral resistance that is offset by an inverse relationship between MSNA and cardiac output [74,75]. Thus, men with greater resting sympathetically-mediated vascular resistance have lower cardiac output to prevent excessive blood pressure. Also, in a young male cohort there was an inverse relationship between resting MSNA burst frequency and forearm vasoconstrictor responsiveness to norepinephrine and tyramine infusions [76]. Therefore, reduced alpha-adrenergic sensitivity balances sympathetic vasoconstriction in young men with higher resting sympathetic outflow.
Conversely, in young women, no relationship exists between MSNA and total peripheral resistance or cardiac output [75]. Consequently, young women have less vasomotor regulation compared to young men. Consistent with this idea, ganglionic blockade with trimethaphan imposed lesser pressure reductions in young women than men, suggesting that women rely less on autonomic support of blood pressure [77]. Beta-2 adrenergic receptors on the vascular smooth muscle likely represent a key mechanism underlying the absence of the MSNA-BP relationship in young women. Indeed, in a cohort of young women Hart and colleagues observed significant positive associations between MSNA burst occurrence and both TPR and MAP during beta adrenergic blockade [78]. Also, forearm NE infusion produced a larger reduction in forearm vascular conductance during beta-adrenergic blockade compared to control conditions in the same group of women [78]. These data also provide an explanation for the observation of lower blood pressure young women than young men.
Ageing modifies sympathetic circulatory regulation in men and women. On a cohort level in both healthy men and women >40 years a moderate positive relationship exists between MSNA and blood pressure [73]. Thus, older individuals with greater MSNA generally have elevated blood pressure. Consistent with this idea, studies have repeatedly observed that compared to young individuals, older men and women exhibit larger reductions in blood pressure following ganglionic blockade with trimethaphan, though these findings are complicated by the observation that older individuals also demonstrate lesser cardiac responses to ganglionic blockade than younger cohorts [70,79]. Nevertheless, the magnitude of the blood pressure reduction with ganglionic blockade is inversely related to resting MSNA, implicating sympathetic control of the vasculature [70,80].
Multiple interactive mechanisms likely mediate the age-related changes in sympathetic circulatory regulation. In older men, no inverse relationship between MSNA and cardiac output was observed, suggesting that an age-related loss may occur in the integrative neuro-hemodynamic relationships that buffer the MSNA-BP relationship in younger healthy individuals [76]. Surprisingly, despite the positive relationship between MSNA and BP in older individuals, it seems that at least in men, there are reductions in alpha-adrenergic sensitivity with age. For instance, compared to their younger counterparts, older men showed lesser reductions in forearm blood flow during low dose tyramine infusion, a drug which increases norepinephrine release [76]. Reduced beta-2 adrenergic receptor mechanisms likely contribute to age-related alterations in the sympathetic control of blood pressure in women. For example, older women demonstrate attenuated increases in forearm blood flow in response to low doses of a non-specific beta-adrenergic agonist (isoproterenol) and a beta-2 adrenergic agonist (terbutaline) [81,82]. Nitric oxide contributes to the beta-2 adrenergic vasodilation in young women, and reductions in nitric oxide bioavailability likely affect the age-related loss in beta-adrenergic vasodilation in women [82]. Common to both men and women are robust increases in resting MSNA and reductions in vascular endothelial function with ageing that likely also contribute to the relationship between resting MSNA and blood pressure in older individuals that is not observed in younger cohorts [53,83]. Although not discussed in detail here, alterations in circulating sex steroid concentrations with ageing contribute to changes in sympathetic vascular control [30,83,84].
Studies investigating the beat-by-beat sympathetic regulation of vasomotor behaviour have provided additional insight to the effects of age and sex on neurocirculatory regulation. When assessing neurovascular transduction in each participant by assessing the slope of the relationship between diastolic pressures regressed against MSNA burst areas of preceding cardiac cycles, Briant and colleagues found that young women demonstrated weaker neurovascular regulation than young men [66]. Interactive sex and age effects were revealed when comparing neurovascular regulation between older and younger cohorts. Briant and colleagues identified that neurovascular transduction was greater in older women compared to younger women but was lower in older than younger men [66]. Similarly, using a transfer function approach that relied on an adapted Poiseuille’s equation for determining the relationship between MSNA, blood pressure, and leg blood flow, Tan et al. identified that sympathetic regulation of the peripheral skeletal muscle vascular was lesser in older than younger men [85]. Combined, these cross-sectional data support the concept that ageing exerts divergent neurocirculatory effects on men and women — neurovascular transduction becomes augmented with age in women but attenuated in men.
