Graphical abstract
Keywords: Antioxidant activity, Gracilaria edulis, Hypnea valentiae, Polyphenol, DPPH, ABTS, FT-IR, GC–MS
Highlights
-
•
Screen the polyphenol compound from red seaweed Gracilaria edulis and Hypnea valentiae.
-
•
Purification the polyphenol compound.
-
•
Detect the antimicrobial activity of polyphenol compound.
-
•
Evaluate the antioxidant activity of polyphenol compound.
-
•
Analyze the FT-IR and GC MS of polyphenol compound.
Abstract
In recent years, seaweeds drew the intense attention of the researchers owing their biological properties with their multi assorted applications to the humans. Red seaweeds are well-known for their biological activities due to enrichment of phenolic residues. The present investigation deals with the portrayal of biological behavior of red algae Gracilaria edulis and Hypnea valentiae. Polyphenol was extracted using methanol in a soxhlet extractor for 6 h. The crude polyphenol compound was partially purified in DEAE cellulose52 column. The total phenolic content present in the polyphenol compound was G. edulis (75.49 ± 0.12 %) and H. valentiae (70.08 ± 0.34 %). The phytochemicals present in the two seaweeds were flavonoids, saponins, tannins, phenolics, alkaloids and steroids. The antimicrobial activity of polyphenol compounds was assessed against seven human pathogens, five plant pathogens and three fungal pathogens. The free radical scavenging activity of polyphenol compound was assayed such as total antioxidant capacity, reducing power, hydrogen peroxide scavenging activity, DPPH, ABTS, hydroxyl-scavenging assay, superoxide anion radical scavenging and nitric oxide. Polyphenol compound was analyzed by FT-IR and GC–MS.
1. Introduction
The different phenolic compounds can act each one alone or through synergistic mechanisms to impart various biological effects. Due to the presence of a richly diverse set of phenolic compounds, different extraction methods are needed for complete characterization of the different types and amounts present in a whole system or complex matrix [1]. Polyphenols, carotenoids and polysaccharides are present in seaweeds and they can be applied in food, pharmaceuticals and cosmetic products as they bring health benefits to consumers [2].
The richness of antioxidant found in many foodstuffs and beverages including fruits, vegetables, tea, coffee and cacao on human health have been recently recognized as the beneficial influence in mankind [3]. Phenolic antioxidant compounds can remove free radical production and intercept the dissemination of autoxidation. Anticholinergic compounds as drugs are used extensively to treat Alzheimer's disease and peptic ulcer [4]. Screening of antioxidant properties of plants and plant-derived compounds requires appropriate methods, which address the mechanism of antioxidant activity and focus on the kinetics of the reactions, including the antioxidants.Antioxidants had a growing interest owing to their protective roles in food and pharmaceutical products against oxidative deterioration and in the body and against oxidative stress-mediated pathological processes [5].
Red algae (Botryoclardia sp. Additionally, Gracilaria sp.) and green algae (C. Sertulaioides and Codium sp.) were present in high antioxidant activity, indicating that these marine algae extracts exert potential natural antioxidant properties [6]. Free radicals are in the form of reactive oxygen and nitrogen species, these can occur, due to oxidative stress brought about by the imbalance of the bodily antioxidant defense system and free-radical formation [7]. Oxidative stress has been linked to cancer, aging, ischemic injury, inflammation and neurodegenerative diseases. Biological molecules of the kind mentioned lipids, proteins, enzymes, DNA and RNA are affected by reactive oxygen species such as superoxide radical, hydroxyl radical, peroxyl radical and nitric oxide radical, leading to cell or tissue injury associated with aging, atherosclerosis carcinogenesis [8].
A group of phenol compounds found in seaweeds called phlorotannins, which function as polymers of phloroglucinol, has been reported to act as strong antioxidant properties and their free radical scavenging ability is more powerful than that of other polyphenols compared to terrestrial plants [9]. Polyphenols synthesized by seaweeds, as one of the largest and most widely distributed groups of seaweed phytochemicals, have gained special attention due to their pharmacological activity and array of health-promoting benefits, as polyphenols play a significant role in the high variety of seaweed biological activities [10,11].
High biological activity of algae is often associated with the presence of powerful and nontoxic natural antioxidants. The antioxidant effect is associated with polyphenols and particularly the phlorotannins, oligomers or polymers of phloroglucinol [11,12]. Some polyphenolic compounds also exhibit antioxidant effects, such as catechins, flavonols and flavonol glycosides, have been identified in methanol extracts of red and brown algae [[13], [14], [15]].
Infrared (IR) spectroscopy was until recently the most frequently used vibrational technique for the study of the chemical composition of polyphenols (phycocolloids). This technique presents two main advantages, it requires minute amounts of sample (mg), and it is a non-aggressive method with reliable accuracy [16]. This study was designed to study the purification, antimicrobial and antioxidant activities of polyphenol compound of G.edulis and H. valentiae red seaweed obtained from Mandapam coastal area, Gulf of Mannar, Tamil Nadu, India.
2. Materials and methods
2.1. Sample collection
The red seaweed is Gracilaria edulis and Hypnea valentiae were collected from the Mandapam (Lat 9°16′ 18.2′′N, Long 79° 10′ 05.04′′ E) a coastal fishing village in Ramanathapuram district, Tamil Nadu, India.
2.2. Extraction of crude polyphenols
In the laboratory, algal sample was rinsed with sterile distilled water, shade dried, cut into small pieces and powdered in a mixer grinder. It was stored in air-tight polypropylene container at room temperature. 100 g of G. edulis and H. valentiae powders were separately extracted with 500-ml methanol in a soxhlet extractor for 6 h. For both, the extraction was repeated twice. The total extract of both was filtered and the obtained filtrate was concentrated under reduced pressure to dryness. The concentrated extract served as the seaweed polyphenol compounds for further analysis [17].
2.3. Purification polyphenol compound
The crude polyphenol compound was further purified by column chromatography and then applied to a DEAE-cellulose 52 column. The collected fractions were estimated for the total phenolic contents [18].
