Abstract
Dendritic cells are potently activated by the synergistic action of CD40 stimulation in conjunction with signaling through toll like receptors, subsequently priming T cells. Cancer vaccines targeting the activation of dendritic cells in this manner show promise in murine models and are being developed for human patients. While the efficacy of vaccines based on CD40 and toll like receptor stimulation has been established, further investigation is needed to understand the mechanism of tumor control and how vaccination alters tumor infiltrating immune cells. In this study we vaccinated mice bearing established murine melanoma tumors with agonistic anti-CD40, polyI:C, and tumor antigen. Vaccination led to increased intratumoral T cell numbers and delayed tumor growth, yet did not require trafficking of T cells from the periphery. Pre-existing intratumoral T cells exhibited an acute burst in proliferation but became less functional in response to vaccination. However, the increased intratumoral T cell numbers yielded increased numbers of effector T cells per tumor. Together, our data indicate that the existing T cell response and intratumoral dendritic cells are critical for vaccination efficacy. It also suggests that circulating T cells responding to vaccination may not be an appropriate biomarker for vaccine efficacy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-020-02841-z.
Keywords: Vaccination, CD40, TLR3, CD8 T cells, Trafficking, Melanoma
Introduction
Recent advances in checkpoint blockade and adoptive cell therapy demonstrate the potential for harnessing adaptive immunity to fight cancer. Immune infiltration of tumors, especially by cytotoxic CD8 T cells, correlates with improved patient prognosis overall and in response to immunotherapy [1, 2]. One approach to induce CD8 T cell expansion in cancer patients is through vaccination. Early human vaccination trials were generally unsuccessful at controlling tumor growth, despite expanding antigen specific T cells [3–7]. More recent trials expanding the range of adjuvants or combining vaccination with other immunotherapies have improved the number of patients with stable or regressing tumors, but the magnitude of induced T cell expansion remains far below what is seen in response to infection in humans or with vaccination in tumor bearing mice [8–11]. As human vaccine trials evolve to incorporate next generation adjuvants and neoantigens, preclinical studies are needed to investigate the best combination of adjuvants for cancer vaccines and characterize their synergistic effects on immune responses.
In vivo, interactions with mature antigen presenting cells, primarily dendritic cells (DC), lead to CD8 T cell activation. One of the key drivers of DC maturation and subsequent T cell activation is stimulation of CD40 on DC, through engagement with CD40L on CD4 T cells [12]. While toll like receptor (TLR) agonists show some ability to promote T cell responses to vaccination [13, 14], the combination of CD40 stimulation and TLR agonists synergize to drive robust antigen specific CD8 T cell expansion [15]. Supporting the notion that activating DC promotes anti-tumor immunity, several studies demonstrate that vaccine approaches combining αCD40 agonistic antibody with TLR agonists are effective in mouse models of cancer [11, 16–19].
Despite the efficacy of vaccination with αCD40, polyinosinic-polycytidylic acid (polyI:C) and antigen in murine tumor models, the mechanistic basis for tumor control is still unclear. Some studies suggest that CD40-mediated activation of myeloid cells is sufficient for tumor control [20–22], while others demonstrate that αCD40 vaccination increases antigen specific CD8 T cell numbers in the spleen and blood, and tumor control is dependent on CD8 T cells [11, 23]. Whether vaccination with αCD40 and polyI:C alters the number and function of intratumoral T cells, and whether CD4 T cells contribute to tumor control after vaccination including MHC class II epitopes are unknown. Interestingly, tumor control with αCD40, polyI:C, and peptide antigen vaccination is dependent on perforin but independent of IFNγ, suggesting that increased cytotoxicity may drive tumor control [11]. Additionally, the relative contribution of vaccination-induced tumor control of pre-existing tumor infiltrating T cells as compared to newly primed T cells arriving from the periphery is unclear.
Here, using a well-established murine model of melanoma, we examine T cell responses within the tumor after vaccination with αCD40, polyI:C, and tumor antigen. Although vaccination expands T cells in the peripheral lymphoid tissues, we demonstrate that vaccination slows tumor growth without requiring trafficking of additional T cells from the periphery. Therefore, we interrogate the proliferation, survival, and function of the pre-existing intratumoral T cells to understand the mechanism by which vaccination delays tumor growth without new T cell infiltration.
Materials and methods
Tumor model
The B16cOVA tumor line was previously generated in our laboratory [24]. B16cOVA cells (4 × 105) were injected subcutaneously between the shoulders of C57BL/6 mice (National Cancer Institute). Tumors were measured by calipers in two directions and reported as tumor area. Mice were euthanized to harvest tumors at indicated time points or upon reaching the maximum allowed size. All mice were treated in accordance with policies established by the University of Virginia Animal Care and Use Committee.