The effects of age and sex on neurovascular transduction are not universal in studies relying on beat-to-beat models of neurovascular transduction, however. For example, Vianna and colleagues found similar neurovascular transduction in young men and women and observed reduced neurovascular transduction with ageing both sexes [62]. In some studies sex differences only emerge when examining vasomotor or depressor responses resulting from cardiac cycles containing no bursts of MSNA. For example, Coovadia and colleagues [86] found that compared to young women, young men demonstrated larger reductions in mean pressure following cardiac cycles without sympathetic bursts during device-guided slow breathing [86]. These findings support the overall concept that men rely more on sympathetic vasomotor control but also raise the possibility that sex differences may exist in asynchronous sympathetic AP discharge which theoretically may support circulatory control during periods without sympathetic bursting activity.
4.3 |. Impact of physical exercise and aerobic fitness on sympathetic neurocirculatory regulation
The link between elevated sympathetic activity and blood pressure with human ageing necessitates the implementation of strategies that modulate sympathetic vascular regulation. Aerobic physical exercise represents a reliable non-pharmacological strategy often employed concomitantly with other behavioural interventions (e.g., dietary) to lower blood pressure in healthy individuals and patients diagnosed with, or at risk of, cardiovascular diseases [1,87]. Reduced sympathetic vasoconstrictor signaling with physical exercise training has been observed consistently in patients with cardiovascular diseases and other risk factors such as metabolic syndrome, obesity, and sleep apnea [88]. The reduction in supine resting MSNA burst frequency likely represents an important mechanism among the multiple factors (e.g., improved endothelial function) contributing to lower blood pressure [88,89]. For example, a cohort of middle-aged hypertensives exhibited large reductions in MSNA burst incidence (~40%) and mean pressure (10 mmHg) after four months of an aerobic intervention [90]. Although aerobic training decreases blood pressure in healthy individuals, this effect may not stem from a sympathoinhibitory effect [88]. Thus, it is plausible that physical exercise may also modulate the vascular responses to sympathetic discharge in healthy and high-cardiovascular risk individuals.
The limited available evidence suggests that greater aerobic fitness may dampen the circulatory adjustments to MSNA, at least in men. When studying the beat-by-beat pressor responses to sympathetic activity in a young male cohort (VO2peak range: ~35 – 60 mL/kg/min), O’Brien and colleagues observed an inverse relationship between aerobic fitness and maximal pressure responses to individual bursts of MSNA [91]. This is consistent with the observation that forearm vascular resistance responses scaled positively to MSNA changes (imposed by simulated orthostatic stress) in sedentary middle-aged men (VO2peak: ~25 mL/kg/min) but no relationship was observed in trained counterparts (VO2peak: ~40 mL/kg/min) [59]. Combined, despite using different approaches, these studies suggest that aerobic fitness modulates the circulatory responses to sympathetic vasoconstrictor stimuli, at least in men. However, the finding that trained and sedentary men exhibit similar blood pressure reductions with trimethaphan do not align with these findings [92]. How these data translate to the impact of physical exercise remains inconclusive. This question is complicated by observations that sympathetic outflow is often reduced by exercise interventions (at least in those with elevated vascular risk) but some cross-sectional data have revealed a positive relationship between aerobic fitness and MSNA burst frequency [71,88].
