Abstract
The SARS Covid-19 pneumonia became a pandemic in 2019 affecting millions worldwide and carried a significant high mortality rate. The common presentation of this novel virus is upper and lower respiratory tract infection. However, its popularity as neuropathogen has increased dramatically. Patient presents a wide range of symptoms. We report a case of Covid-19 encephalitis which was incidentally found to have cerebral venous sinus thrombosis, presented with acute delirium and then developed new onset seizures.
Keywords: COVID encephalitis, Seizures, CVST
1. Introduction
Coronavirus disease 2019 (COVID-19) is a disease with a significantly broad spectrum of presentation and clinical syndromes. This novel infectious disease has been associated with acute respiratory distress syndrome (ARDS), thromboembolic syndrome, severe metabolic syndromes, severe acute tubular necrosis, electrolyte abnormalities, neurologic syndromes and cardiac events, including myocarditis and arrhythmias.1, 2, 3 COVID-19 possesses neuroinvasive potentials, which makes the central nervous system (CNS) an important target.4
Cerebral venous sinus thrombosis (CVST) is an uncommon condition and can occur as a result of various genetic or acquired risk factors. Infections are one of the most important etiological factors in development of cerebral venous sinus thrombosis. Neurological manifestation of COVID-19, encephalitis and thrombotic event CVST have been progressively appreciated.
2. Case report
A 30 years old gentleman presented with history of acute delirium and on arrival developed generalized tonic-clonic seizures. He had history of body aches and fever one week prior to presentation. There was no history of hypertension, diabetes mellitus or drug intoxication.
His presenting GCS was 12/15 (E3, M5, V3). On examination he was afebrile, there were no signs of meningeal irritation. There was no focal deficit and cranial nerve examination was also unremarkable.
The initial laboratory testing including hematology, biochemistry and coagulation profile was normal. CT-Brain without contrast was unremarkable. As patient had history of prior fever and body aches, COVID-PCR was sent immediately (reporting takes 2–3 days). Lumbar puncture was performed and management started on lines of viral encephalitis i.e. IV fluids and antiepileptic were given along with IV acyclovir which was started empirically. Cerebral spinal fluid (CSF) showed WBC 19, mainly lymphocytic 95%, protein 92.8 (<45), Glucose 64.2 (40–70) BSR 110 mg/dL. CSF for gram staining and AFB staining was negative and no growth was obtained on culture. Patient’s GCS improved to 15/15 and seizures were controlled within 48 h after the initiation of therapy.
Meanwhile MRI-brain with contrast was done (on 4th day of admission) which showed partial thrombi of superior sagittal sinus, vein of Galen, bilateral transverse sinus and left proximal sigmoid sinus, sequel of COVID-19. (Fig. 1, Fig. 2 ). He was immediately put on oral anticoagulation, Rivaroxiban 15 mg in morning and evening dose for 21 days followed by 20 mg for 3 months. Before starting anticoagulation thrombophilia, workup was sent. Thrombophilia work up including Lupus anticoagulation, Factor –V Leiden, Anti thrombin III, Protein C and S stands negative (Table 2 ). Later his COVID-PCR nasal swab came out positive and PCR for HSV I and II was not detected in CSF sample.
Fig. 1.
Comparison of T1 with and without contrast images showing bilateral transverse filling defect.
Fig. 2.
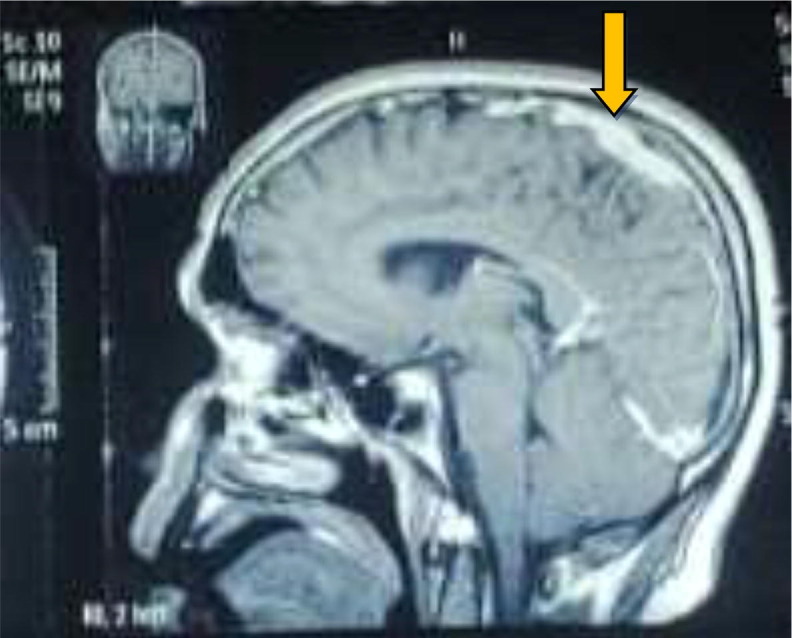
Sagital T1 with contrast showing thrombosis in superior sagittal sinus.
