Abstract
Information on the biodistribution (BD) of cell therapy products (CTPs) is essential for prediction and assessment of their efficacy and toxicity profiles in non-clinical and clinical studies. To conduct BD studies, it is necessary to understand regulatory requirements, implementation status, and analytical methods. This review aimed at surveying international and Japanese trends concerning the BD study for CTPs and the following subjects were investigated, which were considered particularly important: 1) comparison of guidelines to understand the regulatory status of BD studies in a global setting; 2) case studies of the BD study using databases to understand its current status in cell therapy; 3) case studies on quantitative polymerase chain reaction (qPCR) used primarily in non-clinical BD studies for CTPs; and 4) survey of imaging methods used for non-clinical and clinical BD studies. The results in this review will be a useful resource for implementing BD studies.
Keywords: Biodistribution, Cell therapy product, qPCR, Imaging, PET, SPECT, MRI
1. Introduction
Regenerative therapies using cell therapy products (CTPs) have been attracting a great attention in recent years [1]. In particular, application of pluripotent stem cells, such as induced pluripotent stem (iPS) cells, embryonic stem (ES) cells, and somatic stem cells, has a potential to become a novel therapy for refractory diseases. Investigation of the biodistribution (BD) and fate of administered CTPs is essential for prediction and assessment of their efficacy and toxicity profiles. In particular, tumorigenicity arising from undifferentiated pluripotent stem cells and transformed cells is a critical risk associated with CTPs derived from pluripotent stem cells such as iPS cells. To assess this risk, it is useful to understand the BD after administration of cells because it may predict the clinical risk. In this context, BD studies are required in regulatory guidelines issued by multiple regulatory agencies. However, there is no international consensus on the meaning of the BD study or its method, and each agency has its own interpretation. Moreover, it is not possible to analyze BD of cells using the same methods for conventional drug products such as low-molecular-weight compounds and therapeutic antibodies. In addition, the distribution of cells and the toxicity risk of CTPs are expected to change greatly depending on their administration routes and doses, as well as their characteristics. Therefore, these points must be considered when the method for the BD study of cells is discussed. It is useful to understand what kinds of BD studies have been performed for CTPs that are already launched or in development stages. Quantitative polymerase chain reaction (qPCR) for measuring DNA sequences specific to administered cells is primarily used as the method for analyzing BD in non-clinical studies [2,3]. Imaging technologies, such as in vivo imaging system (IVIS) with fluorescence labeling, positron emission tomography (PET) and single photon emission computed tomography (SPECT) with radioisotope labeling, and magnetic resonance imaging (MRI), are used in non-clinical and clinical studies [[2], [3], [4]] as non-invasive tracking methods for BD of cells. For conducting BD studies, it is important to clarify how these methods are used and what their advantages and disadvantages are. Given these circumstances, we surveyed international and Japanese trends concerning the BD study for CTPs and summarized the results in this review. The following subjects that were considered to be of particular importance were investigated and discussed:
-
1)
Surveys and comparison of guidelines on the BD study by different regulatory agencies and an international scientific organization for the study's purpose, contents, conditions, and methods
-
2)
Use of cell therapy product databases for data collection and trend analysis as case studies of BD studies for cell and tissue-based therapies
-
3)
Survey of literatures and guidance documents concerning qPCR methods for BD analysis of CTPs
-
4)
Survey of articles concerning various imaging techniques used for BD studies and their advantages and disadvantages
This review was prepared as a part of MEASURE (Multisite Evaluation Study on Analytical Methods for Non-Clinical Safety Assessment of hUman-derived REgenerative Medical Products), the Japan Agency for Medical Research and Development (AMED)-funded project, which was conducted by the Committee for Non-Clinical Safety Evaluation of Pluripotent Stem Cell-derived Product, Forum for Innovative Regenerative Medicine (FIRM-CoNCEPT), and the Japan National Institute of Health Sciences.
2. Comparison of current regulatory status for BD of cell products in PMDA, FDA, EMA and ISSCR
2.1. Purpose of a survey on BD studies
To understand the efficacy and safety of CTPs, their BD after administration is considered useful. However, there is no internationally accepted consensus on the meaning of the BD study or its method, and each agency has its own interpretation. Given these circumstances, we surveyed and compared the purpose, timing, and content of BD studies described in guidelines on cell therapy [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]] published by June 2018 from the Ministry of Health, Labour and Welfare (MHLW); the Food and Drug Administration (FDA); and the European Medicines Agency (EMA), which are the regulatory agencies in Japan, the United States (US), and European Union (EU), respectively; and International Society for Stem Cell Research (ISSCR), an international science-based organization, to understand the regulatory view of BD studies in a global setting.
2.2. Purpose of BD studies
In a guidance document from FDA, the BD study is viewed as one of the considerations for determination of the cell fate, which refers to the conditions of cells after administration determined by assessment of survival/engraftment and distribution, differentiation/integration, and tumorigenicity of cells [16]. The guidance takes a stance that cellular products are not subject to conventional pharmacokinetic testing primarily used for low-molecular-weight drugs and that assessment of cell fate can help justify the selection of the animal species, the amount of cells to administer, the duration of studies for assessment, and organs to evaluate characterizing the mode of action and collecting safety data. The guideline states that selection of the animal species, the duration of the studies, and organs for toxicity evaluation must be justified to be most suitable for non-clinical studies for determination of cell fate [16].
EMA defines the purpose of the BD study as the assessment of adverse events arising from the risk factors specific to cell therapies, which are survival/engraftment, proliferation, differentiation, migration, and tumorigenicity, and the efficacy of the products. EMA guidelines also state that the doses and the method (route) of administration are assessed through the BD study [26,29]. Since the cellular nature may change depending on the environment of the engraftment site, these guidelines are formulated for the purpose of ensuring the safety by comprehensive assessment of adverse reactions, such as immune response, tumorigenicity, ectopic integration, through assessment of BD and the state of cells where they are localized [26,29].
MHLW does not clarify the role of BD studies as much as FDA or EMA does, although it can be interpreted that MHLW and the Pharmaceuticals and Medical Devices Agency (PMDA, an incorporated administrative agency under MHLW) view BD as post-administration localization. The purpose of BD studies is to estimate post-administration localization and retention period from the results of BD studies to examine the efficacy and safety [[5], [6], [7], [8], [9], [10], [11]]. In addition, from the fact that the guidelines also stipulate clarification of the rationale for the administration method by animal studies, the purpose of BD studies is considered to be clarification of “efficacy,” “administration route” and “safety” [[5], [6], [7], [8], [9], [10], [11]].
ISSCR considers that administered cells have retention and proliferation potentials in the body; therefore, it is necessary to understand the nature and extent of BD, tissue engraftment, and differentiation of cells. ISSCR considers the above items critical for interpretation of efficacy and adverse events and requests safety evaluation by BD studies [39].
The common and different points in guidelines from these three agencies and ISSCR are summarized in Table 1. They commonly place confirmation of safety and efficacy as the purpose and recommend estimation of the durations of survival of cells/tissues and their effects and assessment of the rationale for the administration method, distribution of the cells after administration, engraftment in ectopic sites, etc. By contrast, differences were found between Japan, which places emphasis on assessment of cell distribution, and FDA and EMA, which recommend BD assessment also for the evaluation of the conditions and function of the cells.
Table 1.
The purpose, timing, and content/method of BD studies in guideline documents of ICH founder countries/region and ISSCR.
| Guideline Developer | Purpose | Timing | Content/Method |
|---|---|---|---|
| MHLW (Japan) | Prediction of efficacy and safety and demonstration of rationality of the administration method/route | Although the actual timing is not specified, investigation of the presence of cell engraftment site and its identification are considered necessary before or during conducting the non-clinical safety study at the latest. | Appropriate methods that are technically feasible should be selected case by case. Technologies are selected through discussion with the agency as necessary. |
| FDA (US) | One of the considerations for determination of the cell fate, which becomes the basis for mechanism of action (MOA) and the content of safety study of cell therapy. | Although the actual timing is not specified, BD studies are considered to be conducted for the purpose of examining conditions for non-clinical studies. | Assessment for each cellular product and mode of administration. Methods with the highest sensitivity currently available. Desirable to use multiple animal models. |
| EMA (EU) | Assessment of adverse events and efficacy from BD and cell state of cells where they are localized. | Required as a part of non-clinical data to submit to the agency. | Survival, engraftment, proliferation, differentiation, migration, integration, tumorigenicity, and ability/duration of active humoral factor secretion. Evaluation methods are not specified. Emphasis on advantage of using small animals. |
| ISSCR | Used for interpretation of efficacy and adverse events through understanding of the nature and extent of distribution, tissue engraftment, and differentiation of cells | Although the actual timing is not specified, BD studies are considered to be conducted for the purpose of examining conditions in non-clinical studies. | Long-term survival and integration of cells. Use of highly sensitive methods. Studies in rodents are required. Studies in large animals are recommended. |
2.3. Timing of BD studies
The actual timing of BD studies to be implemented is specified by EMA but not by MHLW, FDA or ISSCR.
MHLW guidelines [[5], [6], [7], [8], [9], [10], [11]] comment on clarifying the target tissues and organs for assessment of the efficacy and safety. This is interpreted to mean that investigation of the presence of a cell engraftment site and its identification are necessary before or during the non-clinical safety study is conducted at the latest. Moreover, it is necessary to determine medical benefit and an administration route based on the presence of cells. Therefore, non-clinical studies should be conducted to determine local (tissue) or systemic distribution of CTPs and investigate the efficacy and safety as necessary.
FDA [16] and ISSCR [39] consider BD as the basis for designing studies to elucidate safety and the mechanism of action. Therefore, BD studies are considered to be conducted for the purpose of examining conditions in non-clinical studies.
EMA requires the BD study as a part of non-clinical data to submit to the agency. Justification is required if the BD study has not been conducted [[28], [29], [30]]. Examples of justifiable reasons for not conducting the BD study are described in the reference [28].
As described so far, all regulatory agencies state that the outcomes and methods of non-clinical studies should be determined based on BD evaluation. The rationale must be presented if BD evaluation is not a part of non-clinical and clinical studies. Therefore, BD evaluation must be referred to regardless of its implementation.
2.4. Methods for BD studies
2.4.1. Outcomes
Outcomes are not specified in the MHLW guidelines, whereas the FDA guidelines only stipulate investigation of BD of cellular products in the product-specific target and non-target tissues. The EMA guidelines state that survival/engraftment, proliferation, differentiation, migration, and tumorigenicity should be assessed. In addition, if the intended function of the product is based on the capacity of cells to secrete bioactive substances, evaluation of the capacity and duration of secretion is requested. ISSCR also requests knowledge of the long-term survival and integration of such cells.
2.4.2. Evaluation methods
For evaluation of safety and efficacy, analysis of the spatial and temporal distribution of cells in the BD study is considered necessary. None of the agencies clearly specifies or recommends the methods for such an analysis while they present examples of methods. The common concept between the three agencies and ISSCR is that the methods used must have the highest sensitivity currently available. The examples of such methods listed by MHLW guidelines and FDA guidance are as follows: magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computed tomography (SPECT), fluorescence imaging (FI), autoradiography (ARG), polymerase chain reaction (PCR), immunohistochemistry (IHC), and in situ hybridization (ISH). Although such methods are not described in EU guidelines, similar methods are described in the Q and A section [[5], [6], [7], [8], [9],16,26,29]. Appropriate methods that are technically feasible should be selected regardless of the guidelines. Additionally, selection of technologies is considered to be through discussion with the regulatory agency as necessary. All guidelines comment on the difficulty of tracking cell products using currently available technologies and stipulate presentation of information to support the method used.