Treatments
Tumor bearing mice were vaccinated i.p. with 100 µg αCD40 (FGK45; BioXcell), 75 µg polyI:C (Invivogen), and 500 µg ovalbumin (Sigma) or 200 µg OVA257-264 (Genscript) at day ten after tumor injection. FTY720 (Sigma) was provided in the drinking water (2 µg/mL) starting at day nine after tumor implantation and supplemented with daily i.p. injection (25 µg) on days nine through twelve. Depletion antibodies (250 µg) for CD8 (2.43; BioXCell) and/or CD4 (GK1.5; BioXCell) were administered i.p. at day eight after tumor injection and again every 4 days for the remainder of the experiment. FTY720 and depletion efficiency were confirmed by flow cytometric analysis of blood in each experiment. LFA blocking antibody (M17/4; BioXCell) was administered i.p. (200 µg) every 2 days. Brefeldin A (Thermo Fisher) was injected i.v. (250 µg) 5 h prior to harvest to block cytokine secretion. For OT1 transfer, spleens were harvested from OT1 mice originally from Taconic and maintained at the University of Virginia.
Sample preparation
Tumors were excised and subjected to manual homogenization. T cells were isolated by density gradient separation with Lympholyte-m (Cedarlane). For in vitro function, isolated T cells were stimulated with plate-bound αCD3 (1 µg/mL; eBioscience) or OVA257 peptide pulsed, CD45-mismatched splenocytes. H-2 Kb/SIINFEKL dextramer (Immudex) was used to identify OVA257 specific CD8. Antibodies and reagents used for staining are listed in Table S1.
Data analysis
Flow samples were collected on Cytoflex S (Beckman Coulter) or Attune NxT (Thermo Fisher) cytometers and analyzed using FlowJo V10. Statistical analyses were performed using Graphpad Prism V8. Tumor outgrowth data is presented as mean ± SEM from one representative experiment and analyzed by Holm-Sidak multiple t tests. All other data is presented as mean ± SD and analyzed by Welch’s t test for two groups or Holm-Sidak multiple t tests for more than two groups or time points. In some cases, pooled data are normalized to the control samples within each experiment to compensate for batch effects and shown as the relative expression of indicated factors. Differences were considered significant when p < 0.05.
Results
Vaccination is effective in established murine melanoma
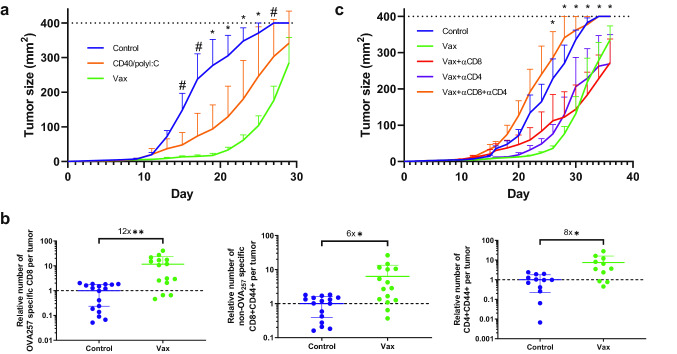
To assess the mechanism by which vaccination with a CD40 agonistic antibody, TLR3 agonist polyI:C, and tumor antigen controls tumor growth, we utilized the B16cOVA melanoma model, which allowed us to assess the immune response to the well-defined pseudo-neoantigen ovalbumin (OVA) expressed by the tumor. When implanted subcutaneously, B16cOVA elicits a modest inflammatory immune response, including both CD8 and CD4 T cells, that diminishes over time and ultimately fails to control the tumor [25]. When tumor bearing mice were vaccinated 3 and 10 days after tumor implantation by i.p. injection of either whole OVA protein or the CD8 immunodominant peptide OVA257-264 (SIINFEKL) in combination with agonistic αCD40 and polyI:C, mice were able to substantially control tumor growth (Fig. S1). To ask whether this immunization regimen was effective in more established tumors and if tumor control required booster immunizations, we gave a single vaccination of αCD40, polyI:C, and ovalbumin 10 days post-tumor implantation. This treatment approach resulted in significantly smaller tumors compared to controls (Fig. 1a), affirming the efficacy of the vaccination approach. To test whether the adjuvants could control tumor growth without antigen, we treated mice with αCD40 and polyI:C alone at 10 days post-implantation. While the adjuvants partially controlled tumor growth, the addition of antigen led to significantly more robust tumor control (Fig. 1a). The tumor control achieved with a single vaccination at day ten therefore provided the opportunity to investigate how vaccination alters the anti-tumor immune response.
Fig. 1.
