Abstract
Background
Most current guidelines do not recommend the serial analysis of tumour marker CA 15.3 in the follow-up of asymptomatic patients treated for early breast cancer (EBC). These guidelines are based on small-scale studies carried out in an era with more limited treatment options than today. In our large academic centre, serial measurements of CA 15.3 are used routinely in the follow-up of EBC, whereas imaging for distant metastases is only carried out on indication.
Patients and methods
In this retrospective single-centre study, patients were included if they were treated for EBC between 1 January 2000 and 1 January 2018, diagnosed with secondary metastatic disease at least 6 months after initial surgery and had CA 15.3 available at the time of diagnosis of metastases. The primary objective was to evaluate the proportion of patients in whom metastatic disease was discovered by an increasing CA 15.3. Information on the method of metastases detection, CA 15.3 evolution and survival was collected after approval of the ethics committee.
Results
At the moment of diagnosis of metastases, 451 of 730 included patients (62%) had CA 15.3 levels above the upper limit of normal (>30 kU/l). In 269 patients (37%), an increasing CA 15.3 was the first sign that led to the diagnosis of metastases. This was most frequent in luminal A-like tumours (48%) and in liver (45%) and bone (41%) localisation of metastases. By contrast, reported symptoms triggered the diagnosis of metastatic disease in 48% of the patients. Median overall survival was significantly longer when the relapse was discovered by CA 15.3 elevation versus those discovered by another trigger (abnormal clinical examination or history, abnormal laboratory tests or an incidental finding) (35 versus 22 months; P = 0.0027).
Conclusion
When CA 15.3 is systematically used in the follow-up of EBC patients, the diagnosis of metastatic disease is made in 37% by a CA 15.3 increase.
Key words: early breast cancer, detection metastases, CA 15.3
Highlights
-
•
When CA 15.3 is routinely followed in patients treated for EBC, CA 15.3 elevation is the first sign of metastases in 37%.
-
•
At the moment of diagnosis of metastases, 62% of the patients had CA 15.3 levels above the upper limit of normal (>30 kU/l).
-
•
Reported symptoms remain the most important indicator and led to the diagnosis of metastases in 48% of the patients.
Introduction
The European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) recommend a regular evaluation with history and physical examination, as well as an annual mammography in the follow-up of patients with early breast cancer (EBC). The serial measurement of tumour markers such as CA 15.3 in the follow-up of these patients is discouraged, due to the lack of data that can produce a survival benefit.1, 2, 3
Contrary to these current guidelines, many oncologists carry out a serial assessment of blood-based tests (like measurement of the tumour marker CA 15.3) as part of routine follow-up in asymptomatic patients with EBC.4, 5, 6, 7 Serial measurement of CA 15.3 after primary treatment of EBC can detect preclinical recurrent disease with lead times of about 2-9 months, but the clinical significance of this finding remains unclear.8, 9, 10
The current guidelines are based on a limited number of studies showing no clear difference in disease-free survival and overall survival.11,12 In the non-randomised study of Joseph et al.,12 126 patients with recurrent breast cancer were identified during a 10-year period. Twenty-seven of them (21%) were classified as an ‘intensive method of detection group’ (liver function tests, tumour markers, chest radiograph, computer tomography scan and bone scan); 99 patients (79%) were classified as the ‘minimal detection group’ (history, physical examination and mammography). There was no significant difference between the time of recurrence detection between the two groups and the method of detection did not significantly affect survival.12 Kokko et al.11 studied the cost of follow-up of 472 EBC patients without distant metastasis after primary treatment in four different schedules in a randomised trial; the mean follow-up was 4.2 years. The four schedules differed in frequency of follow-up visits (every third or sixth month) and in the intensity of diagnostic examinations. Diagnostic examinations were done routinely in one group and only when clinically indicated in the other group. Diagnostic examinations that were carried out routinely included blood tests and CA 15.3 determination every visit, chest radiograph every 6 months, liver ultrasound and bone scan every 2 years. Neither the frequency of visits nor the intensity of diagnostic examinations had any effect on disease-free or overall survival of patients. The more intense schemes even increased the cost of follow-up 2.2 times.11 Kokko et al.13 also carried out another prospective study in which 243 EBC patients were followed after primary treatment until the first relapse. During the 5-year follow-up period, 24% of relapses were discovered, and CA 15.3 was elevated (≥40 IU/l) in 36% of these patients at least once. There was a positive CA 15.3 level in 3% of patients without recurrence. The authors concluded that CA 15.3 is specific, but not sensitive enough to indicate the first relapse earlier than other methods.13
The limitations of these studies are small sample size and a short follow-up period.11 Additionally, they were carried out in the distant past, preceding novel advances in diagnostics and therapies. Nowadays, many of the treatment regimens are more effective and less toxic than the older types of medication.5 Some studies confirmed the validity of serial CA 15.3 assays in the early diagnosis of metastatic disease and showed that CA 15.3 elevation is more often seen in the follow-up of EBC patients who develop metastases.9,14, 15, 16
There is a lot of controversy about this subject and as a result of this, at least one international group, the European Group on Tumour Markers (EGTM), recommends serial CA 15.3 (and carcinoembryonic antigen, CEA) serum measurements for the early detection of recurrence in patients with EBC. This is only advised, however, if the detection of metastatic disease would alter clinical management. They suggest follow-up of asymptomatic women who were treated for EBC and propose to determine tumour markers every 2-4 months during the initial 5 years after diagnosis, then less frequently thereafter. Nevertheless, they acknowledge that the impact of these results on clinical outcome is unclear.17