2.4.3. Animal species
The MHLW guidelines refer to studies only on small animals but not on large animals. FDA and ISSCR recommend studies on large animals in addition to the studies on small animals. By contrast, EMA emphasizes the advantage of evaluating systemic BD in small animals while also stating the importance of similar evaluation in large animals [[26], [27], [28]].
US guidelines do not clearly specify the animal species and number of animals for BD evaluation although they recommend use of multiple animal models (large and small animal models, multiple small animal models, or only large animal models) to evaluate the activity and toxicity of cellular products [16]. It is speculated that implementation of BD studies in multiple animal models is also required because clarification of cell fate is required to justify the duration of the studies on functionality and toxicity of cellular products.
All guidelines comment on the difficulty of extrapolating the results of animal studies to humans. In particular, the following statement is in the MHLW guidance dated June 14, 2016 [12]: Non-clinical safety studies shall be conducted with understanding of the limitation that cellular products with human origin may induce xenogeneic immune response in animals, thereby limiting the information from animal studies. Also in this context, Q and A (No. 56) in Office Memorandum dated March 12, 2008 [13] answers that there is a theoretical possibility that a BD study using products of animal origin and administration of human-derived cells to immunodeficient animals or similar models, if rationale for such studies can be explained, may be accepted. This indicates that using cells of animal origin as a product model is acceptable as one measure for avoiding xenogeneic immune response.
2.4.4. Necessity of performing studies in compliance with GLP
Concerning the grade of BD studies, FDA recognizes that some studies, such as proof-of-concept (POC) studies using animal models and studies that incorporate some endpoints such as cell fate in the safety study, may not fully comply with good laboratory practice (GLP) regulations. From this comment, it is considered that FDA does not require BD studies to be performed always in compliance with GLP [16]. By contrast, ISSCR states that studies may need to be performed in a GLP-certified facility depending on the regulations of the specific country [39].
3. Case studies on BD studies for CTPs
Risks on safety of CTPs, such as ectopic tissue formation and adverse effects on tissues other than the target tissue, are expected to widely vary depending on their administration routes and nature. Therefore, the types of BD studies required for evaluation of safety risk must be discussed in consideration of the nature of CTPs. In doing so, it is useful to learn the trend of BD studies performed for CTPs that are already approved (commercialized) or in development pipeline. In this section, we collected information on BD studies that have been performed from CTP databases of regulatory agencies of different countries and analyzed their trend to understand and discuss the implementation status of BD studies necessary for CTPs. The information was summarized in Supplementary Table 1 and Fig. 1, Fig. 2 were created based on this information. The number of BD studies, whose information was available, was counted. CTPs were picked up from databases by California Institute for Regenerative Medicine (CIRM) of US, Cell and Gene Therapy Catapult of the United Kingdom (CGT Catapult), Paul-Ehrlich-Institut (PEI) of Germany, and Highway Program for Realization of Regenerative Medicine of Japan, from assessment reports of regulatory authorities, and from review articles in scientific journals.
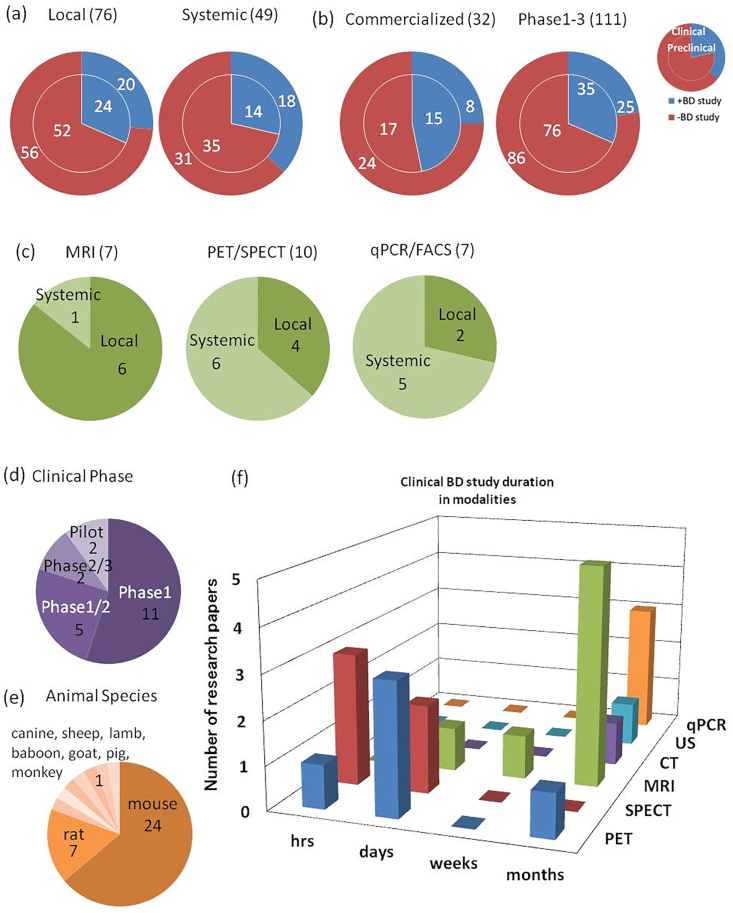
Fig. 1.
Number of biodistribution (BD) studies in cell-based therapeutic product as of September 2018 in comparison among (a) local or systemic administration product, (b) commercialized or product in clinical phases, (c) biodistribution measurement modalities, (d) clinical phases of development, (e) used animal species and (f) measurement modalities and biodistribution study duration (refer to Supplementary Table 1).
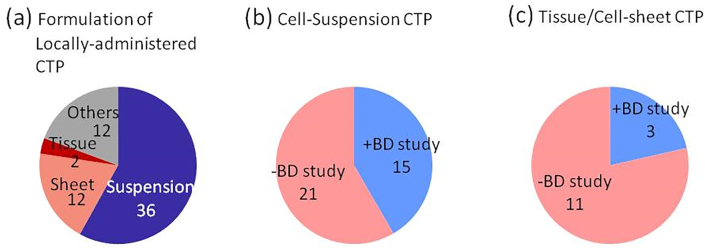
Fig. 2.
Implementation status of clinical biodistribution by formulation in locally administered product group as of September 2018. (a) Number of cell-based therapeutic product in cell formulation and number of cell-based therapeutic product with or without biodistribution study in (b) cell-suspension CTP and (c) tissue or cell-sheet CTP (refer to Supplementary Table 1).
Fig. 1(a) illustrates the implementation status of clinical and non-clinical BD studies by administration route of CTPs. For locally administered products, both clinical and non-clinical BD studies were conducted for approximately 30% of products. For systemically administered products, however, clinical BD studies were conducted for approximately 40% of products. It was speculated that BD studies were conducted more frequently for systemically administered products because they have a higher risk of off-target integration of cells than for locally administered products.
Fig. 1(b) illustrates the implementation status of clinical and non-clinical BD studies separately for CTPs that are commercialized and those in development stages. Clinical BD studies were conducted for one-fourth of both commercialized products and products in development stages. Non-clinical BD studies were reported for only approximately one-third of the products in development stages, whereas they were reported for nearly half of the commercialized products, which may be due to the lack of publication prior to approval, though the difference was not statistically significant (p ≥ 0.05 in Fisher's exact test with Benjamini-Hochberg adjustment).
Fig. 1(c) illustrates administration routes used in clinical BD studies by technology used for evaluation. Six out of 7 products, for which MRI was used for clinical BD studies, were for local administration. Contrarily, 6 out of 10 products, for which PET or SPECT were clinically performed, were for systemic administration. Five out of 7 products, for which qPCR or FACS was applied, were also for systemic administration. MRI was used more often for locally administered products, compared with the other methods (p < 0.05 in chi-square test), presumably because MRI allows for acquisition of local anatomical information; thus, evaluation of accuracy of local administration although MRI takes longer to acquire images than PET and SPECT do and is not suitable for whole body scanning. PET and SPECT are useful technologies for systemically administered products because these technologies have high level of specificity of signal detection and are applicable for evaluation of systemic localization of cells.
Clinical phases when clinical BD studies were performed are illustrated in Fig. 1(d). Phase 1 accounted for half of the cases for which the clinical phase was identified, whereas clinical BD studies in Phase 2 or later accounted for less than a quarter of all the cases. These results indicated that clinical BD studies for CTPs were conducted in the early clinical phase.
Animal species used in non-clinical BD studies are shown in Fig. 1(e). Although approximately 80% of BD studies were performed in rodents, non-clinical BD studies using large animals such as sheep, goats, canines, monkeys, pigs, lambs, and baboons accounted for no more than 20%. Although the ISSCR guidelines in 2016, which are international guidelines for development of CTPs, recommend non-clinical BD studies in large animals, our survey revealed that BD studies are not conducted in large animals for many CTPs currently in development stages.
Fig. 1(f) illustrates the BD evaluation periods for clinical BD studies by evaluation method. Although the evaluation periods for PET and SPECT are relatively short (from several hours to several days) in most cases, BD studies using MRI, CT, ultrasound, or qPCR typically last relatively long (from several weeks to several months). It was inferred that nuclear imaging such as PET and SPECT was applied for BD evaluation in a relatively short period because the half-lives of radioactive tracers are from several hours to several days, whereas evaluation methods without time restraints such as MRI are used for BD evaluation over a relatively long period.
Fig. 2 illustrates the implementation status of clinical BD studies for locally administered products by product type. Approximately half of the locally administered products were cell suspensions, whereas approximately one-third was cell/tissue products such as cell sheets (Fig. 2(a)). Clinical BD studies were performed for nearly half of the cell suspension products but only for less than 20% of cell/tissue products (Fig. 2(b) and (c)). It was speculated that BD studies were performed more frequently for cell suspensions than for cell/tissue products because cell suspensions have a higher risk of migration outside of the administration site.
In this section, we collected and analyzed information on implementation of BD studies from CTPs databases of regulatory agencies of different countries to examine the desirable method of implementing BD studies for CTPs in development stages. The analysis indicated that BD studies were used effectively in development stages of CTPs and can be a source of useful information. It was also suggested that the implementation of BD studies and their evaluation methods (detection methods, duration, animal species, development phase for implementation, etc.) were justified and selected based on the nature of products (administration route, type of products, etc.) and the purpose of evaluation (safety risk evaluation, etc.).
Based on these analyses on the implementation status of BD studies, multiple guidelines, and characterization of methodology for BD evaluation, the desirable way of BD studies should be further discussed among relevant business entities, regulatory agencies, and academic and research institutions engaged in studies in this field.
4. Case studies on cell quantification by qPCR techniques
4.1. Cell quantification by qPCR techniques
The use of qPCR techniques for quantification of mRNA and DNA has been common practice in all areas of life science. Recently, qPCR has also been used for CTPs to determine the numbers of cells and evaluate their biodistribution in vivo. Compared to cell imaging techniques, such as fluorescence imaging, MRI, and PET, the advantages of qPCR-based cell quantification are highly quantitative, sensitive, and of lower cost. On the other hand, one of the disadvantages is that the qPCR-based cell detection is invasive because tissue sampling and the preparation of their homogenates are inevitable. In addition, qPCR cannot distinguish between DNA leaked from dead cells and DNA extracted from living cells, and thus there are difficulties to provide accurate information on the live or death of cells and localization of the living cells.
