Abstract
Background
Pituitary adenoma (PA) accounts for 10–15% of all intracranial neoplasms. Despite their benign nature, PA often shows invasive growth. MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) are a class of non-coding RNAs that play important roles in PA initiation and progression.
Aim
The aim of this study was to find specific profiles of miR-200a and long non-coding RNA (lncRNA) antisense non-coding RNA in the INK4 locus (ANRIL) in PA based on a comparative study using Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analyses of tumor tissue and plasma.
Methods
Plasma and PA tissue samples were obtained from two groups of included patients (15 invasive and 15 non-invasive PA). In addition, plasma samples from patients with invasive PA have collected pre- and post-operation. Plasma and tissue samples subjected to qRT-PCR analyses for the expression levels of miR-200a and lncRNA ANRIL.
Results
The expression levels of miR-200a and lncRNA ANRIL were increased in tissue samples patients with invasive PA than in the patients with non-invasive PA. In addition, the expression levels of circulating miR-200a and lncRNA ANRIL were increased in patients with invasive PA than in patients with non-invasive PA in the pre-operation period. However, the expression level of plasma circulating miR-200a and lncRNA ANRIL was decreased in patients with invasive PA in the post-operation period. Our results depicted a miR-200a and lncRNA ANRIL expression in tissue and plasma samples in the patients with invasive PA. In addition, Receiver Operating Characteristic (ROC) curve was used to evaluate the diagnostic value of these circulating miR-200a and lncRNA ANRIL.
Conclusion
The expression of these tumor-associated ncRNAs has been elevated in the PAs. Therefore, miR-200a and lncRNA ANRIL represents as biomarkers for diagnosis and potential targets for novel invasive PA treatment strategies.
Keywords: Pituitary adenoma, Invasive, miR-200a, lncRNA ANRIL, Circulating, Biomarker, Therapy
1. Introduction
Pituitary adenomas (PAs) are benign, typically slowly progressing tumors of the adenohypophysis and represent 10–15% of all intracranial tumors, occurring in almost 20% of the general population [1]. Despite their benign nature, PA often shows invasive growth. Invasive PAs are aggressive and result in high mortality because medical and radiation therapies are either partially or completely ineffective [2]. Therefore, understanding the molecular pathogenesis of the invasive PA and discovering novel biomarkers and therapeutic targets would facilitate early detection, prognosis, and improve patient survival.
To date, various classes of non-coding RNAs (ncRNAs) with different targets and functions have been identified, and these molecules can be grouped into two major classes: small ncRNAs (<200 nt in length), including microRNAs (miRNAs), and long non-coding RNAs (lncRNAs) (>200 nt in length) [3,4]. It has been proven that miRNAs and lncRNAs play a significant role in various biological processes, including the cell cycle, proliferation, differentiation, and cell apoptosis [3]. Ectopic expression of miRNAs and lncRNAs involved in tumorigenesis has been well described in most tumor types [5]. A series of miRNAs and lncRNAs have been identified to act as promoters of tumor progression in PAs by targeting tumor suppressor genes or interfering with pathways that regulate cell proliferation and affecting tumor invasion, angiogenesis, and apoptosis [[6], [7], [8], [9], [10]]. Previous studies have shown that miR-200a and lncRNA antisense ncRNA gene at the INK4 locus (ANRIL) played a role as an oncogene in a variety of tumors. As shown in Table 1, their abnormally high expression can be observed in various tumor tissues and has multiple functions regulating tumor growth and invasion [[11], [12], [13], [14], [15], [16]].
Table 1.
MiR-200a and lncRNA ANRIL involved in the pathogenesis of various tumors.
| miR-200a | ||||
|---|---|---|---|---|
| Study | Gene-Target | Type of tumor | Biological function | Expression |
| Li et al., 2016 [11] | PTEN | Colorectal cancer | Promotes tumor cell proliferation, invasion, migration, and invasion | Up- regulation |
| Zuo et al., 2018 [12] | ZEB1 | Breast cancer | Promotes tumor cell invasion and migration | Up- regulation |
| Suo et al., 2018 [13] |
PTEN |
Ovarian cancer |
Promotes tumor cell invasion and migration |
Up- regulation |
| LncRNA ANRIL | ||||
| Study |
Gene-Target |
Type of tumor |
Biological function |
Expression |
| Ma et al., 2019 [14] | miR-144 and PBX3 | Hepatocellular carcinoma | Promotes tumor cell proliferation, migration, invasion | Up- regulation |
| Deng et al., 2019 [15] | NF-kB | Gastric cancer | Suppresses apoptosis in tumor cell. Promotes tumor growth and tumor cells migration | Up- regulation |
| Wu et al., 2018 [16] | SOX2 | Nasopharyngeal carcinoma | Promotes cell proliferation and tumor growth | Up- regulation |
Abbreviation: LncRNA, Long non-coding ribonucleic acid; miRNA, microRNA; PTEN, Validated phosphatase and tensin homolog; ZEB1, Zinc finger E-box binding homeobox 1; PBX3, PBX homeobox 3; NF-kB, Nuclear factor-κB; SOX2, SRY-Box transcription factor 2; LncRNA ANRIL, Long non-coding RNA antisense non-coding RNA in the INK4 locus.