Disadvantages of regular blood checks are that they can increase fear in patients, cause false-positive values leading to additional useless investigations and lead to earlier diagnosis of incurable metastatic disease (in which case earlier detection could temporarily decrease a patient's quality of life). Additionally, CA 15.3 is not elevated in all patients with metastatic disease and low CA 15.3 levels may provide false reassurance to the patient.8,10
By contrast, earlier detection of metastases may also have relevant theoretical advantages: (i) a higher detection rate of oligometastatic disease, where an aggressive treatment approach could potentially be related to improved outcome; (ii) earlier detection of metastatic disease, which is potentially genomically more stable, and more responsive to anticancer therapy; (iii) less need for first-line salvage chemotherapy in hormone-sensitive disease; (iv) avoid situations where the diagnosis is made through symptoms like jaundice and severe liver failure precluding the use of proper systemic therapy; and (v) regular blood test and follow-up of the tumour marker can also give a feeling of security and reassurance to the patients, which may impact the quality of life.5,18
In our hospital network, the University Hospitals Leuven, the standard follow-up for patients with EBC is a regular clinical examination carried out by experienced doctors from the multidisciplinary breast centre and a yearly mammography. Systematic imaging for distant metastases is not carried out unless clinically indicated. Given the controversial data on the use of CA 15.3 in follow-up of EBC patients and the experience that this strategy can also have potential benefits, the use of systematic blood sampling including CA 15.3, calcium and liver tests has carried on until present. Omission of this blood sampling was only done in specific situations such as the patient asking for the pros and cons of this strategy and deciding to omit it. In this study, we want to evaluate in our centre how many of the diagnoses of secondary metastatic disease were discovered by abnormal CA 15.3 and in which subgroups this occurs more frequently. We also aimed to assess if the outcome of this group is different from patients in whom symptoms or other abnormal laboratory values triggered the diagnosis of metastases.
Methods
Study design
In this retrospective single-centre study, eligible patients were those treated for EBC at the University Hospitals Leuven between 1 January 2000 and 1 January 2018 and diagnosed with secondary metastatic breast cancer at least 6 months after the initial surgery. The curative surgery of the primary breast cancer must have taken place at the University Hospitals Leuven and the pathological report of the tumour should be available. Patients must have remained in follow-up at the University Hospitals Leuven until the diagnosis of metastatic disease. Patients with only locoregional recurrence of the disease (without distant metastases) were not included. The availability of a CA 15.3 value measured within 2 months before the detection of metastasis was required. This CA 15.3 value needed to be assessed before any systemic therapy for metastatic disease was started. CA 15.3 was measured according to the standard clinical practice using an electrochemiluminescence immunoassay (ECLIA) from Roche (Basel, Switzerland). Patients were excluded when the origin of metastases was unclear (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100203).
Standard follow-up of patients with EBC in the University Hospitals Leuven is carried out every 4 months during the first year after treatment. Every visit includes a thorough history to identify potential symptoms, a physical examination and a routine blood test (calcium, liver tests and CA 15.3). A mammography is carried out once a year, and the radiologist decides if it is necessary to carry out an additional ultrasound or magnetic resonance imaging scan of the breast. In the following 2 years, patients are seen every 4-6 months depending on their risk of relapse. After 3 years, patients visit for a clinical examination every 6 months. After 5 years, the follow-up period is prolonged to 1 year and 10 years after the treatment of EBC, the follow-up interval becomes 2 years (depending on the patient's life expectancy and general condition). Follow-up can be intensified for high-risk patients, defined by the treating oncologist. During the timespan of the study, the majority of patients were not discharged from follow-up with an oncologist.
For all potentially eligible patients the individual electronic medical files were screened for inclusion and exclusion criteria. For patients fulfilling the criteria, the following data were collected for each patient: age of the patient at diagnosis of EBC and at diagnosis of metastatic disease, characteristics of the primary breast tumour [tumour histology, tumour grade, pathological and clinical TNM19 (tumour–node–metastasis) stage, receptor status] and characteristics at the first diagnosis of metastases (localisation of relapse, treatment approach). Sequential laboratory values for CA 15.3, calcium and liver tests until detection of distant metastases were collected. Finally, we gathered information about the long-term follow-up (time and cause of death).
The study was completed in compliance with the principles of the Declaration of Helsinki and approved by the ethics committee of the University Hospitals Leuven (S62467). All data were entered in an anonymised database following the general data protection regulation of the University Hospitals Leuven.
Outcome measures
The primary outcome was the proportion of diagnoses of secondary metastatic disease triggered by a CA 15.3 elevation. A subgroup analysis based on different breast cancer subtypes, stage of EBC, age of the patients at diagnosis of EBC and the localisation of metastases was carried out.