4.2. Target sequences for qPCR detection
For the detection of CTPs in vivo, CTP-specific sequences should be selected as target for qPCR. For example, human specific sequences are the targets when human derived cells are administered to mice and rats. The single copy genes like sex-determining region Y (Sry) and human down syndrome region of chromosome 21 are chosen in several studies [40,41], however, multi-copy genes are more often used as targets for qPCR because larger copy numbers of the targets increase the sensitivity. Thus, Alu elements and α-satellite sequences are preferably selected. Alu elements are short interspersed repetitive elements in primate genomes and consist of approximately 300 bp sequences. Approximately 5 × 105 to 1 × 106 Alu elements are estimated to be interspersed throughout the human genome and this accounts for 10% of human nuclear DNA [42]. Alu elements are suitable for the target sequence of qPCR, however, Alu elements are common in primates, not human specific. Therefore, Alu-based real time PCR (Alu-qPCR) is applicable to detect human or monkey cells dosed to rodents while there are challenges to detect human cells dosed to monkeys by Alu-qPCR in general.
For allogeneic cell therapies, target sequences to detect donor cells are divided into two categories. One is the donor specific transgenic sequences, and the one of which, CTL019, level in blood was determined for chimeric antigen receptor (CAR) T cell immunotherapy [43]. The other is the Y-chromosome specific sequence, e.g. Sry and RNA-binding motif gene on Y chromosome (Rbmy) [40,44]. This is not so versatile, but intact male donor cells can be detected in the female recipient.
4.3. Alu-qPCR
4.3.1. Primers and probes in Alu-qPCR
Reported primers and probes in the Alu-qPCR method are shown in Supplementary Table 2 [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60]]. The Alu element has some subfamilies such as AluS or AluY. Since each subfamily has different copy numbers in the genome and different cross-reactivity with other primates, a wide variety of primers and probes targeting Alu element are designed to fit for purpose. For example, qPCR targeting AluS (No. 4, 7, and 8 in Supplementary Table 2) is relatively sensitive, and, although the copy number is small, targeting the human specific AluYb8 (No. 5 and 6 in Supplementary Table 2) enables to measure human DNA separately from monkey DNA.
4.3.2. Application of Alu-qPCR in quantification and biodistribution of CTPs
Information on DNA extraction method, PCR detection method, primer/probe sequences, and the units for cell quantification was investigated from the literatures on cell quantification using Alu-qPCR (Supplementary Table 3 [46,[48], [49], [50], [51], [52], [53], [54], [55], [56],[58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88]], and the trend was summarized in Fig. 3.
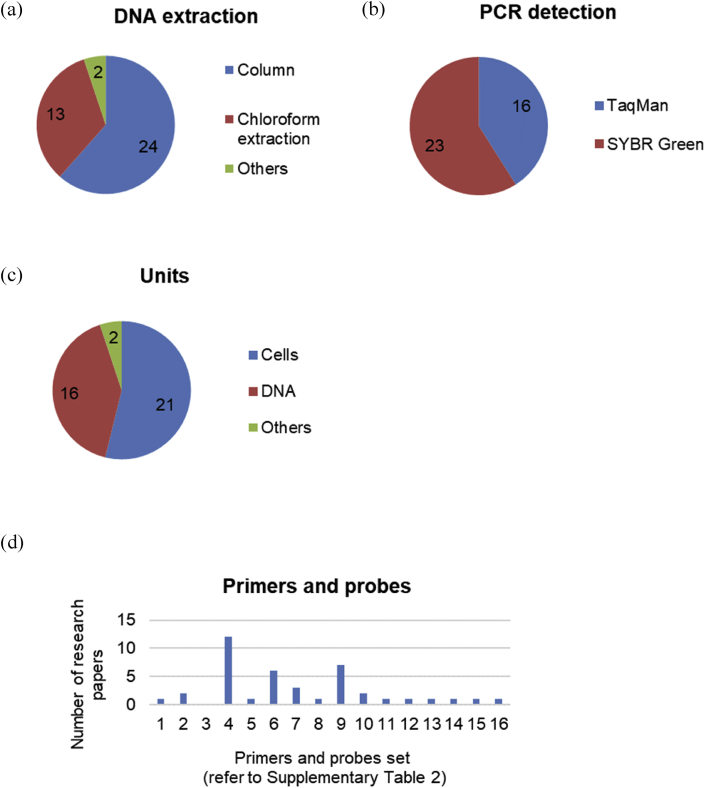
Fig. 3.
Trends of cell quantification using Alu-qPCR methods. (a) DNA extraction, (b) PCR detection, (c) units, and (d) primer and probe sequences (refer to Supplementary Table 3).
For DNA extraction method, anion-exchange chromatography or silica gel membrane technology (column method) were the most frequently applied, followed by chloroform extraction, and ethanol precipitation method (Fig. 3). As for the detection method, SYBR Green method was more frequently used compared to TaqMan probe method. Regarding primers and probe sequences, sequence-4 in Supplementary Table 2 was used most frequently, followed by sequence-6. Respective primers and probe sequences are described in Supplementary Table 2. Looking at the unit used for cell quantification, the cases converted to cell numbers and the cases using DNA amounts without cell conversion were almost equivalent.
As a result of the investigation, there was no standard method for DNA extraction and cell quantification using Alu-qPCR, and it was confirmed that various methods were applied at each experimental facility. In this survey, although the literatures on cellular quantification were examined, the unit used is not necessarily cell numbers and about half of cases is based on DNA calculated from qPCR as it is. And, in some cases, the absolute values (cell numbers) and relative values (human cell numbers per mouse cell numbers, etc.) were applied as a unit for cellular quantification.
4.4. Guidelines and documents related to qPCR
Although the information on the cell quantification by qPCR is still limited, qPCR techniques for detecting nucleic acids are widely applied in the fields of genetically modified organisms (GMO) and gene therapy, and guidelines on qPCR methods have been issued.
Therefore, in order to utilize it for cell quantification, we investigated 2 documents, focusing on the qPCR as an experimental method, 2 guidances in the GMO field and 2 guidances in the field of gene therapy. Acceptance criteria of validation items or assay performance are summarized in Table 2 [[89], [90], [91], [92]].
Table 2.
Comparison of guidelines and documents related to qPCR on acceptance criteria of each items.
| Item | 1) MIQE | 2) Checklist | 4) FDA Guidance | 5) GMO |
|---|---|---|---|---|
| LOD | 95% probability | ≥95% | 95% confidence | ensuring ≤5% false negative results |
| LOQ | – | Item only (Criteria not described) | ≤50 copies of vector/μg genomic DNA | less than 1/10 of the value of the target concentration with CV ≤25% |
| Accuracy (Trueness) | Item only (Criteria not described) | Item only (Criteria not described) | – | RE within ±25% |
| Precision | Item only (Criteria not described) | Item only (Criteria not described) | – | CV ≤25% |
| PCR efficiencya | Item only (Criteria not described) | 90–110% (−3.1 ≥ slope ≥ −3.6) | – | 90–110% −3.1 ≥ slope ≥ −3.6 |
| Correlation coefficient: r2 | Item only (Criteria not described) | 0.99 ≤ r2 ≤ 0.999 | – | r2 ≥ 0.98 |
MIQE, The MIQE Guidelines [89]; Checklist, Checklist for optimization and validation of real-time PCR assays [90]; FDA Guidance, Guidance for Industry: Gene Therapy Clinical Trials – Observing Subjects for Delayed Adverse Events [91]; GMO, Definition of Minimum Performance Requirements for Analytical Methods of GMO Testing [92]; CV, coefficient of variation; LOD, limit of detection; LOQ, limit of quantification; RE, relative error.
PCR efficiency (%) = [10(−1/slope) −1] ×100.
4.4.1. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments [89]
Although the usefulness of qPCR was widely accepted, there was no consensus for the information on qPCR experimental conditions to be described in publications. This situation had impeded the reproducibility of the experiment. Therefore, this guideline was issued with the aim of proposing the minimum information necessary for the evaluation of the qPCR experiment required for paper writing and review. In this guideline, 85 items of information which is essential or desirable to be described in the paper are mentioned in a series of processes from experimental design and nucleic acid extraction to qPCR experiments and their validation and data analysis.
As validation items, specificity, information on the calibration curve (linearity, slope, y intercept, r2), PCR efficiency, variation in quantification cycle (Cq) value in limit of detection (LOD), etc. are listed, but the criteria for them have not been mentioned. As assay performance, PCR efficiency, linearity, LOD and accuracy are listed (Table 2). The terms of Cq and threshold cycle (Ct) have the same definition as the number of cycles required for the fluorescent signal to cross the threshold. The difference merely depends on the manufacturer of equipment. However, MIQE guidelines recommend the use of Cq, and thus, this review follows the recommendation.
4.4.2. Checklist for optimization and validation of real-time PCR assays [90]
This document has been proposed as a checklist for optimization and validation of qPCR method.
Target gene, detection method, oligonucleotide, sample processing, quantification strategies (standard curve method or comparative method) are described as main investigation items for the optimization of qPCR. And for the validation, verification of design of oligonucleotide, verification of amplification, optimization of reaction conditions, PCR characteristics, analytical verification, and internal quality control are described.
4.4.3. Guidance for industry: preclinical assessment of investigational cellular and gene therapy products [16]
Guidance on nonclinical studies in cell therapy or gene therapy is presented in this document issued by the FDA in 2013. For the biodistribution evaluation of CTPs, many measurement methods including qPCR are exemplified, however, required items and criteria for validation are not shown. Regarding gene therapy products, it is recommended to quantify the number of vector copies per μg of genomic DNA at multiple time points after administration by qPCR targeted to the administered vector sequence, but similar to CTPs, specific measurement criteria are not shown. Since this guidance refers to the following guidance (Guidance for Industry Gene Therapy Clinical Trials - Observing Subjects for Delayed Adverse Events) on the tissue collection, it is inferred that the criteria should follow the guidance.
4.4.4. Guidance for industry: gene therapy clinical trials—observing subjects for delayed adverse events [91]
In this guidance issued by the FDA in 2006, guidance for conducting clinical trials of gene therapy are presented, and there is description on the biodistribution evaluation of vectors in nonclinical studies, among which the measurement of administered vector by qPCR is recommended. It is also recommended to indicate that the qPCR method used can specifically detect vector sequences in animal and human tissues at the time of IND submission.
The descriptions are summarized as follows.
-
−
Have a limit of quantitation of ≤50 copies of vector/μg genomic DNA (with 95% confidence)
-
−
Sample and analyze the following panel of tissues, at a minimum: blood, injection site(s), gonads, brain, liver, kidneys, lung, heart, and spleen.
-
−
Use a minimum of three samples per tissue. One sample of each tissue should include a spike of control DNA, including a known amount of the vector sequences. The control will determine the specified PCR assay sensitivity.
-
−
Provide a rationale for the number of replicates for testing per tissue, taking into account the size of the sample relative to the tissue.
4.4.5. Definition of minimum performance requirements for analytical methods of GMO testing [92]
qPCR is a general-purpose analytical method also in the field of GMO. This document defines validation items and criteria for the qPCR as a GMO inspection method and was issued by the European Network of GMO Laboratories (ENGL) in 2008.