In addition, in many biological fluids of the human body, such as blood, urine, saliva, and cerebrospinal fluid, numerous miRNAs and lncRNAs, called circulating, were found [17]. In the human biofluids, miRNAs and lncRNAs circulate in extracellular vesicles (EVs) (apoptotic bodies, microvesicles, exosomes) and bound to macromolecular complexes, like the Argonaute 2 (Ago 2) protein or lipoproteins [17]. Circulating miRNAs and lncRNAs meet the basic conditions for utility as non-invasive biomarkers that may be measured repeatedly and non-invasively in a wide array of tumor types. Circulating miRNAs and lncRNAs may play an important role in the diagnosis and prognosis of various diseases, including PA [18,19]. Our previous study demonstrated that circulating miRNAs in plasma samples could be served as potential diagnostic biomarkers in intracerebral hemorrhage [20]. Although multiple lines of evidence emphasize the significant role of non-coding RNAs in different human tumors, to the best of our knowledge, there are no studies available to date on the role of miR-200a and lncRNA ANRIL in PA. In this study, we examined miR-200a and lncRNA ANRIL expression in invasive and non-invasive PAs, to determine their role in the PA invasion. Furthermore, we detected the expressions in plasma of circulating miR-200 and lncRNA ANRIL to investigate their diagnostic values.
2. Materials and methods
2.1. Patients and samples
Patients (n = 30) diagnosed with PA between February 2020 and July 2020 at the Clinic of the Bashkir State Medical University were analyzed. The PA diagnosis was confirmed based on magnetic resonance imaging (MRI) and hormonal information, clinical manifestation, as well as histopathological analyses and immunohistochemical staining. There were 15, 9, and 6 cases of adrenocorticotropic hormone (ACTH) – secreting PA, prolactin – secreting PA, and growth hormone (GH) – secreting PA, respectively. A total of 15 patients were classified into the non-invasive pituitary tumor group while the other 15 were classified into the invasive adenoma group. Invasive PAs were defined as Hardy–Wilson classification grade III-IV and Knosp classification grades III-IV [1,2] (Fig. 1A, Fig. 1B). Non-invasive PA was defined as the limitation of tumor body within the sellar region, without any compression on peripheral structures (Fig. 1C and D). We excluded patients with cardiovascular diseases, immune diseases, operations, injuries, organ failure, other tumors, and infections in their past medical history since these diseases may influence the levels of non-coding RNAs in our patients. After surgery, PAs tissue was stored at – 80 °C according to standard procedure. Some fragments of each sample were separated and immediately frozen in liquid nitrogen under RNase-free conditions until total RNA extraction. Plasma samples were collected twice: 3 days before medical preparation for the surgical intervention for patients with invasive and non-invasive PA and then 7 days after the surgery for patients with invasive PA, usually on the day of discharge from the hospital. All plasma samples were extracted from ethylenediaminetetraacetic acid (EDTA) tubes and centrifuged. After the first centrifugation for 10 min at 1600 g, the supernatants were carefully removed and transferred to a new tube follow by centrifugation again at 16,000 g for 10 min to remove residual blood cells. Plasma was then stored at −80 °C until further processing. All tissue and plasma samples were collected with approval from all the patients and the ethics committee of the Clinic of the Bashkir State Medical University. Clinical characteristics of the patients with PA are summarized in Table 2.
Fig. 1.
(A) PA grew toward the suprasellar region with invasion into the third ventricle (Hardy–Wilson classification grade IV). (B) PA invaded the left cavernous sinus to surround the cavernous segment of the carotid artery (Knosp classification grades IV). PA without invasive (C, D).
Table 2.
Characteristics of PA patients (n = 30).
| Characteristics | Patients (n = 30) |
|---|---|
| Age (years) | |
| <50 | 12 |
| ≤50 | 18 |
| Median | 47 |
| Gender | |
| Male | 9 |
| Female | 21 |
| Biological behavior | |
| Invasive Non-invasive |
15 15 |
| Temperature, °C | 36.5 ± 0.2 |
| Tumor size (cm) | |
| <5 | 9 |
| ≥5 | 21 |
| Classification | |
| Hardy–Wilson grade I-II | 15 |
| Hardy–Wilson grade III-IV | 15 |
| Knosp grade I-II | 15 |
| Knosp grade III-IV | 15 |
Abbreviation: PA, Pituitary adenoma.
2.2. Total RNA extraction and complementary DNA (cDNA) synthesis
PA tissue was stored in liquid nitrogen immediately after harvesting. Total RNA was extracted from frozen tumor tissue and 200 μL plasma samples (for lncRNA ANRIL) using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. In addition, plasma total RNA (for miR-200a) was isolated from 200 μL plasma samples using the miRNeasy Serum/Plasma Kit (Qiagen) according to the manufacturer's instructions. RNA purity and concentration were determined using a Nano-Drop 2000 (Thermo Scientific) and consistently yielded A260:A280 and A260:A230 ratios close to 2.0. All isolated total RNA was stored at a −80 °C freezer until use. Reverse transcription of the extracted total RNA into cDNA was performed using the Transcriptor First Stand cDNA Synthesis Kit (Roche).
2.3. Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was performed using an ABI 7500 Real-Time PCR machine (Applied Biosystems) and the FastStart Universal SYBR Green Master (Rox) (Roche) according to the manufacturer's instructions. The volume of reaction was 20 μL. U6 for miR-200a and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for lncRNA ANRIL was used as an internal control. The sequence of all primers used in this study is provided in Table 3. Primers were obtained from the Invitrogen. The thermal cycling conditions were 95 °C for 10 min followed by 40 cycles of 95 °C for 20 s, 55 °C for 20 s and 72 °C for 20 s. All qRT-PCR analyses were performed in triplicate. The cycle threshold (CT) value greater than 35 was considered as below the detection threshold of the array.
Table 3.
