Abstract
During prostate cancer treatment with 223Ra. 219Rn (actinon) occurs and may be exhaled by the patient. Nurses and other hospital employees may inhale this radionuclide and its decay products. The alpha-emitting decay products of actinon deposited within a body will irradiate tissues and organs. Therefore. it is necessary to evaluate organ doses of actinon progeny. The purpose of this study is to set up a dosimetric method to assess dose coefficients for actinon progeny. The effective dose coefficients were calculated separately for three modes. The unattached mode which concerned the activity median thermodynamic diameter (AMTD) of 1 nm. and the nucleation and accumulation modes which are represented by activity median aerodynamic diameters (AMAD) of 60 and 500 nm respectively. The recent biokinetic models of actinon progeny developed in the Occupational Intakes of Radionuclides (OIR) publications series of the International Commission of Radiological Protection (ICRP) were implemented on BIOKMOD (Biokinetic Modeling) to calculate the number of nuclear transformations per activity intake of actinon progeny. The organ equivalent and effective dose coefficients were determined using the dosimetric approach of the ICRP. The inhalation dose coefficients of actinon progeny are dominated by the contribution of lung dose. The calculated dose coefficients of 211Pb and 211Bi are 5.78 × 10−8 and 4.84 × 10−9 Sv.Bq−1 for unattached particles (AMTD = 1 nm). and 1.4 × 10−8 and 3.55 × 10−9 Sv.Bq−1 for attached particles (AMAD = 60 nm). and 7.37 × 10−9 and 1.91 × 10−9 Sv.Bq−1 for attached particles (AMAD = 500 nm). These values are much closer to those of the recently published ICRP 137.
Keywords: actinon, 211Pb, 211Bi, inhalation, biokinetic models, dosimetric models, dose coefficient
INTRODUCTION
Actinon (219Rn) is a radioactive noble gas and a decay product of 223Ra in the 235U decay chain. 219Rn decays through the short-lived progeny 215Po. 211Pb. 211Bi and 207Tl to the stable nuclide 207Pb (Fig. 1). In contrast to radon (222Rn) and thoron (220Rn) which leave the soil and building materials and enter into the atmosphere. 219Rn due to its very short half-life (3.96 s) is generally less able to emanate from mineral matrices. Because of typically very low concentrations in the ambient air. exposure to 219Rn and its progeny are usually neglected. Thus. the measurement of 219Rn has not been described in standards such as ICRP 137 or ICRU 88 [1, 3]. Recently. in hospitals cancer treatment with 223Ra (Xofigo) was introduced [4, 5]. 223Ra is injected into patients to fight against bone metastasis of prostate cancer. In the decay chain. 219Rn occurs which may be exhaled by the patient. Secondary exposure of care-takers in the hospital and at home may happen by inhalation of actinon and its decay products.
Fig. 1.

Decay chain of 223Ra with half-lives and decay-energy types [1, 2].
Whereas many papers have been published on dosimetric studies of radon. thoron and their decay products. the International Commission on Radiological Protection (ICRP) has published inhalation dose coefficients of decay products of actinon (211Pb and 211Bi) using the size characteristics of radon progeny [1].
There are several pieces of software for internal dose assessment. However. most of them are commercialized. In the present work. the models for inhalation of actinon progeny have been mathematically implemented using a freely available package ICRP130Models on the recent version of BIOKMOD (version 5.4). and a dosimetric method is established to evaluate inhalation dose of actinon progeny using Microsoft excel. To make it effective. the effective doses and the organ equivalent doses in lung and in other organs of inhaled 219Rn progeny such as 211Pb and 211Bi were determined separately as a function of particle size distribution of three modes. using the human respiratory tract model (HRTM). the human alimentary tract model (HATM) and systemic models developed by ICRP [1, 6, 7]. The aim of this study is to determine the inhalation dose coefficients of actinon decay products. This approach could be used to also determine the dose conversion coefficient of actinon progeny for a real situation of secondary exposures of nurses at hospitals or the members of public at home.
MATERIAL AND METHODS
In this section the internal dose calculations of actinon progeny are introduced. First the deposition fractions in the different regions of HRTM are presented. Then the biokinetic models describing dissolution. absorption and elimination of deposited material in the human body were implemented. and the activities occurring within the organs or tissues were calculated. Finally. the dosimetric model was applied to assess organ equivalent dose and effective dose coefficients with the calculation of radiation weighted S coefficient values. denoted Sw. or specified source and target organs which were derived from the new ICRP voxel computational phantoms for a reference adult [8].
Aerosol fractional deposition in human respiratory tract
Up to now there is no activity size measurement of actinon progeny. Due to its short half-life. 219Rn will probably not be able to escape from the point where it is formed. Therefore. 219Rn and its progeny are very rare in the ambient air. As the lead (211Pb) decay product of actinon has a half-life of 36.1 min. which is much closer to that of the lead (214Pb) progeny of radon (222Rn) with a half-life of 26.8 min. the activity size for radon progeny was used as the deposition fraction in the respiratory tract region. as displayed in Table 1 [1].
Table 1.
Deposition fraction in the HRTM [1]. A breathing rate of 1.2 m3.h−1 is assumed. Geometric standard deviation (GSD) of 1.3 and 2.0 for unattached and attached particles. respectively. a unit of density and a shape factor are used for all modes.
| Deposition fraction (%) | |||
|---|---|---|---|
| Regiona | AMTD (1 nm) | AMAD (60 nm) | AMAD (500 nm) |
| ET1 | 51.91 | 3.85 | 10.68 |
| ET2 | 27.96 | 2.08 | 5.75 |
| BB | 7.93 | 0.93 | 0.60 |
| bb | 10.05 | 6.53 | 1.42 |
| AI | 0.59 | 27.90 | 9.05 |
| Total | 98.43 | 41.29 | 27.51 |
aET1. anterior nasal passage; ET2. posterior nasal passage. pharynx and larynx; BB. bronchial; bb. bronchiolar. AI. alveolar interstitial.
Biokinetic models of actinon progeny
The behavior of inhaled radioactive particles in the respiratory tract is described in the HRTM and some changes have been made in ICRP 130 [6, 7]. The systemic models for actinon progeny (211Pb and 211Bi) and the HATM are described respectively by ICRP 137 and ICRP 100 [1, 9]. The biokinetic models describing inhalation of each actinon progeny are represented in Fig. 2 for 211Pb and Fig. 3 for 211Bi. The dissolution and absorption parameter values of inhaled 211Pb were applied to 211Bi formed in the respiratory tract [7, 10]. The systemic model for bismuth as progeny of lead (bismuth formed within the body) is described in ICRP 137 [1].