2.4. Estimation of total phenolic content
Phenolic contents of crude methanolic extracts were estimated by the method of Senevirathne et al. [18]. 100 μL crude samples were mixed with 2 mL of 2 % sodium carbonate and allowed to stand for 2 min at room temperature in the dark. The absorbance of all sample solutions was measured at 720 nm using spectrophotometer. Gallic acid was used as a standard and a calibration curve was prepared with a range of concentrations from 10 to 200 mg/L. Phenolic content was expressed as gallic acid equivalent per gram (GAE/g) of extract.
2.5. Phytochemical analysis
Tests for phytochemical constituents including flavonoids, saponins, tannins, phenolics, alkaloids and steroid followed the methods described previously [19].
2.6. Antimicrobial assay
Antibacterial activity of polyphenol from G. edulis and H. valentiae was determined against 7 clinical pathogens and three plant pathogens using the disc diffusion method [[20], [21], [22]]. Two holes were placed on MHA plate using well cutter. The 24-h-old cultures were swabbed in Muller Hinton agar (microbiological grade) plates by using a sterile cotton swab aseptically. The disks were loaded with 30 mcg titer value against the control tetracycline 50 mcg. The plates were incubated at 35 °C for 24 h. The results were obtained by measuring the diameter of inhibition zone for each well and expressed in millimeter. The human clinical pathogens were selected for this study, namely, Klebsiella sp, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Serratia sp and Salmonella sp. The five plant pathogens namely, Erwinia amylovora, Listeria monocytogenes, Xanthomonas sp, Erwiniacarotovora and Alkaligens. Fungal cultures were incubated for 2–3 days at room temperature and seeded on Sabouraud Dextrose Agar plates (SDA) for bioassay by the agar disc diffusion method. Whatman No.1 filter paper disc of 6 mm containing seaweed extract was placed on the surface of the plates. After 72 h at 30 °C the plates were observed for the presence of inhibition zones [23]. The three fungal pathogens namely, Rhizopus stolonifer, Aspergillus japonicus and Aspergillus nidulans was obtained from the laboratory in Department of Microbiology, Ayya Nadar Janaki Ammal College, Sivakasi, Tamil Nadu, India.
2.7. In vitro antioxidant activity
2.7.1. Determination of total antioxidant capacity
Total antioxidant activity of polyphenol from G. edulis and H. valentiae was determined according to the method of Prieto et al. [24]. Briefly, 0.3-ml sample was mixed with 3.0 mL reagent solution (0.6-M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixture was incubated at 95 °C for 90 min under water bath. The absorbance of all sample mixtures was measured at 695 nm after 15 min. Ascorbic acid was used as standard.
2.7.2. Determination of reducing power
Reducing power of the polyphenol from G. edulis and H. valentiae was determined by the following method of Yamaguchi et al. [25]. Briefly, 4 mL of reaction mixture, containing samples of different concentrations in the phosphate buffer (0.2 M, pH 6.6) was incubated with potassium ferricyanide (1% w/v) at 50 °C for 20 min. The reaction was terminated by TCA solution (10 % w/v). The solution was then mixed with distilled water and ferric chloride (0.1 % w/v) solution and the absorbance was measured at 700 nm.
2.7.3. Hydrogen peroxide scavenging assay
The free radical scavenging activity of the polyphenol from G. edulis and H. valentiae was determined by hydrogen peroxide assay [26]. Hydrogen peroxide (10 mM) solution was prepared in phosphate buffered saline (0.1 M, pH 7.4). 1 mL of the extract containing samples of different concentrations (100, 250, 500, 750, and 1000 μg) was rapidly mixed with 2 mL of hydrogen peroxide solution. The absorbance was measured at 230 nm in the UV spectrophotometer after 10 min of incubation at 37 °C against a blank (without hydrogen peroxide). The percentage of scavenging of hydrogen peroxide was calculated using the formula
| Percentage scavenging (H2O2) = ((Ao-A1) / Ao) x 100 |
Ao - Absorbance of control; A1 - Absorbance of sample
2.7.4. DPPH radical scavenging assay
The free radical scavenging activity of polyphenol from G. edulis and H. valentiae was measured by the 1-1-Diphenyl-2-picryl-hydrazyl (DPPH) following the method of Blois, [27]. DPPH was used as a reagent, which evidently offers a convenient and accurate method for titrating oxidizable groups of natural (or) synthetic antioxidants. 0.1 mM solution of DPPH in methanol was prepared and 1 mL of this solution was added to 3 mL of seaweed extracts of different concentrations (100, 250, 500, 750, and 1000 μg). After 10 min, absorbance was measured at 517 nm. The percentage-scavenging activity values were calculated using the following formula
| Percentage of Scavengings = ((Ao-A1) / Ao) x 100 |
Where Ao is the absorbance of control and A1 is absorbance of sample turbidity factor.
2.7.5. ABTS inhibition assay
The ability of the extract to scavenge ABTS (2, 2 azino bis (3-etheylbenzothiazoline-6-sulphonicacid) diammonium salt) radical scavenging was determined by the method of Re et al. [28]. ABTS was generated by mixing 5 mL of 7 mM ABTS with 88 μl of 140 mM potassium persulfate under darkness at room temperature for 16 h. The solution was diluted with 50 % ethanol and the absorbance at 734 nm was measured. The ABTS radical cation scavenging activity was assessed by mixing 5 mL ABTS solution (absorbance of 0.7 ± 0.05) with 0.1 mL polyphenol (100, 250, 500, 750, and 1000 μg). The final absorbance was measured at 743 nm using spectrophotometer. The percentage of scavenging was calculated using the following formula,
| % of scavenging = ((Ao-A1) / Ao) x 100 |
Where Ao - Absorbance of control; A1 - Absorbance of sample
2.7.6. Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity was measured by studying the competition between deoxyribose and test compounds for hydroxyl radical generated by Fe3+- Ascorbate EDTA H2O2 system (Fenton reaction) according to the method of Kunchandy and Rao [29]. The hydroxyl radicals attack deoxyribose that eventually results in TBARS formation. The reaction mixture contained in a final volume of 1.0 mL, 100 μl of 2-deoxy-2-ribose (28 mM in potassium phosphate-potassium hydroxide buffer, pH 7.4) 500 μl solutions of various concentrations of polyphenol (100, 250, 500, 750, and 1000 μg) and standard in KH2PO4-KOH buffer (20 mM, pH 7.4), 200 μl of 1.04 mM ethylene diamine tetra acetic acid and 200 μl of 200 μM ferric chloride, 100 μl of 10 mM hydrogen peroxide and 100 μl of 1.0 mM ascorbic acid was incubated at 37 °C for 1 h.