Therapeutic efficacy of vaccination with αCD40/polyI:C/antigen in murine melanoma. a Tumor size in mice vaccinated with αCD40/polyI:C/ovalbumin or treated with αCD40/polyI:C on day 10. n = 5 mice per group. c Relative number of OVA257 specific CD8 T cells identified by MHC I dextramer (left) and non-OVA257 specific antigen experienced CD8 (middle) and CD4 (right) T cells infiltrating tumors at day 21 from mice vaccinated as in (a). Data are pooled from five experiments and normalized to the number of T cells in unvaccinated control tumors within each experiment. c Tumor size in mice vaccinated at day 10 as in a and injected i.p. with depleting antibodies to CD8 and/or CD4 every 4 days starting at day 8. n = 4–7 mice per group. Data were analyzed by Holm-Sidak multiple t tests (ac) or Welch’s t test (b). a Significant difference between all groups at time points indicated by * and Control/Vax at time points indicated by #. c Significant difference between Control/Vax, Control/Vax + αCD8, and Control/Vax + αCD4 at indicated time points. *p < 0.05, **p < 0.01, #p < 0.05
The antigen component of the vaccine includes epitopes recognized by both CD8 and CD4 T cells, so we measured the number of both intratumoral T cell subsets when we observed reduced tumor size and sufficient time had passed for T cells to be activated and traffic to the tumors. At 11 days post-vaccination (21 days after tumor implantation), within the tumors there were increased numbers of OVA257 specific CD8 T cells (twelvefold), antigen experienced CD8 (sixfold) T cells with other specificities, and antigen experienced CD4 (eightfold) T cells (Fig. 1b). We used depleting antibodies to test the relative contribution of CD8 versus CD4 T cells to delaying tumor growth. Tumor control after vaccination was lost when both CD8 and CD4 T cells were depleted, but either subset alone was sufficient to delay tumor growth (Fig. 1c). Thus, both CD8 and CD4 T cells can contribute to the control of tumor after αCD40 and polyI:C mediated vaccination with antigen containing epitopes for both subsets.
Additional T cell infiltration is not required for vaccination to slow tumor growth
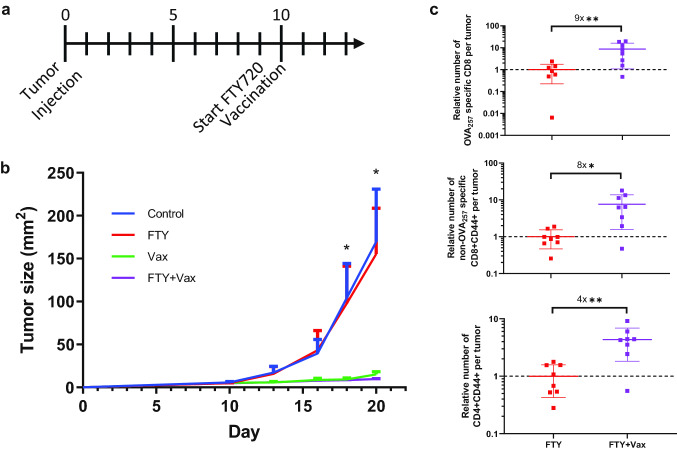
As the spontaneous anti-tumor immune response in untreated mice has a limited ability to control B16cOVA outgrowth, we would predict that vaccination slows tumor growth through expansion or recruitment of more functional T cells to tumors. To test the contribution of new T cell infiltration to tumor control, we used the sphingosine-1-phosphate receptor agonist FTY720 to block T cell egress from lymphoid tissues and thereby block new T cell trafficking into tumors (Fig. S2) [26]. Delaying FTY720 treatment allowed the initial antitumor T cell response to infiltrate the tumor prior to vaccination (Fig. 2a). Contrary to our expectation, vaccination still delayed tumor growth when FTY720 was used to block trafficking of additional T cells into the tumor (Fig. 2b). Separately, we prevented new T cell trafficking into tumors with αLFA1, which blocks T cell extravasation through LFA1 interaction with ICAM on vasculature [27]. Consistent with reports that αLFA1 alters T cell expansion and function in addition to blocking trafficking [28, 29], we observed decreased expansion of antigen specific T cells in the spleens of vaccinated tumor bearing mice (Fig. S3a). Despite the diminished T cell expansion, αLFA1 did not prevent vaccination from delaying tumor growth (Fig. S3b). Together, these data show that vaccination can delay tumor growth through T cells that infiltrated the tumor prior to treatment without the need for recruitment of additional T cells.
Fig. 2.
Additional T cell infiltration is not required for vaccination to slow tumor growth. a Mice bearing B16cOVA tumors were vaccinated and treated with FTY720 as indicated in the timeline and described in the methods. b Tumor size of mice treated with vaccination and FTY720. n = 5 per group. c Relative number of OVA257 specific CD8 T cells (top) and non-OVA257 specific total antigen experienced CD8 (middle) and CD4 (bottom) T cells infiltrating tumors at day 21. Data are pooled from 2 experiments. Each data point is from an individual mouse. Data were analyzed by Holm-Sidak multiple t tests (b) or Welch’s t test (c). b Significant difference between Control/Vax, Control/Vax + FTY, FTY/Vax, and FTY/FTY + Vax at indicated time points. *p < 0.05, **p < 0.01
The vaccine’s ability to slow tumor growth through the pre-infiltrating T cells suggests that the vaccine is acting within the tumor. In support of this, we found that intratumoral DC expressed significantly more CD86 2 days after vaccination and only expressed IL12 (p40) in vaccinated mice (Fig. S4). Thus, intratumoral DC may serve as the intermediary linking vaccination and tumor control dependent on the already infiltrating T cells, and is consistent with previous reports demonstrating that intratumoral DC are critical contributors to vaccination-induced tumor control [30, 31].