The observation that age-related increases in MSNA and blood pressure are greater in women than men highlight the need to study the modulatory influence of aerobic fitness and physical exercise on neurovascular control in women. However, to our knowledge only one investigation has addressed this topic. Recently, Baker and colleagues examined the relationship between aerobic fitness, resting MSNA, and the circulatory responses to intravenous trimethaphan infusion in cohorts of young healthy (VO2max range: ~25 – 50 mL/kg/min) and older healthy, post-menopausal women (VO2max range: ~20 – 40 mL/kg/min) [71]. Compared to the younger group, older women demonstrated greater resting MSNA and larger reductions in blood pressure with ganglionic blockade. Interestingly, the reduction in blood pressure with ganglionic blockade was positively associated with aerobic fitness in young women, but this relationship was not observed in the older cohort. When Baker et al. investigated the autonomic neural mechanisms mediating the positive relationship between aerobic fitness and pressure changes with ganglionic blockade, they found that aerobic fitness was positively related to resting sympathetic activity (MSNA) and parasympathetic activity (the cardiac output responses to ganglionic blockade) in younger women. Conversely, there was no link between fitness, MSNA, and cardiac output responses to ganglionic blockade in older women. Combined, these observations suggest that aerobic fitness influences the autonomic support of blood pressure in young but not older, post-menopausal women. In young women, greater aerobic fitness increases MSNA and parasympathetic activity, resulting in greater cardiac output responses and lesser blood pressure reductions to ganglionic blockade. Whether these findings are attributed to an effect of age, sex hormone status, or some other physiological factor (e.g., blood volume) in women remains unclear from these data. Evidently, further research is require to understand the optimal physiological conditions and exercise paradigms required to expose the neuro- and vaso-protective effects of physical exercise in men and older post-menopausal women.
5 |. Conclusions
Among inhabitants of developed nations, human ageing modifies sympathetic neurocirculatory regulation to produce steady rises in blood pressure across the life course, yet sympathetic vasomotor regulation fundamentally differs between women and men, resulting in sex-specific blood pressure trajectories. Broad inter-individual variability exists in resting MSNA, but generally, young women have lower sympathetic discharge than young men [53,93]. Although MSNA bursts evoke vasoconstriction, healthy young individuals demonstrate no relationship between resting MSNA and blood pressure [16,73]. This explains why individuals with similar blood pressures may have substantially different resting MSNA. The mechanisms buffering the relationship between sympathetic outflow and blood pressure in young healthy individuals differ in men (cardiac output and alpha-adrenergic receptor sensitivity) and women (beta-2 adrenergic vasodilatory mechanisms) [75,76,78,94]. In young men, there is a positive relationship between MSNA and vascular resistance that is offset by an inverse relationship between MSNA and both cardiac output and alpha-adrenergic receptor sensitivity. Conversely, in young women, due to greater contributions from beta-2 adrenergic vasodilatory mechanisms, no relationship exists between MSNA and vascular resistance. Combined, these physiological sex-differences produce lower blood pressure in young women than young men. Sympathetic outflow towards the vasculature increases with ageing, but women demonstrate larger increases in MSNA with age than men [53,73]. Ageing also imposes changes in the integrative neuro-hemodynamic factors that buffer vasoconstriction. Compared to young individuals, older men and women demonstrate a positive relationship between MSNA and blood pressure. In addition to the increase in MSNA with age, this observation likely stems from a loss of the inverse MSNA-cardiac output relationship in men and reduced beta-2 adrenergic vasodilatory mechanisms in women. These physiological alterations drive blood pressure increases with age and explain, at least in part, why women demonstrate larger blood pressure changes and a greater rise in the prevalence of hypertension with age than men. Aerobic physical exercise represents a reliable strategy to lower blood pressure in healthy individuals and patients with elevated vascular risk [87,88]. Evidence regarding the impact of physical exercise interventions on sympathetic neurocirculatory regulation is lacking, but emerging data suggest that greater aerobic fitness may dampen the circulatory adjustments to MSNA, at least in young healthy men and women [71,91]. Future research should explore the optimal physiological conditions and exercise paradigms required to expose the neuro- and vaso-protective effects of physical exercise in men and older post-menopausal women. Also, incorporation of the microneurographic measurement of sympathetic AP discharge, recruitment, and synchronization patterns into studies employing pharmacological or beat-by-beat techniques to quantify neurovascular transduction will likely provide added nuance to our understanding of the interactive effects of age and sex on human sympathetic neurocirculatory regulation.
Highlights.