Sequence of all primers.
| miRNA/LncRNA/Internal control | Primer Sequence (5′-3′) |
|---|---|
| miR-200a | RT: CATCTTACCGGACAGTGCTGGAF: TAACACTGTCTGGTAACGATGTR: ATCGTTACCAGACAGTGTTATT |
| LncRNA ANRIL | RT: TTT TTT TTT TTT TTT TTTF: TGCTCTATCCGCCAATCAGGR: GGGCCTCAGTGGCACATACC |
| U6 | RT: CGCTTCACGAATTTGCGTGTCABF: GCTTCGGCAGCACATATACTAAAATR: CGCTTCACGAATTTGCGTGTCAT |
| GAPDH | RT: TTT TTT TTT TTT TTT TTTF: GTCAAGGCTGAGAACGGGAAR: AAATGAGCCCCAGCCTTCTC |
Abbreviation: LncRNA, Long non-coding ribonucleic acid; miRNA, microRNA; LncRNA ANRIL, Long non-coding RNA antisense non-coding RNA in the INK4 locus; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
2.4. Statistical analyses
Relative levels of the miR-200a and lncRNA ANRIL were quantified using the 2-ΔΔCq method. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were used to estimate the diagnostic power and accuracy, respectively, of circulating miR-200a and lncRNA ANRIL. The Student t-test, ANOVA, chi-square analysis, or Mann-Whitney test was applied, where appropriate. A probability of P < 0.05 and p < 0.001 was considered to indicate a statistically significant difference. The statistical analyses were carried out with the IBM SPSS 22.0 software and the graphs were generated by using Graph-PadPrism 7.0.
3. Results
MiR-200a and lncRNA ANRIL expression levels in invasive and non-invasive PAs tissue.
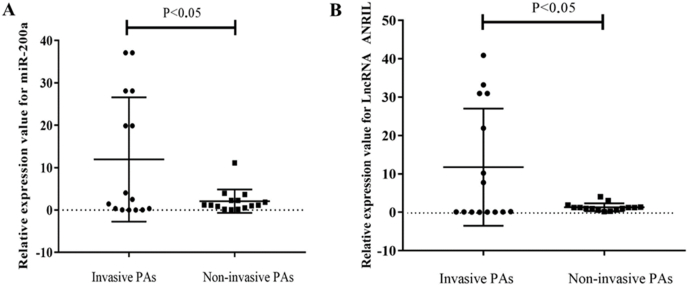
To verify the expression profiles of miR-200a and lncRNA ANRIL in tumor tissue of PA patients, we detected the expression levels of miR-200a and lncRNA ANRIL in 15 patients with in invasive and 15 patients with non-invasive PAs using qRT-PCR. The results of qRT-PCR assay in Fig. 2 show that the expression level of miR-200a (A) and lncRNA ANRIL (B) in tumor tissue of patients with invasive PA was significantly higher than that in non-invasive PA tissue (P < 0.05), and the difference was statistically significant. Our results indicated that increased miR-200a and lncRNA ANRIL levels might play a role in invasive of PA.
Fig. 2.
Determination of miR-200a and lncRNA ANRIL expression levels in invasive and non-invasive PAs tissue by qRT-PCR. The expression level of miR-200a (A) and lncRNA ANRIL (B) in tumor tissue of patients with invasive PA was significantly higher than that in non-invasive PA tissue.
3.1. Expressions of circulating miR-200a and lncRNA ANRIL in invasive and non-invasive PAs
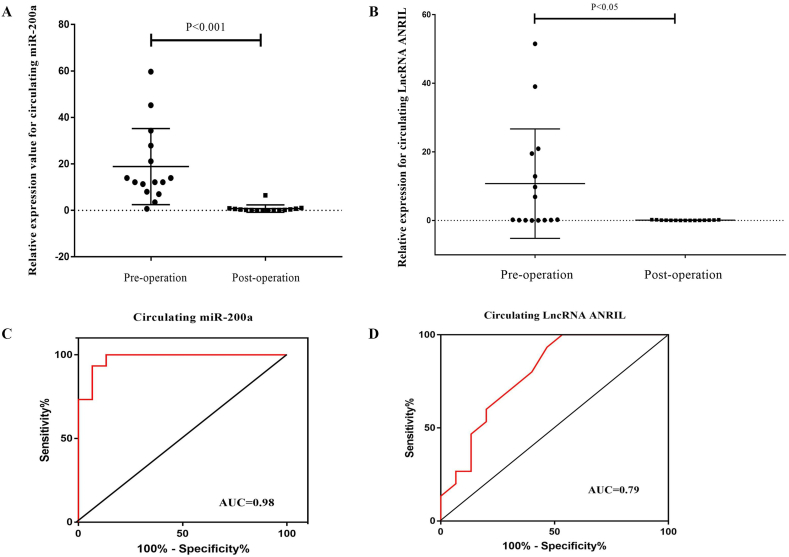
As shown in Fig. 3, the expression level of miR-200a and lncRNA ANRIL in patients with invasive PA was significantly higher than that in patients with non-invasive PA before the operation and the difference was statistically significant (P < 0.001, P < 0.05; Fig. 3A and B). However, the results regarding miR-200a and lncRNA ANRIL expression in plasma from the patients with invasive PA after the operation revealed that the expression level of circulating miR-200a and lncRNA ANRIL was significantly lower than that in plasma of patients with non-invasive PA before the operation (P < 0.05, P < 0.001; Fig. 3C and D). In addition, results showed that expression levels of circulating miR-200a and lncRNA ANRIL were lower in plasma of patients with invasive PA after operation compared to the same patients with invasive PA before operation (P < 0.001, P < 0.05; Fig. 4A, Fig. 4B).
Fig. 3.
Circulating miR-200a and lncRNA ANRIL expression levels in the plasma of patients with invasive and non-invasive PAs. The expression level of miR-200a (A) and lncRNA ANRIL (B) in patients with invasive PA was significantly higher than that in patients with non-invasive PA before operation. The expression level of miR-200a (C) and lncRNA ANRIL (D) in patients with invasive PA after operation was significantly lower than that in patients with non-invasive PA before operation.