Fig. 2.

Inhalation biokinetic compartmental model for lead. It combines the HATM [9]. The systemic model of lead [1] and the HRTM [6, 7]. Extrathoracic region: ET1 = anterior nose), ET2 = posterior nasal passages, larynx, pharynx and mouth. 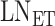 = lymph nodes. Thoracic region: BB = bronchial, bb = bronchiolar, AI = alveolar-interstitial,
= lymph nodes. Thoracic region: BB = bronchial, bb = bronchiolar, AI = alveolar-interstitial,  = lymph nodes) [15]. ALV and INT = alveolar-interstitial. Other soft tissues: ST0 = soft tissue (fast turnover), ST1 = soft tissue (intermediate turnover), ST2 = soft tissue (slow turnover). Other compartments: Oes S = oesophagus slow, stomach contents, small intestine contents, right colon contents, left colon contents, rectosigmoid contents, faeces, sweat, urinary bladder contents, urine, loss in hair skin and nails, plasma. RBC = red blood cells. Bone: cortical surface. Exch Cortical Volume = exchangeable cortical volume, Nonexch Cortical Volume = Nonexchangeable cortical volume. Trabecular Surface. Exch Trabecular Volume = exchangeable trabecular volume, Nonexch Trabecular Volume = nonexchangeable trabecular volume. Liver: Liver1 and Liver2. Kidney: other kidney tissue, urinary path.
= lymph nodes) [15]. ALV and INT = alveolar-interstitial. Other soft tissues: ST0 = soft tissue (fast turnover), ST1 = soft tissue (intermediate turnover), ST2 = soft tissue (slow turnover). Other compartments: Oes S = oesophagus slow, stomach contents, small intestine contents, right colon contents, left colon contents, rectosigmoid contents, faeces, sweat, urinary bladder contents, urine, loss in hair skin and nails, plasma. RBC = red blood cells. Bone: cortical surface. Exch Cortical Volume = exchangeable cortical volume, Nonexch Cortical Volume = Nonexchangeable cortical volume. Trabecular Surface. Exch Trabecular Volume = exchangeable trabecular volume, Nonexch Trabecular Volume = nonexchangeable trabecular volume. Liver: Liver1 and Liver2. Kidney: other kidney tissue, urinary path.
Fig. 3.

Inhalation biokinetic compartmental model for bismuth. It combines the HATM [9]. The systemic model of bismuth [1] and the HRTM [6, 7]. Extrathoracic region: ET1 = anterior nose, ET2 =posterior nasal passages, larynx, pharynx and mouth. 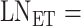 lymph nodes. Thoracic region: BB = bronchial, bb = bronchiolar, AI = alveolar-interstitial,
lymph nodes. Thoracic region: BB = bronchial, bb = bronchiolar, AI = alveolar-interstitial, 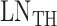 = lymph nodes [15]. Other soft tissues: ST0 = soft tissue (fast turnover), ST1 = soft tissue (intermediate turnover), ST2 = soft tissue (slow turnover). Other compartments: Oes S = oesophagus slow. Stomach contents, small intestine contents, right colon contents, left colon contents, rectosigmoid contents, faeces, sweat, urinary bladder contents, urine, plasma. RBC = red blood cells. Bone: trabecular bone surface, cortical bone surface. Liver: Liver1 and Liver2. Kidney: other kidney tissue, urinary path.
= lymph nodes [15]. Other soft tissues: ST0 = soft tissue (fast turnover), ST1 = soft tissue (intermediate turnover), ST2 = soft tissue (slow turnover). Other compartments: Oes S = oesophagus slow. Stomach contents, small intestine contents, right colon contents, left colon contents, rectosigmoid contents, faeces, sweat, urinary bladder contents, urine, plasma. RBC = red blood cells. Bone: trabecular bone surface, cortical bone surface. Liver: Liver1 and Liver2. Kidney: other kidney tissue, urinary path.
The biokinetic model parameters. i.e. transfer rate. absorption parameter values of the actinon progeny between organs or tissues in the HRTM. HATM and systemic models were taken from ICRP publications [1, 7].
The dynamic behavior of decay products of actinon in the organism can be represented by a number of interconnected compartments with transfer coefficients describing the exchange of material. The transfer of inhaled actinon progeny between compartments can be modelled as systems of coupled. first-order differential equations. These systems have been implemented and solved in BIOKMOD; a computer tool developed by Sanchez using the Wolfram Mathematica programming language [11–13]. The general form of the rate of change of the radionuclide concentration i. can be written as in [14].
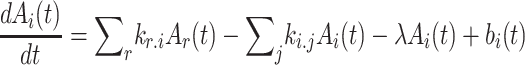 |
(1) |
where  is the retention in compartment i.
is the retention in compartment i. 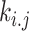 is the transfer coefficient of material from compartment i to compartment j (the first term represents the inputs to the compartment i from the rest of compartments r
is the transfer coefficient of material from compartment i to compartment j (the first term represents the inputs to the compartment i from the rest of compartments r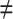 i and the second term represents the outputs from the compartment i to others compartments j
i and the second term represents the outputs from the compartment i to others compartments j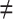 i).
i).  is the physical decay constant and
is the physical decay constant and  is the input from outside. The initial conditions were determined by using the deposition fractions (Table 1). The number of nuclear transformations occurring in source region
is the input from outside. The initial conditions were determined by using the deposition fractions (Table 1). The number of nuclear transformations occurring in source region 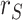 during the commitment period (18250 days for adults) denoted
during the commitment period (18250 days for adults) denoted 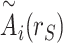 is given:
is given:
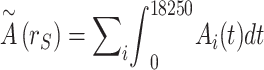 |
(2) |
where 18 250 (50 years) represents the number of days which is the commitment period for an adult. The summation in the equation above is over the association of kinetic compartments i forming source regions rs. These numbers of nuclear transformations for actinon progeny were calculated by using Wolfram Mathematica software. The number of nuclear transformations per activity intake in the source region rs is given in the following equation [8]:
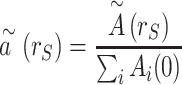 |
(3) |
where 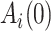 represents the initial deposition fraction in each compartment i.
represents the initial deposition fraction in each compartment i.