Sex-specific blood pressure trajectories exist across the life-course
The sympathetic nervous system represents a critical regulator of blood pressure
Interactive effects of age and sex exist in sympathetic neurocirculatory regulation
Greater aerobic fitness may modulate sympathetic neurocirculatory regulation
Funding:
This work was funded by a National Heart, Lung, and Blood Institute (NHLBI) Grant awarded to M.J.J. (R-35-139854), National Institute of Aging Grant support to S.E.B. (U54 AG044170), and a Natural Sciences and Engineering Research Council of Canada (NSERC) Post-doctoral fellowship awarded to S.A.K. (PDF-532926-2019).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, J. Am. Coll. Cardiol 71 (2017) 1269–1324. [DOI] [PubMed] [Google Scholar]
- [2].Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Abate KH, Akinyemiju TF, Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015, Jama 317 (2017) 165–182. [DOI] [PubMed] [Google Scholar]
- [3].Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Merz CNB, Cheng S, Sex differences in blood pressure trajectories over the life course, JAMA Cardiol 5 (2020) 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure, Hypertension. 42 (2003) 1206–1252. [DOI] [PubMed] [Google Scholar]
- [5].Martins D, Nelson K, Pan D, Tareen N, Norris K, The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III., J. Gender-Specific Med. JGSM Off. J. Partnersh. Women’s Heal. Columbia 4 (2001) 10. [PubMed] [Google Scholar]
- [6].Hollenberg NK, Martinez G, McCullough M, Meinking T, Passan D, Preston M, Rivera A, Taplin D, Vicaria-Clement M, Aging, acculturation, salt intake, and hypertension in the Kuna of Panama, Hypertension 29 (1997) 171–176. [DOI] [PubMed] [Google Scholar]
- [7].Chirinos JA, Segers P, Hughes T, Townsend R, Large-artery stiffness in health and disease: JACC state-of-the-art review, J. Am. Coll. Cardiol 74 (2019) 1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA, Women exhibit a greater age-related increase in proximal aortic stiffness than men, J. Hypertens 19 (2001) 2205–2212. [DOI] [PubMed] [Google Scholar]
- [9].Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N, Neural control of the circulation: how sex and age differences interact in humans, Compr. Physiol 5 (2011) 193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wehrwein EA, Orer HS, Barman SM, Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system, Compr. Physiol (2016). [DOI] [PubMed]
- [11].Smith GD, Watson LP, Pavitt DV, Mathias CJ, Abnormal cardiovascular and catecholamine responses to supine exercise in human subjects with sympathetic dysfunction., J. Physiol 484 (1995) 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Van Lieshout JJ, Wieling W, Wesseling KH, Endert E, Karemaker JM, Orthostatic hypotension caused by sympathectomies performed for hyperhidrosis., Neth. J. Med 36 (1990) 53–57. [PubMed] [Google Scholar]
- [13].Westcott EB, Segal SS, Perivascular innervation: a multiplicity of roles in vasomotor control and myoendothelial signaling, Microcirculation 20 (2013) 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guimarães S, Moura D, Vascular adrenoceptors: an update, Pharmacol. Rev 53 (2001) 319–356. [PubMed] [Google Scholar]
- [15].Shoemaker JK, Badrov MB, Al-Khazraji BK, Jackson DN, Neural Control of Vascular Function in Skeletal Muscle, Compr. Physiol 6 (2016) 303–329. [DOI] [PubMed] [Google Scholar]
- [16].Hagbarth K, Vallbo ÅB, Pulse and respiratory grouping of sympathetic impulses in human muscle nerves, Acta Physiol. Scand 74 (1968) 96–108. [DOI] [PubMed] [Google Scholar]
- [17].Adrian ED, Bronk DW, Phillips G, Discharges in mammalian sympathetic nerves, J. Physiol 74 (1932) 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vallbo ÅB, Hagbarth K-E, Wallin BG, Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system, J. Appl. Physiol 96 (2004) 1262–1269. http://jap.physiology.org/content/jap/96/4/1262.full.pdf. [DOI] [PubMed] [Google Scholar]
- [19].Hart ECJ, Head GA, Carter JR, Wallin G, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW, Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization, Am. J. Physiol. Circ. Physiol 312 (2017) H1031–H1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Charkoudian N, Wallin BG, Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics, Compr. Physiol 4 (2011) 827–850. [DOI] [PubMed] [Google Scholar]