The each item and criteria, except for Table 2, are summarized as follows.
-
−
Applicability: suitable for the actual measurement of analytes, matrices and concentration.
-
−
Practicability: practical from the viewpoints of ease of operations, efficiency of implementation, and costs
-
−
DNA Extraction and Purification: repeatable recovery, fragmentation profile and DNA concentration.
-
−
Purity of DNA extracts: [(measured Ct—extrapolated Ct or diluted sample)] <0.5
-
−
Robustness: The response of an assay with respect to the small changes shall not deviate more than 30%.
-
−
Collaborative trial: Precision (≤35%), Trueness (within ±25%)
4.4.6. Guidelines for validation of qualitative real-time PCR methods [93]
In contrast to the document mentioned above, this guideline was issued with qualitative qPCR for GMO screening. Applicability, practicability, specificity and LOD are indispensable, while trueness, precision, LOQ are unnecessary. Regarding amplification efficiency, the criteria range is wide (−2.9 ≥≧ slope ≥ −3.9), and for robustness the criteria values are not specified.
5. Survey on imaging-based methods for BD studies
From the viewpoint of translational research, application of diagnostic imaging technologies used in the clinical settings for the evaluation of BD of cells is expected. Imaging technology has a potential to realize the non-invasive evaluation and visualization of spatial and temporal distribution of cells in both preclinical and clinical research/study. One of the most promising tool for the analysis of cell BD is nuclear medicine imaging such as PET and SPECT [[94], [95], [96], [97], [98]]. To acquire the PET/SPECT images of cell BD in vivo, cells must be labeled with radioisotopes for PET/SPECT, and the labeled cells are administrated to the patients/subjects, then the whole body scan was performed. The obstacle to use of this technology for a clinical BD research/study is not so high because a few agents for cell labeling such as 111In-oxine and 99mTc-HMPAO has already been used as radiopharmaceuticals in the field of clinical diagnosis [[99], [100], [101], [102], [103], [104]]. The advantages of nuclear medicine techniques are translatability from non-clinical studies to clinical, three-dimensional (3D) imaging; high sensitivity; possibility of quantitative evaluation; and possibility of whole body scanning (Table 3), although the physical half-life of nuclide limits the period of PET/SPECT study [96,97]. By contrast, MRI can visualize the distribution of cells labeled with paramagnetic iron-oxide particles or other labeling agents together with the tissue structure [96,97]. Therefore, MRI allows precise evaluation of cell distribution in soft tissues with high sensitivity and high spatial resolution in addition to the general characteristics of imaging technique such as translatability and 3D imaging (Table 3). Taken together, nuclear medicine imaging techniques allow measurement of a large area in a short period of time and thus have a strength in quantitative measurement of the whole body, whereas MRI enables visualization of local distribution together with precise morphological images [96,97].
Table 3.
Comparison of Pros/Cons among imaging modalities.
| Pros | Cons | Label | Properties of Measures [98] |
|||
|---|---|---|---|---|---|---|
| Sensitivity | Spatial Resolution | |||||
| Imaging Modality | PET/SPECT | High sensitivity 3D image Quantitative Translational Probe variety Molecular specific Whole body scan Good deep part image |
Facility limitation (RI) Lack of anatomical information (⇒Complementary with CT/MRI) Expensive Half life of nuclide Radiation exposure |
Passive diffusion:89Zr-oxine; 111In-oxine; 99mTc-HMPO Transporter:18FDG Reporter: HSV1-tk/18F-FHBG; NIS/123I |
High | Preclinical PET: ~1 mm Clinical PET: 4–6 mm Preclinical SPECT: 1–2 mm Clinical SPECT: 5–8 mm |
| MRI | 3D image Translational Good soft tissue contrast Anatomical information available Good deep part image |
Body motion artifact Low throughput Narrow FOV Expensive |
Negative Contrast Agent: SPIO Positive Contrast Agent:19F, Gd Reporter: Ferritin |
Moderate | Preclinical MRI: 25–250 μm Clinical: 0.5–5 mm, 1–3 mm3 |
|
There are two major strategies to label the cells for cell tracking, direct and indirect labeling method. In the direct labeling method, cells are labeled (incubated) with the labeling agents described below in vitro, and then the labeled cells are injected into animals and monitored the BD of the cells. For the indirect labeling method, reporter gene such as HSV1-tk is transduced into the cells. The cells expressing the reporter gene are then administrated into the subject animals, followed by the radiolabeled substrate to detect the cell distribution.
As for PET imaging-based cell tracking methods, many direct labeling agents were reported including 18F-fluorodeoxyglucose (FDG) [[105], [106], [107], [108], [109], [110], [111], [112], [113]], 89Zr-oxine [[114], [115], [116], [117], [118], [119]], 64Cu-PTSM [120], 64Cu-TETA-anti CD45 [121], and 89Zr-desferrioxamine (DFO)-anti CD45 [121], whereas HSV1-tk/18F-FHBG was used as an indirect labeling agent for the metabolic trapping strategy [[122], [123], [124], [125]] (Table 4). 18F-FDG was the only agent clinically available among the labeling agents reported in the literature [[105], [106], [107], [108], [109], [110], [111], [112], [113]], and was used for the labeling of leukocytes, MSCs, stem cells, and T-lymphocytes. As the physical half-life of Fluorine-18 is 109.8 min, 18F-FDG is considered to be suitable for the evaluation of systemic and local distributions in the early stage up to 4–6 h after administration. In addition, labeling efficiency of cells with 18F-FDG was dependent on cell type, widely ranging from 4% to over 99% [105]. Besides 18F-FDG, a few labeling agents, utilizing the metal nuclides such as 89Zr and 64Cu are reported [[114], [115], [116], [117], [118], [119], [120], [121]]. These nuclides have relatively longer half-lives (89Zr: 3.3 days, 64Cu: 12.7 h) than that of 18F, therefore, these labeling agents allow cell tracking for a longer period (a few days to a week), although their use is currently limited to non-clinical studies at present.
Table 4.
PET imaging-based cell tracking methods.
| General Detection Limit/cells [98] | Labeling strategy | Labeling agent | Availability | Clinical approval of agent | Applicable cell types | Labeling efficiency |
|---|---|---|---|---|---|---|
| ~104 | direct | 18F-FDG | Clinical | Yes | Leukocytes [105] | 72–75% |
| MAK cells [105] | 88% | |||||
| Islets cells [105] | 4–97% | |||||
| Unselected BMCs, enriched CD34+ cells [105] | >99% | |||||
| Non-mobilized peripheral blood CD34+ cells [105] | 6% | |||||
| PHSC [105] | 46–95% | |||||
| Bone Marrow-Derived Stem Cells [106] | NA | |||||
| Cytokine-induced killer (CIK) cells [107] | NA | |||||
| Adipose-derived stem cells [108] | NA | |||||
| T lymphocytes [109] | NA | |||||
| T-lymphoblasts [110] | NA | |||||
| circulating progenitor cells [111,112] | NA | |||||
| WBC [113] | NA | |||||
| direct | 89Zr-oxine | non-clinical (house-made) | No | Dendritic cell: DCs [114] | 40–50% | |
| cytotoxic T cells: CTLs [114] | 10–20% | |||||
| Natural killer: NK [114] | 30–40% | |||||
| Bone Marrow [114] | 10–20% | |||||
| murine myeloma cells [115] | NA | |||||
| direct | 64Cu-PTSM | non-clinical (house-made) | No | C6 glioma cells [120] | 70–85% | |
| direct | 64Cu-TETA- or 89Zr-DFO-antiCD45 | non-clinical (house-made) | No | hPBSCs [121] | NA | |
| indirect | HSV1-tk/18F-FHBG | non-clinical (house-made) | No | CD34-TK75(+)-selected donor T cells [122] | NA | |
| hMSC [123,124] | NA | |||||
| cytolytic T cells: CTLs [125] | NA |
SPECT imaging-based cell tracking methods also include direct and indirect methods (Table 5). A standard level of quantitative sensitivity was around or more than 105 cells. 111In (half-life: 2.8 days), and 99mTc (half-life: 6 h) have been used in the routine clinical diagnosis, and these can be applied to the cell tracking. 111In-oxine and 99mTc-HMPAO approved as radiopharmaceuticals can be applied to the direct labeling agents [[99], [100], [101], [102], [103], [104],[126], [127], [128], [129]], and application of 99mTc-pertechnetate (99mTcO4−) to an indirect labeling method using the sodium-iodide symporter has been studied [130]. These labeling agents were used for labeling of cells such as circulating progenitor cells, bone marrow MSCs, white blood cells, and CD34+ cells. The labeling efficiency of SPECT imaging agents was also dependent on cell types, ranging from around 10%–80% [[99], [100], [101], [102], [103], [104],[126], [127], [128], [129]].
Table 5.
SPECT imaging-based cell tracking methods.
| General Detection Limit/cells [98] | Labeling strategy | Labeling agent | Availability | Clinical approval | Applicable cell types | Labeling efficiency | Cell viability | Efflux from cells |
|---|---|---|---|---|---|---|---|---|
| ~105 | direct | 111In-oxine | Clinical | Yes | circulating progenitor cells [100] | 10–60% | 78–100% | NA |
| BM MSC [99] | 36–53% | >99% | NA | |||||
| White blood cell [101] | NA | NA | NA | |||||
| direct | 111In-tropolone | non-clinical (house-made) | No | canine bone marrow MSC [126] | 80% (0.14 Bq/cell) | 100% (compared with unlabeled cells) | NA | |
| canine bone marrow mononuclear cell and bone marrow stromal cells [127] | < ca. 60% | normal viability and proliferation | NA | |||||
| canine bone marrow stromal cell (BMSC) [128] | BMSC: 92% (0.105 Bq/cell.) | BMSC: 93% after labeling | 111In biologic T1/2 = 14.1 days | |||||
| bone marrow mesenchymal stem cells [129] | 66 ± 5% (38Bq/cell) | viability: equal proliferation: decrease |
retention: 85.3% at 1 h 45.1% at 48 h |
|||||
| indirect | 99mTc-pertechnetate | Clinical | Yes | NIS-expressing adenovirus-transfected canine stem cells [130] | NA | NA | NA | |
| direct | 99mTc-HMPAO | Clinical | Yes | CD34+ cells (Peripheral Blood Bone Marrow Cell) [103] | NA | NA | NA | |
| Stromal vascular factor (MSC) [102] | 30–40% (1 × 10ˆ7 cells) | no apoptosis or necrosis induction; may induce reactive oxygen species (ROSs) | NA | |||||
| White blood cell [104] | 40–80% | NA | <10% for first 1h |
Similar to PET and SPECT, MRI-based cell tracking strategies included direct [[131], [132], [133], [134], [135], [136], [137], [138], [139], [140]] and indirect labeling methods [[141], [142], [143]] (Table 6). Superparamagnetic iron oxide (SPIO) [[131], [132], [133], [134], [135]] and ultrasmall superparamagnetic iron oxide (USPO) [136], as well as 19F-perfluorochemicals (PFCs) [137], gadoteridol (Gd-HP-DO3A) [138] and MnCl2 [139,140] are applicable to labeling of cells such as human acute monocytic leukemia cell lines (THP-1), MSCs, established cell lines, stem cells, primary cells, hMPCs, J774A.1 cells, and K562 cells.
Table 6.