Dosimetric models
This section presents the method used to calculate the radiation-weighting S coefficient. committed equivalent doses in each organ/tissue within the body and effective dose after inhalation of actinon decay products.
Radiation weighted S coefficient
The radiation weighted S coefficient  represents the time-dependent equivalent dose rate in the target tissue rT per unit activity present in source tissue rS.
represents the time-dependent equivalent dose rate in the target tissue rT per unit activity present in source tissue rS. 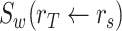 was calculated for each radiation type emitted by the actinon progeny. The general form of the
was calculated for each radiation type emitted by the actinon progeny. The general form of the 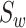 coefficient is given by [8].
coefficient is given by [8].
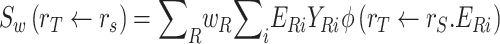 |
(4) |
where 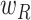 is the radiation weighting factor for radiation type R.
is the radiation weighting factor for radiation type R. 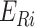 is the energy of the
is the energy of the 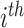 radiation of type R emitted in the nuclear transformations of the radionuclide in joules (J);
radiation of type R emitted in the nuclear transformations of the radionuclide in joules (J); 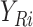 is the yield of the
is the yield of the  radiation of type R per nuclear transformations (Bq.s−1);
radiation of type R per nuclear transformations (Bq.s−1); 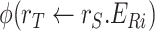 is the specific absorbed fraction denoted as SAF which is defined as the fraction of energy
is the specific absorbed fraction denoted as SAF which is defined as the fraction of energy  of radiation type R emitted within the source tissue/organ rs that is absorbed per mass in the target tissue
of radiation type R emitted within the source tissue/organ rs that is absorbed per mass in the target tissue 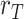 (kg−1). 211Pb decays to the nuclide 211Bi through beta particle emission. In this case the spectral data are used in the calculation of
(kg−1). 211Pb decays to the nuclide 211Bi through beta particle emission. In this case the spectral data are used in the calculation of  instead of mean energy value [8].
instead of mean energy value [8]. 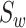 for beta radiation is given by:
for beta radiation is given by:
 |
(5) |
where P(E) is proportional to the probability that the beta particle will be emitted with kinetic energy between E and E + dE. E represents the beta energy. P(E) and E are taken from DECDATA software [16]. The calculation of integral over the beta particle spectrum is made by numerical methods. However. 211Bi decays to the nuclide of 207Tl through alpha particle emission and to 211Po through beta emission. The form of  of alpha emission is given by:
of alpha emission is given by:
 |
(6) |
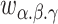 values (radiation weighting factor) are taken form ICRP publication 103 [17]. The specific absorbed fractions values are taken from the electronic data of ICRP 133 [8]. The linear interpolation was done on Microsoft excel to find each corresponding value of
values (radiation weighting factor) are taken form ICRP publication 103 [17]. The specific absorbed fractions values are taken from the electronic data of ICRP 133 [8]. The linear interpolation was done on Microsoft excel to find each corresponding value of 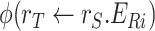 to
to 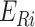 .For the compartment called other soft tissue in the biokinetic models. including several source regions
.For the compartment called other soft tissue in the biokinetic models. including several source regions 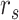 . the specific absorbed fraction
. the specific absorbed fraction 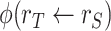 was calculated as [8]:
was calculated as [8]:
 |
(7) |
Committed equivalent dose and committed effective dose
The committed equivalent dose h ( in the target region was calculated for reference adult male
in the target region was calculated for reference adult male  and reference adult female
and reference adult female 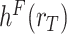 as [8]:
as [8]:
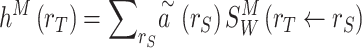 |
(8) |
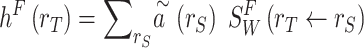 |
(9) |
where 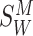 (
(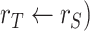 and
and 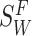 (
(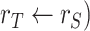 are the S coefficients for male and female respectively. There is an exception for target regions consisting of several target tissues: extrathoracic region. lung. colon and lymphatic nodes. For each target region in those tissues there is an associated fractional weighting factor. The committed equivalent dose for those particular regions was calculated as:
are the S coefficients for male and female respectively. There is an exception for target regions consisting of several target tissues: extrathoracic region. lung. colon and lymphatic nodes. For each target region in those tissues there is an associated fractional weighting factor. The committed equivalent dose for those particular regions was calculated as:
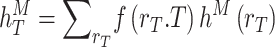 |
(10) |
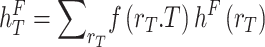 |
(11) |
where 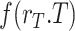 is the fractional weight. the values are taken from ICRP 133 [8]. The committed effective dose coefficient was calculated as [17]:
is the fractional weight. the values are taken from ICRP 133 [8]. The committed effective dose coefficient was calculated as [17]:
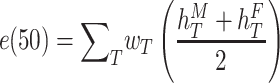 |
(12) |
where 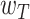 is the weighting factor for tissue T taken from the ICRP publication [17]. Microsoft Excel was used to calculate the radiation weighting S coefficient. the committed equivalent dose coefficient and the committed effective dose coefficient after inhalation of the actinon progeny.
is the weighting factor for tissue T taken from the ICRP publication [17]. Microsoft Excel was used to calculate the radiation weighting S coefficient. the committed equivalent dose coefficient and the committed effective dose coefficient after inhalation of the actinon progeny.
RESULTS AND DISCUSSION
The calculated committed equivalent dose coefficients (male and female) from 211Pb and 211Bi for each target organ/tissue are shown in the Appendix (Tables A1 and A2). In general. the equivalent dose coefficients in the lung and extrathoracic tissues were relatively larger than in other organs for the three particles sizes (radon and thoron progeny as well). For the progeny of 211Pb. the equivalent doses in bronchi basal cells. bronchiole basal cells and ET1 basal cells were the highest for each mode: unattached. nucleation and accumulation respectively. However. for the progeny of 211Bi the ET2 basal cells. bronchiole basal cells and ET2 basal cells were the target regions where the equivalent dose coefficient was the highest for unattached (1 nm). nucleation (60 nm) and accumulation (500 nm) modes. respectively.
The committed equivalent dose in each tissue/organ for actinon progeny in unattached and attached modes are given in Table 2. For both actinon progeny the lung equivalent dose was the highest for unattached and nucleation modes. However. the ET airways (extrathoracic region) equivalent dose was the highest for accumulation mode. The lung dose strongly depends on the percentage of the deposition fraction within the bronchial and bronchiolar tissues. The organs and effective doses (dose coefficients) after inhalation actinon (219Rn) progeny. 211Pb and 211Bi in unattached and attached (nucleation and accumulation) modes are given in Table 2. The dose coefficient of short-lived actinon progeny was the highest for unattached mode (1 nm).