Fig. 4.
The expression levels and ROC curves analysis for circulating miR-200a and lncRNA ANRIL in the plasma of patients with invasive PAs between pre- and post-operation period. The expression level of miR-200a (A) and lncRNA ANRIL (B) in patients with invasive PA after operation was significantly lower than that in patients with invasive PA before operation. The AUCs for miR-200a and lncRNA ANRIL were 0.98 (C) and 0.79 (D), respectively suggesting that miR-200a and lncRNA ANRIL can distinguish patients with invasive PAs between pre- and post-operation period. Note: AUC ≥0, 75 is considered diagnostically significant for the biomarker.
3.2. Diagnostic values of circulating miR-200a and lncRNA ANRIL for invasive PA
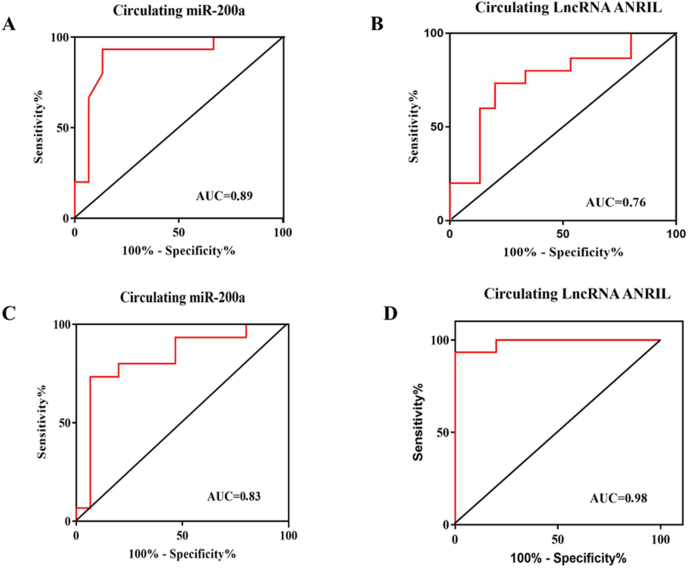
ROC curve analysis were performed to determine the diagnostic sensitivity and specificity of circulating miR-200a and lncRNA ANRIL in plasma in the pre- (Fig. 5A, Fig. 5B) and post-operation (Fig. 5C and D) period. The AUCs for miR-200a were 0.89 (95% CI: 0.76–1.00) and 0.83 (95% CI: 0.66–0.98), for lncRNA ANRIL were 0.76 (95% CI: 0.57–0.93) and 0.98 (95% CI: 0.95–1.00), respectively, suggesting that miR-200a and lncRNA ANRIL, could differentiate patients with invasive PA from patients with non-invasive PA. In additional, ROC curve analysis for miR-200a and lncRNA ANRIL revealed the AUC of 0.98 (95% confidence interval (95% CI) 0, 63–0, 96) and 0.79 (95% confidence interval (95% CI): 0.68–0.83), demonstrated that these circulating non-coding RNAs displayed considerable accuracy in discriminating plasma of patients with invasive PA in the pre-operation period from post-operation period (Fig. 4C and D).
Fig. 5.
ROC curves for circulating miR-200a and lncRNA ANRIL between invasive and non-invasive PA in the pre- (A, B) and post-operation (C, D) period. The AUCs for miR-200a and lncRNA ANRIL were 0.89 (A), 0.76 (B), 0.83 (C) and 0.98 (D), respectively suggesting that miR-200a and lncRNA ANRIL can distinguish invasive PA from non-invasive PA. Note: AUC ≥0, 75 is considered diagnostically significant for the biomarker.
4. Discussion
The aim of this study was to investigate the expression pattern and correlation of miR-200a and lncRNA ANRIL between tumor tissue and plasma in patients with invasive and non-invasive PA. For miR-200a and lncRNA ANRIL, significant correlations between tissue expression and plasma levels were observed in both cohorts. To our knowledge, this is the first comprehensive study on the expression levels of miR-200a and lncRNA ANRIL in PA patients. In additional, our aim was based on checking the change in expression of miR-200 b/c and lncRNA growth arrest-specific transcript 5 (GAS5). However, our current study did not find statistical significant miR-200 b/c and lncRNA GAS5 expression in tumor tissue and plasma of patients with PA. Numerous studies have indicated that members of the miR-200 family (miR-200a, miR-200 b, miR-200c, miR-141, and miR-429) and lncRNA GAS5 play an important role in tumor development and progression. Particularly, miR-200 b and miR-200c can regulate the proliferation, invasion, and migration of tumor cells by inhibiting the transcription of downstream genes (including Rho family GTPase 3 (RhoE), zinc finger E-box-binding homeobox (ZEB) 1, E-cadherin, IKKβ (inhibitor of nuclear factor kappa-B kinase β) control PAI-1 (plasminogen activator inhibitor-1)) [[21], [22], [23], [24]]. Meanwhile, lncRNA GAS5 can bind to miR-200c-3p to promote the apoptosis of A549 cells, thus to promote the progression of acute respiratory distress syndrome (ARDS) [25]. In the future, studies that are more detailed are needed to delineate the underlying mechanisms by which miR-200c and lncRNA GAS5 affect tumor development and progression. Besides, the expression of the lncRNA GAS5 can affect the mechanism of chemotherapy resistance [26]. For instance, Long et al. confirmed the low-expression of lncRNA GAS5 in epithelial ovarian cancer, ascertained its tumor suppressor gene-like role, and discovered its sensitization function of cisplatin (DDP) in epithelial ovarian cancer. Moreover, for the first time, the authors explored the specific mechanisms of epithelial ovarian cancer DDP resistance and tumor progress due to the lncRNA GAS5-E2F4-PARP1-MAPK axis [27]. In addition, in a recent study, Li et al. demonstrated that lncRNA GAS5 can promote the EMT process and invasion-metastasis cascade of breast cancer cells by competitive binding to miR-216 b [28].