- [21].Ottaviani MM, Wright L, Dawood T, Macefield VG, In vivo recordings from the human vagus nerve using ultrasound-guided microneurography, J. Physiol 598 (2020) 3569–3576. [DOI] [PubMed] [Google Scholar]
- [22].WALLIN BG, SUNDLÖF G, ERIKSSON B, DOMINIAK P, GROBECKER H, LINDBLAD LE, Plasma noradrenaline correlates to sympathetic muscle nerve activity in normotensive man, Acta Physiol. Scand 111 (1981) 69–73. [DOI] [PubMed] [Google Scholar]
- [23].Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G, Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover., Hypertension 11 (1988) 3–20. [DOI] [PubMed] [Google Scholar]
- [24].Lambert EA, Schlaich MP, Dawood T, Sari C, Chopra R, Barton DA, Kaye DM, Elam M, Esler MD, Lambert GW, Single-unit muscle sympathetic nervous activity and its relation to cardiac noradrenaline spillover, J. Physiol 589 (2011) 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shoemaker JK, Klassen SA, Badrov MB, Fadel PJ, Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans., J. Neurophysiol 119 (2018) 1731–1744. 10.1152/jn.00841.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tompkins RPR, Melling CWJ, Wilson TD, Bates BD, Shoemaker JK, Arrangement of sympathetic fibers within the human common peroneal nerve: implications for microneurography, J. Appl. Physiol 115 (2013) 1553–1561. http://jap.physiology.org/content/jap/115/10/1553.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McAllen RM, Malpas SC, Sympathetic burst activity: characteristics and significance, Clin. Exp. Pharmacol. Physiol 24 (1997) 791–799. [DOI] [PubMed] [Google Scholar]
- [28].Fagius JAN, Wallin BG, Sundlof G, CHRISTER N, Englesson S, Sympathetic outflow in man after anaesthesia of the glossopharyngeal and vagus nerves, Brain 108 (1985) 423–438. [DOI] [PubMed] [Google Scholar]
- [29].Rea RF, Eckberg DL, Carotid baroreceptor-muscle sympathetic relation in humans, Am. J. Physiol. Integr. Comp. Physiol 253 (1987) R929–R934. [DOI] [PubMed] [Google Scholar]
- [30].Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N, Neural control of the circulation: how sex and age differences interact in humans, Compr. Physiol 5 (2015) 193–215. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4459710/pdf/nihms686810.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mano T, Microneurographic research on sympathetic nerve responses to environmental stimuli in humans, Jpn. J. Physiol 48 (1998) 99–114. [DOI] [PubMed] [Google Scholar]
- [32].Delius W, Hagbarth K, Hongell A, Wallin BG, General characteristics of sympathetic activity in human muscle nerves, Acta Physiol. Scand 84 (1972) 65–81. http://onlinelibrary.wiley.com/store/10.1111/j.1748-1716.1972.tb05158.x/asset/j.1748-1716.1972.tb05158.x.pdf?v=1&t=ip9xdvnk&s=6575e4e9bac12b79784df4f4113e890167528ec2. [DOI] [PubMed] [Google Scholar]
- [33].Sverrisdóttir YB, Rundqvist B, Johannsson G, Elam M, Sympathetic Neural Burst Amplitude Distribution A More Specific Indicator of Sympathoexcitation in Human Heart Failure, Circulation 102 (2000) 2076–2081. http://circ.ahajournals.org/content/circulationaha/102/17/2076.full.pdf. [DOI] [PubMed] [Google Scholar]
- [34].White DW, Shoemaker JK, Raven PB, Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans, Auton. Neurosci 193 (2015) 12–21. http://ac.els-cdn.com/S1566070215300175/1-s2.0-S1566070215300175-main.pdf?_tid=e494eb64-2f24-11e6-8901-00000aacb35f&acdnat=1465574798_ddb6ff3d3508c5e6c40197f4d735e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Macefield VG, Wallin BG, Vallbo AB, The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves, J. Physiol 481 (1994) 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Macefield VG, Wallin BG, Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity, J. Physiol 516 (1999) 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Macefield VG, Wallin BG, Physiological and pathophysiological firing properties of single postganglionic sympathetic neurons in humans, J. Neurophysiol 119 (2017) 944–956. [DOI] [PubMed] [Google Scholar]
