Abstract
Aims
This meta-analysis aims to analyze the association of calcium channel blocker (CCB) use with COVID-19 clinical outcomes.
Methods
PubMed, ProQuest, Science Direct, Scopus, and medRxiv databases were searched systematically in a limited period. The primary outcome was mortality.
Results
A total of 119,298 patients from 31 eligible studies were included. Pooled analysis of the random-effect model revealed CCB was not associated with reduced mortality (OR = 1.21 [95%CI: 0.98–1.49], p = 0.08). Interestingly, subgroup analysis in hypertensive patients revealed significantly reduced mortality (OR = 0.69 [95%CI: 0.52–0.91], p = 0.009).
Conclusion
CCB usage was not associated with the outcome of COVID-19. However, CCB was associated with a decreased mortality rate in hypertensive COVID-19 patients.
Keywords: COVID-19, Calcium channel blocker, Hypertension, Severity, Mortality
1. Introduction
COVID-19 is an emerging infectious disease and currently causes multisectoral problems worldwide. The first case of COVID-19 was reported in December 2019 in Wuhan, China, and has spread rapidly since. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is confirmed as the cause of COVID-19. This virus is relatively identical to severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), which also utilizes the angiotensin-converting enzyme-2 (ACE-2) receptor for host cell entry [1].
ACE-2 receptor is found to be higher in hypertensive patients treated with renin-angiotensin inhibitors [2]. Hence, it is plausible that hypertension (HTN) is the most common morbidity in COVID-19 patients [3]. Based on current guidelines, there are five major antihypertensive drug classes: angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), beta-blockers, calcium channel blockers (CCB), and diuretics [4]. CCBs are one of the most prescribed antihypertensive drugs and act by blocking calcium influx into vascular muscle cells [5].
Previous studies revealed SARS-CoV and MERS-CoV viral entry through their Spike (S) proteins is calcium-dependent [6,7]. Reduction of intracellular and/or extracellular calcium suppresses SARS-CoV and MERS-CoV entry. A recent in vitro study of SARS-CoV-2 demonstrated Nifedipine and Felodipine inhibit epithelial lung cell infection [8]. Another study of 77 COVID-19 patients showed Nifedipine and Amlodipine improve pulmonary blood flow and reduce hypoxia, thus reducing severity and mortality rate [9]. Therefore, CCBs hold promising potential for COVID-19 outcomes, especially those with HTN. This meta-analysis aims to analyze the association of calcium channel blockers usage towards COVID-19 clinical outcomes.
2. Methods
2.1. Study design
We reported this study following the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). Our study has been registered in UMIN Clinical Trials Registry (UMIN000042076).
2.2. Patient and public involvement
No patients or the public were involved in this study.
2.3. Database and literature search strategies
We selected all observational studies or trials involving adult patients with COVID-19 that had any data regarding the use of CCB for comparison groups of primary and secondary outcomes. We excluded any study that had missing required data and not in English literature. A systematic search of the published literature was conducted in a limited period (January 1st – October 15th, 2020). Five different databases (PubMed, MedRxiv, ProQuest, Science Direct, Scopus) were used to perform a systematic search using the keywords “COVID-19″, “coronavirus 2019″, “2019-nCoV”, “SARS-CoV-2″, “antihypertensive”, “calcium channel blocker blocker”, “severity”, “death”, “mechanical ventilation”, and “intensive” in the title, abstract, and medical subject heading (MeSH). Reference lists of the included studies were also screened to identify additional relevant studies.
2.4. Data extraction
Three investigators independently screened and assessed titles and abstracts before full-text retrieval. The full papers that potentially met the inclusion and exclusion criteria were reviewed by the two authors for final inclusion. Subsequently, three investigators extracted the data, including authors, year of publication, location, study design, sex, age, peer-reviewed publication status, severity criteria, type of CCB, use of CCB in each comparison group, and main and additional outcomes measures. All extracted data were recorded with a dedicated form on an Excel spreadsheet.
2.5. Outcome
The primary outcome of our meta-analysis was mortality. The secondary outcomes were severity, admission for intensive care unit (ICU), and mechanical ventilation (MV) usage. We define disease severity criteria based on the World Health Organization (WHO) and the National Health Commission of the People's Republic of China [10]. If the study categorized severity into 3 or 4 groups, we combined the data between mild and moderate groups into one group as non-severe; severe and critical groups into one group as severe.
2.6. Quality assessment and small-study effects
Two authors independently assessed the methodological quality assessment of included studies using the Newcastle-Ottawa Scale (NOS) for non-randomized studies. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology was used to assess the quality of the body of retrieved evidence (GRADEpro Guideline Development Tool [Software]. McMaster University, 2020). Funnel plots were used for the assessment of the symmetrical distribution of the effect size of outcomes. In addition, a regression-based Harbord's test was used to assess small study effects for binary endpoints [11].
2.7. Data analysis
Mantel-Haenszel formula was used for dichotomous variables to calculate the pooled odds ratios (ORs). We used the random-effect model if there was a presence of heterogeneity using the I2 test. I2>50% were considered high. Otherwise, the fixed-effects Mantel–Haenszel model was used. We performed a subgroup analysis based on HTN status, CCB monotherapy or combination therapy, and type of CCB. Sensitivity analysis was done using the leave-one-out method to assess the cause of heterogeneity. Mean and standard deviation were extrapolated from the sample size, median, and interquartile range (IQR), according to Wan et al. [12] The average of the mean and standard deviation between the two groups was calculated using the formula in Table 7.7.a of the Cochrane Handbook [13]. Restricted maximum likelihood random-effects meta-regression was performed for age, sex, cardiovascular disease (CVD), HTN, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), chronic kidney disease (CKD), and smoking status to assess the influence of these covariates. All analyses were performed using Revman v.5.4 and Stata v.16. All p values less than 0.05 in this meta-analysis were statistically significant (except for heterogeneity using p < 0.10).
3. Results
3.1. Baseline characteristics and study selection
Initial search results in 900 records from the PUBMED, Science Direct, ProQuest, Scopus, and Medxriv databases, as shown in Fig. 1 . Twenty-four additional records were acquired from other sources. After duplicate removal, 855 records remained. Title and abstracts were then screened, and a total of 784 records were removed. 71 full texts were then assessed for eligibility, and 36 articles were excluded due to incorrect patient population (n = 8); unavailability of data on CCB use (n = 13); no outcome of interest (n = 15); the outcome was composite of ICU, MV, and death (n = 1); and irrelevant severity criteria (n = 3). Finally, we included 31 eligible studies (119,298 patients) for analysis.
Fig. 1.
Study flow chart (as per PRISMA guideline).
The included studies' baseline characteristics are presented in Table 1, Table 2 . Twenty-seven studies were retrospective, and four studies were prospective observational. Twenty-two studies have already been undergone peer-review [9,[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. Most studies were conducted in China and Italy. Most studies adapted severity criteria based on the National Health Commission of the people's Republic of China. In addition, study that mention or specify the type or administration of CCB is scarce.
Table 1.