Several studies have shown miR-200a as an oncogene, whose deficiency contributes to tumor formation and development [[11], [12], [13]]. Numerous studies have demonstrated the potential role of miR-200a in ovarian cancer. For instance, Shi et al. demonstrated that miR-200a was upregulated in ovarian cancer and significantly associated with the advanced progression of ovarian cancer patients. Overexpressed miR-200a-3p promoted the proliferation of ovarian cancer cells via targeting protocadherin 9 (PCDH9) [29]. In another study, Zhu et al., provide evidence that miR-200a is upregulated in a significant proportion of advanced ovarian carcinomas, and that elevated miR-200a expression facilitates tumor progression such as tumor cell proliferation and invasion [30].
Matrix metalloproteinases (MMPs) play important roles in tumor invasion primarily via extracellular matrix (ECM) degradation and/or the activation of pre-pro-growth factors. Recent studies have demonstrated that miR-200a participate in MMP regulation at the posttranscriptional level and ultimately influence the translation and expression of MMP genes. For instance, Yang et al. demonstrated that miR-200a was overexpressed in bladder cancer cells and promoted tumor invasion, at least in part, by causing an upregulation of the matrix metalloproteinase-2 (MMP-2) expression [31]. Using the Ago-HITS-CLIP technology for transcriptome-wide identification of direct miRNA targets Bracken et al. identified a number of transcription factors, some of which have been previously identified either as miR-200 family targets or have been implicated in epithelial–mesenchymal transition (EMT), invasion, and cancer progression, including matrix metalloproteinases-2, 9, 14 (MMP-2,9, 14) activity [32]. This evidence clearly suggests the role of miR-200a expression in tumor aggressiveness. In the present study, we found that miR-200a is significantly upregulated in invasive PA tissues. This result indicated that miR-200a is possibly involved in the pathogenesis of invasive PA and possibly through the regulation of certain genes like MMP and phosphatase and tensin homolog deleted on chromosome 10 (PTEN), but this requires further research.
Another ncRNA, whose participation in different types of human tumors has been well-established in numerous studies is lncRNA ANRIL [33,34]. In this study, we found that the expression of lncRNA ANRIL also was significantly increased in invasive PA tissues. Consistently, Zhao et al. introduced lncRNA ANRIL as an oncogene and demonstrated that lncRNA ANRIL was highly expressed in thyroid cancer, and siRNA-mediated lncRNA ANRIL silencing in two pore segment channel 1 (TPC1) and SW579 cells inhibited proliferation, invasion, and metastasis via the transforming growth factor beta (TGF-β)/Smad pathway [32]. Likewise, the results of another study demonstrated that knockdown of lncRNA ANRIL inhibited proliferation, migration, invasion, and promoted apoptosis in HepG2 cells through inactivation of nuclear factor kappa B (NF-κB) and Wnt/β-catenin signaling pathways by downregulation of miR-191 [34]. These results collectively suggest that lncRNA ANRIL is a typical oncogene.
In addition, we demonstrated that circulating miR-200a and lncRNA ANRIL in plasma were increased in invasive PA patients before operation. However, the expression level of plasma circulating miR-200a and lncRNA ANRIL was decreased in patients with invasive PA after operation. ROC curve analyses for circulating miR-200a and lncRNA ANRIL revealed AUC of 0.89, 0.76, 0.83, 0.98, 0.98, and 0.79, demonstrated that circulating miR-200a and lncRNA ANRIL displayed considerable accuracy in discriminating plasma of invasive PA patients before operation from the plasma of non-invasive PA patients and the same patients with invasive PA after an operation. As already said before miRNAs and lncRNAs may contribute actively to tumor development and progression, making circulating miRNAs and lncRNAs potential non-invasive biomarkers for tumor prevention, diagnosis, and prognosis. Invasive PA is mainly diagnosed through neuroimaging techniques (MRI or computer tomography (CT)) and tissue biopsy, but they all have limitations [2]. For instance, neuroimaging techniques can only detect established tumors with sufficient mass. Currently, the vast majority of surgical interventions, including biopsy, on the pituitary gland are carried out through the endoscopic endonasal transsphenoidal approach in its various modifications. Over the past decades, there has been a sharp decrease in the number of complications when performing such interventions. However, even with the most modern technologies, it is not always possible to avoid both mild and life-threatening complications. One of the most dangerous is intraoperative bleeding in the tumor bed [35,36]. In addition, repeated surgery and sampling in order to define the real molecular profile of the tumor progression is not always possible. Liquid biopsy is a real-time sampling of biomarkers from biofluids such as ncRNAs [37,38]. Liquid biopsy appears as a promising approach to non-invasive detection, molecularly characterized, and monitoring central nervous system (CNS) tumors progression and invasive PAs in particular. Early detection of tumors plays an important role in reducing mortality and in some cases when it comes to detecting pre-cancer and morbidity from malignant tumors. In this regard, the development of new screening methods, including the detection of molecular biomarkers of early stages of tumorigenesis, is of great importance. In this scenario, it is reasonable to assume that tumor progression will increase the expression level of certain oncogenic circulating ncRNAs, for example, in the bloodstream, in patients with invasive PA [37,38]. As a result, circulating miR-200a and lncRNA ANRIL are becoming candidates of emerging non-invasive biomarkers for invasive PA.