Vaccination sustains T cells within the tumor
We next considered how vaccination could slow tumor growth without new T cell infiltration, despite the apparent exhausted phenotype of intratumoral T cells in this model [32]. The most likely explanation is that vaccination expanded the T cells within the tumor. The number of OVA257 specific (ninefold) and non-OVA257 specific antigen experienced (eightfold) intratumoral CD8 T cells was higher at day eleven after vaccination when trafficking was limited with FTY720 (Fig. 2c). The number of CD4 T cells was also increased in vaccinated tumors (fourfold), although to a lesser extent than the CD8 T cells (Fig. 2c). Surprisingly, we found similar numbers of all three groups of intratumoral T cells after vaccination with or without blocking T cell trafficking (Fig. S5a). Together, these data indicate that vaccination is primarily expanding or preserving both CD8 and CD4 T cells within the tumor.
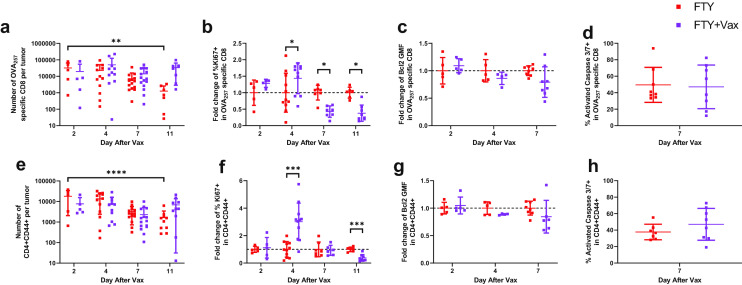
To further understand how vaccination increased the number of both CD8 and CD4 T cells without new infiltration, we measured the number of intratumoral T cells from two to 11 days after vaccination while trafficking was blocked with FTY720. Whereas untreated tumors had fewer OVA257 specific CD8 T cells as time progressed, the number of OVA257 specific CD8 T cells remained steady in the vaccinated tumors (Fig. 3a). Although there were fewer CD4 T cells than OVA257 specific CD8 T cells, they followed the same pattern: sustained numbers in vaccinated tumors and a decline over time in unvaccinated tumors (Fig. 3e). To determine how vaccination sustained intratumoral T cell numbers, we measured their proliferation and survival. There was an increase in the proportion of OVA257 specific CD8 and total CD4 T cells expressing Ki67 at 4 days after vaccination but a decrease in Ki67 expression at later time points, indicating a transient increase in proliferation (Fig. 3b, f). To examine survival, we measured expression of Bcl2 and caspase 3/7 activation. Both OVA257 specific CD8 and total CD4 T cells had no difference in Bcl2 expression at 2 and 4 days after vaccination and a trending decrease in at seven days after vaccination (Fig. 3c, g). Similar activated caspase 3/7 levels indicated no difference in cell death in either OVA257 specific CD8 or total CD4 T cells at 7 days after vaccination (Fig. 3d, h). Together, the Bcl2 and caspase 3/7 data indicate that vaccination does not improve intratumoral T cell survival. In summary, vaccination drives increased proliferation of intratumoral T cells acutely, which may contribute to sustained numbers of both CD8 and CD4 T cells within the tumor.
Fig. 3.
Vaccination sustains T cells within the tumor. Mice bearing B16cOVA tumors were vaccinated at day 10 and began receiving FTY720 at day 9 as described in methods and shown in Fig. 2a. Tumors were harvested and T cells were analyzed at 2, 4, 7, or 11 days after vaccination or from control mice at matching time points. (ae) Number of intratumoral OVA257 specific CD8 a and CD4 e T cells. (bf) Relative proportion of Ki67 expressing OVA257 specific CD8 b and CD4 f T cells within control or vaccinated tumors. (cg) Relative per cell expression of Bcl2 in intratumoral OVA257 specific CD8 c and CD4 g T cells. (dh) Relative caspase 3/7 activation in intratumoral OVA257 specific CD8 d and CD4 h T cells. Data were analyzed by ANOVA with test for linear trend (ae) or Holm-Sidak multiple t tests (b–d, f–h). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Vaccination does not improve T cell function within the tumor
Aside from T cell infiltration, we also predicted that vaccination would improve the function of intratumoral T cells. Supporting this, 11 days after vaccination we found that the intensity of expression of the inhibitory receptor PD-1, which can lead to diminished T cell function when engaged by its ligands PD-L1 or PD-L2 on tumor or other immune cells in the tumor microenvironment, was significantly lower (twofold) on a per cell basis on OVA257 specific CD8 T cells from vaccinated mice (Fig. S7a). To test function, we isolated OVA257 specific CD8 T cells from vaccinated and unvaccinated tumors and co-cultured them in vitro with OVA257-pulsed antigen presenting cells. Contrary to our expectations, significantly fewer OVA257 specific CD8 T cells from the tumors of vaccinated mice produced IFNγ and Granzyme B than those present in the tumors of control mice (Fig. S7b, c). Thus, diminished PD-1 expression did not lead to increased effector function of OVA257 specific CD8 T cells after vaccination. Remarkably, fewer OVA257 specific CD8 T cells from vaccinated mice produced IFNγ than from unvaccinated mice when trafficking was allowed (Fig. S5b), although Granzyme B expression was not significantly different (Fig. S5c). This suggests that after vaccination newly infiltrating T cells are more cytotoxic but not more capable of IFNγ production compared to T cells already present within the tumor.