Characteristics of included studies.
| No | Author | Study Design | Town, Country | Period | Samples (n) | Male (%) | Age (years) | HTN (%) | CVD (%) | DM (%) | CKD (%) | COPD (%) | Smoking (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Li et al., 2020 [14] |
Retrospective observational | Wuhan, China | Jan 15-Mar 15, 2020 | 362 (Mor: 77 vs 285; Sev:173 vs 189) | 52.2 (Mor: 64.9 vs 48.2; Sev: 56.1 vs 48.7) | 66 ± 10.42 (Mor: 72.83 ± 13.2 vs 64.5 ± 10.06; Sev: 69 ± 10.47 vs 63.83 ± 10.09) | 100 (100 vs 100) | 17.1 (Mor: 27.3 vs 14.4; Sev: 22.5 vs 12.2) | 35.1 (Mor: 49.4 vs 31.2; Sev: 43.9 vs 27) | 9.7 (Sev: 17.3 vs 2.6; Mor: 26 vs 5.3) | n/a | n/a |
| 2 | Liu et al., 2020 [15] |
Retrospective observational | Wuhan, China | Jan 25-Mar 15, 2020 | 157 (Sev: 75 vs 82; Mor: 6 vs 151) | n/a | n/a | 100 (n/a) | n/a | n/a | n/a | n/a | n/a |
| 3 | Liu et al., 2020 [41] |
Retrospective observational | Shenzhen, Wuhan, Beijing, China | Dec 27, 2019–Feb 29, 2020 | 78 (38 vs 40) | 55.1 (71.1 vs 40) | 65.2 ± 10.7 (68 ± 9.7 vs 62.5 ± 11.1) | 100 (100 vs 100) | n/a | n/a | n/a | n/a | n/a |
| 4 | Yan et al., 2020 [16] |
Retrospective observational | Zhejiang province, China | Jan 10-Feb 28, 2020 | 610 (128 vs 482) | 51.10 (67.2 vs 46.9) | 48.75 ± 14.19 (55.96 ± 14.34 vs 46.83 ± 13.56) | 22.5 (44.5 vs 16.6) | 2.6 (4.7 vs 2.1) | 9.8 (8.1 vs 16.4) | n/a | n/a | 9.21 (7.9 vs 15.6) |
| 5 | Schneeweis et al., 2020 [52] |
Retrospective observational | USA | Dec 1, 2019–May 30, 2020 | 17137 (102 vs 17035) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 6 | Fosbøl et al., 2020 [21] |
Retrospective observational | Danish, Denmark | Feb 22-May 4, 2020 | 4480 mor(478 vs 4002) sev (576 vs 3904) | 54.3 (n/a) | 72.6 ± 13.3 (n/a) | 100 (100 vs 100) | 16.6 (n/a) | 18.2 (n/a) | n/a | 13 (n/a) | n/a |
| 7 | Yan et al., 2020 [53] |
Retrospective observational | Hainan, China | Jan 22-Mar 13, 2020 | 168 (36 vs 132) | 48.2 (58.3 vs 45.5) | 49.67 ± 19.44 (59.77 ± 13.67 vs 47.67 ± 19.49) | 14.3 (30.6 vs 9.8) | 7.1 (16.7 vs 4.5) | 7.1 (19.4 vs 3.8) | 0.6 (2.8 vs 0) | 6.0 (11.1 vs 4.5) | n/a |
| 8 | Reilev et al., 2020 [54] |
Retrospective observational | Nationwide Denmark | Feb 27-Apr 30, 2020 | 2090 (Mor: 524 vs 1566: ICU: 300 vs 1790) | 54 (ICU: 74 vs 51) | 69 ± 17.80 (ICU: 69.33 ± 19.29) | 55 icu (57 vs 55) | 21 (ICU: 21 vs 21) | 19 (24 vs 19) | 2.9 (6.7 vs 8.9) | 22 (23 vs 19) | n/a |
| 9 | Liabeuf et al., 2020 [17] |
Retrospective observational | Amiens, France | Feb 28-Mar 30, 2020 | 268 (Comp: 116 vs 152) | 58 (Comp: 63 vs 55) | 72.67 ± 17.14 (Comp: 74 ± 17.27 vs 71 ± 17.96) | 57 (Comp: 62 vs 53) | 12 (Comp: 19 vs 7) | 18 (18 vs 18) | 7 (9 vs 6) | 10 (13 vs 7) | 20 (21 vs 18) |
| 10 | Sardu et al., 2020 [18] |
Prospective observational | Naples, Italy | n/a | 62 (ICU: 12 vs 50; MV: 26 vs 36; Mor: 9 vs 53) | 66.1 (n/a) | 58 ± 18 (n/a vs n/a) | 100 (n/a) | 33.9 (n/a) | 25.8 (n/a) | n/a | 16.1 (n/a) | 11.2 (n/a) |
| 11 | Solaimanzadeh et al., 2020 [9] |
Retrospective observational | New York, USA | Feb 27-Apr 13, 2020 | 65 (Mor: 47 vs 18; MV:17 vs 48) | 49.23 (n/a) | 76.02 ± 17.52 (n/a) | 86.15 (n/a) | CHF: 9.23 (n/a) | 58.5 (n/a) | n/a | 23.1 (n/a) | n/a |
| 12 | Zeng et al., 2020 [55] |
Retrospective observational | Wuhan, China | Jan 27- Mar 8, 2020 | 1031 (165 vs 866) | 52.2 (72.8 vs 48.3) | 60.3 ± 14.3 (68.4 ± 12.0 vs 58.7 ± 14.2) | 37.2 (46.6 vs 35.4) | 8.1 (15.7 vs 6.6) | 18.3 (22.4 vs 17.5) | n/a | 3.7 (10.9 vs 2.4) | 10.1 (21.8 vs 7.9) |
| 13 | Zhang et al., 2020 [38] |
Retrospective observational | Wuhan, China | Jan 17-Mar 30, 2020 | 90 (15 vs 75) | n/a | n/a | 100 (n/a) | n/a | n/a | n/a | n/a | n/a |
| 14 | Rath et al., 2020 [22] |
Prospective observational | Tübingen, Germany | Feb–Mar 2020 | 123 (16 vs 107) | 62.6 (75 vs 60.7) | 73 ± 16 (73 ± 6 vs 67 ± 15) | 69.9 (75 vs 69.2) | n/a | 24.3 (31.3 vs 23.4) | n/a | n/a | 8 (0 vs 9) |
| 15 | Conversano et al., 2020 [23] |
Prospective observational | Milan, Italy | Feb 27-Mar 17, 2020 | 191 (42 vs 149) | 68.5 (73.8 vs 67.6) | 60.4 ± 13.7 (75.3 ± 12.9 vs 60.4 ± 13.7) | 50.2 (81 vs 42.3) | 14.6 (21.4 vs 12.8) | 14.6 (26.2 vs 11.4) | 11.4 (12.5 vs 11.2) | 5 (14.3 vs 2.7) | n/a |
| 16 | Giacomelli et al., 2020 [24] |
Retrospective observational | Milan, Italy | Feb 21-Mar 19, 2020 | 233 (48 vs 185) | 30.9 (34.1 vs 18.8) | 60.6 ± 17.64 (70.41 ± 26.55 vs 58.6 ± 17.21) | n/a | n/a | n/a | n/a | n/a | 70 (64.6 vs 70) |
| 17 | Iaccarino et al., 2020 [26] |
Retrospective observational | Italy | Mar 9-Apr 9, 2020 | 1591 (188 vs 1403) | 64 (66.5 vs 63.6) | 66.5 ± 0.4 (79.6 ± 0.8 vs 64.7 ± 0.4) | 54.9 (72.9 vs 52.5) | CAD: 13.6 (29.8 vs 11.4); HF: 11.8 (30.3 vs 9.3) | 16.9 (32.4 vs 14.8) | 5.5 (16.5 vs 4.0) | 7.7 (14.9 vs 6.7) | n/a |