LncRNAs and miRNAs are novel and promising targets to be developed into biomarkers for diagnosis and prognosis as well as treatment options. The interaction between lncRNAs and miRNAs as well as its pathophysiological significance in PAs have recently been reported. For instance, small nucleolar RNA host gene 6 (SNHG6)/miR-944 axis plays a crucial role in regulating proliferation, migration, invasion, and EMT of PA by regulating RAB11A [39]. In another study, Tang et al. showed that AFAP1-AS1 acts as a competing RNA of miR-103a-3p to regulate PA growth via the phosphoinositide 3-kinases (PI3Ks)/RAC-alpha serine/threonine-protein kinase (AKT) signal pathway [40]. The current state of knowledge showed that miRNAs and lncRNAs are not only individually important in the regulation of tumorigenesis but that their cross communication represents a new aspect of tumor pathogenesis and progression. There may also be indirect interaction between miR-200a and lncRNA ANRIL in PA. However, further studies are needed to clarify the exact interaction between miR-200a and lncRNA ANRIL in PA.
The present study has a number of limitations. First, the possible clinical implications of miR-200a and lncRNA ANRIL pattern in tissue and plasma in the detection, diagnosis, and prognosis of invasive PA patients remain to be elucidated using a larger number of patient samples. Second, we tested the change of miR-200a and lncRNA ANRIL in tumor tissue between invasive and non-invasive PA, we did not compare expression with normal PA tissue and unable to evaluate the potential role of these ncRNAs in the development of non-invasive PAs. Unfortunately, in the context of the Covid-19 pandemic, collecting samples was difficult. Besides, it is worth noticing that exists a possible correlation between tumor size, with other markers like p53 and Ki-67, treatment, the potential associations between these circulating ncRNAs profiles, and the gene expression profiles and hormones. If the results are further confirmed the relationship between miR-200a and lncRNA ANRIL levels and protein levels as MMP and PTEN in PA, there is translational clinical potential in the cases presented. Namely, miR-200a and lncRNA ANRIL can serve as potential therapeutic targets of PA; however, further research is needed. All of this is undoubtedly the goal of our future research. In addition, the functions and mechanisms of miR-200a and lncRNA ANRIL in PA require further investigation.
5. Conclusions
This study reveals that the expression levels of miR-200a and lncRNA ANRIL in tumor tissue and plasma of patients with invasive PA were increased. Moreover, the expression of circulating miRNA-200a and lncRNA ANRIL in plasma was reduced after the operation in patients with invasive PA compared with patients non-invasive PA before the operation. In other words, increased expression of miR-200a and lncRNA ANRIL was associated with PA development and invasion. The results in this study suggest that miR-200a and lncRNA ANRIL may become a new target for the treatment and as non-invasive biomarkers of invasive PA.
Funding
The reported study was funded by RFBR and NSFC, project number 21-515-53017.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Ethical approval
All tissue and plasma samples were collected with approval from all the patients and the ethics committee of Clinic of the Bashkir State Medical University (2021.17.02, protocol number 17).
CRediT authorship contribution statement
Ozal Beylerli: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Dinar Khasanov: Data curation, Formal analysis. Ilgiz Gareev: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization. Elvir Valitov: Formal analysis. Andrei Sokhatskii: Formal analysis. Chunlei Wang: Formal analysis. Valentin Pavlov: Formal analysis. Guzel Khasanova: Formal analysis. Aamir Ahmad: Conceptualization, Resources, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that no conflicts of interest exist.
References
- 1.Lithgow K., Batra R., Matthews T., Karavitaki N. Management OF endocrine disease: visual morbidity in patients with pituitary adenoma. Eur. J. Endocrinol. 2019;181:R185–R197. doi: 10.1530/EJE-19-0349. PMID: 31416048] [DOI] [PubMed] [Google Scholar]
- 2.Serioli S., Doglietto F., Fiorindi A., Biroli A., Mattavelli D., Buffoli B., Ferrari M., Cornali C., Rodella L., Maroldi R., Gasparotti R., Nicolai P., Fontanella M.M., Poliani P.L. Pituitary adenomas and invasiveness from anatomo-surgical, radiological, and histological perspectives: a systematic literature review. Cancers. 2019;11:1936. doi: 10.3390/cancers11121936. PMID: 31817110] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu T.X., Rothenberg M.E. MicroRNA. J. Allergy Clin. Immunol. 2018;141:1202–1207. doi: 10.1016/j.jaci.2017.08.034. PMID: 29074454] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., Wang W., Zhu W., Dong J., Cheng Y., Yin Z., Shen F. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int. J. Mol. Sci. 2019;20:5573. doi: 10.3390/ijms20225573. PMID: 31717266] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vishnubalaji R., Shaath H., Elango R., Alajez N.M. Noncoding RNAs as potential mediators of resistance to cancer immunotherapy. Semin. Canc. Biol. 2020;65:65–79. doi: 10.1016/j.semcancer.2019.11.006. PMID: 31733291] [DOI] [PubMed] [Google Scholar]