Definitions for hormone sensitivity and/or human epidermal growth factor receptor 2 (HER2) positivity of breast cancer are based on the ASCO guidelines of 2018. Samples with 1%-100% of tumour nuclei positive for estrogen receptor (ER) or progesterone receptor (PR) are interpreted as positive. A sample is considered negative for ER or PR if <1% of tumour cell nuclei are immunoreactive.20 The HER2 status is initially assessed by immunohistochemistry (IHC) using a semi-quantitative scoring system and confirmed by FISH in all IHC cases with a score of 2+ or 3+.21
The different breast cancer subtypes are defined by the criteria of Brouckaert et al.22 using grade instead of Ki67 positivity, because this was not always available. Luminal A-like tumours are defined as ER-positive and/or PR-positive, HER2-negative and grade 1 or 2; luminal B-like tumours are ER-positive and/or PR-positive, HER2-negative and grade 3. Luminal HER2-like tumours are defined as ER-positive and/or PR-positive, HER2-positive and HER2-like tumours are ER-negative, PR-negative, HER2-positive. Triple-negative tumours are ER-negative, PR-negative, HER2-negative.22,23
Secondary outcomes that were studied are other triggers that led to more investigation and diagnosis of metastatic disease (such as disrupted liver tests and calcium levels, complaints of the patient, abnormal clinical examination or coincidental discovery during radiological examinations or surgery/endoscopy carried out for an unrelated condition).
Furthermore, we investigated the evolution of CA 15.3 towards the moment of diagnosis of metastatic disease. We studied the progressive increase of CA 15.3 by comparing the lowest value (measured at least 6 months after treatment of EBC) with the CA 15.3 value at the moment of diagnosis of metastatic disease. CA 15.3 level at diagnosis of EBC was not taken into account because this is normal in the large majority of patients in such a setting, and an increased CA 15.3 at first diagnosis is often associated with upfront metastatic disease, certainly if levels are in the higher range >40 kU/l.10,24 Exclusion of CA 15.3 levels between primary diagnosis until 6 months afterwards was predetermined, because CA 15.3 can rise (also known as surge) temporarily after the initiation of (neo)adjuvant chemotherapy. The origin of this surge remains unclear, but false-positive CA 15.3 values related to hematopoietic stem cells released in circulation during chemotherapy (certainly if granulocyte colony-stimulating factor is given) or tumour marker shedding caused by tumour cell destruction may contribute.25, 26, 27
The first treatment approach of metastatic disease was registered. An oligometastatic approach was defined as a radical local treatment of all visible (five or fewer) distant metastases.28 Finally, as long-term time-to-event outcome, we recorded overall survival, defined as time between diagnosis of metastases and death due to any cause.
Statistical analysis
Descriptive information on patient characteristics and the primary and secondary outcomes was presented as frequencies with percentages for categorical variables or means with standard deviation for continuous variables.
To study the evolution of CA 15.3 values, individual profiles of CA 15.3 were plotted over the follow-up period before or until first metastasis. A linear mixed model was used to estimate the average evolution of CA 15.3 before metastases. CA 15.3 level was modelled as a function of months before metastasis. A non-linear trend was captured by modelling cubic splines. Random intercept and slope were modelled to deal with repeated measures. The mean evolution is presented in a graph with 95% confidence intervals (CIs).
The Kaplan–Meier method was used for estimating overall survival after metastasis. A Cox proportional hazards model was used to analyse the association between the role of CA 15.3 and risk of death. Analyses were carried out using SAS software (version 9.4 of the SAS System for Windows, SAS Institute, Cary, NC).
Results
Out of 1219 potentially eligible patients with the diagnosis of secondary metastatic breast cancer, 730 patients were selected for the final analysis based on the detailed inclusion and exclusion criteria (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100203). The baseline characteristics of the patients in our study population at the moment of diagnosis of EBC are specified in Table 1.
Table 1.
Patients and baseline characteristics of the 730 secondary metastatic patients, at the moment of early breast cancer
| Sex, n/N (%) | |
| Male | 7/730 (1) |
| Female | 723/730 (99) |
| Mean age at diagnosis of primary breast cancer, yearsa | 56 ± 13 |
| Menopausal status, n/N (%) | |
| Premenopausal | 262/730 (36) |
| Postmenopausal | 436/730 (60) |
| Perimenopausal | 25/730 (3) |
| Not applicable (male) | 7/730 (1) |
| Mean CA 15.3 value at moment of diagnosis of early breast cancer, kU/la | 25 ± 73 |
| Tumour characteristics of primary surgery group, n/N (%) | 548/730 (75) |
| Pathological tumour size n/N (%) | |
| pT1 | 161/548 (29) |
| pT2 | 296/548 (54) |
| pT3 | 88/548 (16) |
| pT4 | 3/548 (1) |
| Pathological nodal status, n/N (%) | |
| pN− | 200/548 (36) |
| pN+ | 348/548 (64) |
| Tumour characteristics of neoadjuvant group, n/N (%) | 182/730 (25) |
| Clinical tumour size, n/N (%) | |
| cT0 | 1/182 (1) |
| cT1 | 9/182 (5) |
| cT2 | 34/182 (19) |
| cT3 | 41/182 (22) |
| cT4 | 97/182 (53) |
| Clinical nodal status, n/N (%) | |
| cN− | 28/182 (15) |
| cN+ | 154/182 (85) |
| Clinical subtype of the primary breast tumour, n/N (%) | |
| Luminal A-like | 264/730 (36) |
| Luminal HER2-like | 52/730 (7) |
| Luminal B-like | 215/730 (30) |
| HER2-like | 56/730 (8) |
| Triple-negative | 143/730 (19) |
| Treatment setting, n/N (%) | |
| Neoadjuvant treatment | 182/730 (25) |
| Luminal A-like | 58/182 (32) |
| Luminal HER2-like | 16/182 (9) |
| Luminal B-like | 32/182 (17) |
| HER2-like | 23/182 (13) |
| Triple-negative | 53/182 (29) |
| Upfront surgery | 548/730 (75) |
| Luminal A-like | 206/548 (38) |
| Luminal HER2-like | 36/548 (7) |
| Luminal B-like | 183/548 (33) |
| HER2-like | 33/548 (6) |
| Triple-negative | 90/548 (16) |
HER2, human epidermal growth factor receptor 2.