An inflection point in tumor growth occurs around 10 days after vaccination, which could suggest that the effects of a single vaccination are only temporary. Therefore, we examined the function of both CD8 and CD4 T cells at 4 and 7 days after vaccination in mice treated with FTY720 to block trafficking of newly primed T cells. In this instance, we gave brefeldin A (BFA) i.v. prior to harvest to block cytokine secretion in situ and thus measure cytokine production by T cells within the tumors. While there was no difference in effector activity in T cells present in the tumors of vaccinated and control mice at day four, a lower proportion of OVA257 specific CD8 produced IFNγ (43% reduction) and Granzyme B (33% reduction) within the tumor 7 days after vaccination (Fig. 4a–c). As in vivo BFA reports steady state cytokine production, we also measured the T cell functional activity by stimulating the T cells isolated from tumors with a low concentration of αCD3 ex vivo. There was a trend for a higher frequency of OVA257-specific CD8 T cells expressing IFNγ at day four but, as seen with in situ assessment, a significant reduction at day seven after vaccination (Fig. S8a). There was no difference in the capability of OVA257 specific CD8 T cells to degranulate, which is required for release of cytotoxic molecules, as measured by surface exposure of CD107a during the ex vivo stimulation (Fig. S8b). Additionally, we saw the same pattern of decreased effector function in intratumoral CD8 T cells that were not specific for OVA257 after vaccination, both in situ and ex vivo (not shown). Together, this data shows that vaccination is not controlling tumor growth by improving CD8 T cell effector molecule expression or degranulation.
Fig. 4.
Vaccination does not improve T cell function within the tumor. Mice bearing B16cOVA tumors were vaccinated at day 10 and began receiving FTY720 at day 9 as described in methods and shown in Fig. 2a. Brefeldin A was administered 5 h prior to harvest to measure in vivo expression of IFNγ and Granzyme B by tumor infiltrating OVA257 specific CD8 and CD4 T cells. a Representative flow plots of naïve T cells from the spleen (top) and OVA257 specific CD8 or CD4 T cells from control (middle) and vaccinated (bottom) tumors. (bc) Relative proportion of OVA257 specific CD8 T cells expressing IFNγ b and Granzyme B c from 3 pooled experiments. (de) Relative proportion of CD4 T cells expressing IFNγ d and Granzyme B e from 3 pooled experiments. Data were analyzed by Holm-Sidak multiple t tests. *p < 0.05, **p < 0.01, ***p < 0.001
While CD8 T cells are important based on their cytotoxic capabilities, CD4 T cells can contribute to the antitumor response through cytokines, and in some cases killing of target cells. In situ, intratumoral CD4 T cells had no significant difference in either IFNγ or Granzyme B expression at day four or day seven after vaccination compared to controls (Fig. 4d, e). Intratumoral CD4 T cells also showed no difference in degranulation based on surface exposure of CD107a during ex vivo stimulation with αCD3 (Fig. S8d). In contrast to both OVA257 specific CD8 and CD4 T cell in situ production of IFNγ, more CD4 T cells expressed IFNγ at both 4 and 7 days after vaccination when stimulated ex vivo (Fig. S8c). Although the vaccinated CD4 T cells did not make more IFNγ within the tumor, they have increased potential to contribute effector cytokines.
Together these data show that vaccination differentially affects CD8 and CD4 T cell function, and that these effects are discrete when examined in the steady state as compared to after restimulation. Importantly, the relative increase in the number of CD8 and CD4 T cells within the tumor found after vaccination outweighs the reduction in function observed in situ, resulting in equivalent or significantly more effector T cells within the tumors of vaccinated mice (Fig. S9), especially at 11 days post-vaccination.
Vaccination does not expand T cell subsets identified by Eomes or Tcf1 expression
Recently, populations of putatively exhausted CD8 T cells that can proliferate and generate more functional CD8 T cells have been separately identified based on two transcription factors: low expression of Eomes and high expression of Tcf1 [33–35]. Therefore, we asked whether a subset of intratumoral CD8 T cells identified by Eomes or Tcf1 expression was responding to vaccination. We found decreased Eomes expression in OVA257 specific CD8 T cells from vaccinated tumors at day 7 after vaccination (Fig. 5a). However, unlike published data, we found that T cells with higher Eomes expression had the highest degree of proliferation in both control and vaccinated mice (Fig. 5b). Further, we found that T cells with higher Eomes expression had increased IFNγ and Granzyme B expression, irrespective of vaccination (Fig. 5c, d). Tcf1 expression declined in OVA257 specific CD8 T cells between day 4 and 7 after vaccination, but was not impacted by vaccination (Fig. 5e). As with Eomes, we found higher Ki67 expression in Tcf1 + cells compared to Tcf1- cells, as expected, and this relationship was also unaltered by vaccination (Fig. 5f). We also found that Tcf1 expression correlated with higher IFNγ and Granzyme B expression, in contrast to previous reports (Fig. 5g, h). In summary, higher expression levels of Eomes and Tcf1 expression correlate with more functional intratumoral CD8 T cells. However, the increase in intratumoral T cells that accompanies vaccination does not result from expansion of a subset of intratumoral CD8 T cells identified by Eomes or Tcf1 expression.