- 6.Wu Z.R., Yan L., Liu Y.T., Cao L., Guo Y.H., Zhang Y., Yao H., Cai L., Shang H.B., Rui W.W., Yang G., Zhang X.B., Tang H., Wang Y., Huang J.Y., Wei Y.X., Zhao W.G., Su B., Wu Z.B. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat. Commun. 2018;9:4624. doi: 10.1038/s41467-018-06853-3. PMID: 30397197] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu D., Zhang Y., Cui H. Long noncoding RNA CCAT2 is activated by E2F1 and exerts oncogenic properties by interacting with PTTG1 in pituitary adenomas. Am J Cancer Res. 2018;8:245–255. [PMID: 29511595] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Z., Zhang Y., Zhang Z., Yang Y., Song T. Effect of miR-106b on invasiveness of pituitary adenoma via PTEN-PI3K/AKT. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2017;23:1277–1285. doi: 10.12659/msm.900092. PMID: 28288092] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renjie W., Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox 5. Canc. Lett. 2015;356(2 Pt B):568–578. doi: 10.1016/j.canlet.2014.10.003. PMID: 25305447] [DOI] [PubMed] [Google Scholar]
- 10.He H., Zhang H., Li Z., Wang R., Li N., Zhu L. miRNA-214: expression, therapeutic and diagnostic potential in cancer. Tumori. 2015;101:375–383. doi: 10.5301/tj.5000318. PMID: 26108246] [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Sun J., Cai Y., Jiang Y., Wang X., Huang X., Yin Y., Li H. MiR-200a acts as an oncogene in colorectal carcinoma by targeting PTEN. Exp. Mol. Pathol. 2016;101:308–313. doi: 10.1016/j.yexmp.2016.10.006. PMID: 27983967] [DOI] [PubMed] [Google Scholar]
- 12.Zou Q., Zhou E., Xu F., Zhang D., Yi W., Yao J. A TP73-AS1/miR-200a/ZEB1 regulating loop promotes breast cancer cell invasion and migration. J. Cell. Biochem. 2018;119:2189–2199. doi: 10.1002/jcb.26380. PMID: 28857253] [DOI] [PubMed] [Google Scholar]
- 13.Suo H.B., Zhang K.C., Zhao J. MiR-200a promotes cell invasion and migration of ovarian carcinoma by targeting PTEN. Eur. Rev. Med. Pharmacol. Sci. 2018;22:4080–4089. doi: 10.26355/eurrev_201807_15398. PMID: 30024595] [DOI] [PubMed] [Google Scholar]
- 14.Ma Y., Zhang H., Li G., Hu J., Liu X., Lin L. LncRNA ANRIL promotes cell growth, migration and invasion of hepatocellular carcinoma cells via sponging miR-144. Anti Canc. Drugs. 2019;30:1013–1021. doi: 10.1097/CAD.0000000000000807. PMID: 31609763] [DOI] [Google Scholar]
- 15.Deng W., Zhang Y., Cai J., Zhang J., Liu X., Yin J., Bai Z., Yao H., Zhang Z. LncRNA-ANRIL promotes gastric cancer progression by enhancing NF-kB signaling. Exp. Biol. Med. 2019;244:953–959. doi: 10.1177/1535370219860207. PMID: 31242038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J.H., Tang J.M., Li J., Li X.W. Upregulation of SOX2-activated lncRNA ANRIL promotes nasopharyngeal carcinoma cell growth. Sci. Rep. 2018;8:3333. doi: 10.1038/s41598-018-21708-z. PMID: 29463902] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anfossi S., Babayan A., Pantel K., Calin G.A. Clinical utility of circulating non-coding RNAs - an update. Nat. Rev. Clin. Oncol. 2018;15:541–563. doi: 10.1038/s41571-018-0035-x. PMID: 29784926] [DOI] [PubMed] [Google Scholar]
- 18.Németh K., Darvasi O., Likó I., Szücs N., Czirják S., Reiniger L., Szabó B., Krokker L., Pállinger É., Igaz P., Patócs A., Butz H. Comprehensive analysis of circulating microRNAs in plasma of patients with pituitary adenomas. J. Clin. Endocrinol. Metab. 2019 doi: 10.1210/jc.2018-02479. jc.2018-02479. [DOI: 10.1210/jc.2018-02479. PMID: 31112271] [DOI] [PubMed] [Google Scholar]
- 19.Wu Z.R., Yan L., Liu Y.T., Cao L., Guo Y.H., Zhang Y., Yao H., Cai L., Shang H.B., Rui W.W., Yang G., Zhang X.B., Tang H., Wang Y., Huang J.Y., Wei Y.X., Zhao W.G., Su B., Wu Z.B. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat. Commun. 2018;9:4624. doi: 10.1038/s41467-018-06853-3. PMID: 30397197] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gareev I., Yang G., Sun J., Beylerli O., Chen X., Zhang D., Zhao B., Zhang R., Sun Z., Yang Q., Li L., Pavlov V., Safin S., Zhao S. Circulating MicroRNAs as potential noninvasive biomarkers of spontaneous intracerebral hemorrhage. World Neurosurg. 2020;133:e369–e375. doi: 10.1016/j.wneu.2019.09.016. PMID: 31525485. [DOI] [PubMed] [Google Scholar]
- 21.Tang Q., Li M., Chen L., Bi F., Xia H. miR-200b/c targets the expression of RhoE and inhibits the proliferation and invasion of non-small cell lung cancer cells. Int. J. Oncol. 2018;53(4):1732–1742. doi: 10.3892/ijo.2018.4493. [DOI] [PubMed] [Google Scholar]