Mean ± standard deviation.
Table 2 shows more information about the discovery and the characteristics of the first distant metastases. Abnormalities in the patients' history (48%) and a CA 15.3 elevation (37%) were the main factors that led to more investigations and subsequent detection of metastatic disease. Most frequent symptoms (Figure 1) reported by the patients were pain (67%) and cough or dyspnoea (28%). Less frequent (4%) triggers for the diagnosis of metastases are an abnormal clinical examination or laboratory changes other than a CA 15.3 elevation (such as disturbed liver tests or hypercalcemia). Accidental findings (e.g. on imaging, surgery or endoscopy carried out for an unrelated medical condition) could reveal metastatic disease in 7% of the patients (Table 2).
Table 2.
Characteristics of the 730 secondary metastatic patients, at the moment of metastatic disease
| Mean age at diagnosis of metastatic disease, yearsa | 60 ± 7 |
| Trigger for further investigation that led to diagnosis of metastases, n/N (%) | |
| History | 348/730 (48) |
| CA 15.3 elevation | 269/730 (37) |
| Accidental finding | 55/730 (7) |
| Clinical examination | 27/730 (4) |
| Other laboratory changes | 31/730 (4) |
| Disturbed laboratory values (other than CA 15.3) that led to the diagnosis of metastases, n/N (%) | |
| Elevated aspartate aminotransferase or alanine aminotransferase | 1/31 (3) |
| Elevated gamma-glutamyltransferase or alkaline phosphatase | 4/31 (13) |
| Elevation of all liver enzymes | 20/31 (65) |
| Hypercalcaemia | 4/31 (13) |
| Elevated carcinoembryonic antigen | 1/31 (3) |
| Elevated lactate dehydrogenase | 1/31 (3) |
| Number of patients in whom diagnosis of metastases was made by CA 15.3 increase, in relation to breast cancer subtype, n/N (%) | |
| Luminal A-like | 128/264 (48) |
| Luminal HER2-like | 23/52 (44) |
| Luminal B-like | 80/215 (37) |
| HER2-like | 17/56 (30) |
| Triple-negative | 21/143 (15) |
| Number of patients in whom diagnosis of metastases was made by CA 15.3 increase, in relation to age at diagnosis of early breast cancer, n/N (%) | |
| ≤50 Years old, diagnosed by CA 15.3 increase | 90/276 (33) |
| >50 Years old, diagnosed by CA 15.3 increase | 179/454 (39) |
| Number of patients in whom diagnosis of metastases was made by CA 15.3 increase, in relation to tumour stage at diagnosis of early breast cancer, n/N (%)19 | |
| Stage 1, diagnosed by CA 15.3 increase | 30/85 (35) |
| Stage 2, diagnosed by CA 15.3 increase | 102/300 (34) |
| Stage 3, diagnosed by CA 15.3 increase | 137/345 (40) |
| Localisation of distant metastases, n/N (%) | |
| Localised in 1 organ | 396/730 (54) |
| Localised in multiple organs | 334/730 (46) |
| Specific localisation per organ (several affected organs jointly are possible), n/N (%) | |
| Bone | 424/730 (58) |
| Lung | 237/730 (32) |
| Lymph nodes | 212/730 (29) |
| Liver | 208/730 (28) |
| Abdominal site (other than liver) | 70/730 (10) |
| Brain | 68/730 (9) |
| Skin | 36/730 (5) |
| Number of patients in whom diagnosis of metastases was made by CA 15.3 increase, in relation to localisation of first distant metastases (several affected organs jointly are possible), n/N (%) | |
| Bone | 173/424 (41) |
| Lung | 70/237 (30) |
| Lymph nodes | 74/212 (35) |
| Liver | 94/208 (45) |
| Abdominal site (other than liver) | 23/70 (33) |
| Brain | 5/68 (7) |
| Skin | 3/36 (8) |
| CA 15.3 level at time of first diagnosis of metastases | |
| Mean value, kU/la | 70 ± 134 |
| CA 15.3 above the ULN (>30 kU/l), n/N (%) | 451/730 (62) |
| >50% Increase compared with lowest value measured between 6 months after treatment of EBC and moment of distant metastases, n/N (%) | 502/730 (69) |
| Without crossing the normal value (≤30 kU/l), n/N (%) | 81/502(16) |
| Crossing the normal value (>30 kU/l), n/N (%) | 421/502 (84) |
| Not showing an increase (≤50% increase compared with lowest value measured between 6 months after treatment of EBC and moment of distant metastases), n/N (%) | 205/730 (28) |
| Increase unknown, n/N (%) | 23/730 (3) |
| CA 15.3 increase above ULN (>30 kU/l) at the moment of diagnosis of metastatic disease, in relation to breast cancer subtype, n/N (%) | |
| Luminal A-like | 193/264 (73) |
| Luminal HER2-like | 38/52 (73) |
| Luminal B-like | 139/215 (65) |
| HER2-like | 25/56 (45) |
| Triple-negative | 56/143 (39) |
| Median interval between treatment of early breast cancer and diagnosis of metastases, in monthsb | 39 (37-42) |
| Diagnosis of metastases was triggered by history (n = 348) | 37 (33-42) |
| Diagnosis of metastases was triggered by CA 15.3 elevation (n = 269) | 44 (39-54) |
| Diagnosis of metastases was triggered by accidental finding (n = 55) | 33 (28-48) |
| Diagnosis of metastases was triggered by clinical examination (n = 27) | 37 (28-77) |
| Diagnosis of metastases was triggered by other laboratory changes (n = 31) | 26 (20-48) |
EBC, early breast cancer; HER2, human epidermal growth factor receptor 2; ULN, upper limit of normal.