Fig. 5.
Vaccination does not improve proliferation or function in a CD8 T cell subset identified by Eomes or Tcf1. Mice bearing B16cOVA tumors were vaccinated at day 10 and began receiving FTY720 at day 9 as described in methods and shown in Fig. 2a. Brefeldin A was administered 5 h prior to harvest. a Expression of Eomes in intratumoral OVA257 specific CD8 T cells. b Expression of Ki67 in in intratumoral OVA257 specific CD8 T cells that do (blue) or do not (green) express Eomes. (cd) Proportion expressing IFNγ (c) and Granzyme B (d) of intratumoral OVA257 specific CD8 T cells that do (blue) or do not (green) express Eomes. e Expression of Tcf1 in intratumoral OVA257 specific CD8 T cells. f Expression of Ki67 in in intratumoral OVA257 specific CD8 T cells that do (orange) or do not (green) express Tcf1. (gh) Proportion expressing IFNγ (g) and Granzyme B (h) of intratumoral OVA257 specific CD8 T cells that do (orange) or do not (green) express Tcf1. Data were analyzed by Holm-Sidak multiple t tests. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Pre-infiltrated CD8 T cells are sufficient for vaccination to delay tumor growth
We next tested if both the pre-infiltrated CD8 and CD4 T cells had the potential to impede tumor growth by combining T cell depletion with FTY720 to block trafficking into the tumor (Fig. 6a). As expected, depleting both CD8 and CD4 T cells in FTY720 treated mice resulted in the loss of vaccination induced tumor control (Fig. 6b). When we depleted only CD4 cells in FTY720 treated mice, vaccination still delayed tumor growth. However, when we blocked trafficking with FTY720 and depleted CD8 T cells, the already infiltrating CD4 T cells were insufficient to slow tumor growth after vaccination. Thus, when T cell trafficking to tumor is prevented, only the pre-infiltrated CD8 T cells could slow tumor growth. Therefore, the CD8 T cells infiltrating the tumor at the time of vaccination play critical contributions to the ability of vaccination to delay tumor growth.
Fig. 6.

Pre-infiltrated CD8 T cells are sufficient for vaccination to delay tumor growth. a Mice bearing B16cOVA tumors were treated with vaccination, FTY720, and depleting antibodies as indicated in the timeline and described in the methods. b Tumor size of mice treated as described in (a). n = 6–7 per group. Data were analyzed by Holm-Sidak multiple t tests with significant difference between FTY/FTY + Vax and FTY/FTY + Vax + αCD4 at indicated time points. *p < 0.05
Discussion
Previously, and in our data, vaccination regimens with αCD40, polyI:C, and antigen control tumor growth and generate large T cell responses observed in the spleen and blood. As T cells are known to become exhausted within the tumor, it was assumed that newly activated T cells were trafficking to the tumor and responsible for controlling tumor growth. However, by blocking trafficking we have shown that the existing CD8 T cells are sufficient for vaccination to slow tumor growth without additional T cell infiltration.
Despite the compelling evidence that vaccination leads to tumor control via pre-existing intratumoral T cells, there are several paradoxes concerning the mechanistic basis of tumor control. First, we predicted that vaccination would lead to more functional T cells within the tumor. Instead, a lower proportion of CD8 T cells from vaccinated tumors expressed IFNγ and Granzyme B in situ acutely after vaccination, and they were no better at degranulating. While a greater proportion of CD4 T cells from vaccinated tumors had the potential to produce more IFNγ ex vivo, they were not more functional in situ within the tumor. The basis for the loss of effector activity by the intratumoral CD8 T cells after vaccination remains unclear. OVA257 specific CD8 T cells expressed less PD-1, which we would expect to improve function within the tumor. Decreased Eomes expression may have contributed to diminished function. However, T-bet, which was highly expressed (not shown), and Tcf1 levels were unaffected by vaccination. Notably, intratumoral T cells from vaccinated mice with intact trafficking were also less functional than from control mice, suggesting that loss of functional activity occurs within the tumor microenvironment. After vaccination, we also did not observe a significant increase in the presence within tumors of Treg, MDSC or TAM populations that could suppress T cell function (data not shown). Critically, however, the decreased function of intratumoral T cells was offset by vaccination sustaining T cell numbers within the tumors, resulting in an equivalency or increase in the number of cytokine-expressing T cells per tumor, even when gauged by in situ function. Thus, the impact of vaccination on T cell persistence in the tumor mitigates their diminished functionality. Functional decrease is not terminal as a second vaccination 7d later can further delay tumor outgrowth, though tumors ultimately escape control (data not shown) more rapidly than when two vaccinations are provided early in tumor growth. It is possible that other effector mechanisms or aspects of T cell biology that we currently do not fully understand are contributing to tumor control.