- 22.Arunkumar G., Deva Magendhra Rao A.K., Manikandan M., Prasanna Srinivasa Rao H., Subbiah S., Ilangovan R., Murugan A.K., Munirajan A.K. Dysregulation of miR-200 family microRNAs and epithelial-mesenchymal transition markers in oral squamous cell carcinoma. Oncol Lett. 2018;15(1):649–657. doi: 10.3892/ol.2017.7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurteau G.J., Carlson J.A., Roos E., Brock G.J. Stable expression of miR-200c alone is sufficient to regulate TCF8 (ZEB1) and restore E-cadherin expression. Cell Cycle. 2009;8(13):2064–2069. doi: 10.4161/cc.8.13.8883. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y., Yang D., Xi L., Chen Y., Fu L., Sun K., Yin J., Li X., Liu S., Qin Y., Liu M., Hou Y. Primed atypical ductal hyperplasia-associated fibroblasts promote cell growth and polarity changes of transformed epithelium-like breast cancer MCF-7 cells via miR-200b/c-IKKbeta signaling. Cell Death Dis. 2018;9(2):122. doi: 10.1038/s41419-017-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H.B., Zi P.P., Shi H.J., Gao M., Sun Q.R. Role of signaling pathway of long non-coding RNA growth arrest-specific transcript 5/microRNA-200c-3p/angiotensin converting enzyme 2 in the apoptosis of human lung epithelial cell A549 in acute respiratory distress syndrome. Zhonghua Yixue Zazhi. 2018;98(41):3354–3359. doi: 10.3760/cma.j.issn.0376-2491.2018.41.013. [DOI] [PubMed] [Google Scholar]
- 26.Lambrou G.I., Hatziagapiou K., Zaravinos A. The non-coding RNA GAS5 and its role in tumor therapy-induced resistance. Int. J. Mol. Sci. 2020;21(20):7633. doi: 10.3390/ijms21207633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long X., Song K., Hu H., Tian Q., Wang W., Dong Q., Yin X., Di W. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J. Exp. Clin. Canc. Res. 2019;38(1):345. doi: 10.1186/s13046-019-1329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Guo X.B., Wei Y.H. LncRNA GAS5 affects epithelial-mesenchymal transition and invasion of breast cancer cells by regulating miR-216b. Eur. Rev. Med. Pharmacol. Sci. 2020;24(9):4873–4881. doi: 10.26355/eurrev_202005_21176. [DOI] [PubMed] [Google Scholar]
- 29.Shi C., Yang Y., Zhang L., Yu J., Qin S., Xu H., Gao Y. MiR-200a-3p promoted the malignant behaviors of ovarian cancer cells through regulating PCDH9. OncoTargets Ther. 2019;12:8329–8338. doi: 10.2147/OTT.S220339. PMID: 31632082] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu C.L., Gao G.S. miR-200a overexpression in advanced ovarian carcinomas as a prognostic indicator. Asian Pac. J. Cancer Prev. APJCP. 2014;15:8595–8601. doi: 10.7314/apjcp.2014.15.20.8595. PMID: 25374174] [DOI] [PubMed] [Google Scholar]
- 31.Yang R., Xu J., Hua X., Tian Z., Xie Q., Li J., Jiang G., Cohen M., Sun H., Huang C. Overexpressed miR-200a promotes bladder cancer invasion through direct regulating Dicer/miR-16/JNK2/MMP-2 axis. Oncogene. 2020;39:1983–1996. doi: 10.1038/s41388-019-1120-z. PMID: 31772330] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bracken C.P., Li X., Wright J.A., Lawrence D.M., Pillman K.A., Salmanidis M., Anderson M.A., Dredge B.K., Gregory P.A., Tsykin A., Neilsen C., Thomson D.W., Bert A.G., Leerberg J.M., Yap A.S., Jensen K.B., Khew-Goodall Y., Goodall G.J. Genome-wide identification of miR-200 targets reveals a regulatory network controlling cell invasion. EMBO J. 2014;33:2040–2056. doi: 10.15252/embj.201488641. PMID: 25069772] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J.J., Hao S., Wang L.L., Hu C.Y., Zhang S., Guo L.J., Zhang G., Gao B., Jiang Y., Tian W.G., Luo D.L. Long non-coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF-β/Smad signaling pathway. Oncotarget. 2016;7:57903–57918. doi: 10.18632/oncotarget.11087. PMID: 27507052] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang D., Bi C., Zhao Q., Ding X., Bian C., Wang H., Wang T., Liu H. Knockdown long non-coding RNA ANRIL inhibits proliferation, migration and invasion of HepG2 cells by down-regulation of miR-191. BMC Canc. 2018;18:919. doi: 10.1186/s12885-018-4831-6. PMID: 30249208] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Dallapiazza R.F., Grober Y., Starke R.M., Laws E.R., Jr., Jane J.A., Jr. Long-tern results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery. 2015;76(1):42–52. doi: 10.1227/NEU.0000000000000563. PMID: 25255271] [DOI] [PubMed] [Google Scholar]
- 36.Koutourousiou M., Gardner P.A., Fernandez-Miranda J.C., Paluzzi A., Wang E.W., Snyderman C.H. Giant pituitary adenomas: advantages and limitations of endoscopic endonasal surgery. J. Neurosurg. 2013;118:621–631. doi: 10.3171/2012.11.JNS121190. PMID: 23289816] [DOI] [PubMed] [Google Scholar]
- 37.Beylerli O., Gareev I., Pavlov V., Chen X., Zhao S. The role of long noncoding RNAs in the biology of pituitary adenomas. World Neurosurg. 2020;137:252–256. doi: 10.1016/j.wneu.2019.10.137. PMID: 31678448] [DOI] [PubMed] [Google Scholar]
- 38.Beylerli O., Beeraka N.M., Gareev I., Pavlov V., Yang G., Liang Y., Aliev G. MiRNAs as noninvasive biomarkers and therapeutic agents of pituitary adenomas. Int. J. Mol. Sci. 2020;21(19):7287. doi: 10.3390/ijms21197287. PMID: 33023145] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao D., Jie Y., Lv Y. LncRNA SNHG6 induces epithelial-mesenchymal transition of pituitary adenoma via suppressing MiR-944. Cancer Biother. Radiopharm. 2020 doi: 10.1089/cbr.2020.3587. PMID: 32935999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang H., Zhu D., Zhang G., Luo X., Xie W. AFAP1-AS1 promotes proliferation of pituitary adenoma cells through miR-103a-3p to activate PI3K/AKT signaling pathway. World Neurosurg. 2019;130:e888–e898. doi: 10.1016/j.wneu.2019.07.032. PMID: 31299308] [DOI] [PubMed] [Google Scholar]