Mean ± standard deviation.
95% Confidence interval.
Figure 1.
Reported symptoms in the group of patients in whom an abnormal history led to the diagnosis of metastatic disease (patients could have more than one symptom).
a Indicates neurological symptoms except epilepsy, headache and dizziness.
Diagnosis of metastatic disease made by a CA 15.3 increase (37% of patients in the whole cohort) occurred more frequently in the luminal subtypes: luminal A-like (48%), luminal HER2-like (44%) and luminal B-like (37%). Interestingly, at the moment of first metastases, 62% of all patients had a CA 15.3 level above the upper limit of normal (ULN) (>30 kU/l); this was also more frequent in the luminal subgroups (Table 2).
Nearly half of the patients (46%) had metastases localised in multiple organs, bone being the most frequent (58%). Metastases in lung (32%), lymph nodes (29%) and liver (28%) are also common. Metastases detection by a CA 15.3 elevation occurred most frequently in cases of liver and bone metastases (45% and 41%) (Table 2).
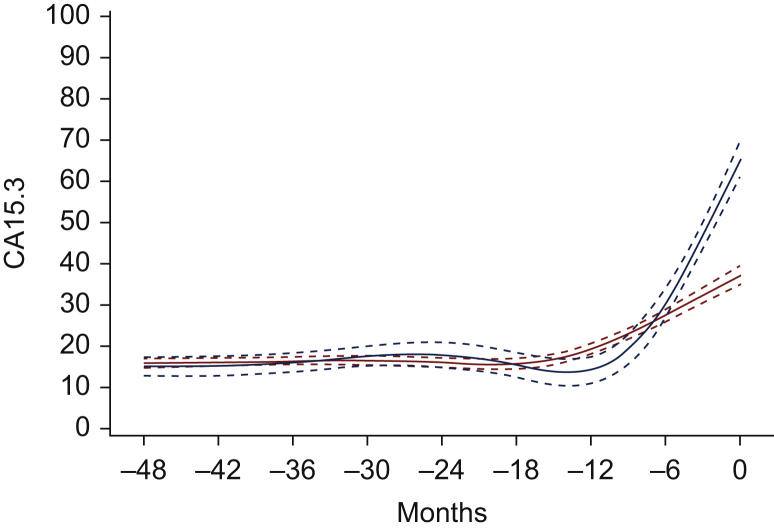
The kinetics of CA 15.3 until the first diagnosis of metastatic disease are mentioned in Table 2 and Figure 2. In 69% of the patients, we noticed an increase of the CA 15.3 level at the moment of diagnosis of metastatic disease of >50% compared with the lowest value (measured at least 6 months after treatment of EBC). For this analysis, 23 of 730 patients were excluded because there were not enough valid measurements of CA 15.3 between the detection of EBC and the diagnosis of metastatic disease. Figure 2 shows a gradual increase in CA 15.3 6-12 months before the first metastases were diagnosed.
Figure 2.
Estimated mean evolution of CA 15.3 before/until first metastasis.
The graph presents the estimated mean evolutions of CA 15.3, with 95% confidence intervals. The blue curve is estimated based on all CA 15.3 measurements before metastasis, hence excluding the value measured at the time of detection of metastasis. The red curve is estimated based on all CA 15.3 measurements until metastasis, hence including also the value measured at metastasis. The minus symbol before the months signifies the number of months prior to the time of diagnosis of metastases.
The median interval between treatment of EBC and diagnosis of secondary metastatic disease is the shortest for patients diagnosed with disturbed liver tests or hypercalcemia (26 months). By contrast, the interval for patients diagnosed by a CA 15.3 increase is the longest (44 months) (Table 2).
Table 3 gives an overview of the treatment approach of metastatic disease. An oligometastatic approach was used in 3% of the patients. Metastases of patients who were treated with an oligometastatic approach in our cohort were all located at one site and the total number of metastases was not higher than three.
Table 3.