The free radical damage imposed on the substrate, deoxyribose (TBARS) was measured by the method of the Yuan and Walsh [30]. 1.0 mL of thiobarbituric acid (1 %) and 1.0 mL of trichloroacetic acid (2.8 %) were added to the test tubes and were incubated at 100 °C for 30 min. After cooling, absorbance was measured at 535 nm against control containing deoxyribose and buffer. The percentage scavenging was determined by the comparing the result of the test compound and control using the following formula,
| Radical scavenging activity (%) = [(A0-A1/ A0) x 100] |
Where A0- Absorbance of control; A1- Absorbance of sample.
2.7.7. Superoxide anion radical scavenging assay
Measurement of superoxide anion scavenging activity of the polyphenol from G. edulis and H. valentiae was done based on the method of Nishimiki et al. [31]. About 1 mL of nitro blue tetrazolium (NBT) solution (156 μM NBT in 100 mM phosphate buffer, pH 7.4), 1 mL of NADH solution (468 μM in 100 mM phosphate buffer, pH 7.4) and 0.1-ml sample at various concentrations (100, 250, 500, 750, and 1000 μg) were mixed and the reaction was started by adding 100 μl of phenazine methosulphate (PMS) solution (60 μM PMS in 100 mM phosphate buffer, pH 7.4). The reaction mixture was incubated at 25 °C for 5 min and the absorbance at 560 nm was measured against blank samples. The percentage-scavenging value was determined as follows.
| Radical scavenging activity (%) = [(A0-A1/ A0) x 100] |
Where A0- Absorbance of control; A1- Absorbance of sample
2.7.8. Nitric oxide radical scavenging assay
Nitric oxide radicals generated from sodium nitroprusside solution at physiological pH interact with oxygen to produce nitrite ions which were measured by the Griess reaction [32]. 2 mL of sodium nitroprusside (10 mm) was mixed with a 1-mL polyphenol with varying concentrations (100, 250, 500, 750, and 1000 μg) in a phosphate buffer (pH 7.4). The mixture was incubated at 25 °C for 150 min. 1 mL of sulphanilic acid reagent (0.33 % sulfanilamide in 20 % acetic acid) was added to 0.5 mL of the incubated solution and allowed to stand for 5 min for completing diazotization. Then, 1 mL of 0.1 % napthyl ethylene diamine dihydrochloride was added and incubated at room temperature for 30 min. Absorbance was read at 540 nm and percentage scavenging was calculated as follows.
| Radical scavenging activity (%) = [(A0-A1/ A0) x 100] |
Where A0- Absorbance of control; A1- Absorbance of sample.
2.8. FT-IR spectrophotometer analysis
Infrared spectra (IR) were also used to identify the phenolic compounds. Seaweed extracts along with the standard gallic acid were tested using SHIMADZU- FT-IR instrument. One milligram of dry sample was mixed with 100 mg of dry potassium bromide (KBr) and then compressed to prepared salt-disc (3-mm diameter). These disks were analyzed under Fourier transform IR-Spectrophotometer. The absorption was read between 400 and 4000 cm−1 [17].
2.9. GC–MS analysis
The G. edulis and H. valentiae polyphenol compounds were characterized by gas chromatograph (GC-2010) interfaced using a quadrupole mass spectrometer (QP-2010) analyzer to determine its chemical constituents using Rtx-5 capillary column (30 m ×0.32 mm ×0.5 μm [33]. Interpretation of mass spectrum analysis was done using database of National Institute Standard and Technology.
3. Results
3.1. Estimation of total phenolic content
The total phenolic content present in the polyphenol compound was G. edulis (75.49 ± 0.12 %) and H. valentiae (70.08 ± 0.34 %) of phenolic content present.
3.2. Phytochemical analysis
Diverse phytochemicals were acknowledged from G. edulis and H. valentiae, polyphenol compound evaluation shown the existence of the following in both the samples flavanoids, saponins, tannin and steroids, whereas phenolics and alkaloids are found only in G. edulis and not in H. valentiae.
3.3. Antimicrobial activity of polyphenol compound against pathogens
The polyphenol compound was assessed against seven human bacterial pathogens and five plant bacterial pathogens. The G. edulis polyphenol compound showed a maximum of 23 mm of inhibition zone against Bacillus subtilis and H. valentiae polyphenol compound showed a maximum of 17 mm of inhibition zone against Klebsiella oxytoca of human bacterial pathogens showed in Table 1. The polyphenol compound was assessed against three fungal pathogens. The G. edulis polyphenol showed higher value 23 mm of inhibition zone against Rhizopus stolonifer and H. valentiae polyphenol compound showed 18 mm of inhibiting zone against Aspergillus nidulans is shown in Table 1.
Table 1.
Antimicrobial activity of polyphenol compound (30 mcg) against human and plant pathogens.
| S. No | Test organisms | Tetracycline −50 mcg (mm) | G. edulis (mm) (30 mcg) | H. valentiae (mm) (30 mcg) |
|---|---|---|---|---|
| Antibacterial activity against Human pathogens | ||||
| 1 | Klebsiella oxytoca | 24 | 21 | 17 |
| 2 | Escherchia coli | 22 | 19 | 12 |
| 3 | Staphylococcus aureus | 24 | 18 | 14 |
| 4 | Pseudomonas aeruginosa | 27 | 16 | 11 |
| 5 | Bacillus subtilis | 28 | 23 | 15 |
| 6 | Serratia sp | 26 | 20 | 13 |
| 7 | Salmonella sp | 25 | 22 | 16 |
| Antibacterial activity against Plant pathogens | ||||
| 1 | Erwinia amylovora | 20 | 17 | 10 |
| 2 | Listeria monocytogenes | 18 | 15 | 12 |
| 3 | Xanthomonas sp | 21 | 14 | 11 |
| 4 | Erwinia carotovora | 23 | 20 | 18 |
| 5 | Alkaligens sp | 25 | 18 | 15 |
| Antifungal activity | ||||
| 1 | Rhizopus stolonifer | 27 | 23 | 16 |
| 2 | Aspergillus japonicus | 23 | 19 | 13 |
| 3 | Aspergillus nidulans | 21 | 17 | 18 |
The polyphenol compound showed a maximum of G. edulis (20 mm) and H. valentiae (18 mm) inhibition zones against Erwinia carotovora of plant pathogens showed in Table 1.
