Abstract
Purpose
To describe acute and chronic retinal ischemic changes following an internal carotid artery pseudoaneurysm stenting procedure, and to review current evidence for risk factors and management of post-procedural retinal ischemic events.
Observation
A 50-year-old man presented with a 3-month history of pulsatile tinnitus, headache, and intermittent blurry vision. A CT angiogram of head and neck showed bilateral cervicopetrous internal carotid artery (ICA) pseudoaneurysms. The patient underwent successful repair with angioplasty and stenting of the flow-limiting high-grade (>95%) stenosis of his left high cervical ICA. On post-operative day 1, the patient reported monocular vision loss with a large central scotoma. He was found to have a central macular area of retinal whitening and multiple areas of perivascular retinal whitening on exam, concerning for retinal artery occlusions secondary to peri-procedural emboli. Dual antiplatelet therapy was started and a stroke evaluation was performed. Two months later, his visual acuity in the affected eye was counting fingers and his left eye fundus examination was notable for multiple areas of scattered hemorrhages, microaneurysms, and retinal exudates in the distribution of prior retinal ischemia. OCT imaging revealed atrophic changes in the left macula. Subsequently, the patient completed stage-2 repair of the left ICA pseudoaneurysm followed by uncomplicated repair of the right ICA. Four months later, his left eye visual acuity and retinal findings remained stable.
Conclusions and Importance
Post-procedure retinal emboli and ischemia are important, vision threatening possible ocular complications for patients undergoing carotid vascular and endovascular procedures.
Keywords: Retinal artery occlusion, Retinal emboli, Internal carotid artery pseudoaneurysm, Endovascular surgery
1. Introduction
Internal carotid artery (ICA) pseudoaneurysms are rare, but can result from carotid dissections secondary to blunt or penetrating trauma, infection, or underlying conditions like fibromuscular dysplasia or connective tissue disease.1,2 A pseudoaneurysm is defined by the loss of integrity of all three layers of the arterial wall (intima, media, adventitia), in contrast to a true aneurysm, in which the arterial wall is intact but expanded. Pseudoaneurysm formation has been reported in approximately 10–40% of cases with carotid artery dissection.3,4 These lesions can cause mass effect on adjacent structures, or potentially result in distal thromboembolism.1,2 Endovascular treatment is the most common technique for management of these lesions, with the goal of providing symptomatic relief and minimizing the risks of stroke and hemorrhage.5 However, cerebral or retinal emboli can result as a potential complication of this intervention, leading to stroke or vision loss. We present a case of retinal emboli and ischemia following internal carotid artery pseudoaneurysm stenting.
2. Case report
A 50-year-old man with a history of bipolar disorder, hypertension, and polysubstance use presented with a 3-month history of bilateral pulsatile tinnitus, headache, and intermittent blurry vision. He was found to have bilateral cervicopetrous ICA pseudoaneurysms on CT angiogram imaging of the brain and neck; given their location, these lesions were attributed to prior trauma. Intermittent blurry vision was thought to be most consistent with intermittent emboli arising from the pseudoaneurysms or associated stenoses. The decision was made to perform staged stent-assisted coiling of the left and then the right ICA in order to prevent further embolic phenomena.