The second paradox concerns the capacity of vaccination to sustain the number of both CD8 and CD4 T cells present in the tumor even when trafficking of T cells into the tumor is blocked. While there was increased proliferation of intratumoral T cells acutely after vaccination, T cells within the tumor were less proliferative at later time points. While both Eomes and Tcf1 correlated with increased proliferation, vaccination did not improve proliferation in a Tcf1 + or Eomes + subset. T cells from vaccinated mice were also no better at surviving within the tumor. We also found that the majority of OVA specific T cells expressed the retention integrin CD103, with no difference after vaccination (not shown). Thus, we currently ascribe the impact of vaccination on T cell numbers to the acute burst in proliferation, although why proliferation is rapidly curtailed remains to be determined.
These mechanistic paradoxes are important as αCD40 and TLR3 agonists are being aggressively developed for clinical implementation. CD40 monoclonal antibodies have shown to be safe in human trials, with limited efficacy on tumor control as a single agent [36–38], and better response rates when combined with chemotherapy or checkpoint blockade [39, 40]. PolyIC:LC has been incorporated into human vaccines that generate T cell responses but not necessarily control tumor growth [8, 9]. Two clinical trials with αCD40, polyIC:LC, and peptide antigens have been initiated for melanoma patients (NCT03597282, NCT04364230), but have not reported outcomes. Based on our data, the existing T cell response may be critical for such vaccination regimens to be effective. Additionally, our data suggest that circulating T cells are not a definitive biomarker for vaccination efficacy, as they may not be required for tumor control.
In summary, we have revealed that vaccination with αCD40, polyI:C, and tumor antigen can delay the growth of established melanoma tumors by sustaining pre-infiltrating CD8 T cells. Intratumoral T cells lose effector function over time after vaccination, yet are more acutely proliferative resulting in a relative increase in the number of effector T cells within the tumors of vaccinated mice. Although vaccination drives T cell expansion in the periphery, our data shows that additional T cell infiltration is unnecessary for tumor control, and that the existing intratumoral CD8 T cells therefore critically contribute to tumor control by this vaccination regimen.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Alexandra Witter and Ms. Marissa Gonzales for assistance and discussions and the Beirne B. Carter Center for Immunology Research for use of the flow cytometry instruments.
Abbreviations
- BFA
Brefeldin A
- DC
Dendritic cells
- polyI:C
Polyinosinic-polycytidylic acid
- TLR
Toll like receptor
Author contributions
All authors conceived the project, designed experiments, interpreted data, and wrote the manuscript. A.D.S. performed experiments and analyzed data. T.N.J.B. supervised and acquired funding.
Funding
This work was funded by National Institutes of Health grant R01CA166458 to Timothy N.J. Bullock. Aaron D. Stevens was supported by National Institutes of Health training Grant T32AI007496.
Data availability
All data relevant to the study are included in the article or uploaded as online supplementary information. Additional information regarding data may be obtained from the authors on reasonable request.
Compliance with ethical standards
Conflict of interests
None declared.
Ethics approval
All mice were treated in accordance with policies established by the University of Virginia Animal Care and Use Committee (protocol 3292).
Consent for publication
All authors have reviewed and approved the submitted manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jäeger E, Bernhard H, Romero P, Ringhoffer M, Arand M, Karbach J, et al. Generation of cytotoxic T-cell responses with synthetic melanoma-associated peptides in vivo: implications for tumor vaccines with melanoma-associated antigens. Int J Cancer. 1996;66:162–169. doi: 10.1002/(SICI)1097-0215(19960410)66:2<162::AID-IJC4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Cormier JN, Salgaller ML, Prevette T, Barracchini KC, Rivoltini L, Restifo NP, et al. Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. Cancer J Sci Am. 1997;3:37–44. [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slingluff CL, Yamshchikov G, Neese P, Galavotti H, Eastham S, Kittlesen D, et al. Phase I trial of a melanoma vaccine with gp100280-288 peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- 7.Marchand M, van Baren N, Weynants P, Brichard V, Dréno B, Tessier M, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–230. doi: 10.1002/(SICI)1097-0215(19990118)80:2<219::AID-IJC10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Melssen MM, Petroni GR, Chianese-Bullock KA, Wages NA, Grosh WW, Varhegyi N, et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. J Immunother Cancer. 2019;7:163. doi: 10.1186/s40425-019-0625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavlick A, Blazquez AB, Meseck M, Lattanzi M, Ott PA, Marron TU, et al. Combined vaccination with NY-ESO-1 protein, poly-ICLC, and montanide improves humoral and cellular immune responses in patients with high-risk melanoma. Cancer Immunol Res. 2020;8:70–80. doi: 10.1158/2326-6066.CIR-19-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callan MFC, Tan L, Annels N, Ogg GS, Wilson JDK, O’Callaghan CA, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Il CH, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69:9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenberger SP, Toes REM, Van Dervoort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD4OL interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 13.