Table 2.
Thrombophilia screening tests.
| Tests | Patient’s results | Normal value |
|---|---|---|
| LUPUS anticoagulant (LA1) | 46 | 35–53 s |
| Antithrombin-III | 80 | 75–125% activity |
| Factor V Leiden | 1.1 | >0.80 |
| ProC Normalise Ratio | 1.5 | >0.80 |
| PCAT | 141.0 | 85–200 s |
| PCAT/O | 43.0 | 35–55 s |
| ANTI Cardiolipin Antibodies IgG | 2 | <10 GPL-U/mL |
| Anti Cardiolipin Antibodies IgM | 1 | <7 GPL-U/mL |
3. Discussion
With the progression of COVID-19, reports of neurological manifestations are increasing. These manifestations can be associated as direct effects of the virus on the nervous system, para-infectious or post-infectious immune-mediated disease, and neurological complications of the systemic effects of COVID-19. Over a 3-weeks period,5 39 (31%) patients had altered mental status, which included 16 (13%) with encephalopathy (of whom seven [6%] had encephalitis), and 23 (18%) with a neuropsychiatric diagnosis, including ten (8%) with psychosis, six (5%) with neurocognitive (dementia-like) syndrome, and four (3%) with an affective disorder. Notably, 77 (62%) patients had a cerebrovascular event: 57 (46%) ischemic strokes, nine (7%) intracerebral hemorrhages, and one (<1%) CNS vasculitis, and ten (8%) other cerebrovascular events are seen in one registry.5
The main concern for SARS-CoV-2 infection concern the routes of entry into the nervous system and the relative contribution of viral infection versus host response to the subsequent damage as with other neurotropic viruses.
One possible mechanism is viral entry to the brain through the olfactory bulb—the only part of the CNS not protected by Dura—could be one conceivable route for SARS-CoV-2, especially given the anosmia in COVID-19.6 This entry route is thought to be used by the herpes simplex virus, the most common cause of sporadic viral encephalitis.7
Alternative entry routes include carriage across the blood–brain barrier, following viraemia, or through infected leukocytes.8 The angiotensin converting enzyme 2 receptor, to which SARS-CoV-2 binds for entry into cells,9 is found in brain vascular endothelium and smooth muscle.10 SARS-CoV-2 replicates in neuronal cells in vitro.11 One proposed criterion for diagnosis of encephalitis is given in (Table 1) according to which our patient falls in probable criteria.
Table 1.
Sars-COV-2 meningitis, encephalitis, myelitis, or CNS vasculitis.18
| Confirmed: |
| 1) SARS-CoV-2 detected in CSF or brain tissue † or evidence of SARS-CoV-2-specific intrathecal antibody; and |
| 2) no other explanatory pathogen or cause found |
| Probable: |
| 1) SARS-CoV-2 detected in respiratory or other non-CNS sample,‡ or evidence of SARS-CoV-2-specific antibody in serum indicating acute infection;§ and |
| 2) no other explanatory pathogen or cause found |
| Possible: |
| Patient meets suspected case definition of COVID-19 according to national or WHO guidance based on clinical symptoms and epidemiological risk factors; in the context of known community SARS-CoV-2 transmission, supportive features include the following: |
| 1) the new onset of either cough, fever, muscle aches, loss of smell or loss of taste |
| 2) lymphopenia or raised D-dimer level |
| 3) and radiological evidence of abnormalities consistent with infection or inflammation(ground glass changes) |
| †Detection in CSF or brain tissue by PCR, culture, or immunohistochemistry, as appropriate.‡ Detection in non-CNS sample by PCR or culture.§Serological evidence of acute infection can be defined as detection of IgM, IgG seroconversion, or an increase of four times in antibody titers in paired acute and convalescent serum samples. |
The treatment of COVID-19-related encephalitis is mainly supportive. A variety of treatments, including high-dose IV steroids, IV immunoglobulin, and immunomodulators (e.g., rituximab), have been tried in various cases, with somewhat limited outcomes.17
CVST is an uncommon condition. There are several cases reported in literature of CVST and COVID 19.