| 18 | Poblador-Plou et al., 2020 [27] |
Retrospective observational | Aragon, Spain | Mar 4-May 17, 2020 | 4412 (771 vs 3641) | 41.3 (52.8 vs 38.8) | 67.7 ± 20.7 (n/a) | 34.5 (28.2 vs 71.8) | CHF: 3.8 (48.2 vs 51.8); AMI: 1.9 (42.7 vs 57.3) | 11.9 (36.4 vs 63.6) | 6.7 (39.19 vs 60.81) | 3.4 (32.4 vs 67.6) | n/a |
| 19 | Selçuk et al., 2020 [28] |
Retrospective observational | Instanbul, Turkey | n/a | 113 (35 vs 78) | 59 (62.9 vs 47.4) | 57 ± 16 (68 ± 13 vs 52 ± 14) | 100 (100 vs 100) | CAD:24.8 (40 vs 17.9); HF: 8 (14.3 vs 5.1); | 42.5 (42.9 vs 42.3) | 11.5 (17.1 vs 9.0) | 20.4 (22.9 vs 19.2) | 8 (11.4 vs 6.4) |
| 20 | Kocayigit et al., 2020 [29] |
Retrospective observational | Sakarya, Turkey | Mar 20-Apr 10, 2020 | 169 (30 vs 139) | 46.7 (50 vs 46) | 65.8 ± 11.7 (73.2 ± 10.5 vs 64.2 ± 11.4) | 100 (100 vs 100) | CAD: 14.8 (26.7 vs 12.2); HF 3.6 (6.7 vs 2.9) | 34.9 (43.3 vs 33.1) | 4.7 (10 vs 3.6) | 10.7 (13.3 vs 10.1) | n/a |
| 21 | Dashti et al., 2020 [56] | Retrospective observational | Boston, Massachusette, USA | Dec 1, 2019–Apr 18, 2020 | 1194 (ICU: 575 vs 619; Mor: 187 vs 1007) | 47.57 (ICU: 57.2 vs 38.6; Mor: 61.5 vs 53.6) | 61.68 ± 18.79 (62.00 ± 20.81 vs 61.33 ± 16.35) | 41.71 (ICU: 31.83 vs 39.08; Mor: 56.68 vs 32.83) | 23.03 (ICU: 19.65 vs 20.10; Mor: 29.41 vs 18.43) | 22.11 (ICU:18.43 vs 20.1; Mor: 29.41 vs 17.5) | 6.01 (ICU: 28.92 vs 29.32; Mor: 21.69 vs 78.31) | n/a | 48.32 ICU (39.83 vs 43.18) mortal (58.3 vs 39.20) |
| 22 | Jackson et al., 2020 [30] | Retrospective observational | Georgia, USA | Mar 1–30, 2020 | 297 (MV: 85 vs 212; Mor: 51 vs 246) | 49.8 (MV: 55.3 vs 47.6; death: 56.9 vs 48.4) | 58.00 ± 17.88 (69.67 ± 10.02 vs 58.83 ± 14.15) | 67.7 (MV: 78.8 vs 63.2; Death: 86.3 vs 63.8) | 24.9 (MV: 32.9 vs 21.7; Mor: 41.2 vs 21.5) | 39.4 (MV: 55.3 vs 33.0; Mor: 54.9 vs 36.2) | 10.44 (10.59 vs 10.38) | 17.2 (MV: 20.0 vs 16.0; Mor: 11.8 vs 18.3) | 28.6 (MV: 30.6 vs 27.8; Mor: 37.3 vs 26.8) |
| 23 | Trifirò et al., 2020 [31] | Retrospective observational | Lombardy and Veneto, Italy | up to Apr 21, 2020 | 42926 (11205 vs 31721) | 62.6 (68.4 vs 60.6) 62.6 (28.5 vs 60.56) | 68.33 ± 16.31 (n/a) | 13.1 (21.4 vs 10.1) | IHD: 10.3 (17.9 vs 7.6) | 17.9 (27.1 vs 14.7) | 2.4 (47.6 vs 52.4) | 3.5 (6.2 vs 2.6) | n/a |
| 24 | Lu et al., 2020 [32] | Retrospective observational | Wuhan and Huanggang, China | Jan 18-Feb 24, 2020 | 1138 (218 vs 920) | 49.9 (59.6 vs 47.6) | 57.33 ± 17.81(70.00 ± 12.69 vs 54.33 ± 18.56) | 32.9 (56.9 vs 27.3) | 9.3 (20.2 vs 6.7) | 15.6 (24.3 vs 13.6) | 3.3 (42.1 vs 57.9) | 6.4 (12.4 vs 5.0) | n/a |
| 25 | Genet et al., 2020 [33] | Retrospective observational | French | Mar 17-Apr 18, 2020 | 201 (66 vs 135) | 32.8 (36.4 vs 31.1) | 86.3 ± 8.0 (86.4 ± 7.6 vs 86.2 ± 8.2) | 62.2 (60.6 vs 63.0) | CAD:23.4 (19.7 vs 25.2); CHF 34.8(36.4 VS 34.1) | 19.4 (25.8 vs 16.3) | n/a | 15.4 (15.2 vs 15.6) | n/a |
| 26 | Rezel-Potts et al., 2020 [57] | Retrospective observational | UK | Jan 29-Jun 25, 2020 | 16866 (921 vs 15945) | 40.3 (50.0 vs 39.7) | n/a | n/a | n/a | n/a | n/a | n/a | 20.8 (33.0 vs 20.1) |
| 27 | Abu-Jamous et al., 2020 [35] | Retrospective observational | London, UK | Jan 1-May 27, 2020 | 1253 (325 vs 928) | n/a | n/a | 30.1 (43.24 vs 24.4) | 13 (28.6 vs 7.5) | 26.2 (31.1 vs 13.7) | 7.9 (18.15 vs 4.32) | 6.9 (13.2 vs 4.6) | n/a |
| 28 | Ferguson et al., 2020 [20] | Retrospective observational | California, USA | Mar 12-May 2, 2020 | 72 (21 vs 51) | 52.8 (61.9 vs 49.0) | 58.13 ± 20.46 (56.63 ± 22.18 vs 60.40 ± 20.06) | 36.1 (52.4 vs 29.4) | CAD: 9.7 (9.5 vs 9.8); HF 6.9: (4.8 vs 7.8) | 27.8 (47.6 vs 19.6) | n/a | 13.9 (14.3 vs 13.7) | 27.4 (31.6 vs 25.6) |
| 29 | Iaccarino et al., 2020b [25] | Retrospective observational | Italy | Mar 9-Apr 29, 2020 | 2378 (395 vs 1983) | 62.6 (73.7 vs 60.4) | 68.21 ± 0.38 (68.9 ± 0.70 vs 68.1 ± 0.43) | 58.5 (65.3 vs 57.2) | 14.3 (15.7 vs 14.1) | 18.2 (22.8 vs 17.3) | 5.5 (16.5 vs 4.0) | 8.5 (10.4 vs 8.1) | n/a |
| 30 | Hippisley-Cox et al., 2020 [34] | Prospective observational | England, Ireland, and Wales | Jan 1-Apr 27, 2020 | 19486 (1286 vs 18200) | 48.12 (73.1 vs 46.4) | 62.18 ± 20.84 (59.19 ± 12.52 vs n/a) | 38.93 (45.4 vs 38.5) | 18.23 (11.0 vs 18.7) | 20.67 (29.5 vs 20) | 4.09 (11.82 vs 17.66) | 7.3 (3.6 vs 7.6) | 36.3 (37.6 vs 38.8) |
| 31 | Higuchi et al., 2020 [19] | Retrospective observational | Osaka, Japan | Feb 20-Jun 10, 2020 | 57 (7 vs 50) | 56.14 (71.4 vs 54.0) | 52.17 ± 26.24 (63.67 ± 11.02 vs 49.43 ± 29.23) | 28.1 (42.9 vs 26) | 8.8 (0 vs 10.2) | 22.8 (28.6 vs 22) | 8.8 (14.3 vs 8) | 7 (0 vs 8) | 42.1 (71.4 vs 38) |
Data are presented as poor outcomes vs. good outcomes.