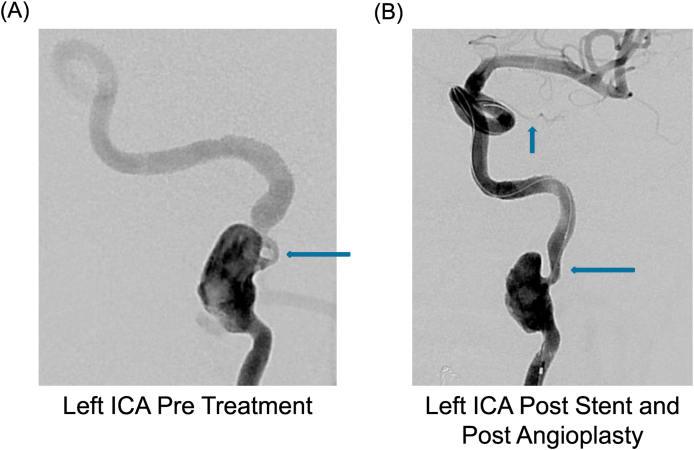
The patient underwent successful repair with angioplasty and stenting of the flow-limiting high-grade (>95%) stenosis of his left high cervical ICA (Fig. 1A) under general anesthesia. Intra-operative angiography demonstrated delayed outflow into the left petrous, cavernous, and supraclinoid internal carotid artery as well as the middle cerebral artery prior to stenting. The left ophthalmic artery was also noted to be small in caliber. The patient was heparinized during the procedure (with therapeutic elevation of activated clotting time from 108 seconds at baseline to 261 seconds following IV heparin) and was already on aspirin and clopidogrel in preparation for intervention. Use of a mechanical distal embolic protection device was not possible due to the skull base location of the stenosis. Following stenting, angiography demonstrated improved intracranial blood flow and no intracranial arterial branch occlusions (Fig. 1B). Trans-stent coiling was deferred until a later date in order to allow the Enterprise stent (Johnson & Johnson) to endothelialize in place and reduce the chance of stent displacement by the coiling catheter.
Fig. 1.
Left sided ICA angiography pre-treatment (A) shows the cervicopetrous junction pseudoaneurysm with severe vessel narrowing (horizontal arrow). Post-stent and post-angioplasty (B), there was a significant decrease in the vessel stenosis (horizontal arrow). The vertical arrow points to the left sided ophthalmic artery, which was noted to be very small.
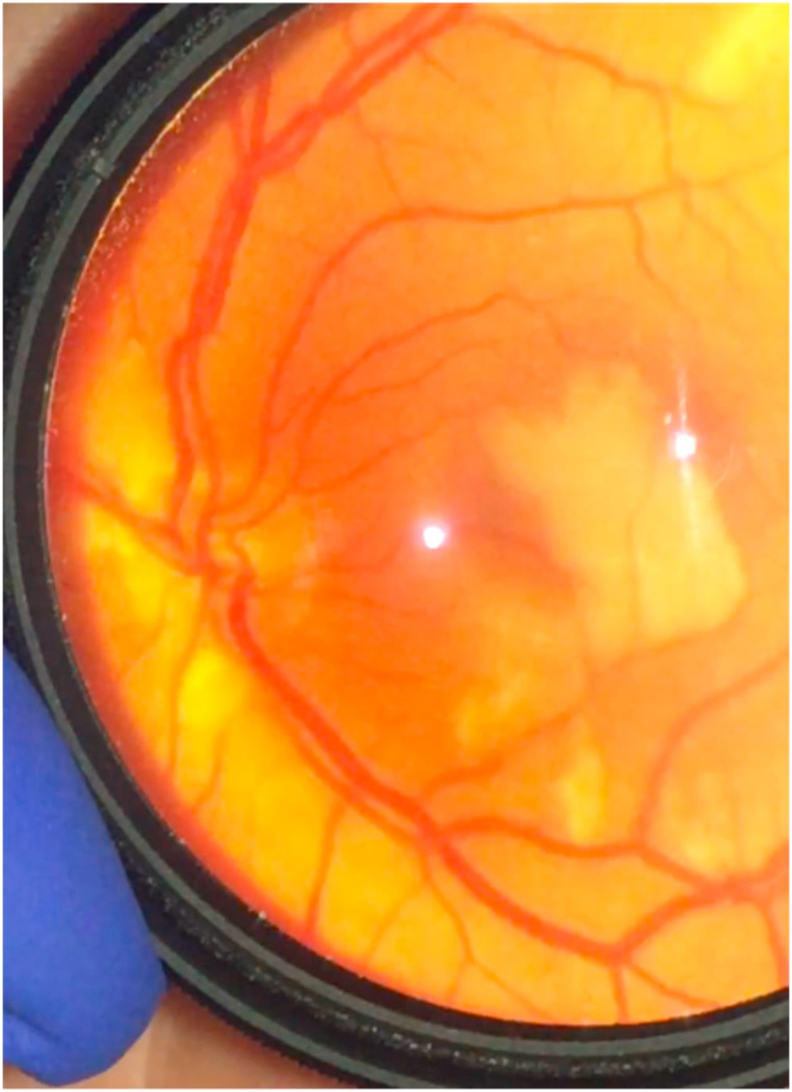