Table 2.
Inhalation dose coefficients (Sv.Bq−1 of 211Pb and 211Bi as a function of particles size in unattached (1 nm) and attached (60 nm and 500 nm)
of 211Pb and 211Bi as a function of particles size in unattached (1 nm) and attached (60 nm and 500 nm)
| Organ | 211Pb | 211Bi | ||||
|---|---|---|---|---|---|---|
| AMTD (1 nm) | AMAD (60 nm) | AMAD (500 nm) | AMTD (1 nm) | AMAD (60 nm) | AMAD (500 nm) | |
| Remainder tissuesa,b | 1.64E-08 | 2.74E-09 | 9.35E-09 | 2.64E-09 | 4.70E-10 | 1.94E-09 |
| Colon | 4.41E-11 | 5.30E-10 | 3.35E-10 | 1.25E-13 | 2.64E-13 | 1.69E-13 |
| Lungs | 4.65E-07 | 1.12E-07 | 5.11E-08 | 3.76E-08 | 2.91E-08 | 1.39E-08 |
| R-Marrow | 2.98E-11 | 3.53E-10 | 2.23E-10 | 8.34E-14 | 1.76E-13 | 1.13E-13 |
| Endost-BS | 2.54E-11 | 3.19E-10 | 2.02E-10 | 4.09E-14 | 8.66E-14 | 5.56E-14 |
| Brain | 1.41E-11 | 1.74E-10 | 1.10E-10 | 3.80E-14 | 8.04E-14 | 5.16E-14 |
| S-Glands | 1.36E-11 | 1.68E-10 | 1.06E-10 | 3.65E-14 | 7.73E-13 | 4.96E-14 |
| Thyroid | 2.72E-11 | 3.24E-10 | 2.04E-10 | 7.62E-14 | 1.61E-13 | 1.03E-13 |
| Breast | 9.10E−12 | 1.11E-10 | 7.02E-11 | 2.52E-14 | 5.35E-14 | 3.43E-14 |
| Liver | 3.35E-11 | 3.98E-10 | 2.51E-10 | 9.89E-14 | 2.09E-13 | 1.34E-13 |
| UB-Wall | 1.18E-11 | 1.58E-10 | 9.98E-11 | 3.26E-14 | 6.90E-14 | 4.43E-14 |
| Gonads | 2.25.E-11 | 2.75E-10 | 1.73E-10 | 6.33E-14 | 1.34E-13 | 8.60E-14 |
| Skin | 1.59E-11 | 1.90E-10 | 1.20E-10 | 4.21E-14 | 8.91E-14 | 5.71E-14 |
| Oesophagus | 9.55E-11 | 4.48E-10 | 3.03E-10 | 1.41E-13 | 2.29E-13 | 1.69E-13 |
| St-stem | 1.53E-10 | 5.57E-10 | 3.89E-10 | 1.29E-13 | 2.68E-13 | 1.74E-13 |
| ET airways | 2.13E-07 | 2.89E-08 | 1.17E-07 | 3.44E-08 | 6.11E-09 | 2.52E-08 |
| Kidneys | 2.66E-10 | 3.53E-10 | 2.23E-09 | 1.32E −13 | 2.81E-13 | 1.80E-13 |
| Adrenals | 3.17E-11 | 3.87E-10 | 2.44E-10 | 9.23E-14 | 1.95E-13 | 1.25E-13 |
| O mucosa | 1.53E-11 | 1.74E-10 | 1.15E-10 | 3.82E-14 | 7.93E-13 | 5.13 E-14 |
| SI-stem | 5.86E-11 | 5.80E-10 | 3.70E-10 | 1.35E −13 | 2.85E-13 | 1.83E-13 |
| Lymphatic N | 1.63E-11 | 1.74E-10 | 1.10E-10 | 4.51E-14 | 9.91E-14 | 6.22E-14 |
| Heart-wall | 3.53E-11 | 3.56E-10 | 2.25E-10 | 8.01E-14 | 1.69E-13 | 1.08E-13 |
| Thymus | 1.76E-11 | 1.76E-10 | 1.11E-10 | 3.68E-14 | 7.80E-14 | 5.00E-14 |
| Spleen | 5.22E-11 | 1.70E-10 | 3.94E-10 | 1.52E-13 | 3.23E-13 | 2.07E-13 |
| Pancreas | 3.30E-11 | 3.97E-10 | 2.50E-10 | 9.49E-14 | 2.01E-13 | 1.28E-13 |
| Prostate/uterus | 9.20E-12 | 1.11E-11 | 1.00E-11 | 3.45E-14 | 7.32E-14 | 4.69E-14 |
| GB-wall | 1.39E-11 | 1.69E-10 | 1.07E-10 | 3.66E-14 | 7.76E-13 | 4.98E-14 |
| Muscle | 1.24E-11 | 1.51E-10 | 9.55E-11 | 3.20E-14 | 6.77E-14 | 4.35E-14 |
| Effective dose | 5.78E-08 | 1.41E-08 | 7.43E-09 | 4.84E-09 | 3.55E-09 | 1.91E-09 |
aRemainder tissues: adrenals. extrathoracic regions of the respiratory tract. gall bladder. heart. kidneys. lymphatic nodes. muscle. oral mucosa. pancreas. prostate (male). small intestine. spleen. thymus and uterus/cervix (female)
bLymphatic N = lymphatic nodes. O mucosa = oral mucosa. R-marrow = red marrow. St-stem = stomach. SI-stem = small intestine. UB-wall = urinary bladder wall. GB-wall = gall bladder wall. ET airways = extra thoracic airways. Endost-BS = endosteal cells. S Glands = Salivary Glands.