MRI-based cell tracking methods.
| General Detection Limit/cells [98] | Labeling strategy | Labeling agent | Physical features of agent | Availability | Clinical approval | Applicable cell types | Labeling efficiency | Cell viability |
|---|---|---|---|---|---|---|---|---|
| ~104 | direct | SPIO: Resovist | iron particle; carboxydextran-coated; 57 nm | Clinical | Yes | rabbit MSC | NA | |
| human monocytic cells (THP-1) [132] | 2.1–22.6 pg/cell | not toxic in a conc. of 0.75 mM Fe | ||||||
| murine MSC [133] | NA | >90% | ||||||
| direct | SPIO: Feridex | iron particle; Dextran-coated; 100–250 nm | Clinical | Yes | rat MSC [134] | 0.6–1.5 pg/cell | >90%, no change from MSC only | |
| rabbit MSC, rat MSC, murine MSC, | NA | NA | ||||||
| direct | SPIO: FeraTrack | iron particle; Dextran-coated; 60–140 nm | non-clinical (commercially available) | No | established cell lines (NIH-3T3 cells, Jurkat cells) | 3.33 ± 0.64 pg Fe/cell (hNSCs, using Metafectene) | 90.6% (hNSC) | |
| primary cells (granulocytes, neural progenitor cells) | ||||||||
| stem cells (hematopoietic stem cells [HSCs], mesenchymal stem cells [MSCs], and embryonic stem cells [ESCs]) | ||||||||
| murine neural progenitor cells and rat granulocytes | ||||||||
| not applicable for natural killer (NK) cells | ||||||||
| NSCs | ||||||||
| direct | USPIO: Ferumoxytol | iron particle carboxymethylether-coated 17–30 nm |
Clinical | Yes | hMSC, ADSCs, hiPS, HEK293 [136] | NA | no significant impact (data not shown) | |
| direct | USPIO: VSOP | citrate-coated very small superparamagnetic iron oxide particles | non-clinical (house-made) | No | human acute monocytic leukemia cell line (THP-1) [132] | 19.6–60.3 pg/cell | not toxic in a conc. of 0.75 mM Fe | |
| direct | SPIO: Endorem | dextran-coated SPIO nanoparticles, 120–180 nm | Clinical | Yes | hMPC [135] | 0.5 pg Fe/cell | 92%, 86%, and 77%,concentration dependent cell morphology did not change | |
| direct | 19F-PFC | hydrophobic emulsion | Clinical | No | human dendritic cells [137] | 3.9 × 1012 ± 3.4 × 101219F/cell | >95% | |
| direct | Gd (HP-DO3A): Gadoteridol/Prohance | chelate | Clinical | Yes | J774A.1, K562 [138] | NA | >90% | |
| direct | MnCl2 | metal ion | non-clinical (commercially available) | No | hESC | NA | NA | |
| human T, NK, B cell [139] | NA | no impact up to a certain concentration | ||||||
| mononuclear cell [140] | NA | 95.4% before transplantation | ||||||
| indirect | Ferritin/Fe ion | metal ion in tissues | non-clinical (house-made) | No | Mouse skeletal myoblasts (C2C12 cells) [140,141] | NA | NA |
The labeling agents used for PET, SPECT, and MRI are powerful tools for the evaluation of cell BD because these agents allow highly sensitive detection of cells in vivo. However, it should be aware that the labeling agents itself leaking from the cells due to exocytosis or cell death would be depicted in the images as well [96,115,128,129]. In order to notice such the phenomena, not only imaging studies but also other conventional ex vivo experiments such as immunohistochemistry (IHC) and fluorescence-activated cell sorting (FACS) are required at preclinical stage, and the multilateral experiments and considerations will lead to better understanding of the cell BD.
6. Conclusions
This review was aimed to conduct a survey of international and Japanese trends concerning the BD study for CTPs. For this purpose, we compared guidelines from multiple regulatory agencies and an international scientific organization for the study's purpose, contents, conditions and methods of BD studies, and collected information from cell therapy product databases and analyzed the trend as case studies. In addition, we examined the literatures and guidance concerning the qPCR method and summarized literature search results of BD studies on various imaging techniques and their advantages and disadvantages. As for guidance, all agencies commonly viewed BD studies as data supporting safety and efficacy of CTPs. Moreover, it was inferred that the agencies expect BD studies to be performed in both non-clinical studies and clinical studies. By contrast, there were differences between agencies in whether to evaluate the cell state and functionality of cells in relation to efficacy and safety, and in use of small or large animals. Survey of the implementation status of BD studies using databases of multiple agencies in Japan, US, and EU indicated that implementation of BD studies and their evaluation methods (detection methods, duration, animal species, development phase, etc.) were justified and selected based on the characteristics of products (administration route, formulation, etc.) and the purpose of evaluation (toxicity risk evaluation, etc.).
Literature survey on the qPCR method indicated that frequently used targets for quantitative detection of cells were sequences specific to administered cells and multicopy sequences that can increase sensitivity. Survey on qPCR conditions (DNA extraction method, detection method, and primer/probe sequences) revealed that even for the Alu elements, which are commonly used in cases of administration of human cells in animals, there were no standard conditions for their measurement. Moreover, different units, such as number of cells and DNA copy numbers, were used as units for quantitative value of cells in different studies. Therefore, it is desirable to have validated qPCR methods for which the ability to detect human cells in animal cells is well characterized, so that they can be applied as applicable to most of relevant therapies under development bringing consistency in the approach. Since 2016, the Public-Private Partnership Initiative FIRM-CoNCEPT/MEASURE [144] has conducted a multi-site study, aiming at evaluating a qPCR-based method for the ability to detect human cells in rodent cells/tissues. This study is in its reporting stage and is expected to be published soon.
We also surveyed literatures on nuclear medicine imaging such as PET and SPECT, and MRI, which are common translational research tools for non-clinical and clinical studies, and summarized the nature and advantages and disadvantages of these methods. It is important to apply these methods with a good understanding of above-mentioned points. We hope this review becomes a useful resource for determining whether or not to conduct the BD study and for selecting the study conditions and methods for performing BD studies.
Declaration of competing interest
All authors declare that they have no competing interests.
Acknowledgments
This work was partly supported by the Japan Agency for Medical Research and Development (AMED), Japan under Grant Number JP19mk0104101 and JP19mk0104080.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2021.06.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Basu J., Assaf B.T., Bertram T.A., Rao M. Preclinical biosafety evaluation of cell-based therapies: emerging global paradigms. Toxicol Pathol. 2015;43(1):115–125. doi: 10.1177/0192623314559104. [DOI] [PubMed] [Google Scholar]
- 2.Brooks A., Futrega K., Liang X., Hu X., Liu X., Crawford D.H.G. Concise review: quantitative detection and modeling the in vivo kinetics of therapeutic mesenchymal stem/stromal cells. Stem Cells Transl Med. 2018;7(1):78–86. doi: 10.1002/sctm.17-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von der Haar K., Lavrentieva A., Stahl F., Scheper T., Blume C. Lost signature: progress and failures in in vivo tracking of implanted stem cells. Appl Microbiol Biotechnol. 2015;99(23):9907–9922. doi: 10.1007/s00253-015-6965-7. [DOI] [PubMed] [Google Scholar]
- 4.Gu E., Chen W.Y., Gu J., Burridge P., Wu J.C. Molecular imaging of stem cells: tracking survival, biodistribution, tumorigenicity, and immunogenicity. Theranostics. 2012;2(4):335–345. doi: 10.7150/thno.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Ministry of Health, Labour and Welfare . September 7, 2012. Guidelines on ensuring the quality and safety of pharmaceuticals and medical devices derived from the processing of autologous human somatic stem cells.https://www.pmda.go.jp/files/000205400.pdf Notification No. 0907-21. [Google Scholar]
- 6.The Ministry of Health, Labour and Welfare . September 7, 2012. Guidelines on ensuring the quality and safety of pharmaceuticals and medical devices derived from the processing of allogeneic human somatic stem cells.https://www.pmda.go.jp/files/000205401.pdf Notification No. 0907-3. [Google Scholar]
- 7.The Ministry of Health, Labour and Welfare . September 7, 2012. Guidelines on ensuring the quality and safety of pharmaceuticals and medical devices derived from processing of autologous human induced pluripotent stem(-like) cells.https://www.pmda.go.jp/files/000205402.pdf Notification No. 0907-4. [Google Scholar]
- 8.The Ministry of Health, Labour and Welfare . September 7, 2012. Guidelines on ensuring the quality and safety of pharmaceuticals and medical devices derived from processing of allogeneic human induced pluripotent stem(-like) cells.https://www.pmda.go.jp/files/000205403.pdf Notification No. 0907-5. [Google Scholar]
- 9.The Ministry of Health, Labour and Welfare . September 7, 2012. Guidelines on ensuring the quality and safety of pharmaceuticals and medical devices derived from the processing of human embryonic stem cells.https://www.pmda.go.jp/files/000205404.pdf Notification N o. 0907-6. [Google Scholar]
- 10.The Ministry of Health, Labour and Welfare . February 8, 2008. Guidelines on ensuring the quality and safety of pharmaceuticals and medical devices derived from processing of autologous human cells and tissues.https://www.pmda.go.jp/files/000205396.pdf Notification No. 0208003. [Google Scholar]
- 11.The Ministry of Health, Labour and Welfare . September 12, 2008. Guidelines on ensuring the quality and safety of pharmaceuticals and medical devices derived from processing of allogeneic human cells and tissues.https://www.pmda.go.jp/files/000205398.pdf Notification No. 0912006. [Google Scholar]
- 12.Technical guidance on the quality of cellular and tissue-based products (human cell/tissue products) and implementation of non-clinical and clinical studies. June 27, 2016. https://www.pmda.go.jp/files/000212850.pdf Office memorandum. [Google Scholar]
- 13.Q&a on the guidelines on ensuring the quality and safety of pharmaceuticals and medical devices derived from processing of allogeneic human cells and tissues. October 3, 2008. https://www.pmda.go.jp/files/000205399.pdf Office memorandum. [Google Scholar]
- 14.Guidelines on ensuring the quality and safety of drugs and medical devices processed from cells and tissues of human or animal origin. December 26, 2000. https://www.pmda.go.jp/files/000205395.pdf Notification No. 1314. [Google Scholar]
- 15.Considerations on detection test of undifferentiated pluripotent stem cells and detection test of transformed cells for quality and safety assessment of human cell/tissue products (draft). https://www.pmda.go.jp/files/000221662.pdf.