On post-operative day 1, the patient had a generalized tonic-clonic seizure in the ICU and also reported monocular central vision loss in the left eye, which, in retrospect, he had noted since the night before. On bedside examination with a near card, the patient's visual acuity was 20/30 in the right eye (OD) and 20/30 in the left eye (OS) with eccentric fixation. Without eccentric fixation, the left eye was counting fingers at approximately 7 feet. Intraocular pressures were within normal limits, and there was no relative afferent pupillary defect in either eye (the left pupil was fixed and asymmetric with posterior synechiae, which were presumably pre-existing and unrelated). Confrontational visual fields were full OD, but there was a central scotoma OS. Ocular motility was normal, and the anterior segment exam with portable slit lamp was otherwise unremarkable. Dilated fundus exam was normal in the right eye. The left posterior segment was notable for a large central macular area of retinal whitening and multiple scattered areas of perivascular retinal whitening along the superior and inferior arcades (particularly at terminal branches) (Fig. 2). There were no obvious intra-vascular emboli. These findings were concerning for likely multiple branch retinal artery occlusions or a cilioretinal artery occlusion from peri-procedural emboli, which may have occurred during or shortly after angioplasty and stenting of the high-grade stenosis just distal to the pseudoaneurysm. The patient underwent aspirin (ARU) and platelet reactivity (PRU P2Y12) testing, with values of 552 and 10, respectively, indicating therapeutic effect of both aspirin and clopidogrel. A CT angiogram of the brain and neck was obtained, which was negative for acute infarct or intracranial hemorrhage. The patient was discharged on dual antiplatelet therapy.
Fig. 2.
Left eye dilated fundus exam photograph taken at bedside in the ICU on post-operative day 1.
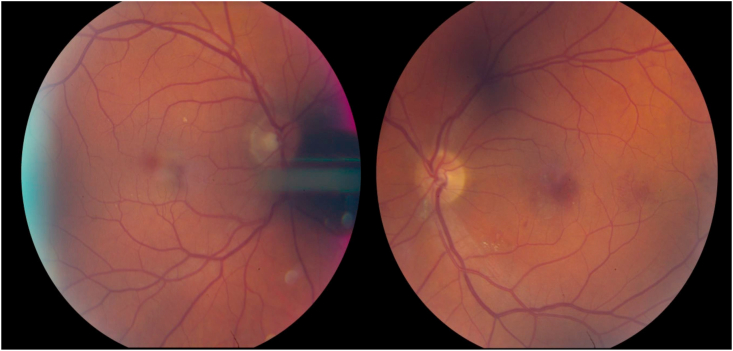
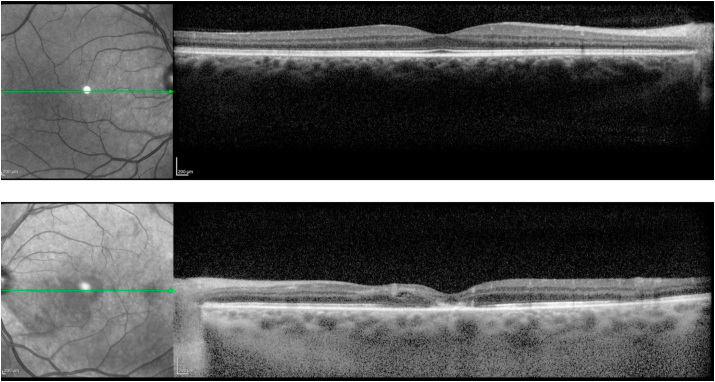
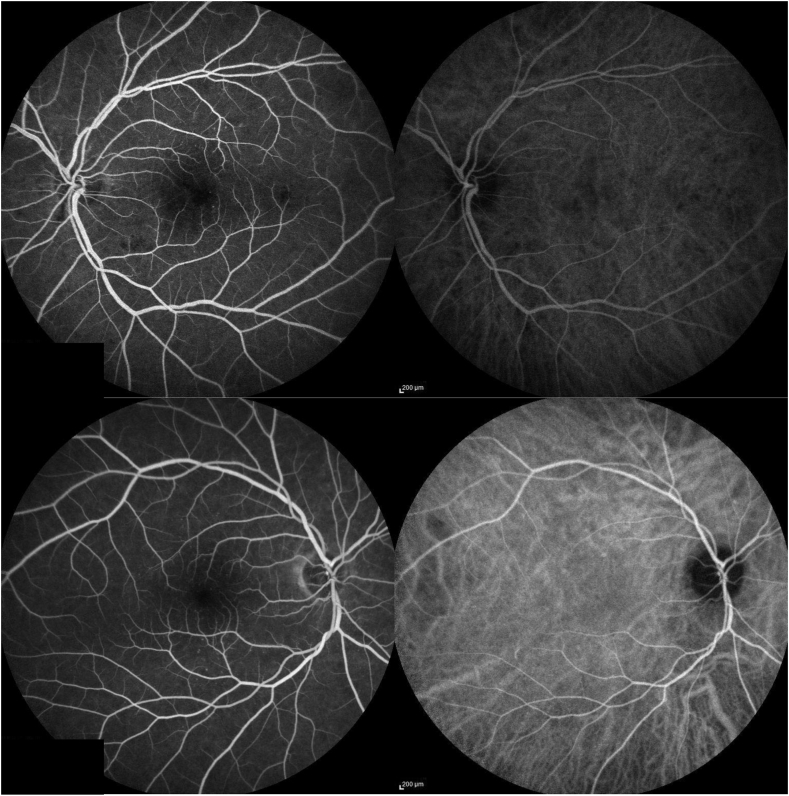