In this study. the lung equivalent dose for 211Pb (4.65 × 10−7 Sv.Bq−1) of the unattached mode was 4–9 times larger than the values (1.12 × 10−7 and 5.06 × 10−8 Sv.Bq−1) of nucleation and accumulation. For 211Bi. the lung equivalent dose (3.76 × 10−8 Sv.Bq−1) of the unattached mode was 1–3 times larger than those (2.91 × 10−8 and 1.39 × 10−8 Sv.Bq−1) of the other modes. For actinon progeny. organs such as kidneys. colon. stomach and oesophagus received relatively high doses compared to other organs. The inhalation dose coefficients of 211Pb and 211Bi for adults are 5.78 × 10−8 and 4.84 × 10−9 Sv.Bq−1 for unattached particles (1 nm). and 1.41 × 10−8 and 3.55 × 10−9 Sv.Bq−1 for attached particles (nucleation mode 60 nm) and 7.43 × 10−9 and 1.91 × 10−9 Sv.Bq−1 for attached particles (accumulation mode 500 nm). Those effective dose coefficients are in comparison with the values of 6.6 × 10−8 and 4.8 × 10−9 Sv.Bq−1 in the unattached mode (1 nm). 2.2 × 10−8 and 1.5 × 10−9 Sv.Bq−1 in the nucleation mode (60 nm) and 7.4 × 10−9 and 5.3 × 10−10 Sv.Bq−1 in the accumulation mode (500 nm) for 211Pb and 211Bi. respectively. calculated by ICRP 137 [1]. In Table 3 effective dose coefficients of actinon progeny calculated in this study were compared to those of ICRP 137 and the differences were found to be in the range −36 to 0.4% for 211Pb and 0.8–260% for 211Bi. The calculations were made using assumptions of the Occupational Intakes of Radionuclides (OIR) publications series. The differences found between the dose coefficients would come from the calculation pattern (the numerical integration of equation (5). the calculation of specific absorbed fractions 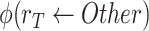 for the compartments denoted other soft tissues and the linear interpolation of specific absorbed fractions) and some details about the treatment of decay products formed in the respiratory tract. Apart of 211Bi. all other progeny radionuclides formed in the respiratory tract (207Tl and 211Po) after inhalation of 211Pb were neglected. In addition. the bound parameter values (transfer rate from the bound-state compartments to the body fluids as shown in Fig. 2) were also neglected for bismuth formed in the respiratory tract. Inhalation dose coefficients for actinon progeny were also calculated by Stabin and Siegel [19]. The dose coefficients were 3.46 × 10−11 and 1.76 × 10−9 Sv.Bq−1 for 211Pb and 211Bi. respectively [18]. One should note that Stabin and Siegel assessed the dose coefficients for one particles size mode (AMAD 5 μm) with the aerosol type ‘M’ in a particular situation of exposure. The dose coefficients (inhalation) of actinon progeny of tissues other than the lungs are ~5% of the total effective dose in the unattached and nucleation modes. while for the accumulation mode the coefficient is ~15% of the total effective dose. Overall the effective dose of inhaled actinon progeny was dominated by the lung equivalent dose. The extra-thoracic equivalent dose was of the same order of magnititude or lower than that to the lungs. However its contribution to the effective dose was quite low, because it is one of the 13 remainder organs (equivalent dose of remainder tissues is the arithmetic mean of the 13 equivalent doses of the remainder tissues) [17].
for the compartments denoted other soft tissues and the linear interpolation of specific absorbed fractions) and some details about the treatment of decay products formed in the respiratory tract. Apart of 211Bi. all other progeny radionuclides formed in the respiratory tract (207Tl and 211Po) after inhalation of 211Pb were neglected. In addition. the bound parameter values (transfer rate from the bound-state compartments to the body fluids as shown in Fig. 2) were also neglected for bismuth formed in the respiratory tract. Inhalation dose coefficients for actinon progeny were also calculated by Stabin and Siegel [19]. The dose coefficients were 3.46 × 10−11 and 1.76 × 10−9 Sv.Bq−1 for 211Pb and 211Bi. respectively [18]. One should note that Stabin and Siegel assessed the dose coefficients for one particles size mode (AMAD 5 μm) with the aerosol type ‘M’ in a particular situation of exposure. The dose coefficients (inhalation) of actinon progeny of tissues other than the lungs are ~5% of the total effective dose in the unattached and nucleation modes. while for the accumulation mode the coefficient is ~15% of the total effective dose. Overall the effective dose of inhaled actinon progeny was dominated by the lung equivalent dose. The extra-thoracic equivalent dose was of the same order of magnititude or lower than that to the lungs. However its contribution to the effective dose was quite low, because it is one of the 13 remainder organs (equivalent dose of remainder tissues is the arithmetic mean of the 13 equivalent doses of the remainder tissues) [17].
Table 3.
Inhalation dose coefficients (Sv Bq−1) calculated in the present work and comparison to the results of ICRP 137
| Mode | Dose coefficients (Sv/Bq) | |||||
|---|---|---|---|---|---|---|
| Present study | ICRP-137 | Differences (%) | ||||
| 211Pb | 211Bi | 211Pb | 211Bi | 211Pb | 211Bi | |
| Unattached | 5.78E-08 | 4.84E-09 | 6.6E-08 | 4.8E-09 | −12 | 0.8 |
| Nucleation | 1.41E-08 | 3.55E-09 | 2.2E-08 | 1.5E-09 | −36 | 136 |
| Accumulation | 7.43E-09 | 1.91E-09 | 7.4E-09 | 5.3E-10 | 0.4 | 260 |
CONCLUSION
This work presents internal dose calculations of inhaled actinon progeny. The effective dose coefficients were calculated separately for three modes. using biokinetic and dosimetric models developed in the recent OIR publications series of ICRP. The biokinetic models of 211Pb and 211Bi have been implemented and solved in BIOKMOD using the approach developed by Sanchez is described in references [11, 12]. The inhalation actinon progeny provided the highest dose to the lungs and ET airways. The calculations indicated that the most exposed region of the lung tissues for 211Pb was the bronchial tissue for the unattached and attached fractions respectively for particle sizes of 1 nm and 500 nm and the bronchiolar tissue for the attached fraction of 60 nm. However. the most exposed region of the lung tissues for 211Bi was the bronchiolar tissue for unattached fraction (1 nm) and bronchioles for attached fractions (60 nm and 500 nm particles size). The inhalation dose coefficients of actinon progeny found in this work were much closer to those of ICRP 137. Furthermore. in order to work out the dose conversion coefficient for actinon (219Rn) decay products. a study of activity size distributions and measurement of activity concentration of actinon progeny is recommended to be conducted in hospitals during the treatment of prostate cancer metastasis with 223Ra.
CONFLICT OF INTEREST
None declared.
Table A1.