Abbreviations, AF: atrial fibrillation; AMI: acute myocardial infarction; CAD: coronary artery disease; CHF: congestive heart failure; CKD: chronic kidney disease; Comp: composite; CVD: cardiovascular disease; DM: diabetes mellitus; HF: heart failure; HTN: hypertension; ICU: intensive care unit; IHD: ischemic heart disease; Mor: mortality; MV: mechanical ventilation; n/a: not available; Sev: severity.
Table 2.
CCB characteristics, outcomes, and quality of the included studies.
| No | Author | Samples with CCB (%) | CCB administration | CCB type | CCB monotherapy/combination | LOS/follow up (days) | Outcome | Severity criteria | NOS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Li et al., 2020 [14] |
69.1 | n/a | n/a | Mono and/or comb | 19.3 ± 11.06 (Mor 17 ± 18.13 vs 19.33 ± 9.69; Sev: 21.3 ± 14.95 vs 18 ± 9.71) | Sev, Mor | COVID-19 guideline of China (5th ed) | 9 |
| 2 | Liu et al., 2020 [15] |
52.9 | n/a | n/a | Mono | n/a | Sev, Mor | Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline (7th ed) | 8 |
| 3 | Liu et al., 2020 [41] |
50 | n/a | n/a | n/a | n/a | Sev | NHC of China | 9 |
| 4 | Yan et al., 2020 [16] |
14.6 | n/a | n/a | n/a | n/a (21.22 ± 10.02 vs 19.07 ± 18.09) | Sev | NHC of China | 7 |
| 5 | Schneeweis et al., 2020 [52] |
0.7 | n/a | DHP | Mono | follow up 30 days | Sev, MV, ICU | Hospitalization for ARDS | 7 |
| 6 | Fosbøl et al., 2020 [21] |
10.9 | n/a | n/a | Mono and/or comb | follow up 30 days | Sev, Mor | ICD-10 diagnosis code B972A according to WHO criteria | 9 |
| 7 | Yan et al., 2020 [53] |
4.2 | n/a | n/a | n/a | 16.58 ± 7.98 (14.27 ± 25.55 vs 16.67 ± 6.73) | Sev | NHC of China | 8 |
| 8 | Reilev et al., 2020 [54] |
19 | n/a | n/a | n/a | follow up 30 days | Mor, ICU | – | 8 |
| 9 | Liabeuf et al., 2020 [17] |
21 | n/a | n/a | n/a | n/a | Mor, ICU | – | 9 |
| 10 | Sardu et al., 2020 [18] |
27.4 | n/a | n/a | n/a | n/a | Mor, ICU, MV | – | 7 |
| 11 | Solaimanzadeh et al., 2020 [9] |
36.9 | more than one dose | DHP (amlodipin-nifedipin) | n/a | n/a | Mor, MV | – | 7 |
| 12 | Zeng et al., 2020 [55] |
19.0 | n/a | DHP | n/a | n/a | Mor | – | 7 |
| 13 | Zhang et al., 2020 [38] |
71.1 | chronic | DHP (amlodipine, nifedipine, other) | Mono | n/a | Mor | – | 7 |
| 14 | Rath et al., 2020 [22] |
21.1 | n/a | n/a | n/a | 30 days | Mor | – | 7 |
| 15 | Conversano et al., 2020 [23] |
13.01 | n/a | n/a | n/a | 28 ± 2.53 | Mor | – | 8 |
| 16 | Giacomelli et al., 2020 [24] |
15.5 | n/a | n/a | n/a | 40 ± 3.25 (44 ± 2.50 vs 11 ± 3.77) | Mor | – | 7 |
| 17 | Iaccarino et al., 2020 [26] |
14.5 | n/a | n/a | n/a | n/a | Mor | – | 8 |
| 18 | Poblador-Plou et al., 2020 [27] |
5.4 | n/a | DHP | n/a | follow up 30 days | Mor | – | 9 |
| 19 | Selçuk et al., 2020 [28] |
30.1 | n/a | n/a | Mono and/or comb | 8.6 (10 ± 6 vs 8 ± 4) | Mor | – | 9 |
| 20 | Kocayigit et al., 2020 [29] |
40.8 | n/a | n/a | Mono and/or comb | n/a | Mor, ICU | – | 8 |
| 21 | Dashti et al., 2020 [56] | 31.9 | chronic | n/a | n/a | 9.73 ± 8.87 (ICU: 13.23 ± 10.40 vs 6.47 ± 5.42) | Mor, ICU | – | 8 |
| 22 | Jackson et al., 2020 [30] | 29.3 | chronic | DHP | n/a | n/a | Mor, MV | – | 8 |
| 23 | Trifirò et al., 2020 [31] | 16.6 | Chronic, 3 month prior | n/a | Mono and/or comb with ACEi/ARB | 23 ± 18.5 (n/a) | Mor, ICU | – | 8 |
| 24 | Lu et al., 2020 [32] | 11.7 | n/a | n/a | n/a | 27.67 ± 10.39 (18.00 ± 8.21 vs 29.00 ± 9.65) | Mor | – | 7 |
| 25 | Genet et al., 2020 [33] | 16.4 | Chronic, 1 week prior | n/a | n/a | 23.4 ± 10.0 (10.0 ± 6.0 vs 30) | Mor | – | 8 |
| 26 | Rezel-Potts et al., 2020 [57] | 10.5 | Chronic, 6 months | n/a | Mono | follow up 30 days | Mor | – | 9 |
| 27 | Abu-Jamous et al., 2020 [35] | 3.0 | newly administered during admission | n/a | n/a | follow up 21 days | Mor | – | 8 |
| 28 | Ferguson et al., 2020 [20] | 18.1 | n/a | n/a | n/a | 8.17 ± 7.16 (19.33 ± 14.37 vs 5.67 ± 4.58) | ICU | – | 8 |
| 29 | Iaccarino et al., 2020b [25] | 8.1 | n/a | n/a | n/a | n/a | ICU | – | 8 |
| 30 | Hippisley-Cox et al., 2020 [34] | 16.9 | Chronic, 3 or more prescription, including 90 days prior to cohort entry | n/a | Mono and/or comb with ACEi/ARB | n/a | ICU | – | 8 |
| 31 | Higuchi et al., 2020 [19] | 15.8 | n/a | n/a | n/a | 8.33 ± 5.32 (n/a) | MV | – | 8 |
Data are presented as poor outcomes vs. good outcomes. Chronic use of CCB represents medication prior to admission.
Abbreviations, ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor blocker; comb: combination therapy; DHP: dihydropyridine; ICU: intensive care unit; LOS: length of stay; mono: monotherapy; Mor: Mortality; MV: mechanical ventilation; n/a: not available; NHC: National Health Commission; NOS: Newcastle Ottawa Scale; Sev: Severity; WHO: World Health Organization.