Long-term follow-up (treatment, mortality and survival rates) of the 730 secondary metastatic patients
| Treatment, n/N (%) | |
| Oligometastatic approach | 21/730 (3) |
| Luminal A-like | 6/21 (29) |
| Luminal HER2-like | 3/21 (14) |
| Luminal B-like | 1/21 (5) |
| HER2-like | 2/21 (9) |
| Triple-negative | 9/21 (43) |
| Number of patients within the oligometastatic approach group in whom CA 15.3 elevation was the trigger for diagnosis of metastases | 6/21 (29) |
| Number of patients within the group in whom CA 15.3 was the trigger for diagnosis of metastases, who received oligometastatic approach | 6/271 (2) |
| Mortality, n/N (%) | |
| Overall mortality | 570/730 (78) |
| Breast cancer-related mortality | 528/570 (93) |
| Other cause of mortality | 28/570 (5) |
| Reason of mortality unknown | 14/570 (2) |
| Mortality in group in whom CA 15.3 was the trigger for the first diagnosis of metastases, n/N (%) | |
| Overall mortality | 196/269 (73) |
| Breast cancer-related mortality | 185/196 (94) |
| Other cause of mortality | 7/196 (4) |
| Reason of mortality unknown | 4/196 (2) |
| Median overall survival in relation to detection method of metastases, in monthsa | |
| Median overall survival in group in whom CA 15.3 was the trigger for the diagnosis of metastases (n = 269) | 35 (29-39) |
| Luminal A-like (n = 128) | 40 (32-46) |
| Luminal HER2-like (n = 23) | 33 (18-42) |
| Luminal B-like (n = 80) | 34 (26-40) |
| HER2-like (n = 17) | 21 (10-60) |
| Triple-negative (n = 21) | 14 (5-26) |
| Median overall survival in groups in whom CA 15.3 was NOT the trigger for the diagnosis of metastases (n = 461) | 22 (18-26) |
| Luminal A-like (n = 137) | 36 (29-46) |
| Luminal HER2-like (n = 29) | 25 (15-37) |
| Luminal B-like (n = 135) | 27 (20-30) |
| HER2-like (n = 39) | 32 (14-44) |
| Triple-negative (n = 121) | 12 (9-16) |
HER2, human epidermal growth factor receptor 2.
95% Confidence interval.
Overall survival was significantly longer when the relapse was discovered by a CA 15.3 elevation versus those discovered by another trigger [median 35 versus 22 months; hazard ratio 0.77 (95% confidence interval 0.645-0.913); P = 0.0027]. Patients with triple-negative breast cancer had the worst prognosis in both groups. By contrast, patients with luminal A-like breast cancer had the best prognosis in both groups (Table 3, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100203).
Discussion
Most current guidelines do not recommend the determination of CA 15.3 in the follow-up of asymptomatic patients treated for EBC. In practice, however, considering the limited amount of discouraging evidence opposed to the potential benefits, serial determinations of CA 15.3 are commonly used in the follow-up of patients with EBC.
Our study shows in more detail how far CA 15.3 contributes to the diagnosis of secondary metastatic disease: it led to the diagnosis of secondary distant metastases in 37% of the whole secondary metastatic population in our centre. CA 15.3 seems most sensitive for detecting liver and bone metastases (Table 2), confirming prior studies.14,29 Bone and liver metastases are among the most frequent sites of metastases (especially in luminal disease) and are more difficult to find by clinical examination. Serial CA 15.3 measurements in the follow-up have the potential to contribute to earlier detection of this site of metastases, that can sometimes be immediately life-threatening if discovered too late (e.g. liver failure or hypercalcemia).
At the moment of diagnosis of recurrent disease, 62% of the patients had a CA 15.3 level above the ULN. This indicates that nearly two-thirds of secondary metastatic breast cancers are associated with increased CA 15.3, especially in the luminal subgroups (Table 2). These findings are also described in the literature. Bensouda et al.30 found that 62% of patients had a CA 15.3 increase at diagnosis of metastatic disease and ER/PR positivity was strongly correlated with an elevated CA 15.3.30 Also, the group of Geng et al.14 confirmed that cases with luminal subtypes exhibited a higher percentage of elevated CA 15.3 and CEA levels compared with non-luminal subtypes.14 This can be explained by the fact that luminal subtypes show a higher expression in mucin 1 genes and CA 15.3 is derived from proteolytic shedding of the extracellular domain of mucin 1 glycoprotein.31 Additionally, it is hypothesised that less differentiated subtypes lack certain tumour antigens (like CA 15.3) and as such, have less potential for spreading CA 15.3 in circulation.14,32 In contrast to the localisation of the metastases or the presence of a luminal subtype, the patients' age at diagnosis of EBC and disease stage could not be identified as potential clinical factors associated with metastatic recurrence detected by CA 15.3 alone (Table 2).
The kinetic evaluation of CA 15.3 before the diagnosis of metastases also displays interesting patterns. In 69% of the patients, an increase of the CA 15.3 level of >50% compared with the lowest value was seen at the moment of recurrent disease. The curves in Figure 2 show that an increase in CA 15.3 can be seen 6-12 months before the diagnosis of metastatic disease.
Furthermore, we should highlight the importance of reported symptoms that led to the diagnosis of metastatic disease in 48% of the patients. Most frequent symptoms were pain (67%), cough or dyspnoea (28%). This is in line with findings of a recent Danish study, which examined the recurrences of breast cancer at scheduled outpatient visits. The patients treated for EBC who developed distant relapse reported pain (58%) and dyspnoea (23%) most often. The pain was most often caused by bone metastases.33 In our patients with bone metastases, 63% reported pain. Our study confirms that a detailed history-taking remains the most important tool to discover distant metastases.
An oligometastatic approach was used in 21 patients (3%); a tumour marker elevation triggered the diagnosis in only 6 of them. These low numbers and the absence of a control group do not support the conclusion that sequential CA 15.3 measurements more frequently allow an oligometastatic approach. Data were collected between 2000 and 2018, a time frame in which an oligometastatic approach was not used that frequently, so it is probable that they do not adequately reflect the patients in whom an oligometastatic approach is considered useful. It may still be that CA 15.3 measurement allows detection of metastatic disease that is genomically more stable compared with late metastatic disease, and as such, is potentially more responsive to anticancer therapy. Only prospective randomised data can provide solid proof for this hypothesis.