3.4. Free radical scavenging activity of polyphenol compound
The in vitro antioxidant activity of G. edulis and H. valentiae was present in total antioxidant capacity, reducing power, hydrogen peroxide scavenging activity, DPPH, ABTS, hydroxyl-scavenging assay, superoxide anion radical scavenging and nitric oxide showed in Table 2.
Table 2.
% of in vitro antioxidant activity of polyphenol compound.
| S. No | Anti Oxidant activities | G. edulis | H. valentiae |
|---|---|---|---|
| 1 | Total antioxidant capacity | 82.93 ± 0.48 % | 78.12 ± 0.22 % |
| 2 | Reducing power | 80.56 ± 0.36 % | 75.09 ± 0.39 % |
| 3 | Hydrogen peroxide scavenging activity | 77.46 ± 0.40 % | 73.18 ± 0.32 % |
| 4 | DPPH | 74.16 ± 0.49 % | 61.41 ± 0.27 % |
| 5 | ABTS | 62.33 ± 0.66 % | 56.84 ± 0.41 % |
| 6 | Hydroxyl scavenging assay | 68.23 ± 0.55 % | 60.09 ± 0.37 % |
| 7 | Superoxide anion radical scavenging | 71.73 ± 0.57 % | 59.75 ± 0.17 % |
| 8 | Nitric oxide | 76.13 ± 0.44 % | 68.25 ± 0.31 % |
3.5. FT-IR spectrophotometer analysis
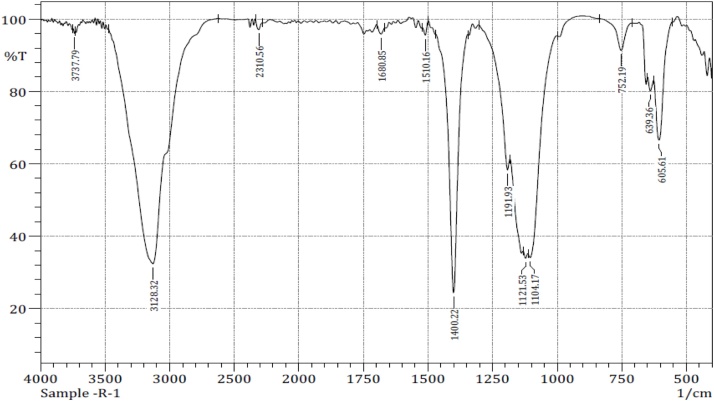
The FT-IR spectrum was purified polyphenol compound of G. edulis is shown in Fig. 1. The strong peak at 3383.87 cm−1 explained N—H stretching vibration (amide group) even the signal at 2360.71 and 2337.56 cm−1 cleared the C—H stretching vibration. The signal at 1406.97 cm−1 contributed C—H stretching vibration and 1637.45 cm−1 contributed C O stretching vibration. The signal was 1046.31 and 716.51 concern weak stretching vibration. The H. valentiae polyphenol compound present in a strong peak at the 3128.32 cm−1 explained C—H stretching vibration (aromatics group), even the signal at 1680.85 cm−1 cleared the C C stretching alkenes. The signal at 1510.16 and 1400.22 cm−1 contributed C—C stretching (in ring) vibration aromatics. The signal was 752.19, 639.36, 605.61 cm-1 concern C-Cl stretching the alkyl halide vibration shown in Fig. 2.
Fig. 1.
FT-IR spectrum analysis of polyphenol compound from G.edulis.
Fig. 2.
FT- IR spectrum of polyphenol compound fromHypneavalentiae.
3.6. GC–MS analysis
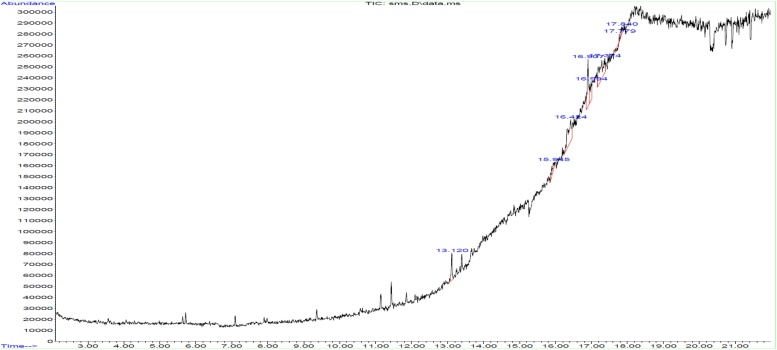
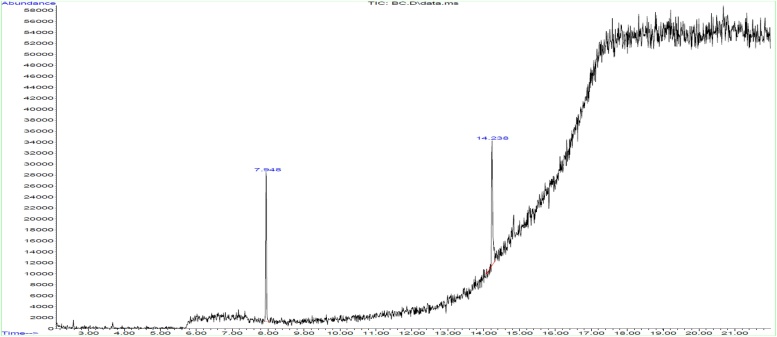
GC–MS analysis shows the polyphenol compound of G. edulis and H. valentiae in the following peaks were obtained for partially purified product of polyphenol 7.948 (Diethyl Phthalate), 13.120 (9-Octadecenoic acid), 14.238 (1H-Benzimidazole, 5,6-dimethyl), 15.945 (Benzene, 2-[(tert-butyldimethylsilyl) oxy]-1-isopropyl-4-methyl-), 16.424 (5-Methyl-2-trimethylsilyloxy-acetophenone), 16.907 (1H-Indole, 2-methyl-3-phenyl-), 16.994 (Benzo[h]quinoline, 2,4-dimethyl-), 17.374 (1,2-Benzisothiazol-3-amine tbdms), 17.779 (diethyl bis (trimethylsilyl) ester) and 17.840 (5-Methyl-2-phenylindolizine) shown in Fig. 3, Fig. 4.