Committed equivalent dose coefficients (Sv.Bq−1) for target region after inhalation 211Pb as decay product of actinon (reference adult male and female). f(rT,T)Mh(rT) and f(rT,T)Fh(rT) are committed equivalent dose coefficients for adult male and female respectively
| Target tissuea | Unattached | Nucleation | Accumulation | |||
|---|---|---|---|---|---|---|
| f(rT.T)hM(rT) | f(rT.T)Fh(rT) | f(rT.T)hM(rT) | f(rT.T)Fh(rT) | f(rT.T)hM(rT) | f(rT.T)Fh(rT) | |
| O-mucosa | 1.45E-11 | 1.62E-11 | 1.67E-10 | 1.82E-10 | 1.10E-10 | 1.21E-10 |
| Oesophagus | 1.16E-10 | 7.56E-11 | 4.15E-10 | 4.81E-10 | 2.91E-10 | 3.15E-10 |
| St-stem | 1.46E-10 | 1.61E-10 | 5.15E-10 | 6.01E-10 | 3.61E-10 | 4.18E-10 |
| SI-stem | 4.79E-11 | 6.95E-11 | 4.58E-10 | 7.04E-10 | 2.93E-10 | 4.48E-10 |
| RC-stem | 1.95E-11 | 1.68E-11 | 2.32E-10 | 1.97E-10 | 1.46E-10 | 1.24E-10 |
| LC-stem | 1.66E-11 | 1.88E-11 | 2.02E-10 | 2.30E-10 | 1.27E-10 | 1.45E-10 |
| RS-stem | 7.07E-12 | 9.41E-12 | 8.61E-11 | 1.15E-10 | 5.43E-11 | 7.24E-11 |
| ET1-bas | 8.19E-08 | 9.47E-08 | 1.45E-08 | 1.68E-08 | 6.03E-08 | 6.98E-08 |
| ET2-bas | 1.16E-07 | 1.34E-07 | 1.23E-08 | 1.43E-08 | 4.84E-08 | 5.61E-08 |
| LN-ET | 2.02E-12 | 1.14E-12 | 8.38E-12 | 8.94E-12 | 1.07E-11 | 7.23E-12 |
| Bronch-bas | 4.85E-08 | 5.40E-08 | 5.89E-09 | 6.61E-09 | 4.45E-09 | 4.99E-09 |
| Bronch-sec | 2.02E-07 | 2.24E-07 | 2.25E-08 | 2.51E-08 | 1.72E-08 | 1.92E-08 |
| Bchiol-sec | 1.97E-07 | 2.05E-07 | 7.67E-08 | 7.98E-08 | 2.53E-08 | 2.64E-08 |
| AI | 2.86E-10 | 3.61E-10 | 3.95E-09 | 4.86E-09 | 2.09E-09 | 2.58E-09 |
| LN-Th | 3.19E-12 | 1.20E-11 | 8.92E-12 | 1.60E-11 | 5.80E-12 | 1.10E-11 |
| R-marrow | 2.60E-11 | 3.36E-11 | 3.13E-10 | 3.94E-10 | 1.97E-10 | 2.49E-10 |
| Endost-BS | 2.21E-11 | 2.88E-11 | 2.79E-10 | 3.61E-10 | 1.76E-10 | 2.28E-10 |
| Brain | 1.31E-11 | 1.53E-11 | 1.61E-10 | 1.88E-10 | 1.01E-10 | 1.19E-10 |
| Eye-lens | 8.57E-12 | 9.85E-12 | 1.02E-10 | 1.22E-10 | 6.47E-11 | 7.67E-11 |
| P-gland | 8.44E-12 | 1.43E-11 | 7.00E-11 | 1.76E-10 | 5.56E-11 | 1.11E-10 |
| Tongue | 2.13E-11 | 1.50E-11 | 2.57E-10 | 1.77E-10 | 1.64E-10 | 1.14E-10 |
| Tonsils | 5.62E-12 | 1.45E-11 | 6.68E-11 | 1.76E-10 | 4.27E-11 | 1.12E-10 |
| S-glands | 1.31E-11 | 1.43E-11 | 1.61E-10 | 1.76E-10 | 1.02E-10 | 1.11E-10 |
| Thyroid | 2.44E-11 | 3.01E-11 | 2.94E-10 | 3.54E-10 | 1.85E-10 | 2.24E-10 |
| Breast | 1.31E-11 | 5.09E-12 | 1.61E-10 | 6.15E-11 | 1.02E-10 | 3.88E-11 |
| Thymus | 1.61E-11 | 1.91E-11 | 1.67E-10 | 1.85E-10 | 1.06E-10 | 1.18E-10 |
| Ht-wall | 2.79E-11 | 4.27E-11 | 3.05E-10 | 4.07E-10 | 1.93E-10 | 2.58E-10 |
| Adrenals | 2.95E-11 | 3.40E-11 | 3.60E-10 | 4.15E-10 | 2.27E-10 | 2.61E-10 |
| Liver | 2.89E-11 | 3.81E-11 | 3.45E-10 | 4.52E-10 | 2.18E-10 | 2.85E-10 |
| Pancreas | 2.99E-11 | 3.62E-11 | 3.60E-10 | 4.35E-10 | 2.27E-10 | 2.74E-10 |
| Kidneys | 2.45E-10 | 2.89E-10 | 3.24E-09 | 3.83E-09 | 2.05E-09 | 2.42E-09 |
| Spleen | 4.70E-11 | 5.75E-11 | 5.62E-10 | 6.88E-10 | 3.55E-10 | 4.34E-10 |
| GB-wall | 1.33E-11 | 1.45E-11 | 1.62E-10 | 1.78E-10 | 1.03E-10 | 1.12E-10 |
| Ureters | 5.62E-12 | 1.43E-11 | 6.66E-11 | 1.76E-10 | 4.21E-11 | 1.11E-10 |
| UB-wall | 1.07E-11 | 1.30E-11 | 1.43E-10 | 1.73E-10 | 9.02E-11 | 1.09E-10 |
| Ovaries | 0.00E+00 | 3.02E-11 | 0.00E+00 | 3.68E-10 | 0.00E+00 | 2.32E-10 |
| Testes | 1.48E-11 | 0.00E+00 | 1.82E-10 | 0.00E+00 | 1.15E-10 | 0.00E+00 |
| Prostate | 1.31E-11 | 0.00E+00 | 1.61E-10 | 0.00E+00 | 1.02E-10 | 0.00E+00 |
| Uterus | 0.00E+00 | 5.31E-12 | 0.00E+00 | 6.15E-11 | 5.97E-11 | 3.89E-11 |
| LN-Sys | 1.47E-11 | 1.80E-11 | 1.57E-10 | 1.92E-10 | 9.96E-11 | 1.21E-10 |
| Skin | 1.43E-11 | 1.77E-11 | 1.67E-10 | 2.15E-10 | 1.06E-10 | 1.36E-10 |
| Adipose | 1.14E-11 | 1.43E-11 | 1.24E-10 | 1.52E-10 | 7.91E-11 | 9.69E-11 |
| Muscle | 1.12E-11 | 1.37E-11 | 1.37E-10 | 1.66E-10 | 8.64E-11 | 1.05E-10 |
aO-mucosa = oral mucosa, St-stem = stomach, RC-stem = right colon, RC-stem = rectosigmoid colon, ET2-bas = ET2 basal cells, Bronch-bas = bronchi basal cells, Bchiol-sec = bronchiolar secretory cells, LN-Sys = lymph nodes thoracic, Endost-BS = endosteal cells, eye-lens = lens of eye, S-glands = salivary glands, Ht-wall = heart wall, GB-wall = gall bladder, UB-wall = urinary bladder.