In contrast with some previously described studies, we could show a statistically significant difference in median overall survival between the group in whom diagnosis of metastatic disease was triggered by a CA 15.3 increase compared with the group in whom metastases were discovered by another trigger.11,12 Lead time bias probably contributes at least partially to the prolonged overall survival in patients in whom metastases were discovered by CA 15.3. Additionally, the unequal distribution of the different molecular subtypes could influence this result. No solid conclusions can be drawn on impact on survival based on our study results.
The present data do not allow us to conclude that metastases were discovered earlier by a CA 15.3 elevation in comparison with other triggers (Table 2). Most likely the tumours detected by a CA 15.3 increase are more differentiated and slower evolving tumours.14,32 This can also contribute to the longer overall survival in this subgroup.
It is important to realise that an elevation of CA 15.3 does not always lead to the diagnosis of metastatic disease. Benign conditions (infection, inflammation, trauma) and other malignancies may give rise to increased marker concentrations.8,10,34 Additionally, there are also attempts to find other, more sensitive and specific blood biomarkers that can give an earlier diagnosis of distant breast cancer metastases. Research is ongoing about the use of circulating tumour cells and (personalised) circulating tumour DNA profiling for detection of recurrence in breast cancer.35 Further research is needed to establish if combinations of different markers can further improve sensitivity and accuracy.
Costs of CA 15.3 measurement should be considered when discussing the pros and cons of this approach. CA 15.3 determination is considered as a relatively inexpensive and easily performed test. Repeated measurements during the follow-up of patients with EBC and additional examinations that are carried out when a relapse is suspected, however, contribute to higher costs.6 In Belgium, a single CA 15.3 determination incurs an average cost of €22.5 for the society. In-depth analysis of the financial consequences of CA 15.3 screening is unfortunately not available.
Besides the financial cost, a major emotional impact should be considered when using CA 15.3 in the follow-up of EBC. It can give a feeling of safety to the patient, but can also cause a (sometimes unnecessary) feeling of anxiety.10 A discussion with the patients about the advantages and disadvantages of measuring the CA 15.3 values is crucial.
Some limitations of our study design should be noted. Our study is a retrospective single-centre study, without a control group in whom CA 15.3 was not systematically measured. The only way to prove that the follow-up of CA 15.3 leads to a better outcome and cost-efficacy is a prospective randomised trial in our current treatment era of targeted therapies. It is unlikely that this type of study will be carried out in the near future, as finding sponsorship may be particularly challenging.
In conclusion, in a population undergoing serial measurements of CA 15.3 in the follow-up of EBC, increased CA 15.3 values are the first sign of secondary metastatic breast cancer in about one-third of patients, while increased values are present irrespective of other signs or symptoms in about two-thirds of patients with secondary metastatic breast cancer. This retrospective dataset cannot be used to establish clinical utility for CA 15.3 monitoring and additional prospective randomised trials are needed to demonstrate clinical utility. The decision to use CA 15.3 in the follow-up of EBC patients should be made together by the treating physician and patient after discussing potential advantages and disadvantages.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Cardoso F., Kyriakides S., Ohno S. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 2.Harris L., Fritsche H., Mennel R. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 3.Khatcheressian J.L., Hurley P., Bantug E. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:961–965. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 4.Hahn E.E., Hays R.D., Kahn K.L., Litwin M.S., Ganz P.A. Use of imaging and biomarker tests for posttreatment care of early-stage breast cancer survivors. Cancer. 2013;119:4316–4324. doi: 10.1002/cncr.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry N.L., Hayes D.F., Ramsey S.D., Hortobagyi G.N., Barlow W.E., Gralow J.R. Promoting quality and evidence-based care in early-stage breast cancer follow-up. J Natl Cancer Inst. 2014;106:dju034. doi: 10.1093/jnci/dju034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsey S.D., Henry N.L., Gralow J.R. Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol. 2015;33:149–155. doi: 10.1200/JCO.2014.55.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating N.L., Landrum M.B., Guadagnoli E., Winer E.P., Ayanian J.Z. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007;25:1074–1081. doi: 10.1200/JCO.2006.08.6876. [DOI] [PubMed] [Google Scholar]
- 8.Duffy M.J. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 9.Wojtacki J., Kruszewski W.J., Sliwińska M. Elevation of serum Ca 15-3 antigen: an early indicator of distant metastasis from breast cancer. Retrospective analysis of 733 casesPrzegl Lek. 2001;58:498–503. [PubMed] [Google Scholar]
- 10.Duffy M.J., Evoy D., McDermott E.W. CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411:1869–1874. doi: 10.1016/j.cca.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Kokko R., Hakama M., Holli K. Follow-up cost of breast cancer patients with localized disease after primary treatment: a randomized trial. Breast Cancer Res Treat. 2005;93:255–260. doi: 10.1007/s10549-005-5199-2. [DOI] [PubMed] [Google Scholar]