- 16.US Food and Drug Administration . November 2013. Guidance for industry: preclinical assessment of investigational cellular and gene therapy products.https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm376521.pdf [Google Scholar]
- 17.US Food and Drug Administration . June 2015. Considerations for the design of early-phase clinical trials of cellular and gene therapy products; guidance for industry.https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm564952.pdf [Google Scholar]
- 18.US Food and Drug Administration . March 1998. Guidance for industry: guidance for human somatic cell therapy and gene therapy.https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm081670.pdf [Google Scholar]
- 19.US Food and Drug Administration . October 2010. Guidance for industry: cellular therapy for cardiac disease.https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm164345.pdf [Google Scholar]
- 20.US Food and Drug Administration . July 2020. Regulatory considerations for human cells, tissues, and cellular and tissue-based products: minimal manipulation and homologous use; guidance for industry and food and drug administration staff.https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm585403.pdf [Google Scholar]
- 21.US Food and Drug Administration . November 2017. Same surgical procedure exception under 21 CFR 1271.15(b): questions and answers regarding the scope of the exception; guidance for industry.https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/tissue/ucm419926.pdf [Google Scholar]
- 22.US Food and Drug Administration . February 2019. Evaluation of devices used with regenerative medicine advanced therapies; guidance for industry.https://www.fda.gov/media/120266/download [Google Scholar]
- 23.US Food and Drug Administration . February 2019. Expedited programs for regenerative medicine therapies for serious conditions; guidance for industry.https://www.fda.gov/media/120267/download [Google Scholar]
- 24.US Food and Drug Administration . September 2017. Deviation reporting for human cells, tissues, and cellular and tissue-based products regulated solely under section 361 of the public health service act and 21 CFR part 1271; guidance for industry.https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm574889.pdf [Google Scholar]
- 25.US Food and Drug Administration . January 2011. Guidance for industry: potency tests for cellular and gene therapy products.https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm243392.pdf [Google Scholar]
- 26.The European parliament and the council of the European union . 2004. Directive 2001/83/EC of of the european parliament and of the council of 6 November 2001 on the community code relating to medicinal products for human use.https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/directive-2001/83/ec-european-parliament-council-6-november-2001-community-code-relating-medicinal-products-human-use_en.pdf [Google Scholar]
- 27.European Medicines Agency . 11 January 2007. Guideline on cell-based medicinal products.https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-human-cell-based-medicinal-products_en.pdf [Google Scholar]
- 28.European Medicines Agency . 14 January 2011. Reflection paper on stem-cell based medicinal products.https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-stem-cell-based-medicinal-products_en.pdf [Google Scholar]
- 29.European Medicines Agency . 11 February 2013. Guideline on the risk-based approach according to annexes I, part IV of directive 2001/83/EC applied to advanced therapy medicinal products.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-risk-based-approach-according-annex-i-part-iv-directive-2001/83/ec-applied-advanced-therapy-medicinal-products_en.pdf [Google Scholar]
- 30.European Medicines Agency . October 2010. Guideline on the minimum quality and non-clinical data for certification of advanced therapy medicinal products 15.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-minimum-quality-non-clinical-data-certification-advanced-therapy-medicinal-products_en.pdf [Google Scholar]
- 31.European Medicines Agency . 8 April 2010. Reflection paper on in-vitro cultured chondrocyte containing products for cartilage repair of the knee.https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-vitro-cultured-chondrocyte-containing-products-cartilage-repair-knee_en.pdf [Google Scholar]
- 32.The European parliament and the council of the European Union. Regulation (EC) No 1394/2007 of the European parliament and of the council of 13 November 2007 on advanced therapy medicinal products and amending directive 2001/83/EC and regulation (EC) No 726/2004. 13 November 2007. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32007R1394&from=EN [Google Scholar]
- 33.The European parliament and the council of the European Union . Regulation (EC) No 726/2004 of the European parliament and of the council of 31 March 2004 laying down community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. 31 March 2004. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_2004_726/reg_2004_726_en.pdf [Google Scholar]
- 34.The Commission of the European Communities . 24 July 2009. Commission regulation (EC) No 668/2009 of 24 July 2009 implementing regulation (EC) No 1394/2007 of the European parliament and of the council with regard to the evaluation and certification of quality and non-clinical data relating to advanced therapy medicinal products developed by micro, small and medium-sized enterprises.https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:194:0007:0010:EN:PDF [Google Scholar]
- 35.European Medicines Agency . 26 July 2018. Guideline on quality, non-clinical and clinical aspects of 5 medicinal products containing genetically modified cells.https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-non-clinical-clinical-aspects-medicinal-products-containing-genetically_en.pdf [Google Scholar]
- 36.European Medicines Agency . 20 November 2008. Guideline on safety and efficacy follow-up and risk management of advanced therapy medicinal products.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-safety-efficacy-follow-risk-management-advanced-therapy-medicinal-products_en.pdf [Google Scholar]
- 37.European Medicines Agency . 22 October 2009. Guideline on xenogeneic cell-based medicinal products.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-xenogeneic-cell-based-medicinal-products_en.pdf [Google Scholar]
- 38.European Medicines Agency . 21 July 2016. Guideline on potency testing of cell based immunotherapy medicinal products for the treatment of cancer.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-potency-testing-cell-based-immunotherapy-medicinal-products-treatment-cancer-revision-1_en.pdf [Google Scholar]
- 39.International society for stem cell research . 12 May 2016. Guidelines for stem cell research and clinical translation.https://www.isscr.org/docs/default-source/all-isscr-guidelines/guidelines-2016/isscr-guidelines-for-stem-cell-research-and-clinical-translationd67119731dff6ddbb37cff0000940c19.pdf?sfvrsn=4 [Google Scholar]
- 40.Hibino N., Duncan D.R., Nalbandian A., Yi T., Qyang Y., Shinoka T. Evaluation of the use of an induced puripotent stem cell sheet for the construction of tissue-engineered vascular grafts. J Thorac Cardiovasc Surg. 2012;143(3):696–703. doi: 10.1016/j.jtcvs.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song P., Xie Z., Guo L., Wang C., Xie W., Wu Y. Human genome-specific real-time PCR method for sensitive detection and reproducible quantitation of human cells in mice. Stem Cell Rev. 2012;8(4):1155–1162. doi: 10.1007/s12015-012-9406-3. [DOI] [PubMed] [Google Scholar]
- 42.Rowold D.J., Herrera R.J. Alu elements and the human genome. Genetica. 2000;108(1):57–72. doi: 10.1023/a:1004099605261. [DOI] [PubMed] [Google Scholar]
- 43.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng K., Gupta S. Quantitative tools for assessing the fate of xenotransplanted human stem/progenitor cells in chimeric mice. Xenotransplantation. 2009;16(3):145–151. doi: 10.1111/j.1399-3089.2009.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicklas J.A., Buel E. Development of an Alu-based, real-time PCR method for quantitation of human DNA in forensic samples. J Forensic Sci. 2003;48(5):936–944. [PubMed] [Google Scholar]
- 46.Shim G., Lee S., Han J., Kim G., Jin H., Miao W. Pharmacokinetics and in vivo fate of intra-articularly transplanted human bone marrow-derived clonal mesenchymal stem cells. Stem Cell Dev. 2015;24(9):1124–1132. doi: 10.1089/scd.2014.0240. [DOI] [PubMed] [Google Scholar]
- 47.Nicklas J.A., Buel E. Simultaneous determination of total human and male DNA using a duplex real-time PCR assay. J Forensic Sci. 2006;51(5):1005–1015. doi: 10.1111/j.1556-4029.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 48.McBride C., Gaupp D., Phinney D.G. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by realtime PCR. Cytotherapy. 2003;5(1):7–18. doi: 10.1080/14653240310000038. [DOI] [PubMed] [Google Scholar]
- 49.Ray D.A., Han K., Walker J.A., Batzer M.A. Laboratory methods for the analysis of primate mobile elements. Methods Mol Biol. 2010:628153–628179. doi: 10.1007/978-1-60327-367-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker J.A., Kilroy G.E., Xing J., Shewale J., Sinha S.K., Batzer M.A. Human DNA quantitation using Alu element-based polymerase chain reaction. Anal Biochem. 2003;315(1):122–128. doi: 10.1016/s0003-2697(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 51.Schneider T., Osl F., Friess T., Stockinger H., Scheuer W.V. Quantification of human Alu sequences by real-time PCR–an improved method to measure therapeutic efficacy of anti-metastatic drugs in human xenotransplants. Clin Exp Metastasis. 2002;19(7):571–582. doi: 10.1023/a:1020992411420. [DOI] [PubMed] [Google Scholar]
- 52.Creane M., Howard L., O'Brien T., Coleman C.M. Biodistribution and retention of locally administered human mesenchymal stromal cells: quantitative polymerase chain reaction-based detection of human DNA in murine organs. Cytotherapy. 2017;19(3):384–394. doi: 10.1016/j.jcyt.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Mira E., Lacalle R.A., Gomez-Mouton C., Leonardo E., Manes S. Quantitative determination of tumor cell intravasation in a real-time polymerase chain reaction-based assay. Clin Exp Metastasis. 2002;19(4):313–318. doi: 10.1023/a:1015563031769. [DOI] [PubMed] [Google Scholar]
- 54.van der Horst E.H., Leupold J.H., Schubbert R., Ullrich A., Allgayer H. Taqman®-based quantification of invasive cells in the chick embryo metastasis assay. Biotechniques. 2004;37(6):940–942. doi: 10.2144/04376ST02. 4, 6. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W., Wu M., Menesale E., Lu T., Magliola A., Bergelson S. Development and qualification of a high sensitivity, high throughput Q-PCR assay for quantitation of residual host cell DNA in purification process intermediate and drug substance samples. J Pharmaceut Biomed Anal. 2014;100:145–149. doi: 10.1016/j.jpba.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 56.Stevanato L., Corteling R.L., Stroemer P., Hope A., Heward J., Miljan E.A. c-MycERTAM transgene silencing in a genetically modified human neural stem cell line implanted into MCAo rodent brain. BMC Neurosci. 2009;10:86. doi: 10.1186/1471-2202-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kojima T., Watanabe Y., Hashimoto Y., Kuroda S., Yamasaki Y., Yano S. In vivo biological purging for lymph node metastasis of human colorectal cancer by telomerase-specific oncolytic virotherapy. Ann Surg. 2010;251(6):1079–1086. doi: 10.1097/SLA.0b013e3181deb69d. [DOI] [PubMed] [Google Scholar]