Table A2.
Committed equivalent dose coefficients (Sv.Bq−1) for target region after inhalation 211Bi as decay product of actinon (reference adult male and female). f(rT,T)hM(rT) and f(rT,T)hF(rT) are committed equivalent dose coefficients for adult Male and Female respectively.
| Target Tissue | Unattached | Nucleation | Accumulation | |||
|---|---|---|---|---|---|---|
| f(rT,T)hF(rT) | f(rT,T)Mh(rT) | f(rT,T)Fh(rT) | f(rT,T)Mh(rT) | f(rT,T)Fh(rT) | f(rT,T)Mh(rT) | |
| O-mucosa | 3.96E-14 | 3.68E-14 | 8.19E-14 | 7.66E-14 | 5.31E-14 | 4.96E-14 |
| Oesophagus | 1.51E-13 | 1.32E-13 | 2.46E-13 | 2.13E-13 | 1.81E-13 | 1.58E-13 |
| St-stem | 1.39E-13 | 1.20E-13 | 2.89E-13 | 2.49E-13 | 1.87E-13 | 1.61E-13 |
| SI-stem | 1.61E-13 | 1.09E-13 | 3.40E-13 | 2.32E-13 | 2.18E-13 | 1.49E-13 |
| RC-stem | 4.74E-14 | 5.36E-14 | 1.00E-13 | 1.13E-13 | 6.43E-14 | 7.27E-14 |
| LC-stem | 5.38E-14 | 4.77E-14 | 1.14E-13 | 1.01E-13 | 7.31E-14 | 6.48E-14 |
| RS-stem | 2.69E-14 | 2.09E-14 | 5.70E-14 | 4.43E-14 | 3.65E-14 | 2.84E-14 |
| ET1-bas | 6.02E-09 | 5.20E-09 | 1.06E-09 | 9.20E-10 | 4.43E-09 | 3.83E-09 |
| ET2-bas | 3.09E-08 | 2.67E-08 | 5.50E-09 | 4.75E-09 | 2.27E-08 | 1.96E-08 |
| LN-ET | 2.42E-15 | 2.81E-15 | 4.59E-15 | 3.82E-15 | 3.12E-15 | 3.13E-15 |
| Bronch-bas | 4.84E-09 | 4.34E-09 | 1.36E-09 | 1.22E-09 | 1.31E-09 | 1.18E-09 |
| Bronch-sec | 2.03E-08 | 1.82E-08 | 5.68E-09 | 5.11E-09 | 5.49E-09 | 4.94E-09 |
| Bchiol-sec | 1.41E-08 | 1.36E-08 | 2.19E-08 | 2.11E-08 | 7.18E-09 | 6.91E-09 |
| AI | 8.98E-12 | 7.34E-12 | 9.93E-10 | 8.12E-10 | 4.82E-10 | 3.94E-10 |
| LN-Th | 2.96E-15 | 1.91E-15 | 5.11E-15 | 3.79E-15 | 3.37E-15 | 2.44E-15 |
| R-marrow | 9.31E-14 | 7.37E-14 | 1.97E-13 | 1.56E-13 | 1.26E-13 | 1.00E-13 |
| Endost-BS | 4.57E-14 | 3.62E-14 | 9.67E-14 | 7.66E-14 | 6.21E-14 | 4.92E-14 |
| Brain | 4.09E-14 | 3.51E-14 | 8.67E-14 | 7.43E-14 | 5.56E-14 | 4.77E-14 |
| Eye-lens | 2.38E-14 | 2.00E-14 | 5.04E-14 | 4.22E-14 | 3.23E-14 | 2.71E-14 |
| P-gland | 3.79E-14 | 1.82E-14 | 8.01E-14 | 3.53E-14 | 5.14E-14 | 2.37E-14 |
| Tongue | 3.82E-14 | 5.43E-14 | 8.02E-14 | 1.14E-13 | 5.17E-14 | 7.35E-14 |
| Tonsils | 3.79E-14 | 1.66E-14 | 8.01E-14 | 3.50E-14 | 5.14E-14 | 2.25E-14 |
| S-glands | 3.78E-14 | 3.52E-14 | 8.01E-14 | 7.46E-14 | 5.14E-14 | 4.79E-14 |
| Thyroid | 8.33E-14 | 6.92E-14 | 1.76E-13 | 1.46E-13 | 1.13E-13 | 9.39E-14 |
| Breast | 1.52E-14 | 3.53E-14 | 3.21E-14 | 7.46E-14 | 2.06E-14 | 4.79E-14 |
| Thymus | 3.82E-14 | 3.55E-14 | 8.08E-14 | 7.50E-14 | 5.18E-14 | 4.81E-14 |
| Ht-wall | 9.08E-14 | 6.97E-14 | 1.92E-13 | 1.48E-13 | 1.23E-13 | 9.47E-14 |
| Adrenals | 9.86E-14 | 8.61E-14 | 2.09E-13 | 1.82E-13 | 1.34E-13 | 1.17E-13 |
| Liver | 1.12E-13 | 8.58E-14 | 2.37E-13 | 1.82E-13 | 1.52E-13 | 1.17E-13 |
| Pancreas | 1.04E-13 | 8.61E-14 | 2.20E-13 | 1.82E-13 | 1.41E-13 | 1.17E-13 |
| Kidneys | 1.44E-13 | 1.22E-13 | 3.05E-13 | 2.57E-13 | 1.95E-13 | 1.65E-13 |
| Spleen | 1.68E-13 | 1.37E-13 | 3.55E-13 | 2.91E-13 | 2.28E-13 | 1.87E-13 |
| GB-wall | 3.80E-14 | 3.54E-14 | 8.04E-14 | 7.49E-14 | 5.16E-14 | 4.81E-14 |
| Ureters | 3.78E-14 | 1.66E-14 | 8.01E-14 | 3.50E-14 | 5.14E-14 | 2.25E-14 |
| UB-wall | 3.58E-14 | 2.95E-14 | 7.57E-14 | 6.24E-14 | 4.86E-14 | 4.00E-14 |
| Ovaries | 8.62E-14 | 0.00E+00 | 1.83E-13 | 0.00E+00 | 1.17E-13 | 0.00E+00 |
| Testes | 0.00E+00 | 4.05E-14 | 0.00E+00 | 8.57E-14 | 0.00E+00 | 5.50E-14 |
| Prostate | 0.00E+00 | 3.53E-14 | 0.00E+00 | 7.46E-14 | 0.00E+00 | 4.79E-14 |
| Uterus | 1.52E-14 | 0.00E+00 | 3.21E-14 | 0.00E+00 | 2.06E-14 | 0.00E+00 |
| LN-Sys | 4.16E-14 | 3.39E-14 | 8.80E-14 | 7.17E-14 | 5.65E-14 | 4.60E-14 |
| Skin | 4.77E-14 | 3.66E-14 | 1.01E-13 | 7.73E-14 | 6.47E-14 | 4.96E-14 |
| Adipose | 3.15E-14 | 2.55E-14 | 6.64E-14 | 5.39E-14 | 4.27E-14 | 3.46E-14 |