- [38].Salmanpour A, Shoemaker JK, Baroreflex mechanisms regulating the occurrence of neural spikes in human muscle sympathetic nerve activity, J. Neurophysiol 107 (2012) 3409–3416. http://jn.physiology.org/content/jn/107/12/3409.full.pdf. [DOI] [PubMed] [Google Scholar]
- [39].Salmanpour A, Brown LJ, Shoemaker JK, Spike detection in human muscle sympathetic nerve activity using a matched wavelet approach, J. Neurosci. Methods 193 (2010) 343–355. http://ac.els-cdn.com/S0165027010004954/1-s2.0-S0165027010004954-main.pdf?_tid=a7017282-6089-11e5-addb-00000aab0f6b&acdnat=1442858183_d0c750e9e8faf7ea221edd3888239559. [DOI] [PubMed] [Google Scholar]
- [40].Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK, Sympathetic neural activation: an ordered affair, J. Physiol 588 (2010) 4825–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Klassen SA, Moir ME, Limberg JK, Baker SE, Nicholson WT, Curry TB, Joyner MJ, Shoemaker JK, Asynchronous action potential discharge in human muscle sympathetic nerve activity, Am. J. Physiol. Circ. Physiol (2019). 10.1152/ajpheart.00258.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Klassen SA, Limberg JK, Baker SE, Nicholson WT, Curry TB, Joyner MJ, Shoemaker JK, The role of the paravertebral ganglia in human sympathetic neural discharge patterns., J. Physiol 596 (2018) 4497–4510. 10.1113/JP276440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Klassen SA, Shoemaker JK, Action potential subpopulations in human muscle sympathetic nerve activity: discharge properties and governing mechanisms, Auton. Neurosci. Accepted N (2020). [DOI] [PubMed]
- [44].Klassen SA, Moir ME, Usselman CW, Shoemaker JK, Heterogeneous baroreflex control of sympathetic action potential subpopulations in humans, J. Physiol (2020). [DOI] [PubMed] [Google Scholar]
- [45].Salmanpour A, Brown LJ, Steinback CD, Usselman CW, Goswami R, Shoemaker JK, Relationship between size and latency of action potentials in human muscle sympathetic nerve activity, J. Neurophysiol 105 (2011) 2830–2842. http://jn.physiology.org/content/jn/105/6/2830.full.pdf. [DOI] [PubMed] [Google Scholar]
- [46].Klassen SA, De Abreu S, Greaves DK, Kimmerly DS, Arbeille P, Denise P, Hughson RL, Normand H, Shoemaker JK, Long-duration bed rest modifies sympathetic neural recruitment strategies in male and female participants, J. Appl. Physiol 124 (2017) 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Henneman E, Somjen G, Carpenter DO, Functional significance of cell size in spinal motoneurons, J. Neurophysiol 28 (1965) 560–580. [DOI] [PubMed] [Google Scholar]
- [48].Badrov MB, Olver TD, Shoemaker JK, Central vs. peripheral determinants of sympathetic neural recruitment: insights from static handgrip exercise and postexercise circulatory occlusion, Am. J. Physiol. Integr. Comp. Physiol 311 (2016) R1013–R1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ott EP, Baker SE, Holbein WW, Shoemaker JK, Limberg JK, Effect of varying chemoreflex stress on sympathetic neural recruitment strategies during apnea, J. Neurophysiol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Grassi G, Seravalle G, Mancia G, Sympathetic activation in cardiovascular disease: evidence, clinical impact and therapeutic implications, Eur. J. Clin. Invest 45 (2015) 1367–1375. [DOI] [PubMed] [Google Scholar]
- [51].Malpas SC, Sympathetic nervous system overactivity and its role in the development of cardiovascular disease, Physiol. Rev 90 (2010) 513–557. [DOI] [PubMed] [Google Scholar]
- [52].Barretto ACP, Santos AC, Munhoz R, Rondon MUPB, Franco FG, Trombetta IC, Roveda F, de Matos LNJ, Braga AMW, Middlekauff HR, Increased muscle sympathetic nerve activity predicts mortality in heart failure patients, Int. J. Cardiol 135 (2009) 302–307. [DOI] [PubMed] [Google Scholar]
- [53].Keir DA, Badrov MB, Tomlinson G, Notarius CF, Kimmerly DS, Millar PJ, Shoemaker JK, Floras JS, Influence of Sex and Age on Muscle Sympathetic Nerve Activity of Healthy Normotensive Adults, Hypertension 76 (2020) 997–1005. [DOI] [PubMed] [Google Scholar]
- [54].Badrov MB, Lalande S, Olver TD, Suskin N, Shoemaker JK, Effects of aging and coronary artery disease on sympathetic neural recruitment strategies during endinspiratory and end-expiratory apnea, Am. J. Physiol. Circ. Physiol 311 (2016) H1040–H1050. [DOI] [PubMed] [Google Scholar]