At follow up 6 weeks later in the clinic, visual acuity was 20/20 OD and counting fingers at 5 ft OS (without eccentric fixation), with persistent left central scotoma. Intraocular pressures, pupillary responses, and ocular motility remained normal. There were no anterior segment findings suggestive of ischemia. The left fundus examination had evolved, with the multiple areas of prior retinal whitening now replaced with scattered hemorrhages in the same distribution, microaneurysms, and a few retinal exudates (Fig. 3). Optical coherence tomography (Spectralis, Heidelberg Engineering, Heidelberg, Germany) revealed atrophic changes in the left macula with thinning and photoreceptor dropout involving the fovea (Fig. 4). Fluorescein and indocyanine green angiography (Spectralis, Heidelberg Engineering, Heidelberg, Germany) revealed a few scattered microaneurysms, but no major retinal arterial occlusion, neovascularization, or evidence of choroidal infarction (Fig. 5). Shortly thereafter, the patient completed stage-2 repair with trans-stent coiling of the left ICA pseudoaneurysm, followed two months later by uncomplicated staged Atlas (Stryker Neurovascular, Fremont, CA, USA) stent-assisted coiling of the right ICA pseudoaneurysm. His left eye visual acuity and retinal findings remained stable 4 months later.
Fig. 3.
Ultra-widefield color fundus photographs obtained two months post-operatively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Optical coherence tomography reveals normal retinal structure and anatomy of the right eye and distortion, atrophic thinning, and photoreceptor layer drop out in the left eye.
Fig. 5.
Fluorescein and indocyanine green angiography of the left eye (top) and the right eye (bottom). The left-sided images in each pair are fluorescein angiography, the right-sided images in each pair are indocyanine green angiography.