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 14.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific t-cell response to vaccination and poly(I:C)/TLR3 signaling. J Immunother. 2005;28:220–228. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 15.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llopiz D, Dotor J, Zabaleta A, Lasarte JJ, Prieto J, Borrás-Cuesta F, et al. Combined immunization with adjuvant molecules poly(I:C) and anti-CD40 plus a tumor antigen has potent prophylactic and therapeutic antitumor effects. Cancer ImmunolImmunother. 2008;57:19–29. doi: 10.1007/s00262-007-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, et al. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009;182:5217–5224. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- 19.Bialojan A, Sohl J, Rausch J, Aranda Lopez P, Denny M, Langguth P, et al. Transcutaneous immunization with CD40 ligation boosts cytotoxic T lymphocyte mediated antitumor immunity independent of CD4 helper cells in mice. Eur J Immunol. 2019;49:2083–2094. doi: 10.1002/eji.201848039. [DOI] [PubMed] [Google Scholar]
- 20.Lum HD. In vivo CD40 ligation can induce T cell-independent antitumor effects that involve macrophages. J LeukocBiol. 2006;79:1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 21.Medina-Echeverz J, Ma C, Duffy AG, Eggert T, Hawk N, Kleiner DE, et al. Systemic agonistic anti-CD40 treatment of tumor-bearing mice modulates hepatic myeloid-suppressive cells and causes immune-mediated liver damage. Cancer Immunol Res. 2015;3:557–566. doi: 10.1158/2326-6066.CIR-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;80-(331):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer ImmunolImmunother. 2012;61:1307–1317. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang ML, Lukens JR, Bullock TNJ. Cognate memory CD4 + T cells generated with dendritic cell priming influence the expansion, trafficking, and differentiation of secondary CD8 + T cells and enhance tumor control. J Immunol. 2007;179:5829–5838. doi: 10.4049/jimmunol.179.9.5829. [DOI] [PubMed] [Google Scholar]
- 25.Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8+ T cells. J Immunother. 2010;33:769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J BiolChem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 27.Woods AN, Wilson AL, Srivinisan N, Zeng J, Dutta AB, Peske JD, et al. Differential expression of homing receptor ligands on tumor-associated vasculature that control CD8 effector T-cell entry. Cancer Immunol Res. 2017;5:1062–1073. doi: 10.1158/2326-6066.CIR-17-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutigliano JA, Johnson TR, Hollinger TN, Fischer JE, Aung S, Graham BS. Treatment with anti-LFA-1 delays the CD8+ cytotoxic-T-lymphocyte response and viral clearance in mice with primary respiratory syncytial virus infection. J Virol. 2004;78:3014–3023. doi: 10.1128/jvi.78.6.3014-3023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setoguchi K, Schenk AD, Ishii D, Hattori Y, Baldwin WM, Tanabe K, et al. LFA-1 antagonism inhibits early infiltration of endogenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. Am J Transplant. 2011;11:923–935. doi: 10.1111/j.1600-6143.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulko V, Liu X, Krco CJ, Harris KJ, Frigola X, Kwon ED, et al. TLR3-stimulated dendritic cells up-regulate B7–H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol. 2009;183:3634–3641. doi: 10.4049/jimmunol.0900974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azuma M, Takeda Y, Nakajima H, Sugiyama H, Ebihara T, Oshiumi H, et al. Biphasic function of TLR3 adjuvant on tumor and spleen dendritic cells promotes tumor T cell infiltration and regression in a vaccine therapy. Oncoimmunology. 2016;5:e1188244. doi: 10.1080/2162402X.2016.1188244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gemta LF, Siska PJ, Nelson ME, Gao X, Liu X, Locasale JW, et al. Impaired enolase 1 glycolytic activity restrains effector functions of tumor-infiltrating CD8+ T cells. SciImmunol. 2019 doi: 10.1126/sciimmunol.aap9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;80-(338):1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral Tcf1 + PD-1 + CD8 + T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50(195–211):e10. doi: 10.1016/j.immuni.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J ClinOncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 37.Sanborn RE, Gabrail NY, Bhardwaj N, Gordon MS, O’Hara M, Khalil D, et al. Abstract LB-194: first-in-human phase I study of the CD40 agonist mAb CDX-1140 and in combination with CDX-301 (rhFLT3L) in patients with advanced cancers: Interim results. Cancer Res Am Assoc Cancer Res (AACR) 2019 doi: 10.1158/1538-7445.am2019-lb-194. [DOI] [Google Scholar]
- 38.Grilley-Olson JE, Curti BD, Smith DC, Goel S, Gajewski T, Markovic S, et al. SEA-CD40, a non-fucosylated CD40 agonist: interim results from a phase 1 study in advanced solid tumors. J ClinOncol. 2018;36:3093–3093. doi: 10.1200/jco.2018.36.15_suppl.3093. [DOI] [Google Scholar]
- 39.Vonderheide RH, Burg JM, Mick R, Trosko JA, Li D, Shaik MN, et al. Phase i study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. 2013 doi: 10.4161/onci.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bajor DL, Mick R, Riese MJ, Huang AC, Sullivan B, Richman LP, et al. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. 2018 doi: 10.1080/2162402X.2018.1468956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplementary information. Additional information regarding data may be obtained from the authors on reasonable request.