3.2. Quality assessment and small study effects
Overall, the quality of the study showed good and fair methodology based on NOS assessment (Table 2). However, most studies did not assess exposure before measuring outcome and might not have adequate time-frames for outcome owing to their cross-sectional design.
Grading of Recommendations Assessment, Development, and Evaluation (GRADE) showed a very low certainty of the evidence for the effect of CCB on mortality, severity, ICU admission, and mechanical ventilation outcomes (Supplementary Table 1).
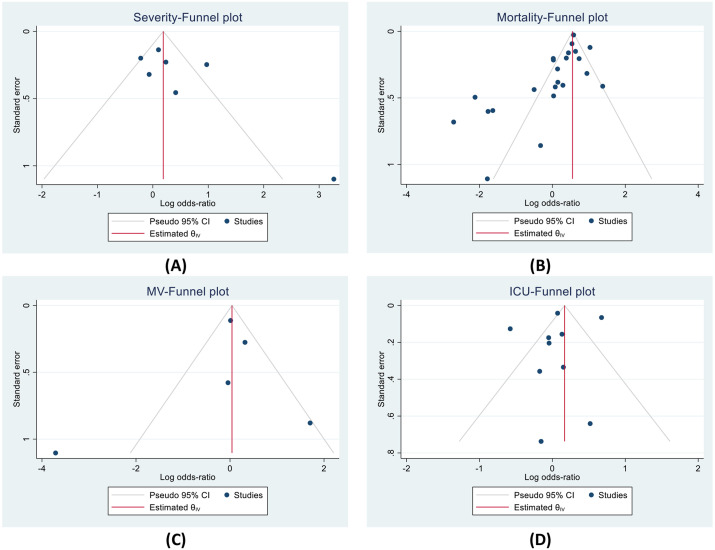
Funnel plots for severity, mortality, and MV showed a qualitatively asymmetrical appearance, but not for ICU outcome (Fig. 2 ). Regression-based Harbord's test also showed that the presence of small-study effects in mortality outcome (P < 0.001). No indication of small-study effects for ICU outcome (P: 0.879). We did not conduct Harbord's regression test of severity and MV outcome due to the lack of included studies (<10 studies).
Fig. 2.
Funnel plots indicated small study effects for (A) severity, (B) mortality, and (C) MV; but not for (D) ICU outcome. ICU: intensive care unit; MV: mechanical ventilation.
3.3. Calcium channel blocker use and mortality
A total of 23 studies described the mortality outcome in CCB use. Random-effects pooled analysis revealed that CCB use was not associated with mortality, as shown in Fig. 3 A (OR = 1.21 [95%CI: 0.98 to 1.49], p = 0.08; I2 = 84%, p < 0.001). Sensitivity analysis by removing Abu-Jamous et al. [35] showed similar result with reduced heterogeneity (OR = 1.33 [95%CI: 1.10 to 1.62], p = 0.004; I2 = 80%, p < 0.001).
Fig. 3.
Forest plot of CCB use and mortality outcome. (A) CCB use was not associated with mortality in all included studies. (B) CCB use was associated with decreased mortality in the hypertensive subgroup. CCB: calcium channel blocker.
Subgroup analysis of 10 studies in hypertensive patients revealed that CCB users had significant lower mortality rate, as shown in Fig. 3B (OR = 0.69 [95%CI: 0.52 to 0.91], p = 0.009; I2 = 64%, p = 0.005). Sensitivity analysis by removal of Abu-Jamous et al. [35] showed that heterogeneity could be reduced with a consistent result (OR = 0.78 [95%CI: 0.66 to 0.92], p = 0.003; I2 = 13%, p = 0.32).
In addition, random-effects meta-regression analysis demonstrated that the association between CCB use and decreased mortality in hypertensive patients was not significantly affected by age (p = 0.242), sex (p = 0.850), CVD (p = 0.302), DM (p = 0.459), CKD (p = 0.901), COPD (p = 0.218), and smoking (p = 0.644).
A Subgroup analysis based on use of dihydropyridine (DHP) CCB demostrated no significant different in mortality rate (OR = 0.85 [95%CI: 0.40 to 1.79], p = 0.67; I2 = 88%, p < 0.001) (Supplementary Fig. 1A). Using CCB as monotherapy was mentioned in three studies (Supplementary Fig. 1B) and exhibited no significant difference between two groups (OR = 0.45 [95%CI: 0.07 to 2.77], p = 0.39; I2 = 88%, p < 0.001). Mixed usage of CCB as monotherapy or combination therapy also showed similar result (OR = 1.33 [95%CI: 0.95 to 1.85], p = 0.39; I2 = 88%, p < 0.001) (Supplementary Fig. 1C).
3.4. Calcium channel blocker use and severity
A total of 19,603 COVID-19 patients from 7 studies were analyzed for COVID-19 severity outcome. Random-effects pooled analysis showed CCB use was not associated with severity outcome, as shown in Supplementary Fig. 2A (OR = 1.36 [95%CI: 0.92 to 2.02], p = 0.12; I2 = 74%, p < 0.001). Sensitivity analysis by removing Yan H et al. [16] showed a similar result with reduced heterogeneity (OR = 1.14 [95%CI: 0.81 to 1.60], p = 0.44; I2 = 58%, p = 0.04).
Subgroup analysis of hypertensive patients in four studies showed no significant difference in COVID-19 severity between CCB users and non-CCB users (OR = 1.05 [95%CI: 0.77 to 1.42], p = 0.78; I2 = 24%, p = 0.26) (Supplementary Fig. 2B). When the study by Yan H et al. [16] was removed, sensitivity analysis showed a similar result with lower heterogeneity (OR = 1.19 [95%CI: 0.85 to 1.67], p = 0.31; I2 = 0%, p = 0.63).
When analyzing CCB usage as monotherapy, pool analysis of two studies showed no difference between groups, as projected in Supplementary Fig. 3A (OR = 1.08 [95%CI: 0.84 to 1.38], p = 0.55; I2 = 0%, p = 0.63). Analysis of CCB usage for monotherapy and combination therapy also showed similar results (2 studies; OR = 0.99 [95%CI: 0.64 to 1.55], p = 0.98; I2 = 55%, p = 0.13) (Supplementary Fig. 3B). We did not perform subgroup analysis on DHP or non-DHP groups due to a lack of included studies with CCB type.
3.5. Calcium channel blocker use and ICU admission
The ICU admission of CCB users was analyzed from a total of 85,780 COVID-19 patients from ten studies. A pooled analysis shown in Supplementary Fig. 4A using the random-effect model showed no significant differences between CCB users and non-CCB users for ICU admission (OR = 1.05 [95%CI: 0.78 to 1.41], p = 0.75; I2 = 91%, p < 0.001). Removing of Hippisley-Cox et al. [34] showed a consistent result with heterogeneity reduction (OR = 0.94 [95%CI: 0.75 to 1.17], p = 0.56; I2 = 69%, p = 0.001).
Subsequently, when analyzing hypertensive patients in four studies, the fixed-effect pooled analysis also showed no significant differences between the two groups, as shown in Supplementary Fig. 4B (OR = 0.97 [95%CI: 0.73 to 1.28], p = 0.83; I2 = 0%, p = 0.95). Furthermore, subgroup analysis based on monotherapy or combination therapy of CCB also demonstrated no different result in a pooled analysis, as shown in Supplementary Fig. 5 (OR = 1.28 [95%CI: 0.77 to 2.14], p = 0.34; I2 = 97%, p < 0.001). Nevertheless, sensitivity analysis by removing Hippisley-Cox et al. [34] demonstrated a major reduction of heterogeneity but with a similar result (3 studies; OR = 1.07 [95%CI: 0.99 to 1.16], p = 0.10; I2 = 0%, p = 0.49). Subgroup analysis on DHP or non-DHP group was not performed due to insufficient included study.