- 12.Joseph E., Hyacinthe M., Lyman G.H. Evaluation of an intensive strategy for follow-up and surveillance of primary breast cancer. Ann Surg Oncol. 1998;5:522–528. doi: 10.1007/BF02303645. [DOI] [PubMed] [Google Scholar]
- 13.Kokko R., Holli K., Hakama M. Ca 15-3 in the follow-up of localised breast cancer: a prospective study. Eur J Cancer. 2002;38:1189–1193. doi: 10.1016/s0959-8049(01)00429-4. [DOI] [PubMed] [Google Scholar]
- 14.Geng B., Liang M.M., Ye X.B., Zhao W.Y. Association of CA 15-3 and CEA with clinicopathological parameters in patients with metastatic breast cancer. Mol Clin Oncol. 2015;3:232–236. doi: 10.3892/mco.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahrami-Ahmadi A., Makarian F., Mortazavizadeh M.R., Yazdi M.F., Mehdi Chamani M. Symptomatic metastasis prediction with serial measurements of CA 15.3 in primary breast cancer patients. J Res Med Sci. 2012;17:850–854. [PMC free article] [PubMed] [Google Scholar]
- 16.De La Lande B., Hacene K., Floiras J.L., Alatrakchi N., Pichon M.F. Prognostic value of CA 15.3 kinetics for metastatic breast cancer. Int J Biol Markers. 2002;17:231–238. doi: 10.1177/172460080201700403. [DOI] [PubMed] [Google Scholar]
- 17.Molina R., Barak V., van Dalen A. Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol. 2005;26:281–293. doi: 10.1159/000089260. [DOI] [PubMed] [Google Scholar]
- 18.Jager W., Eibner K., Loffler B., Gleixner S., Krämer S. Serial CEA and CA 15-3 measurements during follow-up of breast cancer patients. Anticancer Res. 2000;20:5179–5182. [PubMed] [Google Scholar]
- 19.Amin M.B., Edge S.B., Greene F.L., AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 8th ed. Springer; Chicago: 2018. [Google Scholar]
- 20.Allison K.H., Hammond M.E.H., Dowsett M. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP Guideline Update. J Clin Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 21.Ahn S., Woo J.W., Lee K., Park S.Y. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med. 2020;54:34–44. doi: 10.4132/jptm.2019.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouckaert O., Rudolph A., Laenen A. Reproductive profiles and risk of breast cancer subtypes: a multi-center case-only study. Breast Cancer Res. 2017;19:119. doi: 10.1186/s13058-017-0909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldhirsch A., Winer E.P., Coates A.S. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniele A. Clinical usefulness of cancer antigen 15-3 in breast cancer patients before and after surgery. Open Breast Cancer J. 2013;5:1–6. [Google Scholar]
- 25.Wu S.C., Chou F.F., Rau K.M. Clinical significance of a serum CA 15-3 surge and the usefulness of CA 15-3 kinetics in monitoring chemotherapy response in patients with metastatic breast cancer. Breast Cancer Res Treat. 2010;124:879–882. doi: 10.1007/s10549-010-1117-3. [DOI] [PubMed] [Google Scholar]
- 26.Kim H.S., Park Y.H., Park M.J. Clinical significance of a serum CA15-3 surge and the usefulness of CA15-3 kinetics in monitoring chemotherapy response in patients with metastatic breast cancer. Breast Cancer Res Treat. 2009;118:89–97. doi: 10.1007/s10549-009-0377-2. [DOI] [PubMed] [Google Scholar]
- 27.Pentheroudakis G., Malamou-Mitsi V., Briasoulis E. The neutrophil, not the tumor: serum CA 15-3 elevation as a result of granulocyte--colony-stimulating factor-induced neutrophil MU1C overexpression and neutrophilia in patients with breast carcinoma receiving adjuvant chemotherapy. Cancer. 2004;101:1767–1775. doi: 10.1002/cncr.20581. [DOI] [PubMed] [Google Scholar]
- 28.Kent C.L., McDuff S.G.R., Salama J.K. Oligometastatic breast cancer: where are we now and where are we headed?-A narrative review. Ann Palliat Med. 2021;10:5954–5968. doi: 10.21037/apm-20-1128. [DOI] [PubMed] [Google Scholar]
- 29.Molina R., Jo J., Zanon G. Utility of C-erbB-2 in tissue and in serum in the early diagnosis of recurrence in breast cancer patients: comparison with carcinoembryonic antigen and CA 15.3. Br J Cancer. 1996;74:1126–1131. doi: 10.1038/bjc.1996.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bensouda Y., André F., Boulet T. [Prevalence of elevated serum CA 15-3 at time of metastatic relapse of breast cancer and correlation with hormone receptor status] Bull Cancer. 2009;96:923–928. doi: 10.1684/bdc.2009.0919. [DOI] [PubMed] [Google Scholar]
- 31.Park S., Ahn H.K., Park L.C. Implications of different CA 15-3 levels according to breast Cancer subtype at initial diagnosis of recurrent or metastatic breast cancer. Oncology. 2012;82:180–187. doi: 10.1159/000336081. [DOI] [PubMed] [Google Scholar]
- 32.Yerushalmi R., Tyldesley S., Kennecke H. Tumor markers in metastatic breast cancer subtypes: frequency of elevation and correlation with outcome. Ann Oncol. 2012;23:338–345. doi: 10.1093/annonc/mdr154. [DOI] [PubMed] [Google Scholar]
- 33.Saltbæk L., Horsboel T.A., Offersen B.V. Patterns in detection of recurrence among patients treated for breast cancer. Breast Cancer Res Treat. 2020;184:365–373. doi: 10.1007/s10549-020-05847-4. [DOI] [PubMed] [Google Scholar]
- 34.Li X., Xu Y., Zhang L. Serum CA153 as biomarker for cancer and noncancer diseases. Prog Mol Biol Transl Sci. 2019;162:265–276. doi: 10.1016/bs.pmbts.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Coombes R.C., Page K., Salari R. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25:4255–4263. doi: 10.1158/1078-0432.CCR-18-3663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.