- 58.Nehmann N., Wicklein D., Schumacher U., Muller R. Comparison of two techniques for the screening of human tumor cells in mouse blood: quantitative real-time polymerase chain reaction (qRT-PCR) versus laser scanning cytometry (LSC) Acta Histochem. 2010;112(5):489–496. doi: 10.1016/j.acthis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Nicklas J.A., Buel E. An Alu-based, MGB Eclipse real-time PCR method for quantitation of human DNA in forensic samples. J Forensic Sci. 2005;50(5):1081–1090. [PubMed] [Google Scholar]
- 60.Funakoshi K., Bagheri M., Zhou M., Suzuki R., Abe H., Akashi H. Highly sensitive and specific Alu-based quantification of human cells among rodent cells. Sci Rep. 2017;7:13202. doi: 10.1038/s41598-017-13402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nedaeinia R., Sharifi M., Avan A., Kazemi M., Nabinejad A., Ferns G.A. Inhibition of microRNA-21 via locked nucleic acid-anti-miR suppressed metastatic features of colorectal cancer cells through modulation of programmed cell death 4. Tumour Biol. 2017;39(3) doi: 10.1177/1010428317692261. [DOI] [PubMed] [Google Scholar]
- 62.Herrero A., Prigent J., Lombard C., Rosseels V., Daujat-Chavanieu M., Breckpot K. Adult-derived human liver stem/progenitor cells infused 3 days postsurgery improve liver regeneration in a mouse model of extended hepatectomy. Cell Transplant. 2017;26(2):351–364. doi: 10.3727/096368916X692960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abd-Elhalim D.M., El-Wazir Y.M. Do the human umbilical cord blood CD34+ progenitor cells home in the pancreas and kidney of diabetic mice? Int J Diabetes Dev Ctries. 2016;36(1):70–74. [Google Scholar]
- 64.Ammar H.I., Sequiera G.L., Nashed M.B., Ammar R.I., Gabr H.M., Elsayed H.E. Comparison of adipose tissue- and bone marrow- derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats. Stem Cell Res Ther. 2015:6148. doi: 10.1186/s13287-015-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Preston Campbell J., Mulcrone P., Masood S.K., Karolak M., Merkel A., Hebron K. TRIzol and Alu qPCR-based quantification of metastatic seeding within the skeleton. Sci Rep. 2015;5:12635. doi: 10.1038/srep12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toupet K., Maumus M., Luz-Crawford P., Lombardo E., Lopez-Belmonte J., van Lent P. Survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis. PLos One. 2015;10(1) doi: 10.1371/journal.pone.0114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prigent J., Herrero A., Ambroise J., Smets F., Deblandre G.A., Sokal E.M. Human progenitor cell quantification after xenotransplantation in rat and mouse models by a sensitive qPCR assay. Cell Transplant. 2015;24(8):1639–1652. doi: 10.3727/096368914X681955. [DOI] [PubMed] [Google Scholar]
- 68.Zalucha J.L., Jung Y., Joseph J., Wang J., Berry J.E., Shiozawa Y. The role of osteoclasts in early dissemination of prostate cancer tumor cells. J Cancer Stem Cell Res. 2015;3 doi: 10.14343/jcscr.2015.3e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abellaneda J.M., Martinez-Alarcon L., Quereda J.J., Herrero-Medrano J.M., Mendonca L., Mrowiec A. Validation of a quantitative polymerase chain reaction method for human alu gene detection in microchimeric pigs used as donors for xenotransplantation. Transplant Proc. 2015;47(1):132–135. doi: 10.1016/j.transproceed.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Luo J., Weaver M.S., Cao B., Dennis J.E., Van Biber B., Laflamme M.A. Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo. Stem Cells Transl Med. 2014;3(6):734–744. doi: 10.5966/sctm.2013-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim Y.S., Kim J.Y., Shin D.M., Huh J.W., Lee S.W., Oh Y.M. Tracking intravenous adipose-derived mesenchymal stem cells in a model of elastase-induced emphysema. Tuberc Respir Dis (Seoul) 2014;77(3):116–123. doi: 10.4046/trd.2014.77.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kienast Y., Klein C., Scheuer W., Raemsch R., Lorenzon E., Bernicke D. Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer Res. 2013;19(24):6730–6740. doi: 10.1158/1078-0432.CCR-13-0081. [DOI] [PubMed] [Google Scholar]
- 73.Toupet K., Maumus M., Peyrafitte J.A., Bourin P., van Lent P.L., Ferreira R. Long-term detection of human adipose-derived mesenchymal stem cells after intraarticular injection in scid mice. Arthritis Rheum. 2013;65(7):1786–1794. doi: 10.1002/art.37960. [DOI] [PubMed] [Google Scholar]
- 74.Abellaneda J.M., Ramis G., Martinez-Alarcon L., Majado M.J., Quereda J.J., Herrero-Medrano J.M. Generation of human-to-pig chimerism to induce tolerance through transcutaneous in utero injection of cord blood-derived mononuclear cells or human bone marrow mesenchymals cells in a preclinical program of liver xenotransplantation: preliminary results. Transplant Proc. 2012;44(6):1574–1578. doi: 10.1016/j.transproceed.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 75.Schubert A., Hawighorst T., Emons G., Grundker C. Agonists and antagonists of GnRH-I and -II reduce metastasis formation by triple-negative human breast cancer cells in vivo. Breast Cancer Res Treat. 2011;130(3):783–790. doi: 10.1007/s10549-011-1358-9. [DOI] [PubMed] [Google Scholar]
- 76.Liu W., Guan M., Hu T., Gu X., Lu Y. Re-Expression of AKAP12 inhibits progression and metastasis potential of colorectal carcinoma in vivo and in vitro. PLos One. 2011;6(8) doi: 10.1371/journal.pone.0024015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koch M., Hussein F., Woeste A., Grundker C., Frontzek K., Emons G. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res Treat. 2011;128(2):337–346. doi: 10.1007/s10549-010-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shoji M., Oskowitz A., Malone C.D., Prockop D.J., Pochampally R. Human mesenchymal stromal cells (MSCs) reduce neointimal hyperplasia in a mouse model of flow-restriction by transient suppression of anti-inflammatory cytokines. J Atheroscler Thromb. 2011;18(6):464–474. doi: 10.5551/jat.6213. [DOI] [PubMed] [Google Scholar]
- 79.De Paepe M.E., Mao Q., Ghanta S., Hovanesian V., Padbury J.F. Alveolar epithelial cell therapy with human cord blood-derived hematopoietic progenitor cells. Am J Pathol. 2011;178(3):1329–1339. doi: 10.1016/j.ajpath.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen Q.T., Olson E.S., Aguilera T.A., Jiang T., Scadeng M., Ellies L.G. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci U S A. 2010;107(9):4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee W.J., Chen W.K., Wang C.J., Lin W.L., Tseng T.H. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2008;226(2):178–191. doi: 10.1016/j.taap.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 83.Sher Y.P., Chou C.C., Chou R.H., Wu H.M., Wayne Chang W.S., Chen C.H. Human kallikrein 8 protease confers a favorable clinical outcome in non-small cell lung cancer by suppressing tumor cell invasiveness. Cancer Res. 2006;66(24):11763–11770. doi: 10.1158/0008-5472.CAN-06-3165. [DOI] [PubMed] [Google Scholar]
- 84.Lee R.H., Seo M.J., Reger R.L., Spees J.L., Pulin A.A., Olson S.D. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madsen M.A., Deryugina E.I., Niessen S., Cravatt B.F., Quigley J.P. Activity-based protein profiling implicates urokinase activation as a key step in human fibrosarcoma intravasation. J Biol Chem. 2006;281(23):15997–16005. doi: 10.1074/jbc.M601223200. [DOI] [PubMed] [Google Scholar]
- 86.Munoz J.R., Stoutenger B.R., Robinson A.P., Spees J.L., Prockop D.J. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102(50):18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zijlstra A., Mellor R., Panzarella G., Aimes R.T., Hooper J.D., Marchenko N.D. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res. 2002;62(23):7083–7092. [PubMed] [Google Scholar]
- 88.Jin H., Lee W.J., Lee S., Yi T., Song S.U., Shim G. Enhanced survival of human mesenchymal stem cells following co-delivery with glucagon-like peptide-1 analogue in fibrin gel. J Pharm Investig. 2015;45(2):143–149. [Google Scholar]
- 89.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 90.Raymaekers M., Smets R., Maes B., Cartuyvels R. Checklist for optimization and validation of real-time PCR assays. J Clin Lab Anal. 2009;23(3):145–151. doi: 10.1002/jcla.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.US Food and Drug Administration . November 2006. Guidance for industry: gene therapy clinical trials - observing subjects for delayed adverse events.https://www.ngvbcc.org/pdf/gtclin.pdf;jsessionid=64AFB5DB8805374241D9FFBBD0137CCF [Google Scholar]
- 92.European Network of GMO Laboratories . Joint Research Centre; 2015. Definition of minimum performance requirements for analytical methods of GMO testing.http://gmo-crl.jrc.ec.europa.eu/doc/mpr%20report%20application%2020_10_2015.pdf [Google Scholar]
- 93.Broeders S., Huber I., Grohmann L., Berben G., Taverniers I., Mazzara M. Guidelines for validation of qualitative real-time PCR methods. Trends Food Sci Technol. 2014:37115–37126. [Google Scholar]
- 94.Scarfe L., Brillant N., Kumar J.D., Ali N., Alrumayh A., Amali M. Preclinical imaging methods for assessing the safety and efficacy of regenerative medicine therapies. NPJ Regen Med. 2017;228 doi: 10.1038/s41536-017-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leahy M., Thompson K., Zafar H., Alexandrov S., Foley M., O'Flatharta C. Functional imaging for regenerative medicine. Stem Cell Res Ther. 2016;7(1):57. doi: 10.1186/s13287-016-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naumova A.V., Modo M., Moore A., Murry C.E., Frank J.A. Clinical imaging in regenerative medicine. Nat Biotechnol. 2014:32804. doi: 10.1038/nbt.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stacy M.R., Sinusas A.J. Emerging imaging modalities in regenerative medicine. Curr Pathobiol Rep. 2015;3(1):27–36. doi: 10.1007/s40139-015-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen P.K., Riegler J., Wu J.C. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14(4):431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gholamrezanezhad A., Mirpour S., Bagheri M., Mohamadnejad M., Alimoghaddam K., Abdolahzadeh L. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38(7):961–967. doi: 10.1016/j.nucmedbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Schachinger V., Aicher A., Dobert N., Rover R., Diener J., Fichtlscherer S. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118(14):1425–1432. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- 101.Roca M., de Vries E.F.J., Jamar F., Israel O., Signore A. Guidelines for the labelling of leucocytes with 111In-oxine. Eur J Nucl Med Mol Imag. 2010;37(4):835–841. doi: 10.1007/s00259-010-1393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verma V.K., Beevi S.S., Tabassum A., Kumaresan K., Kamaraju R.S., Arbab A.S. In vitro assessment of cytotoxicity and labeling efficiency of 99mTc-HMPAO with stromal vascular fraction of adipose tissue. Nucl Med Biol. 2014;41(9):744–748. doi: 10.1016/j.nucmedbio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 103.Vrtovec B., Poglajen G., Lezaic L., Sever M., Socan A., Domanovic D. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128(11 Suppl 1):S42–S49. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- 104.de Vries E.F., Roca M., Jamar F., Israel O., Signore A. Guidelines for the labelling of leucocytes with 99mTc-HMPAO. Inflammation/infection taskgroup of the european association of nuclear medicine. Eur J Nucl Med Mol Imaging. 2010;37(4):842–848. doi: 10.1007/s00259-010-1394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu C., Ma G., Li J., Zheng K., Dang Y., Shi X. In vivo cell tracking via 18F-fluorodeoxyglucose labeling: a review of the preclinical and clinical applications in cell-based diagnosis and therapy. Clin Imaging. 2013;37(1):28–36. doi: 10.1016/j.clinimag.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 106.Sood V., Mittal B.R., Bhansali A., Singh B., Khandelwal N., Marwaha N. Biodistribution of 18F-FDG-labeled autologous bone marrow-derived stem cells in patients with type 2 diabetes mellitus: exploring targeted and intravenous routes of delivery. Clin Nucl Med. 2015;40(9):697–700. doi: 10.1097/RLU.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 107.Wang H., Cao F., Li J., Li Y., Liu X., Wang L. Homing of cytokine-induced killer cells during the treatment of acute promyelocytic leukemia. Int J Hematol. 2014;100(2):165–170. doi: 10.1007/s12185-014-1618-7. [DOI] [PubMed] [Google Scholar]