Fig. 3.
GC–MS spectrum analysis of polyphenol compound from G.edulis.
Fig. 4.
GC–MS spectrum analysis of polyphenol compound from Hypnea valentiae.
4. Discussion
The MeOH extracts of red seaweed H. valentiae exhibited higher yield (6.5 g/100 g dry sample) followed by J. rubens and H. musciformis (5.3 and 4.8 g/100 g dry sample, respectively) [34]. Gracilaria manilaensis polyphenol extracts were prepared by soxhlet extraction using organic solvents hexane (0.56 %), dichloromethane (0.63 %), chloroform (1.25 %), ethyl acetate (1.19 %), acetone (1.24 %), methanol (3.12 %) and absolute ethanol (2.53 %) [35]. The polyphenol from Padina boergesenii was estimated at 5 g/100 mL of dry weight in polyphenol. The recovered polyphenol from Padina boergesenii was estimated at the fresh weight 0.8 ± 0.01 mg/100 mL and dry weight 0.5 ± 0.01 mg/100 mL [36]. The analysis of phenolic compounds is affected by their source, the extraction and purification techniques employed, the sample particle size, the storage conditions, and the presence of interfering substances in extracts such as fatty acids or pigments [37]. In this study red seaweeds G. edulis and H. valentiae polyphenol was extracted using hot water and crude polyphenol were partially purified using in DEAE cellulose52.
The presence of phytochemical constituents in G. edulis and H. valentia is flavonoids, saponins, tannins, phenolics, alkaloids and steroids. Similarly, the phytochemicals present in G. corticata are alkaloids, terpenoids, flavonoids, tannins, polyphenols, saponins, cardiac glycosides and quinine [38]. The G. corticata possess higher total phenol content (4.00 ± 0.35 mg GAE/g) compared to G. edulis (3.4 ± 0.21 mg GAE/g). G. corticata (3.33 ± 0.12 mg CE/g DW) and G. edulis (2.6 ± 0.08 mg CE/g DW) extract significantly varied in total flavonoid content respectively [39]. The ethanol, acetone, methanol and water extracts of Hypnea valentiae were subjected to a phytochemical analysis of fifteen chemical compounds (alkaloids terpenoids, steroids, tannin, saponins, flavonoids, phlobatannins, glycosides, anthraquninones, chloride, carbohydrate, reducing sugar, amino acid, protein and phenolic compound) [40].
The antimicrobial activity of red seaweed polyphenol from G. edulis and H. valentiae was found against human, plant and fungal pathogens. Antibacterial activity of 70 % methanolic and DMSO extracts of G. corticata and G. edulis was found effective against seafood-borne pathogens E. coli, Photobacterium sp. Pseudomonas fluorescens, Staphylococcus aureus and Bacillus subtilis [39]. Antibacterial activity of red seaweed H. valentiae was against pathogenic bacterial Escherichia coli, Bacillus, Streptococcous, Entrobacter and Pseudomonas. No inhibition zone as formed on all pathogenic bacteria's [40]. The antibacterial activity proved chloroform: methanol (2:1 v/v) extract residue of the experimental brown algae. The brown algae showed maximum antibacterial activity of E. coli (2.2 ± 0.063) and Staphylococcus aureus (6.2 ± 0.128) respectively [41].
The total antioxidant capacity of polyphenol from G.edulis was found to be 82.93 ± 0.48 % and H. valentiae78.12 ± 0.22 %. Similarly, the seaweed is presented in total antioxidant activity of P. gymnospora (1.92 ± 0.05 mg), G. lithophila (1.54 ± 0.07 mg) and H. valentiae (1.27 ± 0.05 mg) respectively [42]. The total antioxidant activity of methanolic extract was U. lactuca showed 0.91 ± 0.09 mg GAE/g [43]. The total antioxidant of the G. jasminoides polyphenolic compounds was expressed as the number of equivalents of ascorbic acid. The total antioxidant activity was dose-dependently increased [44].
Reducing power of H. musciformis (Abs700 nm 1.46) H. valentiae (Abs700 nm 0.48) and J. rubens (Abs700 nm 0.45,) respectively [34]. The reducing power of MeOH (Abs700 nm 0.07–0.74) and EtOAc extracts (Abs700 nm 0.013–0.467) of red seaweed Kappaphycus alvarezii extracts were reported to be higher than n-hexanic extract (Abs700 nm 0.017–0.16 at 0.5–5 mg/mL) [45]. The reducing power of T. ornata increases with the increasing concentration. The reducing power of the samples from T. ornata was 0.2 ± 0.04 to 0.72 ± 0.07 [17]. The polyphenol from G. edulis 80.56 ± 0.36 % and H. valentiae75.09 ± 0.39 %. The reducing capacities of various concentrations of polyphenol were compared with standard compound, which implies that as the concentration increases the reducing power of the extracts was also increased.
The polyphenol compound from seaweed can be a potential source of antioxidants with protective and useful effects [46]. The hydrogen peroxide scavenging activity of polyphenol compound of G.edulis is 77.46 ± 0.40 % and H. valentiae73.18 ± 0.32 % was estimated. Similarly, many species of seaweed possess scavenging ability of hydrogen peroxide [47]. The strongest H2O2 scavenging effect of H. musciformis EtOAc fraction can be explained due to the presence of hydrophilic phenolics [18]. H2O2 scavenging activity (%) at 1 mg/mL of the MeOH extracts/fractions of the red seaweeds are recorded in H. musciformis (43.01 ± 0.81 mg/mL), H. valentiae (32.75 ± 1.03 mg/mL) and J. rubens (27.63 ± 1.36 mg/mL) [34]. The C. Socialis phenolic compounds have been identified with interesting antibacterial activity against methicillin-resistant Staphylococcus aureus [48].