3.6. Calcium channel blocker use and need for mechanical ventilation
A total of five studies described the need for mechanical ventilation in COVID-19 and CCB users. Random-effects pooled analysis showed there was no association between CCB usage and the need for MV, as shown in Supplementary Fig. 6 (OR = 0.97 [95%CI: 0.47 to 2.00], p = 0.94; I2 = 76%, p = 0.002). Sensitivity analysis by removing Solaimanzadeh et al. [9] showed the consistent result with reduced heterogeneity (OR = 1.19 [95%CI: 0.87 to 1.73], p = 0.37; I2 = 34%, p = 0.21).
Subgroup analysis in hypertensive patients could not be done due to a lack of the included study. Therefore a subgroup analysis was done based on DHP and non-DHP CCB. Pooled analysis revealed no significant different between groups (OR = 0.71 [95%CI: 0.29 to 1.76], p = 0.46; I2 = 84%, p = 0.002) (Supplementary Fig. 7). Removing a study by Solaimanzadeh et al. [9] also showed consistent results with reduced heterogeneity (OR = 1.06 [95%CI: 0.85 to 1.33], p = 0.59; I2 = 6%, p = 0.30).
4. Discussion
Our meta-analysis showed no significant impact of CCB usage in COVID-19 outcomes, including mortality, severity, ICU admission, and need for MV. To the authors’ knowledge, our meta-analysis of 31 studies is the first meta-analysis on the elaboration of the antihypertensive medication and COVID-19 outcomes, specifically in CCB usage. The impact remains non-significant even after conducting subgroup analysis based on HTN status, CCB type, and CCB use as monotherapy or combination therapy in each outcome. Nevertheless, CCB is beneficial for COVID-19 patients with hypertension by reducing the mortality rate. It is worthy to note that the heterogeneity of our analysis for the effect estimates was high, and the certainty of the evidence was very low due to the high risk of bias, inconsistency, and indirectness. Even though our meta-analysis demonstrated no benefit/harm in terms of primary or secondary outcomes, integrating adjustments of several confounding variables is crucial, which might result in a different conclusion.
HTN is one of the most common comorbidities in COVID-19. Patients with HTN have a higher risk of acute respiratory disease and chronic lower respiratory disease, independent of age, sex, smoking status, and BMI [36]. The previous meta-analysis also exhibited that HTN increases composite poor outcomes, composed of death, disease progression, acute respiratory distress syndrome (ARDS), and need for ICU care in patients with COVID-19 [37]. CCB as one of the most used anti-HTN worldwide and highly recommended in the guideline might also become crucial in this COVID-19 issue besides ACEi or ARB use [4].
Previous studies only provide limited and contrasting evidence for CCB use and COVID-19 clinical outcomes. A systematic review by Zaki et al. [36] mentioned that CCB are beneficial for COVID-19 patients. A clinical and in vitro study by Zhang et al. [38] showed a beneficial effect of CCB in COVID-19 patients from suppression of SARS-CoV-2 replication in cells. However, the blocking mechanism is not apparent. Therefore, further investigations of CCBs efficacy on post-entry virus replication in vitro and clinically are needed. A multicenter retrospective study showed a significant reduction of COVID-19 severity, especially in elderly patients (adjusted OR = 0.287, 95% CI: 0.114–0.723) [39]. A meta-analysis on septic patients also demonstrated that preadmission CCB use is significantly associated with the improvement of sepsis outcomes. Preadmission CCB use was associated with a significantly lower 30-day mortality in septic shock. The long-term prognosis of sepsis was also improved by preadmission use of CCB [40].
In contrast, a study by Liu et al. [41] showed a different conclusion. A comparison of severity in those who received antihypertensive agents in COVID-19 patients, such as ACEi, ARB, CCB, and beta-blockers to those who did not take any HTN medication showed no significant difference, except for ARB. However, consideration is needed since the sample size was relatively small and limited number of ARB users. While the different result was also reported in a living systematic review and meta-analysis on CVD drugs and COVID-19 outcomes conducted by Asiimwee et al. [42] Their pooled analysis showed that CCB use was associated with increased risk of hospitalization, severity, and mortality in COVID-19. However, their subgroup analysis and adjusted effect estimates showed different results, indicating a lack of statistical robustness [42]. It is suggested that CCB adverse effects might also occur in patients with underlying cardiac or metabolic disorders. Furthermore, CCB had a significantly increased risk of developing COVID-19 symptoms in hypertensive patients (OR = 1.73, 95% CI 1.2–2.3) [16].
Currently, there is still no adequate evidence that successfully explains the underlying mechanism of how CCB altering the poor outcomes of COVID-19. However, a previous case by Lodhi et al. reported that CCB might lead to acute respiratory distress syndrome by two potential mechanisms [43]. First, CCB could lead to alveolar collapse by inhibiting type II pneumocyte secretion, namely endothelin-1-stimulated surfactant [44]. Second, the vasodilatory properties that work selectively on the precapillary may cause excessive fluid accumulation in the alveolar space [45].
One important finding in our study is that CCB could decrease the mortality rate in hypertensive COVID-19 patients. The previous meta-analysis showed that HTN increases the mortality rate in COVID-19 patients and may be explained due to viral infection via ACE2 expression [37]. CCB action, however, could inhibit viral entry without interfering ACE2 expression or activity [2]. Current evidence about the protective mechanism of CCB in COVID-19 remains scarce. However, we suggest several mechanisms of CCB in reducing the mortality rate in COVID-19 patients. First, CCB blocks calcium influx, therefore inhibits viral entry. MERS-CoV and SARS-CoV utilize calcium ions to fuse in cell membranes via Spike protein [6,7]. This protein is also found in SARS-CoV-2; hence it is plausible SARS-CoV-2 also utilizes calcium for viral entry. A recent study by Straus et al. showed that dihydropyridines CCB could inhibit SARS-CoV-2 entry in lung epithelial cells [8]. Second, calcium is potentially protective in preventing multiple-organ failure development in COVID-19 patients. One study linked unsaturated fatty acids and tissue injury in COVID-19 patients; thus, calcium and albumin supplementation is recommended to bind unsaturated fatty acids [46]. Considering CCB usage may pseudo-increase serum calcium, CCB may prevent further injury and organ failure. Third, CCB could induce pulmonary smooth muscle relaxation causing pulmonary vasodilatation and improve hypoxia conditions in COVID-19 patients [9]. Finally, another study showed that nifedipine has an anti-inflammatory effect by suppressing the production of IL-1α, IL-6, and IFN-γ from peripheral blood mononuclear cells, which IL-6 and IFN-γ are known as mediators of cytokine storm in COVID-19 [47,48].
4.1. Clinical implication
Our result supports current guidelines for diagnosing and managing CVD during the COVID-19 pandemic by the European Society of Cardiology (ESC) to continue CCB medication based on existing ESC/European Society of Hypertension (ESH) guideline recommendations [49]. Moreover, we also provide evidence to the previous expert recommendation to use CCB as an alternative in COVID-19 patients with hypertension [2,50,51].