It has several risk factors including genetic and acquired. CVST has a favorable prognosis if diagnosed and treated early. SARS-CoV-2 is known to produce a thrombophilic state. Early indicators suggest that cerebrovascular disease in COVID-19 might be due to a coagulopathy. SARS-CoV-2 can cause damage to endothelial cells, activating inflammatory and thrombotic pathways.9 Endothelial cell infection or monocyte activation, up regulation of tissue factors, and the release of micro particles, which activate the thrombotic pathway and cause microangiopathy, might occur for SARS-CoV-2 as for other viruses.12, 13 Monocyte activation is postulated to constitute part of the secondary haem phagocytic lymphohistiocytosis described in severe COVID-19.14 Thrombocytopenia with elevated D-dimer and C-reactive protein in severe COVID-19 and stroke are consistent with a virus-associated micro angiopathic process.15 Endothelial dysfunction can potentially lead to microvascular and macrovascular complications in the brain, as described systemically.16
Our patient presented with neurological symptoms rather than with usual respiratory symptoms. He had a history of self-limited symptoms of fever and cough 1 week earlier. He responded to the initial empirical treatment of encephalitis and later was incidentally found to have CVST on further workup. CVST has either occurred as a direct effect of COVID-19 infection or has occurred secondary to COVID-19 encephalitis. To date, no case report has shown occurrence of CVST secondary to COVID-19 encephalitis which makes our case report unique.
Nevertheless, in the COVID-19 pandemic era, a high index of suspicion of encephalitis and CVST should be considered, particularly in patients with headache, acute delirium, seizures, focal deficit; whether they present with respiratory symptoms of SARS-CoV-2 infection or not.
4. Conclusion
It is not inaccurate to express that COVID-19 has the wide range of central and peripheral nervous system associations, although primarily it is known to involve respiratory system; that brings attention to our case report.
Encephalitis is more frequently seen in viral infections. However, hypercoagulable states and cerebrovascular disease, which have been seen rarely for some acute viral infections, either as part of encephalitis or direct association with virus are an important neurological complication of COVID-19.
Majority of neurological complications, particularly encephalitis and stroke, has long term effects. It has significantly increased lifelong patient morbidity as well as burden on health care system so prompt diagnosis and management can reduce patient’s lifelong disability to a great extent.
Ethical statement
It is hereby stated that nothing has been found in this case report which against the International Ethical Guidelines for Biomedical Research involving human subjects. Any change in the protocol be notified to the committee prior to approval. All the informed consents should be retained for future reference.
Consent for publication
Patient consented for the study and publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Liu K., Chen Y., Lin R., Han K. Clinical features of Covid-19 in elderly patient: a comparison with young and middle age patients. J Infect. 2020;80:14–18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y., Xu X., Chen Z., et al. Central nerveous system involvement after the infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steardo L., Steardo L., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020;229:e13473. doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varatharaj A,Thomas N, Ellul M et al; UK-wide surveillance of neurological and neuropsychiatric complications of COVID-19: the first 153 patients. SSRN. 2020. [DOI] [PMC free article] [PubMed]
- 6.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Perlman SSevere acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon T. Encephalitis, and infectious encephalopathies.in: Donaghy M Brain's diseases of the nervous system. 12th ed. Oxford University Press, Oxford, 2009.
- 8.Desforges M ,Le Coupanec A ,Dubeau P ,et al.Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019; 12: 12. [DOI] [PMC free article] [PubMed]
- 9.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004; 203: 631-637. [DOI] [PMC free article] [PubMed]
- 11.Chu H, Chan JF-W, Yuen TT-T, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020. [DOI] [PMC free article] [PubMed]
- 12.Lopes da Silva R. Viral-associated thrombotic microangiopathies. Hematol Oncol Stem Cell Ther. 2011;4:51–59. doi: 10.5144/1658-3876.2011.51. [DOI] [PubMed] [Google Scholar]
- 13.Brisse E, Wouters CH, Andrei G, Matthys P, How viruses contribute to the pathogenesis of hemophagocytic lymphohistiocytosis. Front Immunol. 2017; 8;1102. [DOI] [PMC free article] [PubMed]
- 14.Mehta P ,McAuley DF ,Brown M ,Sanchez E ,Tattersall RS ,Manson JJ;COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395: 1033-1034 [DOI] [PMC free article] [PubMed]
- 15.Li Y ,Wang M ,Zhou Y ,et al, Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. [DOI] [PMC free article] [PubMed]
- 16.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh R., Dubey S., Finsterer J., Chatterjee S., Ray B.K. SARS-CoV-2 associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44-year old woman without comorbidities: a case report. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020. [DOI] [PMC free article] [PubMed]