- [55].Sundlöf G, Wallin BG, Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age, J. Physiol 274 (1978) 621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Al-Khazraji BK, Shoemaker JK, The human cortical autonomic network and volitional exercise in health and disease, Appl. Physiol. Nutr. Metab 43 (2018) 1122–1130. [DOI] [PubMed] [Google Scholar]
- [57].Delius W, Hagbarth K, Hongell A, Wallin BG, Manoeuvres affecting sympathetic outflow in human skin nerves, Acta Physiol. Scand 84 (1972) 177–186. . [DOI] [PubMed] [Google Scholar]
- [58].Hissen SL, Taylor CE, Sex differences in vascular transduction of sympathetic nerve activity, Clin. Auton. Res (2020) 1–12. [DOI] [PubMed]
- [59].Notarius CF, Murai H, Morris BL, Floras JS, Effect of fitness on reflex sympathetic neurovascular transduction in middle-age men., Med. Sci. Sports Exerc 44 (2012) 232–237. [DOI] [PubMed] [Google Scholar]
- [60].Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ, Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans, Am. J. Physiol. Circ. Physiol 304 (2013) H759–H766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wallin BG, Nerhed C, Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man, J. Auton. Nerv. Syst 6 (1982) 293–302. [DOI] [PubMed] [Google Scholar]
- [62].Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ, Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity, Am. J. Physiol. Circ. Physiol 302 (2012) H2419–H2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Taylor JA, Williams TD, Seals DR, Davy KP, Low-frequency arterial pressure fluctuations do not reflect sympathetic outflow: gender and age differences, Am. J. Physiol. Circ. Physiol 274 (1998) H1194–H1201. [DOI] [PubMed] [Google Scholar]
- [64].Zamir M, Goswami R, Liu L, Salmanpour A, Shoemaker JK, Myogenic activity in autoregulation during low frequency oscillations, Auton. Neurosci 159 (2011) 104–110. [DOI] [PubMed] [Google Scholar]
- [65].Zamir M, Goswami R, Salzer D, Shoemaker JK, Role of vascular bed compliance in vasomotor control in human skeletal muscle, Exp. Physiol 92 (2007) 841–848. [DOI] [PubMed] [Google Scholar]
- [66].Briant LJB, Burchell AE, Ratcliffe LEK, Charkoudian N, Nightingale AK, Paton JFR, Joyner MJ, Hart EC, Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control, J. Physiol 594 (2016) 4753–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Steinback CD, Fraser GM, Usselman CW, Reyes LM, Julian CG, Stickland MK, Chari RS, Khurana R, Davidge ST, Davenport MH, Blunted sympathetic neurovascular transduction during normotensive pregnancy, J. Physiol 597 (2019) 3687–3696. [DOI] [PubMed] [Google Scholar]
- [68].Ninomiya I, Malpas SC, Matsukawa K, Shindo T, Akiyama T, The amplitude of synchronized cardiac sympathetic nerve activity reflects the number of activated pre-and postganglionic fibers in anesthetized cats, J. Auton. Nerv. Syst 45 (1993) 139–147. [DOI] [PubMed] [Google Scholar]
- [69].Klassen SA, Wiggins CC, Senefeld JW, Does the broad nature of sympathetic discharge affect our understanding regarding the impact of intermittent hypoxia on neurovascular transduction?, J. Physiol (2020). [DOI] [PubMed] [Google Scholar]
- [70].Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR, Altered autonomic support of arterial blood pressure with age in healthy men, Circulation 104 (2001) 2424–2429. [DOI] [PubMed] [Google Scholar]
- [71].Baker SE, Limberg JK, Scruggs ZM, Curry TB, Nicholson WT, Barnes JN, Joyner MJ, Greater Influence of Aerobic Fitness on Autonomic Support of Blood Pressure in Young Women Than in Older Women, Hypertension 75 (2020) 1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Joyner MJ, Wallin BG, Charkoudian N, Sex differences and blood pressure regulation in humans, Exp. Physiol 101 (2016) 349–355. [DOI] [PubMed] [Google Scholar]
- [73].Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK, Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity, Hypertension 45 (2005) 522–525. [DOI] [PubMed] [Google Scholar]