4.2. Limitations
Publication bias or small study effects was noted in several outcomes. There was also substantial heterogeneity across studies. Most included studies did not adequately report data on the administration of CCB, specific CCB type, and CCB use as monotherapy or combination therapy. Non-CCB users, which was used as a comparator was not homogenous since the non-CCB users may be composed of those who were in hypertensive medication and not. The majority of studies did not describe the status of blood pressure control in hypertensive patients. This should be addressed since uncontrolled blood pressure might affect the poor outcome. Most of the included studies in this meta-analysis were retrospective observational, with relatively small sample size, and not adequately matched/adjusted for confounders. Thus, the included studies were subject to potential confounders that may weaken or strengthen the effect estimate. The result of the meta-regression has to be interpreted cautiously due to the known limitations of such analysis. Some of the included studies were published at the preprint server. In addition, most of the studies included were from China, which ethnic and geographical differences might distort the analysis of the results.
5. Conclusion
CCB usage was not associated with the outcome of COVID-19. However, CCB usage was associated with a decreased mortality rate in COVID-19 patients with hypertension. Further prospective cohorts with methodologically analysis sound matching/adjustment or randomized controlled trials are required before a definitive conclusion can be drawn.
Ethical approval and consent to participate
Not applicable.
Availability of data and materials
All data underlying the results are available as part of the article and no additional source data are required.
Funding
No funding was received for the production of this manuscript.
Authors’ contributions
MYA conceptualization, idea, investigate, check, and revised the manuscript. EPBM conceptualization, idea, data screening and extracting, analysis, writing, and editing the manuscript. IM conceptualization, idea, investigate, check and revise the manuscript. KL screen, extract, and analyze the data, write and edit the manuscript. DN screen, extract, and analyze the data, write and edit the manuscript. DAR extract and analyze the data, write and check the manuscript. IS extract data, write, check, and edit the manuscript. MQA screen, extract, and analyze the data, write and edit the manuscript. All the authors have read and approved the final manuscript.
Trial registry
UMIN Clinical Trial Registry (UMIN000042076).
Declaration of competing interest
The authors declare no competing interest in this article.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2021.102210.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21:224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA - J Am Med Assoc. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams B., Mancia G., Spiering W., Rosei E.A., Azizi M., Burnier M., et al. ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. 2018. [DOI] [PubMed] [Google Scholar]
- 5.Wang A.L., Iadecola C., Wang G. New generations of dihydropyridines for treatment of hypertension. J Geriatr Cardiol. 2017;14:67–72. doi: 10.11909/j.issn.1671-5411.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straus M.R., Tang T., Lai A.L., Flegel A., Bidon M., Freed J.H., et al. Ca 2+ ions promote fusion of Middle East respiratory syndrome coronavirus with host cells and increase infectivity. J Virol. 2020;94 doi: 10.1128/jvi.00426-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai A.L., Millet J.K., Daniel S., Freed J.H., Whittaker G.R. The SARS-CoV fusion peptide forms an extended bipartite fusion platform that perturbs membrane order in a calcium-dependent manner. J Mol Biol. 2017;429:3875–3892. doi: 10.1016/j.jmb.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straus M.R., Bidon M., Tang T., Whittaker G.R., Daniel S. FDA approved calcium channel blockers inhibit SARS CoV 2 infectivity in epithelial lung cells. bioRxiv. 2020 doi: 10.1101/2020.07.21.214577. [DOI] [Google Scholar]
- 9.Solaimanzadeh I. Nifedipine and amlodipine are associated with improved mortality and decreased risk for intubation and mechanical ventilation in elderly patients hospitalized for COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . 2020. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19)https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) [Google Scholar]
- 11.Harbord R.M., Egger M., Sterne J.A.C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 12.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M., et al. Cochrane; 2019. Cochrane Handbook for systematic reviews of interventions.https://training.cochrane.org/handbook version 6.0 (updated July 2019) (toegang verkry 20 Mei 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X., Liu Y., Chen K., Yan S., Bai X., Li J., et al. Efficacy of ACEIs/ARBs versus CCBs on the progression of COVID-19 patients with hypertension in Wuhan: a hospital-based retrospective cohort study. J Med Virol. 2021;93:854–862. doi: 10.1002/jmv.26315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan H., Valdes A.M., Vijay A., Wang S., Liang L., Yang S., et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID-19: a large case-control study. Clin Pharmacol Ther. 2020;1–32 doi: 10.1002/cpt.2047. [DOI] [PubMed] [Google Scholar]
- 17.Liabeuf S., Moragny J., Bennis Y., Batteux B., Brochot E., Schmit J.L., et al. Association between renin–angiotensin system inhibitors and COVID-19 complications. Eur Hear J - Cardiovasc Pharmacother. 2020;2 doi: 10.1093/ehjcvp/pvaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Is COVID-19 an endothelial disease? Clinical and basic evidence. Clin Basic Evid. 2020;9:1–26. doi: 10.20944/PREPRINTS202004.0204.V1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higuchi T., Nishida T., Iwahashi H., Morimura O., Otani Y., Okauchi Y., et al. Early clinical factors predicting the development of critical disease in Japanese patients with COVID-19: a single-center, retrospective, observational study. J Med Virol. 2020:1–8. doi: 10.1002/jmv.26599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson J., Rosser J.I., Quintero O., Scott J., Subramanian A., Gumma M., et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March–April 2020. Emerg Infect Dis. 2020;26:1679–1685. doi: 10.3201/eid2608.201776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fosbøl E.L., Butt J.H., Østergaard L., Andersson C., Selmer C., Kragholm K., et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA, J Am Med Assoc. 2020;324:168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rath D., Petersen-Uribe Á., Avdiu A., Witzel K., Jaeger P., Zdanyte M., et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 2020;14:1–9. doi: 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conversano A., Melillo F., Napolano A., Fominskiy E., Spessot M., Ciceri F., et al. Renin-angiotensin-aldosterone system inhibitors and outcome in patients with SARS-CoV-2 pneumonia: a case series study. Hypertension. 2020;76:E10–E12. doi: 10.1161/HYPERTENSIONAHA.120.15312. [DOI] [PubMed] [Google Scholar]
- 24.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M., et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iaccarino G., Grassi G., Borghi C., Carugo S., Fallo F., Ferri C., et al. Gender differences in predictors of intensive care units admission among COVID-19 patients: the results of the SARS-RAS study of the Italian Society of Hypertension. PloS One. 2020;15 doi: 10.1371/journal.pone.0237297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iaccarino G., Grassi G., Borghi C., Ferri C., Salvetti M., Volpe M. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian society of hypertension. Hypertension. 2020;76:366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- 27.Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., Poncel-Falcó A., Bliek-Bueno K., Cano-Del Pozo M., et al. Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID study in Spain. Int J Environ Res Publ Health. 2020;17:1–14. doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selçuk M., Çınar T., Keskin M., Çiçek V., Kılıç Ş., Kenan B., et al. Is the use of ACE inb/ARBs associated with higher in-hospital mortality in Covid-19 pneumonia patients? Clin Exp Hypertens. 2020;42:738–742. doi: 10.1080/10641963.2020.1783549. [DOI] [PubMed] [Google Scholar]