The DPPH radical scavenging assay for the G. edulisis 74.16 ± 0.49 % and H. valentiae61.41 ± 0.27 %. Also, DPPH radicals scavenging activities in the methanolic extracts of red seaweeds G. corticata (44.32 %), G. dura (33.03 %), G. debilis (53.34 %), G. fergusonii (23.99 %) and G. salicornia (53.43 %) [49]. MeOH extract of H. musciformis (15.4 %), J. rubens (17.7 %) showed significantly higher DPPH-scavenging activities than H. valentiae (7.7 %) [34]. DPPH, T. ornata was shown high activity (84.27 ± 2.17) % scavenging activity on DPPH compared with standard Gallic acid [17]. The significant free radical scavenging activities in DPPH radical scavenging antioxidant assays compared to the standard ascorbic acid [44].
The ABTS inhibition assay for the polyphenol from G.edulisis 62.33 ± 0.66 % and H. valentiae56.84 ± 0.41 %. Similarly, EtOAc fraction of H. musciform is registered with significantly higher ABTS scavenging activity (63.3 %) followed by H. valentiae (27.9 %) and J.rubens (11.0 %) [34]. Likewise, G. edulis showed significantly higher ABTS.+ free radical scavenging activity (40.24 %) than G. corticata (32.65 %) [39]. Equally, The ABTS radical scavenging activity in red seaweeds could be due to the presence of high carotenes and other pigments with long hydrocarbon chain and animated compounds [50].
The hydroxyl-scavenging assay G.edulis is 68.23 ± 0.55 % and H. valentiae60.09 ± 0.37 %. Likewise, the extracts of red seaweeds, Gracilaria verrucosa, G. textorii, Grateloupia filicina and Polysiphonia japonica also reported potentially high hydroxyl-scavenging activity [51]. The hydroxyl scavenging assay T. ornata exhibited the inhibition of about (70.12 ± 2.03 %), but this is lower than the standard gallic acid (1 000 μg/mL) whose inhibition is 44.92 ± 1.97 % [17]. The superoxide anion radical scavenging assay for the G.edulisis 71.73 ± 0.57 % and H. valentiae59.75 ± 0.17 %. Similarly, the superoxide radical scavenging activity from Ulva pertusa was found to be 23.4–93.8% [52]. The nitric oxide scavenging assay for the G. edulis is 76.13 ± 0.44 % and H. valentiae68.25 ± 0.31 %. Likewise, G. corticata exhibited higher nitric oxide radical scavenging activity (36.78 %) than G. edulis 35.25 % [39]. The suppression of nitric oxide release may be attributed to a direct nitric oxide scavenging effect. The T.ornata had scavenging activity of 39.8 ± 2.52 % [17].
The FT- IR was performed for extracted polyphenol compound from G. edulis and H. valentiae observe in powder form at 3383.87 cm−1 explained N—H stretching vibration (amide group) and The strong peak at 3128.32 cm−1 explained C—H stretching vibration (aromatics group). Similarly, the FT-IR spectrum of G. corticata and G. edulis confirmed the presence of free hydroxyl alcohol, alkyne, esters, conjugated ketone, cyclic alkene, alkane, ester sulfate, the skeleton of galactons, 3,6-anhydro- l-galactose, carboxylic acid aldehyde, agarocolloides, secondary alcohol and disulfides [39]. Likewise, the FT-IR spectrum of the T. ornata also recorded the same number of peaks lying between 1026.16, 3414.12, 1028.09, and 3394.83 cm-1 respectively. The absorption peaks observed for hydroxyl groups (around 3300–3500 cm-1) and aromatic ring (around 1450–1470 cm-1 and 2850–2960 cm−1) in the spectra of T. ornata also suggested the presence of phenolic compounds [17].
GC- MS analysis was performed for extracted polyphenol compound from G. edulis and H. valentiae in a highly complex nature. Similarly, theGC-MS shows of G. corticata exposed that the presence of 17 secondary compounds (6 from hexanic, 4 from acetonic and 7 from methanolic extracts). Among the 17 compounds, 8 compounds (1 from acetonic and 6 from methanolic and 1 from all three extracts) possessed bioactive properties based on the literature [38]. Also, GC–MS analysis of bioactive compound from methanol extract of sea weed, H. valentiae. The active principles with their retention time (28.74), molecular formula, molecular weight (390) and Peak area (15.20) were determined [40]. Equally, GC–MS analysis revealed phytochemical compounds including sulfurous acid, 2-ethylhexyl isohexyl ester, pentatriacontane, eugenol and phthalic acid [39].
5. Conclusion
The red seaweed G.edulis and H. valentiae were collected from the Mandapam (Lat 9°16′ 18.2′′N, Long 79° 10′ 05.04′′ E) is a coastal fishing village of Ramanathapuram, Tamil Nadu. Red seaweeds act as a reservoir of various biologically active substances. The polyphenol compound from red algae carries potential assets as it may have a strong pharmaceutical value in the future. Additionally, also the cultivation of these red seaweeds will pave the attention of small-scale sector through which pharmaceutical concerns gain the true value of G. edulis and H. valentiae and a prolific product can reach mankind soon.
Conflict of Interest
The authors declare no conflict of interest.
CRediT authorship contribution statement
Shunmugiah Mahendran: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Pandiaraj Maheswari: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Vanaraj Sasikala: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Jeba jaya Rubika: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Jeyaraj Pandiarajan: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
We here wish to state that this work has been self funded by the authors.
Handling Editor: Dr. Aristidis Tsatsakis
Contributor Information
Shunmugiah Mahendran, Email: mahi.ran682@gmail.com.
Jeyaraj Pandiarajan, Email: pandiarajan_sf424@anjaconline.org.