- 108.Elhami E., Goertzen A.L., Xiang B., Deng J., Stillwell C., Mzengeza S. Viability and proliferation potential of adipose-derived stem cells following labeling with a positron-emitting radiotracer. Eur J Nucl Med Mol Imaging. 2011;38(7):1323–1334. doi: 10.1007/s00259-011-1753-9. [DOI] [PubMed] [Google Scholar]
- 109.Grabner A., Kentrup D., Edemir B., Sirin Y., Pavenstadt H., Schlatter E. PET with 18F-FDG-labeled T lymphocytes for diagnosis of acute rat renal allograft rejection. J Nucl Med. 2013;54(7):1147–1153. doi: 10.2967/jnumed.112.109231. [DOI] [PubMed] [Google Scholar]
- 110.Eriksson O., Sadeghi A., Carlsson B., Eich T., Lundgren T., Nilsson B. Distribution of adoptively transferred porcine t-lymphoblasts tracked by 18F-2-fluoro-2-deoxy-d-glucose and position emission tomography. Nucl Med Biol. 2011;38(6):827–833. doi: 10.1016/j.nucmedbio.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y., Thorn S., DaSilva J.N., Lamoureux M., DeKemp R.A., Beanlands R.S. Collagen-based matrices improve the delivery of transplanted circulating progenitor cells: development and demonstration by ex vivo radionuclide cell labeling and in vivo tracking with positron-emission tomography. Circ Cardiovasc Imaging. 2008;1(3):197–204. doi: 10.1161/CIRCIMAGING.108.781120. [DOI] [PubMed] [Google Scholar]
- 112.Doyle B., Kemp B.J., Chareonthaitawee P., Reed C., Schmeckpeper J., Sorajja P. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med. 2007;48(10):1708–1714. doi: 10.2967/jnumed.107.042838. [DOI] [PubMed] [Google Scholar]
- 113.Pellegrino D., Bonab A.A., Dragotakes S.C., Pitman J.T., Mariani G., Carter E.A. Inflammation and infection: imaging properties of 18F-FDG-labeled white blood cells versus 18F-FDG. J Nucl Med. 2005;46(9):1522–1530. [PubMed] [Google Scholar]
- 114.Sato N., Wu H., Asiedu K.O., Szajek L.P., Griffiths G.L., Choyke P.L. 89Zr-oxine complex PET cell imaging in monitoring cell-based therapies. Radiology. 2015;275(2):490–500. doi: 10.1148/radiol.15142849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Charoenphun P., Meszaros L.K., Chuamsaamarkkee K., Sharif-Paghaleh E., Ballinger J.R., Ferris T.J. [89Zr] Oxinate4 for long-term in vivo cell tracking by positron emission tomography. Eur J Nucl Med Mol Imag. 2015;42(2):278–287. doi: 10.1007/s00259-014-2945-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Asiedu K.O., Koyasu S., Szajek L.P., Choyke P.L., Sato N. Bone marrow cell trafficking analyzed by 89Zr-oxine positron emission tomography in a murine transplantation model. Clin Cancer Res. 2017;23(11):2759–2768. doi: 10.1158/1078-0432.CCR-16-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weist M.R., Starr R., Aguilar B., Chea J., Miles J.K., Poku E. PET of adoptively transferred chimeric antigen receptor T cells with 89Zr-oxine. J Nucl Med. 2018;59(10):1531–1537. doi: 10.2967/jnumed.117.206714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Asiedu K.O., Ferdousi M., Ton P.T., Adler S.S., Choyke P.L., Sato N. Bone marrow cell homing to sites of acute tibial fracture: 89Zr-oxine cell labeling with positron emission tomographic imaging in a mouse model. EJNMMI Res. 2018;8(1):109. doi: 10.1186/s13550-018-0463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Man F., Lim L., Volpe A., Gabizon A., Shmeeda H., Draper B. In vivo PET tracking of 89Zr-labeled Vγ9Vδ2 T cells to mouse xenograft breast tumors activated with liposomal alendronate. Mol Ther. 2019;27(1):219–229. doi: 10.1016/j.ymthe.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adonai N., Nguyen K.N., Walsh J., Iyer M., Toyokuni T., Phelps M.E. Ex vivo cell labeling with 64Cu–pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc Natl Acad Sci. 2002;99(5):3030–3035. doi: 10.1073/pnas.052709599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tarantal A.F., Lee C.C., Kukis D.L., Cherry S.R. Radiolabeling human peripheral blood stem cells for positron emission tomography (PET) imaging in young rhesus monkeys. PLos One. 2013;8(10) doi: 10.1371/journal.pone.0077148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eissenberg L.G., Rettig M.P., Ritchey J.K., Prior J.L., Schwarz S.W., Frye J. [18F]FHBG PET/CT imaging of CD34-TK75 transduced donor T cells in relapsed allogeneic stem cell transplant patients: safety and feasibility. Mol Ther. 2015;23(6):1110–1122. doi: 10.1038/mt.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Willmann J.K., Paulmurugan R., Rodriguez-Porcel M., Stein W., Brinton T.J., Connolly A.J. Imaging gene expression in human mesenchymal stem cells: from small to large animals. Radiology. 2009;252(1):117–127. doi: 10.1148/radiol.2513081616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roelants V., Labar D., de Meester C., Havaux X., Tabilio A., Gambhir S.S. Comparison between adenoviral and retroviral vectors for the transduction of the thymidine kinase PET reporter gene in rat mesenchymal stem cells. J Nucl Med. 2008;49(11):1836–1844. doi: 10.2967/jnumed.108.052175. [DOI] [PubMed] [Google Scholar]
- 125.Yaghoubi S.S., Jensen M.C., Satyamurthy N., Budhiraja S., Paik D., Czernin J. Non-invasive detection of therapeutic cytolytic T cells with [18F]FHBG positron emission tomography in a glioma patient. Nat Clin Pract Oncol. 2009;6(1):53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin Y., Kong H., Stodilka R.Z., Wells R.G., Zabel P., Merrifield P.A. Determining the minimum number of detectable cardiac-transplanted 111In-tropolone-labelled bone-marrow-derived mesenchymal stem cells by SPECT. Phys Med Biol. 2005;50(19):4445–4455. doi: 10.1088/0031-9155/50/19/001. [DOI] [PubMed] [Google Scholar]
- 127.Wisenberg G., Lekx K., Zabel P., Kong H., Mann R., Zeman P.R. Cell tracking and therapy evaluation of bone marrow monocytes and stromal cells using SPECT and CMR in a canine model of myocardial infarction. J Cardiovasc Magn Reson. 2009;11(1):11. doi: 10.1186/1532-429X-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blackwood K.J., Lewden B., Wells R.G., Sykes J., Stodilka R.Z., Wisenberg G. In vivo SPECT quantification of transplanted cell survival after engraftment using 111In-tropolone in infarcted canine myocardium. J Nucl Med. 2009;50(6):927–935. doi: 10.2967/jnumed.108.058966. [DOI] [PubMed] [Google Scholar]
- 129.Yoon J.K., Park B.N., Shim W.Y., Shin J.Y., Lee G., Ahn Y.H. In vivo tracking of 111In-labeled bone marrow mesenchymal stem cells in acute brain trauma model. Nucl Med Biol. 2010;37(3):381–388. doi: 10.1016/j.nucmedbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 130.Lee A.R., Woo S.K., Kang S.K., Lee S.Y., Lee M.Y., Park N.W. Adenovirus-mediated expression of human sodium-iodide symporter gene permits in vivo tracking of adipose tissue-derived stem cells in a canine myocardial infarction model. Nucl Med Biol. 2015;42(7):621–629. doi: 10.1016/j.nucmedbio.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 131.Zhang R., Li J., Xin L., Xie J. In vivo magnetic resonance imaging of iron oxide-labeled, intravenous-injected mesenchymal stem cells in kidneys of rabbits with acute ischemic kidney injury: detection and monitoring at 1.5 T. Ren Fail. 2015;37(8):1363–1369. doi: 10.3109/0886022x.2015.1073542. [DOI] [PubMed] [Google Scholar]
- 132.Ludwig A., Poller W.C., Westphal K., Minkwitz S., Lättig-Tünnemann G., Metzkow S. Rapid binding of electrostatically stabilized iron oxide nanoparticles to THP-1 monocytic cells via interaction with glycosaminoglycans. Basic Res Cardiol. 2013;108(2):328. doi: 10.1007/s00395-013-0328-2. [DOI] [PubMed] [Google Scholar]
- 133.Ariza de Schellenberger A., Kratz H., Farr T.D., Lowa N., Hauptmann R., Wagner S. Labeling of mesenchymal stem cells for MRI with single-cell sensitivity. Int J Nanomed. 2016;11:1517–1535. doi: 10.2147/IJN.S101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moraes L., Vasconcelos-dos-Santos A., Santana F.C., Godoy M.A., Rosado-de-Castro P.H., Jasmin Neuroprotective effects and magnetic resonance imaging of mesenchymal stem cells labeled with SPION in a rat model of huntington’s disease. Stem Cell Res. 2012;9(2):143–155. doi: 10.1016/j.scr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 135.Azzabi F., Rottmar M., Jovaisaite V., Rudin M., Sulser T., Boss A. Viability, differentiation capacity, and detectability of super-paramagnetic iron oxide-labeled muscle precursor cells for magnetic-resonance imaging. Tissue Eng Part C Methods. 2015;21(2):182–191. doi: 10.1089/ten.tec.2014.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Castaneda R.T., Khurana A., Khan R., Daldrup-Link H.E. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J Vis Exp. 2011;(57) doi: 10.3791/3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ahrens E.T., Helfer B.M., O’Hanlon C.F., Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014;72(6):1696–1701. doi: 10.1002/mrm.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Filippi M., Boido M., Pasquino C., Garello F., Boffa C., Terreno E. Successful in vivo MRI tracking of MSCs labeled with gadoteridol in a spinal cord injury experimental model. Exp Neurol. 2016;282:66–77. doi: 10.1016/j.expneurol.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 139.Aoki I., Takahashi Y., Chuang K.H., Silva A.C., Igarashi T., Tanaka C. Cell labeling for magnetic resonance imaging with the T1 agent manganese chloride. NMR Biomed. 2006;19(1):50–59. doi: 10.1002/nbm.1000. [DOI] [PubMed] [Google Scholar]
- 140.Odaka K., Aoki I., Moriya J., Tateno K., Tadokoro H., Kershaw J. In vivo tracking of transplanted mononuclear cells using manganese-enhanced magnetic resonance imaging (MEMRI) PLos One. 2011;6(10) doi: 10.1371/journal.pone.0025487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Campan M., Lionetti V., Aquaro G.D., Forini F., Matteucci M., Vannucci L. Ferritin as a reporter Tene for in vivo tracking of stem cells by 1.5-t cardiac MRI in a rat model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;300(6):H2238–H2250. doi: 10.1152/ajpheart.00935.2010. [DOI] [PubMed] [Google Scholar]
- 142.Naumova A.V., Reinecke H., Yarnykh V., Deem J., Yuan C., Murry C.E. Ferritin overexpression for noninvasive magnetic resonance imaging–based tracking of stem cells transplanted into the heart. Mol Imaging. 2010;9(4):201–210. [PMC free article] [PubMed] [Google Scholar]
- 143.Naumova A.V., Yarnykh V.L., Balu N., Reinecke H., Murry C.E., Yuan C. Quantification of MRI signal of transgenic grafts overexpressing ferritin in murine myocardial infarcts. NMR Biomed. 2012;25(10):1187–1195. doi: 10.1002/nbm.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sato Y., Bando H., Di Piazza M., Gowing G., Herberts C., Jackman S. Tumorigenicity assessment of cell therapy products: the need for global consensus and points to consider. Cytotherapy. 2019;21(11):1095–1111. doi: 10.1016/j.jcyt.2019.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.