- 29.Kocayigit I., Kocayigit H., Yaylaci S., Can Y., Erdem A.F., Karabay O. Impact of antihypertensive agents on clinical course and in-hospital mortality: analysis of 169 hypertensive patients hospitalized for COVID-19. Rev Assoc Med Bras. 2020;66(2):71–76. doi: 10.1590/1806-9282.66.S2.71. [DOI] [PubMed] [Google Scholar]
- 30.Jackson B.R., Gold J.A.W., Natarajan P., Rossow J., Neblett Fanfair R., da Silva J., et al. Predictors at admission of mechanical ventilation and death in an observational cohort of adults hospitalized with COVID-19. Clin Infect Dis. 2020:ciaa1459. doi: 10.1093/cid/ciaa1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trifirò G., Massari M., Da Cas R., Menniti Ippolito F., Sultana J., Crisafulli S., et al. Renin–angiotensin–aldosterone system inhibitors and risk of death in patients hospitalised with COVID-19: a retrospective Italian cohort study of 43,000 patients. Drug Saf. 2020:12. doi: 10.1007/s40264-020-00994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Q Bin, Jiang W.L., Zhang X., Li H.J., Zhang X.A., Zeng H.L., et al. Comorbidities for fatal outcome among the COVID-19 patients: a hospital-based case-control study. J Infect. 2020 doi: 10.1016/j.jinf.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genet B., Vidal J.-S., Cohen A., Boully C., Beunardeau M., Harlé L., et al. COVID-19 in-hospital mortality and use of renin-angiotensin system blockers in geriatrics patients. J Am Med Dir Assoc. 2020;21:1539–1545. doi: 10.1016/j.jamda.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hippisley-Cox J., Young D., Coupland C., Channon K.M., Tan P.S., Harrison D.A., et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abu-Jamous B., Anisimovich A., Baxter J., Mackillop L., McCarthy A., Khan R.T., et al. Associations of comorbidities and medications with COVID-19 outcome: a retrospective analysis of real-world evidence data. medRxiv. 2020 doi: 10.1101/2020.08.20.20174169. 2020.08.20.20174169. [DOI] [Google Scholar]
- 36.Zaki N., Alashwal H., Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: a systematic review. Diabetes Metab Syndr. 2020;14:1133–1142. doi: 10.1016/j.dsx.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. JRAAS - J Renin-Angiotensin-Aldosterone Syst. 2020;21 doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L., Sun Y., Zeng H.-L., Peng Y., Jiang X., Shang W.-J., et al. Calcium channel blocker amlodipine besylate is associated with reduced case fatality rate of COVID-19 patients with hypertension. medRxiv. 2020:2020. doi: 10.1101/2020.04.08.20047134. 04.08.20047134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan F., Huang F., Xu J., Yang P., Qin Y., Lv J., et al. Antihypertensive drugs are associated with reduced fatal outcomes and improved clinical characteristics in elderly COVID-19 patients. Cell Discov. 2020;6:77. doi: 10.1038/s41421-020-00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding X., Cui Y., Zhu Y., Liang H., Wang D., Li L., et al. Association between prior calcium channel blocker use and mortality in septic patients: a meta-analysis of cohort studies. Res Sq. 2020 doi: 10.21203/rs.3.rs-41244/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Huang F., Xu J., Yang P., Qin Y., Cao M., et al. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.03.20.20039586. 2020.03.20.20039586. [DOI] [Google Scholar]
- 42.Asiimwe I.G., Pushpakom S., Turner R.M., Kolamunnage-dona R. Cardiovascular drugs and COVID-19 clinical outcomes : a living systematic review and meta- analysis. medRxiv. 2020 doi: 10.1101/2020.10.07.20208918. 2020.10.07.20208918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lodhi F.A.K., Shogren S.L., Vedre J.G., Haque N., Reriani M., Ali R. Calcium channel blocker toxicity causing acute respiratory distress syndrome: a commonly used drug triggering a life-threatening condition. Wis Med J. 2020;119:66–68. [PubMed] [Google Scholar]
- 44.Magdalan J., Antończyk A., Kowalski K., Przewłocki M., Kochman K., Wasylko-Smolarek M. Severe pulmonary complications of massive intoxication with calcium channel blockers and isosorbide mononitrate--a case report. Przegl Lek. 2004;61:405–407. [PubMed] [Google Scholar]
- 45.Humbert V.H., Munn N.J., Hawkins R.F. Noncardiogenic pulmonary edema complicating massive diltiazem overdose. Chest. 1991;99:258–259. doi: 10.1378/chest.99.1.258. [DOI] [PubMed] [Google Scholar]
- 46.El-Kurdi B., Khatua B., Rood C., Snozek C., Cartin-Ceba R., Singh V.P. Mortality from coronavirus disease 2019 increases with unsaturated fat and may Be reduced by early calcium and albumin supplementation. Gastroenterology. 2020;159:1015–1018. doi: 10.1053/j.gastro.2020.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumori A., Nishio R., Nose Y. Calcium channel blockers differentially modulate cytokine production by peripheral blood mononuclear cells. Circ J. 2010;74:567–571. doi: 10.1253/circj.CJ-09-0467. [DOI] [PubMed] [Google Scholar]
- 48.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.European Society of cardiology ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. Eur Heart J. 2020:1–115. [Google Scholar]
- 50.Aydin H. Calcium channel blockers and the renin-angiotensin system in covid-19, response to: drugs and the renin-angiotensin system in covid-19. BMJ. 2020;369:m1313. doi: 10.1136/bmj.m1313. [DOI] [PubMed] [Google Scholar]
- 51.Ciulla M.M. Switching to another antihypertensive effective drug when using ACEIs/ARBs to treat arterial hypertension during COVID-19. Eur Heart J. 2020;41:1856. doi: 10.1093/eurheartj/ehaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneeweiss M.C., Leonard S., Weckstein A., Schneeweiss S., Rassen J. Renin-Angiotensin-Aldosterone-System inhibitor use in patients with COVID-19 infection and prevention of serious events: a cohort study in commercially insured patients in the US. medRxiv. 2020 doi: 10.1101/2020.07.22.20159855. 2020.07.22.20159855. [DOI] [Google Scholar]
- 53.Yan S., Song X., Lin F., Zhu H., Wang X., Li M., et al. medRxiv; 2020. Clinical characteristics of coronavirus disease 2019 in hainan, China. 2020.03.19.20038539. [DOI] [Google Scholar]
- 54.Reilev M., Kristensen K.B., Pottegaard A., Lund L.C., Hallas J., Ernst M.T., et al. Characteristics and predictors of hospitalization and death in the first 9,519 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. medRxiv. 2020 doi: 10.1101/2020.05.24.20111823. 2020.05.24.20111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng H., Zhang T., He X., Du Y., Tong Y., Wang X., et al. Impact of hypertension on progression and prognosis in patients with COVID-19: a retrospective cohort study in 1031 hospitalized cases in wuhan, China. medRxiv. 2020 doi: 10.1101/2020.06.14.20125997. 2020.06.14.20125997. [DOI] [Google Scholar]
- 56.Dashti H.T., Bates D.W., Roche E., Fiskio J., Mora S., Demler O.V. Clinical characteristics and severity of COVID-19 disease in patients from boston area hospitals. medRxiv. 2020 doi: 10.1101/2020.07.27.20163071. 2020.07.27.20163071. [DOI] [Google Scholar]
- 57.Rezel-Potts E., Douiri A., Chowienczyk Frcp P.J., Gulliford Ffph M.C., Rezel-Potts Addison House E. Antihypertensive medications and COVID-19 diagnosis and mortality: population-based case-control analysis in the United Kingdom. medRxiv. 2020 doi: 10.1101/2020.09.25.20201731. 2020.09.25.20201731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.