3. Discussion
While cerebral emboli resulting in stroke are one of the well-recognized possible complications from carotid endovascular procedures or carotid artery endarterectomy (CAE),6 retinal emboli and ischemia resulting in vision loss are another potential source of significant postoperative impairment. To the best of our knowledge, this case represents the first report of retinal emboli and ischemia specifically in the context of cervicopetrous junction ICA nonatherosclerotic pseudoaneurysm repair, despite dual antiplatelet therapy and intraprocedural heparinization. However, numerous prior reports have documented the rate of retinal embolization following cervical carotid artery stenting (CAS) or CAE for the treatment of atherosclerotic carotid stenosis. In a study of 61 consecutive patients with severe carotid artery stenosis (70–99%), 4.9% of patients who were treated with CEA and 16.9% of patients who underwent CAS exhibited postoperative retinal emboli.7 One of the CAS patients suffered a decrease in visual acuity and visual field (with multiple retinal emboli), while the other patients with retinal emboli were visually asymptomatic. Two other reports found that retinal embolization occurred in 4% (6/118) and 15% (5/33) of patients after CAS, with the majority also asymptomatic for vision change or visual field defect.8,9 Of note, while it is most likely that the pattern of multiple areas of macular ischemia observed in this patient resulted from a shower of tiny emboli (too small to visualize at bedside) secondary to the carotid pseudoaneurysm stenting procedure, an alternate explanation would be a period of intra-operative severe relative hypoperfusion of the retina that most prominently affected the branch and terminal arterioles, with subsequent reperfusion after a period of irreversible ischemia, as demonstrated on the fluorescein angiogram obtained later in clinic.
Unfortunately, there is no established, evidence-based effective treatment for acute retinal artery occlusion, including central retinal artery occlusion (CRAO). Conservative treatment options (aspirin, topical beta-blockers, anterior chamber paracentesis, ocular massage) offer little benefit. Intra-arterial thrombolysis (IAT) has been explored for CRAO therapy, but not for branch retinal artery occlusion or distal retinal emboli such as in this case. There is still limited and variable data about the efficacy of IAT, though it may be a promising therapeutic in the future for patients if treated within the first few hours of symptom onset, before irreversible retinal ischemia occurs.10, 11, 12, 13 All patients with an acute retinal artery occlusion should undergo an urgent, complete stroke evaluation and should be referred directly to the nearest American Heart Association Comprehensive Stroke Center in the region.
Although rare, it is important that patients be informed about the risk of permanent vision loss as a result of carotid vascular and endovascular procedures. In addition, patients with pre-operative visual symptoms (as in this case) should ideally be referred for ophthalmology evaluation prior to proceeding with endovascular surgical repair. Patients with new visual symptoms post-operatively should be promptly evaluated by an ophthalmologist following a carotid intervention with a dilated examination, fluorescein angiogram, optical coherence tomography, and visual field testing to monitor any areas of retinal ischemia. Patients with significant vision loss should also be referred for polycarbonate protective lenses and low vision resources, given their monocular status.
Patient consent
Written consent to publish this case was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
This study was supported in part by That Man May See, Inc., San Francisco, CA, an unrestricted grant from Research to Prevent Blindness, New York, NY, and The National Eye Institute (EY002162, Core Grant for Vision Research), Bethesda, MD.
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The authors have no relevant financial disclosures or conflicts of interest.
Contributor Information
Zesemayat K. Mekonnen, Email: zesemayat.mekonnen@ucsf.edu.
Lesley A. Everett, Email: lesley.everett@ucsf.edu.
Steven W. Hetts, Email: steven.hetts@ucsf.edu.
Armin R. Afshar, Email: Armin.Afshar@ucsf.edu.