References
- 1.Ahmad S., Sulaiman M.R., Saimon W., Yee C.F., Matanjun P. Proximate compositions and total phenolic contents of selected edible seaweed from Semporna, Sabah, Malaysia. Borneo Sci. 2014;31:85–95. [Google Scholar]
- 2.Shibata H., Kimura T.I., Hashimoto S., Kimura K., Makimo T., Aiyama R., Ueyama S., Yokokura T. Structural study of fucoidan from cladosi-phonokamuranus. Glycoconju Gate J. 1999;16:19–26. doi: 10.1023/a:1006945618657. [DOI] [PubMed] [Google Scholar]
- 3.Gulcin Ilhami. Antioxidant activity of food constituents: an overview. Arch. Toxicol. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 4.Taslimi Parham, Gulcin Ilhami. Antioxidant and anticholinergic properties of olivetol. J. Food Biochem. 2018;42(3):e12516. [Google Scholar]
- 5.Gulcin Ilhami. Antioxidants and antioxidant methods: an updated overview. Arch. Toxicol. 2020;94(3):651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- 6.Yanti Fendy, Koesnoto Heryanto, Sutanto Andre, Hwang Jae-Kwan. Antioxidant potentials of marine red and green algae extracts in-vitro. Sch. Acad. J. Pharm. 2015;4(3):177–180. [Google Scholar]
- 7.Wong C.K., Ooi V.E.C., Ang P.O. Protective effect of seaweeds against liver injury caused by carbon tetra chloride in rats. Chemosphere. 2000;41:173–176. doi: 10.1016/s0045-6535(99)00407-5. [DOI] [PubMed] [Google Scholar]
- 8.Keli-chen W., Geoff P., Richard Bennett N., Youngping B. Antioxidant activities of extracts from five anti-viral medicinal plants. J. Ethnopharmacol. 2005;96:201–205. doi: 10.1016/j.jep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Ahn G.N., Kim K.N., Cha S.H., Song C.B., Heo M.S., Jeon Y.J. Antioxidant activities of phlorotannins purified from Ecoklonia cava on free radicals scavenging using ESR and H2O2 mediated DNA damage. Eur. Food Res. Technol. 2007;226(2):71–79. [Google Scholar]
- 10.Wijesekara I., Kim S.K., Li Y.X., Li Y.X. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011;46:2219–2224. [Google Scholar]
- 11.Mekinic I.G., Skroza D., Simat V., Hamed I., Cagalj M., Perkovic Z.P. Phenolic content of brown algae (Pheophyceae) species: extraction, identification and quantification. Biomolecules. 2019;9:244. doi: 10.3390/biom9060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heffernan N., Brunton N.P., FitzGerald R.J., Smyth T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs. 2015;13:509–528. doi: 10.3390/md13010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vega J.A.G.F., Güenaga L., Figueroa F.L., Gomez-Pinchetti J.L. Antioxidant activity of extracts from marine macroalgae, wild-collected and cultivated, in an integrated multi-trophic aquaculture system. Aquaculture. 2020;522:1–10. [Google Scholar]
- 14.Santoso J., Yoshie Y., Suzuki T. The distribution and profile of nutrients and catechins of some Indonesian seaweeds. Fish. Sci. 2002;68:1647–1648. [Google Scholar]
- 15.Yoshie-Stark Y. Distribution of flavonoids and related compounds from seaweeds in Japan. J. Tokyo Univ. Fish. 2003;89:1–6. [Google Scholar]
- 16.Pereira L., Sousa A., Coelho H., Amado A.M., Ribeiro-Claro P.J.A. Use of FT-IR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003;20(4–6):223–228. doi: 10.1016/s1389-0344(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 17.Vijayabaskar P., Shiyamala V. Antioxidant properties of seaweed polyphenol from Turbinariaornata (Turner) J. Agardh, 1848. Asian Pac. J. Trop. Biomed. 2012;34:90–98. [Google Scholar]
- 18.Senevirathne M., Kim S.H., Siriwardhana N., Ha J.H., Lee K.W., Jeon Y.J. Antioxidant potential of Ecklonia cava on reactive oxygen species scavenging, metal chelating, reducing power and lipid peroxidation inhibition. Food Sci. Technol. Int. 2006;12:27–38. [Google Scholar]
- 19.Kamba A.S., Hassan L.G. Phytochemical screening and antimicrobial activities of Euphorbia balsamifera leaves, stems and root against some pathogenic microorganisms. Afr. J. Pharm. Pharmacol. 2010;4:645–652. [Google Scholar]
- 20.Eruygur N., Koçyigit U.M., Taslimi P., Atas M., Tekin M., Gulcin I. In vitro antioxidant, antimicrobial, anticholinesterase and antidiabetic activities of Turkish endemic Achillea cucullata (Asteraceae) from ethanol extract. South Afr. J. Bot. 2019;120:141–145. [Google Scholar]
- 21.Koksal Ekrem, Tohma Hatice, Kılıc Omer, Alan Yusuf, Aras Abdülmelik, Gulcin Ilhami, Bursal Ercan. Assessment of antimicrobial and antioxidant activities of Nepeta trachonitica-Analysis of its phenolic compounds using HPLC-MS/MS. Sci. Pharm. 2017;85(2):1–14. doi: 10.3390/scipharm85020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tohma Hatice, Köksal Ekrem, Kılıc Omer, Alan Yusuf, Yılmaz Mustafa Abdullah, Gulcin Ilhami, Bursal Ercan, Alwasel Saleh H. RP-HPLC/MS/MS analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia L. species. Antioxidants. 2016;38(5):1–15. doi: 10.3390/antiox5040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Masry H.A., Fahmy H.H., Abdelwahed A.S.H. Synthesis and antimicrobial activity of some new benzimidazole derivatives. Molecules. 2000;5:1429–1438. [Google Scholar]