- [74].Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG, Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation, J. Physiol 568 (2005) 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hart EC, Joyner MJ, Wallin BG, Charkoudian N, Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors, J. Physiol 590 (2012) 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N, Age-related differences in the sympathetic-hemodynamic balance in men, Hypertension 54 (2009) 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR, Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men, Circulation 111 (2005) 494–498. [DOI] [PubMed] [Google Scholar]
- [78].Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ, Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors, J. Physiol 589 (2011) 5285–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, Joyner MJ, Aging enhances autonomic support of blood pressure in women, Hypertension 63 (2014) 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Baker SE, Limberg JK, Dillon GA, Curry TB, Joyner MJ, Nicholson WT, Aging Alters the Relative Contributions of the Sympathetic and Parasympathetic Nervous System to Blood Pressure Control in Women, Hypertension 72 (2018) 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Harvey RE, Barnes JN, Charkoudian N, Curry TB, Eisenach JH, Hart EC, Joyner MJ, Forearm vasodilator responses to a β-adrenergic receptor agonist in premenopausal and postmenopausal women, Physiol. Rep 2 (2014) e12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Harvey RE, Ranadive SM, Limberg JK, Baker SE, Nicholson WT, Curry TB, Barnes JN, Joyner MJ, Forearm vasodilatation to a β2-adrenergic receptor agonist in premenopausal and postmenopausal women, Exp. Physiol 105 (2020) 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Stanhewicz AE, Wenner MM, Stachenfeld NS, Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan, Am. J. Physiol. Circ. Physiol 315 (2018) H1569–H1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Baker SE, Limberg JK, Ranadive SM, Joyner MJ, Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones, Am. J. Physiol. Integr. Comp. Physiol 311 (2016) R1271–R1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tan CO, Tamisier R, Hamner JW, Taylor JA, Characterizing sympathetic neurovascular transduction in humans, PLoS One 8 (2013) e53769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Coovadia Y, Adler TE, Steinback CD, Fraser GM, Usselman CW, Sex differences in dynamic blood pressure regulation: beat-by- beat responses to muscle sympathetic nerve activity, Am. J. Physiol. Circ. Physiol 319 (2020) H531–H538. [DOI] [PubMed] [Google Scholar]
- [87].Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, Thompson PD, Williams MA, Lauer MS, Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nut, Circulation 111 (2005) 369–376. [DOI] [PubMed] [Google Scholar]
- [88].Carter JR, Ray CA, Sympathetic neural adaptations to exercise training in humans, Auton. Neurosci 188 (2015) 36–43. [DOI] [PubMed] [Google Scholar]
- [89].Eskurza I, Monahan KD, Robinson JA, Seals DR, Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing, J. Physiol 556 (2004) 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Laterza MC, de Matos LDNJ, Trombetta IC, Braga AMW, Roveda F, Alves MJNN, Krieger EM, Negrão CE, Rondon MUPB, Exercise training restores baroreflex sensitivity in never-treated hypertensive patients, Hypertension 49 (2007) 1298–1306. [DOI] [PubMed] [Google Scholar]
- [91].O’Brien MW, Ramsay D, Johnston W, Kimmerly DS, Aerobic fitness and sympathetic responses to spontaneous muscle sympathetic nerve activity in young males, Clin. Auton. Res (2020) 1–9. [DOI] [PubMed]
- [92].Jones PP, Shapiro LF, Keisling GA, Quaife RA, Seals DR, Is autonomic support of arterial blood pressure related to habitual exercise status in healthy men?, J. Physiol 540 (2002) 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sundlöf G, Wallin BG, The variability of muscle nerve sympathetic activity in resting recumbent man, J. Physiol 272 (1977) 383. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1353564/pdf/jphysiol00793-0168.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG, Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans, J. Physiol 572 (2006) 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]