| Muscle | 3.51E-14 | 2.90E-14 | 7.43E-14 | 6.13E-14 | 4.77E-14 | 3.93E-14 |
O-mucosa = oral mucosa, St-stem = stomach, RC-stem = right colon, RC-stem = rectosigmoid colon, ET2-bas = ET2 basal cells, Bronch-bas = bronchi basal cells, Bchiol-sec = bronchiolar secretory cells, LN-Sys = lymph nodes thoracic, Endost-BS = endosteal cells, Eye-lens = lens of eye, RS-stem = rectosigmoid colon, S-glands = salivary glands, Ht-wall = heart wall, GB-wall = gall bladder, UB-wall = urinary bladder.
Contributor Information
Hamadou Issa, Nuclear Physics Laboratory. Faculty of Science. University of Yaoundé I. P.O. Box 812 Yaoundé. Cameroon.
Atangana Bingana Martin Serge, Nuclear Physics Laboratory. Faculty of Science. University of Yaoundé I. P.O. Box 812 Yaoundé. Cameroon.
Saïdou, Nuclear Physics Laboratory. Faculty of Science. University of Yaoundé I. P.O. Box 812 Yaoundé. Cameroon; Nuclear Technology Section. Institute of Geological and Mining Research. P.O. Box 4110 Yaoundé. Cameroon.
References
- 1. ICRP . Occupational intakes of radionuclides: Part 3. ICRP publication 137. Ann ICRP 2017;46:46. [DOI] [PubMed] [Google Scholar]
- 2. Chu S-Y-F. Ekström L-P. Firestone R-B. WWW Table of Radioactive Isotopes. database version 2-28-1999 from http://nucleardata.nuclear.lu.se/nucleardata/toi/
- 3. ICRU . Measurement and reporting of radon exposures. ICRU report 88. Ann ICRU 2012;12:1. [DOI] [PubMed] [Google Scholar]
- 4. Paker C, Nilsson S, Heinrich D et al. Alpha emitter Radium-223 and survival in metastatic prostate. N Engl J Med 2013;369:213–23. [DOI] [PubMed] [Google Scholar]
- 5. Hosono M, Ikebuchi H, Nakumura Y et al. Introduction of the targeted alpha therapy (with radium-223) into clinical practice in Japan; learnings and implementation. Ann Nucl Med 2019;33:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ICRP . Human respiratory tract model for radiological protection. ICRP publication 66. Ann ICRP 1994;24:1. [PubMed] [Google Scholar]
- 7. ICRP . Occupational intakes of radionuclides: Part 1. ICRP publication 130. Ann ICRP 2015;44:1. [DOI] [PubMed] [Google Scholar]
- 8. ICRP . The ICRP computational framework for the internal dose assessment for reference adults: Specific absorbed fractions. ICRP publication 133. Ann ICRP 2016;45:1. [DOI] [PubMed] [Google Scholar]
- 9. ICRP . Human alimentary tract model ICRP publication 100. Ann ICRP 2006;36. [DOI] [PubMed] [Google Scholar]
- 10. ICRP . Age-dependent doses to members of the public from intake of radionuclides: Part 4 inhalation dose coefficients. ICRP publication 71. Ann ICRP 1995;25:1. [PubMed] [Google Scholar]
- 11. Sanchez G. Biokmod: A mathematica toolbox for modelling biokinetic systems. Math Educ Res 2005;10:184. [Google Scholar]
- 12. Montse M, Guillermo S-L, Maria A-L. Internal dosimetry tool for the implementation and use of new ICRP/OIR models: A caesium study. Radiat Prot Dosimetry 2020;1–9. [DOI] [PubMed] [Google Scholar]
- 13. Wolfram Research. Inc . Mathematica. Version 10.3. Champaign. IL: Wolfram Research. Inc, 2015. [Google Scholar]
- 14. Guillermo S-L, Jesus L-F. Mathematical techniques for solving analytically large compartmental systems. Health Phys 2003;85:184–93. [DOI] [PubMed] [Google Scholar]
- 15. Wei B-L. Internal dosimetry- a review of progress. Jpn J Health Phys 2018;53:72–99. [Google Scholar]
- 16. ICRP . Nuclear decay data for Dosimetric calculations. ICRP publication 107. Ann ICRP 2008;38:1. [DOI] [PubMed] [Google Scholar]
- 17. ICRP . The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP 2007;37:1. [DOI] [PubMed] [Google Scholar]
- 18. Stabin M-G, Siegel J-A. Radiation dose and hazard assessment of potential contamination events during use of 223Ra dichloride in radionuclide therapy. Health Phys 2015;109:212–7. [DOI] [PubMed] [Google Scholar]