- 24.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphor molybdenum complex specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi T., Takamura H., Matoba T., Terao J. HPLC method for evalution of the free radical scavenging activity of foods by using 1, 1- Diphenyl-2-Picryl hydrozl. Biosci. Biotechnol. Biochem. 1998;62:111–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- 26.Gulcin T., Irfan K.O., Kufrevioglu M., Okatay M., Buyukokuroglu M.E. Atioxidant, antimicrobial, antiuclcer and analgesic activities of nettle. J. Etnopharmacol. 2004;90:205–215. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 28.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorizing assay. Free Radic. Biol. Med. 1999;26(9):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 29.Kunchandy Rao. Antioxidant properties of dried kayamonori, a brown algae Scytosiphonlomentaria. Food Chem. 1990;89:617–622. [Google Scholar]
- 30.Yuan Y.V., Walsh N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006;44:1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Nishimiki M., Rao N.A., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–853. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 32.Gulcin I. Antioxidant and antiratical activities of L- Carnitine. Life Sci. 2006;78:803–811. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]
- 33.Tao Xiaoyun, Sun Hongnan, Chen Jian, Li Luning, Wang Yin, Sun Aidong. Analysis of polyphenols in apple pomace using gas chromatography-mass spectrometry with derivatization. Int. J. Food Prop. 2014;17(8):1818–1827. [Google Scholar]
- 34.Chakraborty Kajal, Joseph Deepu. Nammunayathuputhenkotta Krishnankartha Praveen, Antioxidant activities and phenolic contents of three red seaweeds (Division: rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015;52(4):1924–1935. doi: 10.1007/s13197-013-1189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdullah Nor Salmi, Muhamad Shamsul, Omar Ibrahim Che, Abdullah Hasmah. Radical scavenging activity and total phenolic content of Gracilariamanilaensis extracts. Technol. Sci. Soc. Sci. Human. Int. Conf. 2012;2012:1–7. [Google Scholar]
- 36.Rajamani K., Somasundaram S.T., Manivasagam T., Balasubramanian T., Anantharaman P. Hepatoprotective activity of brown alga Padina boergesenii against CCl4 induced oxidative damage in wistar rats. Asian Pac. J. Trop. Med. 2010;7:696–701. [Google Scholar]
- 37.Shahidi F., Naczk M. CRC Press; Boca Raton, FL, USA: 2003. Phenolics in Food and Nutraceuticals. ISBN 9780367395094. [Google Scholar]
- 38.Rajkumara Gopalan, Bhavan Periyakali Saravana, Srinivasan Veeran, Udayasuriyan Rajendran. Phytochemical screenings of the marine red alga, Gracilariacorticata. Noble Int. J. Sci. Res. 2017;1(8):90–97. [Google Scholar]
- 39.Arulkumar Abimannan, Rosemary Thomas, Paramasivam Sadayan. Ramaswamy Babu Rajendran, Phytochemical composition, in vitro antioxidant, antibacterial potential and GC-MS analysis o1f red seaweeds (Gracilariacorticata and Gracilariaedulis) from Palk Bay, India. Biocatal. Agric. Biotechnol. 2018;15:63–71. [Google Scholar]
- 40.Rama Devi P., Vasudhevan I., Babu C., Muthu Ganga M., Balakrishnan C.P. Phytochemical analysis and bioactive compound separation of sea weed, Hypneavalentiae. Int. J. Res. Advent Technol. 2018;6(11):3192–3197. [Google Scholar]
- 41.Rajasulochana P., Krishnamoorthy P., Dhamotharan R. Isolation, identification of bromophenol compound and antibacterial activity of Kappaphycussp. Int. J. Pharma Bio Sci. 2012;3(2):176–186. [Google Scholar]
- 42.Saranya C., Parthiban C., Anantharaman P. Evaluation of antibacterial and antioxidant activities of seaweeds from Pondicherry coast. Adv. Appl. Sci. Res. 2014;5(4):82–90. 2014. [Google Scholar]
- 43.Meenakshi S., Manicka D.G., Tamilmozhi S., Arumugam M., Balasubramanian T. Total Flavanoid and in vitro antioxidant activity of two seaweeds of Rameshwaram coast. Glob. J. Pharmacol. 2009;3(2):59–62. [Google Scholar]
- 44.Uddin Riaz, Saha Moni Rani, Subhan Nusrat, Hossain Hemayet, Jahan Ismet Ara, Akter Raushanara, Alam Ashraful. HPLC-Analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv. Pharm. Bull. 2014;4(3):273–281. doi: 10.5681/apb.2014.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S.K., Ganesan K., Rao P.V.S. Antioxidant potential of solvent extracts of Kappaphycusalvarezii (Doty) Doty an edible seaweed. Food Chem. 2008;107:289–295. [Google Scholar]
- 46.Valdes L., Cuervo A., Salazar N., Ruas-Madiedo P., Gueimonde M., Gonzalez S. The relationship between phenolic compounds from diet and microbiota, impact on human health. Food Funct. 2015;6(8):2424–2439. doi: 10.1039/c5fo00322a. [DOI] [PubMed] [Google Scholar]
- 47.Sriwardhana N., Lee K.W., Kim S.H., Ha J.W., Jeon Y.J. Antioxidant activity of Hijikiafusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci. Technol. Int. 2003;9(5):339–348. [Google Scholar]
- 48.Lavoie S., Sweeney-Jones A.M., Mojib N., Dale B., Gagaring K., McNamara C.W., Quave C.L., Soapi K., Kubanek J. Antibacterial oligomeric polyphenols from the green alga Cladophora socialis. J. Org. Chem. 2019;84:5035–5045. doi: 10.1021/acs.joc.8b03218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar M., Kumari P., Trivedi N., Shukla M.K., Gupta V., Reddy C.R.K., Jha B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 2011;23:797–810. [Google Scholar]
- 50.Chew Y.L., Lim Y.Y., Omar M., Khoo K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT Food Sci. Technol. 2008;41:1067–1072. [Google Scholar]
- 51.Heo S.J., Cha S.H., Lee K.W., Jeon Y.J. Antioxidant activities of red algae from Jeju Island. Korean Soc. Phycol. 2006;21:149–156. [Google Scholar]
- 52.Qi H., Zhang Q., Zhao T., Hu R., Zhangc K., Li Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulvapertusa (Chlorophyta) Bioorg. Med. Chem. Let. 2006:2441–2445. doi: 10.1016/j.bmcl.2006.01.076. [DOI] [PubMed] [Google Scholar]