References
- 1.Pearson S.E., Choi S.S. Pseudoaneurysm of the internal carotid artery: a case report and review of the literature. Arch Otolaryngol Head Neck Surg. 2005;131(5):454–456. doi: 10.1001/archotol.131.5.454. May. [DOI] [PubMed] [Google Scholar]
- 2.Cruciata G., Parikh R., Pradhan M., Shah J., Greif E., Stein E.G. Internal carotid artery dissection and pseudoaneurysm formation with resultant ipsilateral hypoglossal nerve palsy. Radiology case reports. 2017;12(2):371–375. doi: 10.1016/j.radcr.2017.01.016. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daou B., Hammer C., Chalouhi N. Dissecting pseudoaneurysms: predictors of symptom occurrence, enlargement, clinical outcome, and treatment. J Neurosurg. 2016;125(4):936–942. doi: 10.3171/2015.10.JNS151846. Oct. [DOI] [PubMed] [Google Scholar]
- 4.Paraskevas K.I., Batchelder A.J., Naylor A.R. Fate of distal false aneurysms complicating internal carotid artery dissection: a systematic review. Eur J Vasc Endovasc Surg : the official journal of the European Society for Vascular Surgery. 2016;52(3):281–286. doi: 10.1016/j.ejvs.2016.03.021. Sep. [DOI] [PubMed] [Google Scholar]
- 5.Hwang C.J., Moonis G., Hurst R.W., Hockstein N., Bigelow D. Bilateral petrous internal carotid artery pseudoaneurysms presenting with sensorineural hearing loss. AJNR. American journal of neuroradiology. 2003;24(6):1139–1141. Jun-Jul. [PMC free article] [PubMed] [Google Scholar]
- 6.McPhee J.T., Schanzer A., Messina L.M., Eslami M.H. Carotid artery stenting has increased rates of postprocedure stroke, death, and resource utilization than does carotid endarterectomy in the United States, 2005. J Vasc Surg. 2008;48(6):1442–1450. doi: 10.1016/j.jvs.2008.07.017. Dec. 1450 e1441. [DOI] [PubMed] [Google Scholar]
- 7.Song G., Sun R., Chen Y.F. Retinal embolization after carotid endarterectomy and stenting for carotid artery stenosis. J Clin Neurosci : official journal of the Neurosurgical Society of Australasia. 2015;22(8):1298–1302. doi: 10.1016/j.jocn.2015.01.033. Aug. [DOI] [PubMed] [Google Scholar]
- 8.Vos J.A., van Werkum M.H., Bistervels J.H., Ackerstaff R.G., Tromp S.C., van den Berg J.C. Retinal embolization during carotid angioplasty and stenting: periprocedural data and follow-up. Cardiovasc Intervent Radiol. 2010;33(4):714–719. doi: 10.1007/s00270-009-9775-4. Aug. [DOI] [PubMed] [Google Scholar]
- 9.Wilentz J.R., Chati Z., Krafft V., Amor M. Retinal embolization during carotid angioplasty and stenting: mechanisms and role of cerebral protection systems. Cathet Cardiovasc Interv : official journal of the Society for Cardiac Angiography & Interventions. 2002;56(3):320–327. doi: 10.1002/ccd.10232. Jul. [DOI] [PubMed] [Google Scholar]
- 10.Hakim N., Hakim J. Intra-arterial thrombolysis for central retinal artery occlusion. Clin Ophthalmol. 2019;13:2489–2509. doi: 10.2147/OPTH.S232560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page P.S., Khattar N.K., White A.C. Intra-arterial thrombolysis for acute central retinal artery occlusion: a systematic review and meta-analysis. Front Neurol. 2018;9:76. doi: 10.3389/fneur.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultheiss M., Hartig F., Spitzer M.S. Intravenous thrombolysis in acute central retinal artery occlusion - a prospective interventional case series. PloS One. 2018;13(5) doi: 10.1371/journal.pone.0198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayreh S.S., Jonas J.B. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol. 2000;129(6):786–795. doi: 10.1016/s0002-9394(00)00384-6. Jun. [DOI] [PubMed] [Google Scholar]